INTRODUCTION
A three lipid-component parallel artificial membrane permeability assay (PAMPA) system was previously devised and evaluated (1). Denoted A-PAMPA for anionic-PAMPA, A-PAMPA was designed to mimic the lipid composition of the enterocyte's plasma membrane and included 1,2-dioleoyl-sn-glycero-3-[phospho-l-serine] (PS18:1) as an anionic lipid component. A-PAMPA also consists of 1,2-dioleoyl-sn-glycero-3-phosphocholine (PC18:1) and cholesterol. Metoprolol flux across A-PAMPA was measured, as well as across three other PAMPA systems (1). Results indicated that metoprolol transport across A-PAMPA was dominated by an ion-pair-mediated mechanism (i.e., metoprolol-PS18:1 complex).
The objective in the present study was to compare the performance of this three lipid-component PAMPA system to Caco-2 monolayers, in terms of screening for drug permeability values. Several development laboratories employ PAMPA membranes in candidate selection, in part because of other experimental approaches, such as Caco-2 and MDCK monolayers, are more labor-intensive and expensive (2–8). However, A-PAMPA has not been previously evaluated to identify drugs that are likely to exhibit high permeability in Caco-2 BCS testing.
MATERIALS AND METHODS
Materials
PC18:1 and PS18:1 were purchased from Avanti Polar Lipids (Alabaster, AL). Hydrophobic microfilter plates (polyvinylidene fluoride, 0.45 μm) were purchased from Millipore Corporation (Bedford, MA). Caco-2 cells were obtained from ATCC (Rockville, MD). Drug compounds were either USP- or commercial-grade.
Permeability Measurements
A-PAMPA preparation and permeability methods follow previous descriptions (1,5). Hydrophobic filters were impregnated with 5 μL of membrane solution consisting of lipid in n-dodecane (5% w/v), where lipid consisted of PC18:1 (2.6%w/v), PS18:1 (0.9%w/v), and cholesterol (1.5%w/v). Permeability was calculated as described previously, using Eq. 3 in Palm et al. (7). Mass balance ranged from 85–100%.
Caco-2 studies followed the cell culture and permeability methods previously described (9,10). Permeability was calculated as described previously (9,10). Mass balance ranged between 80–100%.
RESULTS AND DISCUSSION
Comparison of Drug Permeabilities
Permeability across A-PAMPA and Caco-2 are given at Table I. Figure 1 plots Caco-2 compound permeability versus A-PAMPA compound permeability. There was generally good agreement between permeability values from the two methods (linear r2 = 0.82, excluding coumarin and naproxen, which provided the highest permeabilities in both assays). Results here for A-PAMPA follow previous comparisons of PAMPA permeabilities to Caco-2 permeabilities (2,3,11–16). As suggestive of Fig. 1 and noted previously (8), A-PAMPA permeability values were generally several-fold lower than Caco-2 permeability values.
Table I.
A-PAMPA and Caco-2 Permeability, Using Metoprolol Tartrate as a Reference Compound
| Compound | A-PAMPA permeability (±SEM) ×106 [cm/s] | Caco-2 permeability (±SEM) ×106 [cm/s] | BCS classification according to A-PAMPA | BCS classification according to Caco-2 | Human percent dose absorbed (literature reference) |
|---|---|---|---|---|---|
| Acylovir | 0.0841 (±0.0019) | 1.24 (±0.24) | Low | Low | 20 (20,21) |
| Atenolol | 2.41 (±0.38) | 2.62 (±0.17) | High | Low | 50 (21,22) |
| Buproprion | 18.5 (±0.1) | 66.4 (±0.6) | High | High | 87 (23) |
| Carbamazepine | 27.4 (±0.3) | 95.7 (±5.1) | High | High | 97 (24) |
| Chlorpheniramine | 8.57 (±0.07) | 16.0 (±1.9) | High | Low | 80 (25) |
| Coumarin | 48.4 (±0.2) | 141 (±6) | High | High | 100 (26) |
| Disopyramide | 2.71 (±0.26) | 4.24 (±0.05) | High | Low | 85.3 (27,28) |
| Furosemide | 0.572 (±0.135) | 3.33 (±0.07) | Low | Low | 61 (21,29) |
| Hydrocholothiazide | 0.497 (±0.040) | 0.710 (±0.063) | Low | Low | 70 (30) |
| Indomethacin | 12.4 (±0.4) | 38.4 (±2.2) | High | Low | 100 (25,28) |
| Inulin | 0.360 (±0.006) | 0.921 (±0.101) | Low | Low | 0 (31) |
| Ketoprofen | 12.6 (±0.5) | 50.5 (±0.5) | High | High | 100 (23,30) |
| Mannitol | 0.018 (±0.025) | 2.81 (±0.16) | Low | Low | 16 (32,33) |
| Metoprolol | 1.53 (±0.05) | 40.0 (±1.4) | High | High | 95 (25) |
| Naproxen | 41.8 (±0.2) | 126 (±4) | High | High | 98 (30) |
| Piroxicam | 29.2 (±0.1) | 116 (±1) | High | High | 100 (8,24,34) |
| Propranolol | 4.29 (±0.13) | 49.5 (±1.2) | High | High | 100 (21,33) |
| Ranitidine | 0.672 (±0.087) | 0.405 (±0.031) | Low | Low | 52 (35) |
| Theophylline | 8.58 (±0.06) | 61.0 (±0.9) | High | High | 96 (36) |
| Verapamil | 9.40 (±0.09) | 32.9 (±1.0) | High | Low | 98 (30,37) |
Fig. 1.
Comparison between Caco-2 permeability and PAMPA permeability. The PAMPA membrane included and an anionic component, such that this system is denoted A-PAMPA. In general, high permeability in PAMPA corresponded with high permeability across Caco-2 monolayers
Corti et al. (16) showed a good correlation of PAMPA and Caco-2 permeabilities (r2 = 0.813), where PAMPA membrane employed Lipoid E80 (1.70%), n-octanol (96.2%), and cholesterol (2.10%). Lipoid E80 is fat-free egg lecithin and consists of largely (about 80%) phosphatidyl choline (17). A-PAMPA has some compositional similarity to the membranes in Corti et al. (16). However, since oleic acid is a modest fatty acid component in Lipoid E80 (about 28% of all fatty acid), the phosphatidyl choline in Lipoid E80 is largely not PC18:1. Lipoid E80 also lacks PS18:1, which was included in the present three lipid-component PAMPA system as an anionic lipid component and an important contributor to ion-pair-mediated drug transport (1).
Of the 21 compounds studied by Corti et al. and the 20 compounds studied here, there were 11 common compounds tested in both studies: acyclovir, atenolol, furosemide, hydrochlorotiazide, ketoprofen, metoprolol tartrate, naproxen, propranolol, ranitidine, theophylline, and verapamil. Among these drugs, naproxen showed the highest permeability here and in Corti et al. Likewise, acyclovir showed the lowest permeability. In general, permeability values from Corti et al. were several-fold larger than values from A-PAMPA here. Permeability values from A-PAMPA exhibited a wider range, which differed 500-fold between naproxen and acyclovir. Naproxen and acyclovir permeabilities in Corti et al. differed less than sixfold, although differences were easily detected.
PAMPA to Anticipate BCS Results
These results suggest that three lipid-component PAMPA system can be used to identify drugs that are likely to exhibit high permeability in Caco-2 BCS testing (18). Prior to subjecting a compound to BCS-based permeability assessment, it is typically desirable to know whether the compound is likely to show high permeability. Because a BCS-regulatory submission requires a validated permeability method, a more facile method, such as A-PAMPA, has potential role in rapidly assessing whether a drug will likely show high permeability across Caco-2 monolayers.
In order to assess the utility of A-PAMPA to anticipate Caco-2 BCS classification results, each compound was classified as either high or low permeability. Table II shows the concordance between A-PAMPA classification results and Caco-2 classification results, employing metoprolol as the lower limit of high permeability (18). Overall, six compounds exhibited lower permeability than metoprolol in A-PAMPA, with the remaining 14 (including metoprolol itself) showing high permeability. Of the 20 compounds, 15 were correctly predicted by A-PAMPA. Six compounds predicted by A-PAMPA to have low permeability showed low Caco-2 permeability. Nine compounds predicted by A-PAMPA to have high permeability (of 14 such compounds) showed high Caco-2 permeability. No compound was predicted by A-PAMPA to have low permeability but showed high Caco-2 permeability.
Table II.
Comparison of BCS Classification of A-PAMPA and Caco-2, Using Metoprolol Tartrate as a Reference Compound
| Caco-2 classification result | |||
|---|---|---|---|
| Low | High | ||
| A-PAMPA classification result | Low | 6 | 0 |
| High | 5 | 9 | |
Entries in table denote number of compounds (out of 20)
However, five compounds predicted by A-PAMPA to have high permeability showed low Caco-2 permeability and were atenolol, chlorphineramine, disopyramide, indomethacin, and verapamil. As noted in Table I, the human percent dose absorbed of atenolol, chlorphineramine, disopyramide, indomethacin, and verapamil are 50%, 80%, 85.3%, 100%, and 98%, respectively. Hence, the disconcordance between A-PAMPA and Caco-2 classifications for indomethacin and verapamil were Caco-2 classification error. Meanwhile, the disconcordance for atenolol is A-PAMPA classification error. The absorption of atenolol is low and incomplete (19). Likewise, but to a lesser extent given their fraction dose absorbed nears that of metoprolol, the disconcordance for chlorpheniramine and disopyramide was also A-PAMPA classification error.
From these five cases, A-PAMPA permeability “over-estimated” BCS permeability classification based on Caco-2 permeability. These results support the use of A-PAMPA to screen compounds for subsequent BCS classification, with the expectation that only compounds that are likely to exhibit high permeability are subjected to BCS Caco-2 assay. One possible explanation for the mis-specification of atenolol, chlorphineramine, and disopyramide by A-PAMPA may be possible ion pairing between these weakly basic drug and PS18:1. Such ion pairing increases the permeability of metoprolol (1), but may perhaps have an even greater effect on atenolol, chlorphineramine, and disopyramide.
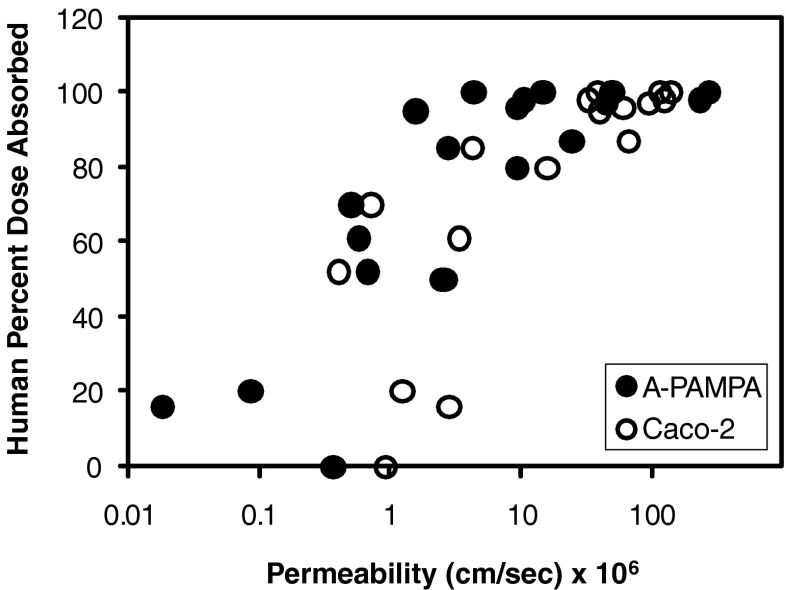
Figure 2 plots human percent dose absorbed versus A-PAMPA permeability for the 20 compounds in Table I. Corresponding Caco-2 results are also plotted. The A-PAMPA profile is generally pushed towards the left, reflecting the lower A-PAMPA permeability of each compound, compared to Caco-2 permeability.
Fig. 2.
Plots of human percent dose absorbed versus A-PAMPA permeability and Caco-2 permeability. For each of the 20 compounds, A-PAMPA permeability was generally less than corresponding Caco-2 permeability, typically by several-fold
Based on human-fraction-dose absorbed data, A-PAMPA assay correctly classified all ten high permeability drugs as high permeability. Among the ten low-permeability drugs, four (atenolol, buproprion, chlorpheniramine, and disopyramide) was incorrectly classified as high permeability. The Caco-2 assay incorrectly classified three drugs: buproprion, indomethacin, and verapamil.
CONCLUSIONS
A three lipid-component PAMPA system was previously devised to mimic the lipid composition of the enterocyte's plasma membrane. The objective was to compare A-PAMPA system to Caco-2 monolayers, in terms of screening for drug permeability values. Results show good agreement for 20 drugs between PAMPA permeabilities and Caco-2 permeabilities, including concordance between PAMPA BCS classification and Caco-2 BCS classification. There was a modest tendency for the PAMPA system to “over-estimate” BCS permeability classification, such that the system appears to have utility to anticipate compounds that are likely to exhibit high permeability in a BCS Caco-2 assay.
Acknowledgments
Z.S. Teksin was supported by NATO Science Fellowship Program by The Scientific and Technical Research Council of Turkey (TUBITAK) and Gazi University in Ankara, Turkey. This work was support in part by National Institutes of Health grant DK67530.
References
- 1.Teksin ZS, Hom K, Balakrishnan A, Polli JE. Apparent ion pair-mediated transport of metoprolol across a three lipid component PAMPA system. J Control Rel. 2006;116:50–57. doi: 10.1016/j.jconrel.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 2.Kerns EH, Li D, Petusky S, Farris M, Ley R, Jupp P. Combined application of parallel artificial membrane permeability assay and Caco-2 permeability assays in drug discovery. J Pharm Sci. 2004;93(6):1440–1453. doi: 10.1002/jps.20075. [DOI] [PubMed] [Google Scholar]
- 3.Avdeef A, Strafford M, Block E, Balogh MP, Chambliss W, Khan I. Drug absorption in vitro model: filter-immobilized artificial membranes. 2. Studies of the permeability properties of lactones in piper methysticum Forst. Eur J Pharm Sci. 2001;14:271–280. doi: 10.1016/S0928-0987(01)00191-9. [DOI] [PubMed] [Google Scholar]
- 4.Wohnsland F, Faller B. Permeability pH profile and high-throughput alkane/water log P artificial membranes. J Med Chem. 2001;44:923–930. doi: 10.1021/jm001020e. [DOI] [PubMed] [Google Scholar]
- 5.Seo PR, Teksin ZS, Kao JPY, Polli JE. Lipid composition effect on permeability across PAMPA. Eur J Pharm Sci. 2006;29:259–268. doi: 10.1016/j.ejps.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Sugano K, Nabuchi Y, Machida M, Asoh Y. Permeation characteristics of a hydrophilic basic compound across a bio-mimetic artificial membrane. Int J Pharm. 2004;275:271–278. doi: 10.1016/j.ijpharm.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Palm K, Luthman K, Ros J, Grasjo J, Artursson P. Effect of molecular charge on intestinal epithelial drug transport: pH-dependent transport of cationic drugs. J Pharmacol Exp Ther. 1999;291:435–443. [PubMed] [Google Scholar]
- 8.Hwang K, Martin NE, Jiang L, Zhu C. Permeation prediction of M100240 using the parallel artificial membrane permeability assay. J Pharm Sci. 2003;6(3):315–320. [PubMed] [Google Scholar]
- 9.Polli JE, Ginski MJ. Human drug absorption kinetics and comparison to Caco-2 monolayer permeabilities. Pharm Res. 1998;15:47–52. doi: 10.1023/A:1011992518592. [DOI] [PubMed] [Google Scholar]
- 10.Tolle-Sander S, Grill A, Joshi H, Kapil R, Persiani S, Polli JE. Characterization of dexloxiglumide in vitro biopharmaceutic properties and active transport. J Pharm Sci. 2003;92:1968–1980. doi: 10.1002/jps.10428. [DOI] [PubMed] [Google Scholar]
- 11.Bermejo M, Avdeef A, Ruiz A, Nalda R, Ruell JA, Tsinman O, Gonzalez I, Fernandez C, Sanchez G, Garrigues TM, Merino V. PAMPA-a drug absorption in vitro model 7. Comparing rat in situ, Caco-2, and PAMPA permeability of fluoroquinolones. Eur J Pharm Sci. 2004;21:429–441. doi: 10.1016/j.ejps.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Fujikawa M, Ano R, Nakao K, Shimizu R, Akamatsu M. Relationships between structure and high-throughput screening permeability of diverse drugs with artificial membranes: application to prediction of Caco-2 cell permeability. Bioorg Med Chem. 2005;13:4721–4732. doi: 10.1016/j.bmc.2005.04.076. [DOI] [PubMed] [Google Scholar]
- 13.Avdeef A, Artursson P, Neuhoff S, Lazorova L, Grasjö J, Tavelin S. Caco-2 permeability of weakly basic drugs predicted with the double-sink PAMPA pKa(flux) method. Eur J Pharm Sci. 2005;24:333–349. doi: 10.1016/j.ejps.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Masungi C, Mensch J, Van Dijck A, Borremans C, Willems B, Mackie C, Noppe M, Brewster M. Parallel artificial membrane permeability assay (PAMPA) combined with a 10-day multiscreen Caco-2 cell culture as a tool for assessing new drug candidates. Pharmazie. 2008;63:194–199. [PubMed] [Google Scholar]
- 15.Koljonen M, Rousu K, Cierny J, Kaukonen A, Hirvonen J. Transport evaluation of salicylic acid and structurally related compounds across Caco-2 cell monolayers and artificial PAMPA membranes. Eur J Pharm Biopharm. 2008;70:531–538. doi: 10.1016/j.ejpb.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 16.Corti G, Maestrelli F, Cirri M, Zerrouk N, Mura P. Development and evaluation of an in vitro method for prediction of human drug absorption II. Demonstration of the method suitability. Eur J Pharm Sci. 2006;27:354–362. doi: 10.1016/j.ejps.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Lipoid E 80 product brochure. Lipoid GmbH, Ludwigshafen. 2006.
- 18.Guidance for Industry, Waiver of In Vivo Bioavailability and Bioequivalence Studies for Immediate-Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System. August 2000, CDER/FDA. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070246.pdf (accessed 1/12/2010).
- 19.Vogelpoel H, Welink J, Amidon GL, Junginger HE, Midha KK, Moller H, Olling M, Shah VP, Barends DM. Biowaiver monographs for immediate release solid oral dosage forms based on biopharmaceutics classification system (BCS) literature data: verapamil hydrochloride, propranolol hydrochloride, and atenolol. J Pharm Sci. 2004;93:1945–1956. doi: 10.1002/jps.20131. [DOI] [PubMed] [Google Scholar]
- 20.de Miranda P, Blum MR. Pharmacokinetics of acyclovir after intravenous and oral administration. J Antimicrob Chemother. 1983;12(Suppl. B):29–37. doi: 10.1093/jac/12.suppl_b.29. [DOI] [PubMed] [Google Scholar]
- 21.Chiou WL, Jeong HY, Chung SM, Wu TC. Evaluation of using dog as an animal model to study the fraction of oral dose absorbed of 43 drugs in humans. Pharm Res. 2000;17(2):135–40. doi: 10.1023/A:1007552927404. [DOI] [PubMed] [Google Scholar]
- 22.Artursson P, Karlsson J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2)cells. Biochem Biophys Res Com. 1991;175(3):880–5. doi: 10.1016/0006-291X(91)91647-U. [DOI] [PubMed] [Google Scholar]
- 23.Irvine JD, Takahashi L, Lockhart K, Cheong J, Tolan JW, Selick HE, et al. MDCK (Madin-Darby canine kidney) cells: A tool for membrane permeability screening. J Pharm Sci. 1999;88(1):28–33. doi: 10.1021/js9803205. [DOI] [PubMed] [Google Scholar]
- 24.Zakeri-Milania P, Valizadeha H, Tajerzadehc H, Azarmia Y, Islambolchilara Z, Barzegara S, et al. Predicting human intestinal permeability using single-pass intestinal perfusion in rat. J Pharm Pharm Sci. 2007;10:368–79. [PubMed] [Google Scholar]
- 25.Dollery Sir C. Therapeutic drugs. Livingstone: Churchill; 1991. [Google Scholar]
- 26.Ritschel WA, Brady ME, Tan HS. First-pass effect of coumarin in man. Int J Clin Pharmacol Biopharm. 1979;17(3):99–103. [PubMed] [Google Scholar]
- 27.Lima JJ, Haughey DB, Leier CV. Disopyramide pharmacokinetics and bioavailability following the simultaneous administration of disopyramide and 14Cdisopyramide. J Pharm Biopharm. 1984;12(3):289–313. doi: 10.1007/BF01061722. [DOI] [PubMed] [Google Scholar]
- 28.Hardman JG, Limbird LE. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 9. New York: McGraw-Hill; 1996. [Google Scholar]
- 29.Ponto LL, Schoenwald RD. Furosemide (frusemide). A pharmacokinetic/pharmacodynamic review (Part I) Clin Pharmacokin. 1990;18(5):381–408. doi: 10.2165/00003088-199018050-00004. [DOI] [PubMed] [Google Scholar]
- 30.Zhu C, Jiang L, Chen TM, Hwang KK. A comparative study of artificial membrane permeability assay for high-throughput profiling of drug absorption potential. Eur J Med Chem. 2002;37(5):399–407. doi: 10.1016/S0223-5234(02)01360-0. [DOI] [PubMed] [Google Scholar]
- 31.Roberfroid MB. Concepts in functional foods: The case of inulin and oligofructose. J Nutrition. 1999;129(7):1398–401. doi: 10.1093/jn/129.7.1398S. [DOI] [PubMed] [Google Scholar]
- 32.Li C, Wainhaus S, Uss A, Cheng K. High-throughput screening using Caco-2 Cell and PAMPA systems. In: Ehrhardt C, Kim KJ, editors. In drug absorption studies in situ, in vitro and in silico models. Springer US; 2008. p. 418–429.
- 33.Yee S. In vitro permeability across Caco-2 cells (colonic) can predict in vivo(small intestinal) absorption in man-fact or myth. Pharm Res. 1997;14(6):763–6. doi: 10.1023/A:1012102522787. [DOI] [PubMed] [Google Scholar]
- 34.Yazdanian M, Glynn SL, Wright JL, Hawi A. Correlating partitioning and Caco-2 cell permeability of structurally diverse small molecular weight compounds. Pharm Res. 1998;15(9):1490–4. doi: 10.1023/A:1011930411574. [DOI] [PubMed] [Google Scholar]
- 35.Grant SM, Langtry HD, Brogden RN. Ranitidine: An updated review of its pharmacodynamic and pharmacokinetic properties and therapeutic use in peptic ulcer disease and other allied diseases. Drugs. 1989;37(6):801–70. doi: 10.2165/00003495-198937060-00003. [DOI] [PubMed] [Google Scholar]
- 36.Hendeles L, Weinberger M, Bigley L. Absolute oral bioavailability of oral theophylline. Am J Hosp Pharm. 1977;34:525–7. [PubMed] [Google Scholar]
- 37.Balimane PV, Han YH, Chong S. Current industrial practices of assessing permeability and P-glycoprotein interaction. AAPS J. 2006;8(1):E1–13. doi: 10.1208/aapsj080101. [DOI] [PMC free article] [PubMed] [Google Scholar]