Abstract
In recent years, a variety of biomaterial implantable devices has been developed. Of particular significance to pharmaceutical sciences is the progress made on the development of drug/implantable device combination products. However, the clinical application of these devices is still a critical issue due to the host response, which results from both the tissue trauma during implantation and the presence of the device in the body. Accordingly, the in vivo functionality and durability of any implantable device can be compromised by the body response to the foreign material. Numerous strategies to overcome negative body reactions have been reported. The aim of this review is to outline some key issues of biomaterial/tissue interactions such as foreign body response and biocompatibility and biocompatibility assessment. In addition, general approaches used to overcome the in vivo instability of implantable devices are presented, including (a) biocompatible material coatings, (b) steroidal and nonsteroidal anti-inflammatory drugs, and (c) angiogenic drugs. In particular, strategies to overcome host response to glucose biosensors are summarized.
Key words: biocompatible coating for implantable devices; foreign body response (FBR); glucose biosensor; tissue compatibility assessment, drug device combination products
INTRODUCTION
The technological progress achieved in the recent years in areas such as biomaterials, biotechnology, cell and molecular biology, tissue engineering, and polymer science as in other related fields has resulted in a significant increase in the use of devices for medical/pharmaceutical applications, e.g. artificial organs (1), biosensors (2–6), catheters (7), heart valves (8), and scaffolds for tissue engineering (9,10). Of particular significance to pharmaceutical sciences is the development of drug/implantable device combination products, e.g., drug-eluting stents (11) and glucose monitoring biosensors (4–6). However, there are still some important challenges to be overcome since implantable devices typically experience a loss of functionality over time following implantation. Limited in vivo functionality and longevity is a critical issue, resulting either from the normal homeostatic response to the implantation injury, tissue or blood/device interface interactions, or even to a lack of biocompatibility (12–17).
A foreign body response based on nonspecific protein adsorption, immune, and inflammatory cells occurs under normal physiological conditions in order to protect the body from the foreign object. Reactions of both the implant on the host blood/tissue and of the host on the implantable device must be understood to avoid health complications to the patient and/or device failure. The degree to which the homeostatic mechanisms are perturbed, the pathophysiological conditions created, and resolution of the inflammatory response can be considered a measure of the host reaction, which ultimately determines the relative compatibility of the device (18–20). Although it is convenient to separate homeostatic mechanisms into blood–material or tissue–material interactions, it is noteworthy that many of components or mechanisms involved in homeostasis are a part of the same physiologic continuum (21,22).
The focus of this review is the tissue–material interactions. Some key concepts of biomaterial–tissue interactions are emphasized in the first part of the review such as foreign body response (FBR) and biocompatibility and biocompatibility assessment, whereas the second part emphasizes general approaches to overcome the in vivo implantable device instability. These approaches include (a) biocompatible material coatings, (b) steroidal and nonsteroidal anti-inflammatory drugs, and (c) angiogenic drugs. In the last part of the review, a specific example where tissue response to a subcutaneous (s.c.) implant (biosensor) has been successfully overcome through the use of a drug eluting biocompatible coating is summarized.
FOREIGN BODY RESPONSE
Following intramuscular and s.c. implantation, a tissue/device interface is immediately created, and nonspecific adsorption of blood and tissue fluid proteins onto the implant surface is usually induced (21,23,24). The degree and extent of the FBR depends on the properties of the device, such as (a) composition, (b) contact duration, (c) degradation rate, (d) morphology, (e) porosity, (f) roughness, (g) shape, (h) size, (i) sterility, and (j) surface chemistry (16,17,21).
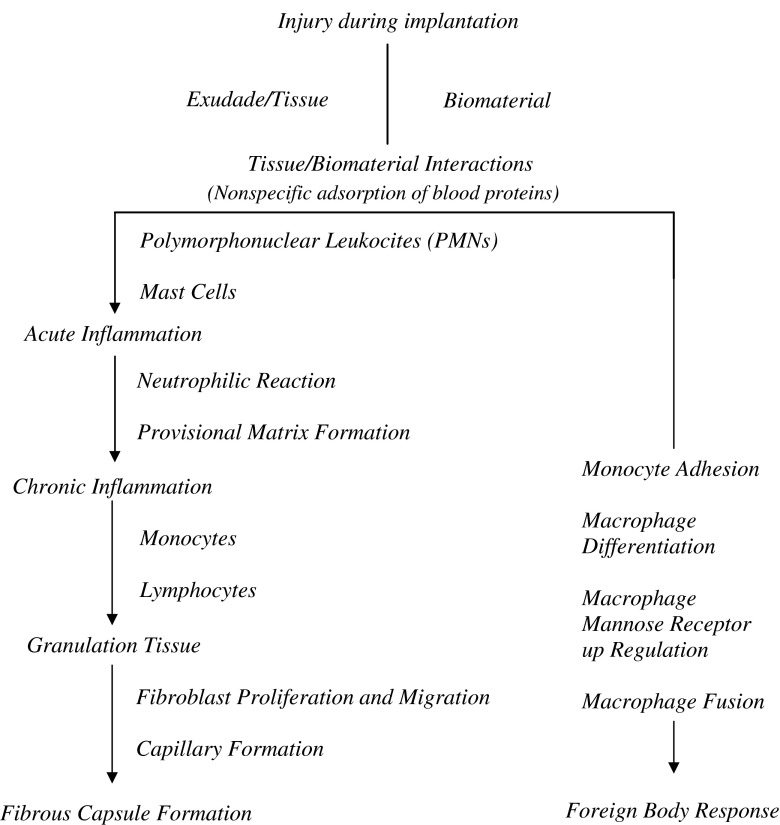
Device implantation and the associated tissue injury trigger a cascade of inflammatory and wound healing responses that are typical of a FBR. The inflammatory response comprises an initial acute phase and a subsequent chronic phase (23). The acute phase lasts from hours to days and is marked by fluid and protein exudation as well as a neutrophilic reaction. The acute phase is mostly responsible for the provisional matrix formation and cleaning of the wound site. Vessels dilate and excess blood flows into the injury site (18,25–28). Numerous blood and tissue proteins such as cytokines and growth factors are released, and leukocytes adhere to the endothelium of the blood vessels and infiltrate the injury site. Monocytes are then called into the site and these differentiate into macrophages (29). Persistent inflammatory stimuli, such as the continual presence of the biomaterial/medical device, lead to chronic inflammation. Chronic inflammation is histologically less uniform when compared to acute inflammation, and the wound healing response is generally dependent on the size and/or degree of injury (23). This phase is generally characterized by the presence of monocytes, macrophages, and lymphocytes, as well as the proliferation of blood vessels and connective tissue to restructure the affected area (26–30). The formation of blood vessels is essential to wound healing, supplying necessary nutrients (31). Eventually, the granulation tissue is replaced by an extracellular matrix (ECM). The ECM acts not only as a physical scaffold but also as a crucial modulator of the biological processes, including differentiation, development regeneration, repair, as well tumor progression (32). The end stage of the FBR involves walling off the implant by a vascular and collagenous fibrous capsule that is typically 50–200 µm in thickness (27,30,32). This fibrous wall confines the implant and consequently prevents it from interacting with the surrounding tissue (Fig. 1).
Fig. 1.
Sequence of events involved in the FBR to an implantable device. Note that overlap and simultaneous occurrence of these events occurs (based on (21,33))
BIOCOMPATIBILITY
Biocompatibility reflects the nature and degree of interaction between biomaterials and host tissue and is one of the critical concerns in biomaterials research (12,13,34). Biocompatibility can be defined as the ability of a material to perform with an appropriate host response in a specific application (14–17,21,33). Biocompatibility reflects a set of complex characteristics, and various implications and extensions of this definition have been reported (21,33). In summary, biocompatibility consists basically of two elements: (a) biosafety, i.e., appropriate host response not only systemic but also local (the surrounding tissue), the absence of cytotoxicity, mutagenesis, and/or carcinogenesis, and (b) biofunctionality, i.e., the ability of material to perform the specific task for which it is intended (16,21).
BIOCOMPATIBILITY ASSESSMENT (TISSUE COMPATIBILITY)
Biocompatibility of a biomaterial cannot be completely evaluated by a single test or method but rather requires a schedule of methods (35–39). Biocompatibility studies on an implantable device require complex in vitro and in vivo experiments to test the local and systemic effects of the material on the host (33,35,36). Evaluation of biocompatibility and biofunctionality of materials is performed mainly by methods based on the assessment of cytotoxicity, mutagenesis and/or carcinogenesis, and cell function (35,36). In fact, the extent of nonspecific protein absorption (biofouling) can be used to evaluate the degree of biocompatibility of the implant (36).
In vitro cell culture tests are often used to screen the tissue compatibility of implantable devices. The objective of cell culture techniques in biocompatibility assessments is (a) to simulate the biological response of the body environment on which the biomaterial is placed and (b) predict its functional performance. The method allows direct investigation of cell–biomaterial interactions and provides some insight into the cellular mechanisms controlling host response to the implanted biomaterial. Three primary cell culture assays are used to evaluate biocompatibility: (a) direct contact, (b) agar diffusion, and (c) elution (also known as extract dilution) (35,36). These assays are described in the US Pharmacopeia and in standards published by the American Society for Testing and Materials, the British Standards Institute, and the International Standards Organization (ISO) (40–43). These are morphological assays, meaning that the outcome is measured by observation of changes in cell morphology.
To standardize the methods and compare the results of these assays, it is important to carefully control: (a) the number of cells, (b) the growth phase of the cells (period of frequent cell replication), (c) the cell type, (d) the duration of exposure, (e) the test sample size (e.g., geometry, density, shape, thickness), and (f) the surface area of test sample. It is worth mentioning that cell lines that have been developed for growth in vitro have been preferred to primary cells that are freshly harvest from live organisms because these cell lines have improved reproducibility and reduced variability among laboratories (35,36). Specifically, L-929 mouse fibroblast cell line has been extensively used for testing biomaterials. Initially, L-929 cells were selected because they are easy to maintain in culture and produce results that have a high correlation with specific animal bioassays. In addition, fibroblasts are appropriate for these assays because they are one of the early cells to populate a healing wound and are often the major cell in the tissues that adhere to implanted devices.
Cell lines from other tissues or species may also be used. Ultimately, the selection of a cell line should be based upon the type of assay, the investigator’s experience, and measurement endpoints (viability, enzymatic activity, species receptors, etc.). These in vitro tests include positive and negative control materials, extraction conditions, and choice of cell lines and cell media. Important aspects of the test procedures include tests on extracts and on direct and indirect contents. Such tests are a sensitive, reliable, convenient, and reproducible screening method (35,36,40–43).
Relevant to the overall in vivo assessment of tissue compatibility of a biomaterial or device is knowledge of the chemical composition of the materials and the conditions of tissue exposure (including nature, degree, frequency, and duration of exposure). General principles that may apply to the biological evaluation of materials and devices are described on Table I (36).
Table I.
Biomaterials and Components Relevant to In Vivo Assessment of Tissue Compatibility
| The material(s) of manufacture |
| Intended additives, process contaminants, and residues |
| Leachable substances |
| Degradation products |
| Other components and their interactions in the final product |
| The properties and characteristics of the final product |
Table II identifies the ISO 10993-1 and US Food and Drug Administration Agency (FDA) categories for selection of biomedical methods, categorized by body contact and contact duration (36,40). The biological response tests, prior to clinical testing, which are included in the ISO 10993 and FDA documents are indicated in Table III (33,36,40,44).
Table II.
ISO 10993-1 and FDA Categories for Selection of Biological Response Test Methods
| Tissue contact |
|---|
| Surface devices |
| Skin |
| Mucosal membranes |
| Breached or compromised surfaces |
| External communicating devices |
| Blood path, indirect |
| Tissue/bone/dentin communicating |
| Circulating blood |
| Implant devices |
| Tissue/bone |
| Blood |
| Contact duration |
| Limited, ≤24 h |
| Prolonged, >24 h and <30 days |
| Permanent, >30 days |
Table III.
ISO 10993-1 and FDA Biological Response Test (In Vivo Tests for Tissue Compatibility)
| Initial evaluation steps |
| Cytotoxicity |
| Sensitization |
| Irritation |
| Intracutaneous reactivity |
| Systemic toxicity (acute toxicity) |
| Subchronic toxicity (subacute toxicity) |
| Genotoxicity |
| Implantation |
| Hemocompatibility |
| Supplementary evaluation steps |
| Chronic toxicity |
| Carcinogenicity |
| Reproductive and developmental toxicity |
| Biodegradation |
POSSIBLE SOLUTIONS TO OVERCOME FOREIGN BODY RESPONSE
To overcome the limited in vivo functionality and longevity of implantable devices, some important approaches have been reported and are summarized below.
Biocompatible Material Coatings
The use of biocompatible materials for coating implantable devices is based on their ability to mask the underlying surface. Masking is achieved by producing a hydrophilic interface between the device surface and the tissue fluids, thereby minimizing tissue reactions induced by device implantation (45). The formation of these biocompatible layers improves implantable device/host tissue interactions and consequently improves device functionality and life span (44–49).
Various natural, synthetic, and semisynthetic materials are currently utilized in the fabrication of implantable device coatings. Naturally occurring materials include (a) alginate (50), (b) chitosan (51,52), (c) collagen (53,54), (d) dextran (55), and (e) hyaluronan (56). These methods offer the advantage of being very similar to macromolecular substances that the biological environment is prepared to recognize and to deal with metabolically. On the other hand, serious disadvantages are (a) natural polymers are frequently immunogenic, (b) these polymers typically decompose or undergo pyrolytic modification at temperatures below their melting point, thereby precluding the convenience of high-temperature thermoplastic processing methods (such as melt extrusion) during the manufacturing of the implant, and (c) since they are derived from animal or plant sources, natural variability in macromolecular structure are expected (44). Numerous synthetic polymeric materials have been employed as coating materials, e.g., poly(lactic-acid) and poly(lactic co-glycolic acid) (PLGA) (57,58), poly(ethylene-glycol), 2-hydroxyethyl methacrylate (59), poly(ethylene glycol) (PEG) (60), and poly(vinyl-alcohol) (PVA) (61,62). Knowledge of the physical and chemical properties of the polymer is a useful tool to rationalize the choice of the coating material (16,44).
Hydrogel-type coatings have been applied in a broad range of biomaterial devices (63,64). These include poly(hydroxyl ethyl methacrylate) (59), PEG (60), and PVA (65–74). Hydrogels are three-dimensional polymeric networks, which adsorb and retain large amounts of water and are highly permeable to small molecules. The use of hydrogel coatings allow for the diffusion of analytes, such as glucose, through the water-swollen gel layer. The degree of analyte diffusion can be readily modulated by controlling the cross-link density of the gel, which in turn controls the water content of the gel and the openness of the polymer network (44,46). Another advantage is that their mechanical properties are similar to soft body tissue (16,44). However, despite these advantages, several potential drawbacks need to be considered, as poor adhesion to the substrate; less than acceptable mechanical strength for some applications; and for chemically cross-linked material, the safety of the chemical agents used. Furthermore, a number of studies have reported biocompatibility issues (12,13). In addition, it has been shown that biological reactions that are adverse for a material in one application may not be adverse for the same material in a different application. Similarly, a material found to be safe in one application may not be safe in another application (33). Table IV summarizes properties and applications of the most commonly used polymers in biomedical field.
Table IV.
Chemical Names, Properties, and Applications of Most Commonly Used Polymers in Biomedical Applications
| Component | Properties | Some applications |
|---|---|---|
| Phospholipid-based biomimicry | ||
| Phospholipid, phospholipid-containing or phospholipid-like materials | Device surface mimics cell’s own membrane; fragile and difficult to deposit | Coating (47) |
| Phospholipid-modified polymers (proteins + PHEMA) | High water content | Coating (47) |
| Natural derivates | ||
| Albumin | Immobilization of glucose oxidase (in combination with glutaraldehyde) | Glucose biosensor functionality (47) |
| Cellulose | After hydroxylation is able to decrease complement activation, not long-term stability | Coating (47) |
| Collagen | Extracellular matrix component | Porous sponge scaffolds (44) |
| Synthetic polymers | ||
| PE | Strength, lubricity | Orthopedic implants and catheters (45) |
| PP | Chemical inertness and rigidity | Drug delivery, meshes, and sutures (45) |
| Pluronics® surfactants (PEO–PPO–PEO) | Decrease biofouling and sensor passivation | Coating (47) |
| Perfluorosulfonic acid (Nafion®) | Decrease biofouling, uncured Nafion® (not treated with FeCl3) leads to high inflammatory response, not long-term stability | Coating (47) |
| Hydrogels | ||
| PHEMA | Negligible protein adsorption | Coating (45,47) |
| PEO, PEG | Negligible protein adsorption | Coating (45,47) |
| PVA | Surfactant and gel-forming properties | Emulsifier in drug encapsulation process and matrix for sustained drug delivery (45,47) |
| PLA and PLGA | Negligible protein adsorption | Coating (44,47) |
PE poly(ethylene), PP poly(propylene), PEO poly(ethylene oxide), PPO polypropylene oxide, PHEMA poly(hydroxyethyl methacrylate), PLA poly(lactic acid), PLGA poly(lactic co-glycolic acid), PVA poly(vinyl-alcohol)
Steroidal and Nonsteroidal Anti-inflammatory Drugs
FRB at the implant site may be minimized and/or controlled with the use of steroidal and nonsteroidal anti-inflammatory drugs. Glucocorticoids have been used because of their ability to suppress the immune response by inhibiting the formation and secretion of inflammatory mediators such as prostaglandins and leukotrienes. By inhibiting these inflammatory mediators, the glucocorticoids can lead to diminished release of inflammatory cells at the injury site, decreasing capillary permeability, and suppressing fibroblast proliferation (74). Since long-term systemic use of these drugs leads to unwanted side effects, localized and sustained delivery of anti-inflammatory drugs has been investigated. Drug-filled reservoirs (device itself and/or device coating), e.g., microspheres, nanoparticles, hydrogels, microspheres embedded in hydrogel matrices (smart hydrogels), have been investigated as means for the delivery of drugs to the implant site (65–73,75–79).
Angiogenic Drugs
Biosensor functionality and longevity can be compromised by a biofouling response and the formation of an avascular fibrous capsule around the device that greatly decreases both the transport of analyte from the tissue to the sensor and the diffusion of reaction products from the sensor to surrounding (80,81). Therefore, controlling fibrotic encapsulation at the implant site would appear to be critical to achieve a functional and extended life-time biosensor in vivo. One approach to improve the analyte transport around the implant is the promotion of angiogenesis. This can be achieved by inducing new blood vessel formation in the vicinity of the sensor using growth factors such as the vascular endothelial growth factor (VEGF) (67,71,82–84). It is noteworthy that well-vascularized tissue at the implant site is also critical for healing the trauma caused during implantation (28,31). Another issue associated with the use of corticosteroid drugs to treat the inflammation process is that these drugs also downregulate endogenous VEGF, thereby inhibiting angiogenesis (85,86). Accordingly, a two-pronged approach (control of inflammation and induction of angiogenesis) may be necessary (71).
GLUCOSE BIOSENSORS
Monitoring blood glucose concentrations is important for an adequate insulin regimen for patients with diabetes. Currently, glucose monitoring depends on finger pricking and external monitoring several times per day (87–90). Accordingly, the pain and inconvenience of such routine reduces patient compliance. Implantable glucose sensors are a promising solution to this problem, and numerous researchers are attempting to develop implantable glucose sensors (90–92). At present, six minimally invasive blood glucose monitoring systems have been approved by the FDA (93). Nevertheless, the longest in vivo functional lifetime of a marketed system is 7 days, and frequent calibration is required with handheld glucose meters. The s.c. tissue is regarded as an appropriate site for biosensor implantations since it provides easy access for surgical procedures (insertion and/or removal). In addition, it has been reported that the glucose level in the s.c. tissue is directly related to the blood glucose concentration (80). Despite outstanding advances in the in vitro functionality of such sensors, a reliable long-term and continuous glucose monitoring in vivo has not as yet been achieved due to the gradual loss of sensor functionality following implantation. Numerous investigators have suggested that glucose diffusion is negatively influenced by nonspecific protein adsorption from the tissue fluid to the sensor surface (80,93,94). Moreover, the fibrous capsule usually formed around the sensor can restrict the transport of glucose molecules and/or other low molecular weight analytes (93).
PVA HYDROGEL/ANTI-INFLAMMATORY DRUG LOADED-PLGA MICROSPHERES
Microspheres have been utilized for localized and controlled drug delivery. However, their utility for implantable devices is hindered by some factors: (a) multiple injections of microspheres carrying different drugs in the vicinity of the implant are difficult, (b) multiple injections will induce additional trauma, and (c) a constant zero-order release might be more desirable than a typical triphasic one. As summarized in a recent review (95), a versatile drug eluting/biomaterial coating combination for implantable devices, a PVA hydrogel containing entrapped drug-loaded PLGA microspheres, has been developed. These PLGA microsphere/PVA hydrogel composites show promise as coatings for controlling the inflammatory response following device implantation and have the ability to mask the underlying device surface.
LOCAL AND CONTROLLED DELIVERY OF DEXAMETASONE
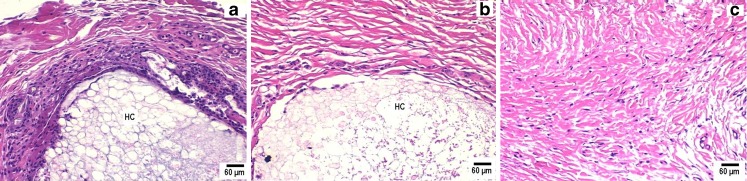
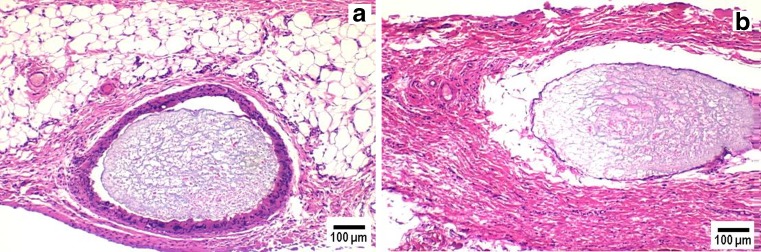
Dexamethasone (a potent anti-inflammatory drug)-loaded PLGA microsphere/PVA hydrogel composites achieved localized drug delivery with approximate zero-order release kinetics successfully control negative tissue reactions at the implant site by reducing the level of inflammation-mediating cells when compared to those implants not containing dexamethasone (67). Pharmacodynamic effects were evaluated by histopathological examination of s.c. tissue surrounding implanted composites using a rat model (Figs. 2 and 3). All animal studies were conducted at the University of Connecticut in accordance with Institutional Animal Care and Use Committee (IACUC) guidelines using an approved protocol (number E2901201). Tissue samples surrounding composites without entrapped drug showed a large number of neutrophils in the initial acute inflammatory phase. A chronic inflammatory reaction was observed by days 21 and 28 and was characterized by a dense network of fibrous tissue together with lymphocytes and macrophages (Fig. 3). A band of fibrous connective tissue with accompanying deposition of collagen encapsulated the composites, which is the usual reaction of the body to the continuous presence of foreign material. One the other hand, tissue surrounding composites containing dexamethasone were similar to normal s.c. tissue with only a few neutrophils present and with no evidence of fibrous encapsulation until day 21 (Fig. 2). At day 28, which was when the drug had been exhausted from the composites, there was evidence of increased number of neutrophils. This and subsequent studies with composites that released drug for both shorter (7 days) (72) and longer (3 months) (73,95) durations have indicated that dexamethasone release will be required throughout the lifetime of the implant. Once dexamethasone release was completed, the body was able to recognize the implant as a foreign body, and subsequent inflammatory and immunogenic reactions were observed (67,71–73,95).
Fig. 2.
Pharmacodynamic changes in representative tissue sections on day 3 from s.c. tissue of rats implanted with PLGA microsphere/PVA hydrogel composites: a without dexamethasone and b with dexamethasone compared with control untreated tissue sections (c). Inflammation-mediating cells and normal cells are stained purple and pink, respectively (hematoxylin and eosin stain). Hydrogel composites are marked HC
Fig. 3.
Pharmacodynamic changes in representative tissue sections on day 21 from s.c. tissue of rats implanted with PLGA microsphere/PVA hydrogel composites: a without dexamethasone and b with dexamethasone. Inflammation-mediating cells and normal cells are stained purple and pink, respectively (hematoxylin and eosin stain). The white area surrounding the hydrogel composite in b is an artifact due to tissue detachment during sectioning. Hydrogel composites are marked HC
CONCURRENT DELIVERY OF DEXAMETASONE AND VGEF
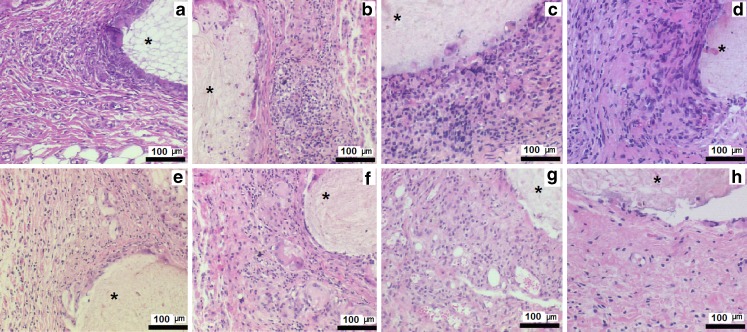
As mentioned above, corticosteroid drugs prevent angiogenesis by inhibiting or downregulating endogenous VEGF (69–71). Therefore, control of the inflammatory response, along with a method of inducing neo-angiogenesis, may be important to achieve implantable biosensor functionality (69–71). It is worth to mention that systemic administration of protein growth factors is ineffective due to their rapid degradation and consequent inability to achieve adequate concentration at the local site. Accordingly, VEGF-loaded PLGA microspheres have been investigated to achieve neo-angiogenesis in the implant site (71). A combination of dexamethasone and VEGF-loaded PLGA microsphere/PVA hydrogel composites was also investigated for concurrent localized delivery. Pharmacodynamic effects were evaluated by histopathological examination of s.c. tissue surrounding implanted composites using a rat model (Fig. 4). All animal studies were conducted at the University of Connecticut in accordance with IACUC guidelines using an approved protocol (number E2901201).
Fig. 4.
Pharmacodynamics changes in representative subcutaneous tissue sections of rats implanted with PLGA microsphere/PVA hydrogel composites (asterisk) containing VEGF alone over week 1 (a), week 2 (b), week 3 (c), and week 4 (d) postimplantation and dexamethasone and VEGF combination week 1 (e); week 2 (f); week 3 (g), and week postimplantation (h). Inflammation-mediating cells and normal cells are stained purple and pink, respectively (hematoxylin and eosin stain)
The hydrogel composites were capable of simultaneously releasing VEGF and dexamethasone with approximately zero-order kinetics. The ability of exogenous VEGF to induce neo-angiogenesis in the s.c. tissue was evaluated after staining the blood vessels with α-smooth muscle actin (immunochemistry). The composites were successful in controlling the implant/tissue interface by suppressing inflammation and fibrosis as well as facilitating neo-angiogenesis at a fraction of their typical oral or i.v. bolus doses. Implants containing VEGF showed a significantly higher number of blood vessels at the end of the 4-week study irrespective of the presence of dexamethasone. Thus, localized concurrent elution of VEGF and dexamethasone could overcome the anti-angiogenic effects of the dexamethasone and be used to engineer inflammation free and well-vascularized tissue in the vicinity of the implant.
CONCLUSIONS
The appropriate selection of biocompatible coating materials for use with implantable devices can minimize the negative body’s response while maintaining implantable device functionality and longevity. Development of drug/implantable device combination products provides an exciting strategy for the controlled and localized delivery of tissue-response modifying drugs. Anti-inflammatory agents released at the local site have been the most successful in preventing inflammation and fibrosis. Research has also focused on the release of growth factors at the implant site of biosensors for the purpose of inducing blood vessel growth to ensure adequate healing process and an analyte supply for biosensors. Drug-loaded PLGA microsphere/PVA hydrogel coatings, developed for implantable glucose biosensors, can be easily tuned by incorporating different types of PLGA microspheres and two or more drugs can be simultaneously delivered (69–71). It is anticipated that these efforts to develop biocompatible material coating for glucose biosensors will also assist in the development of a variety of long-term implantable devices in the near future.
Acknowledgments
The authors thank TATRC (Grant # W81XWH0710688) and NIH (Grant # R21HL90458-01) for financial support.
References
- 1.Prokop A. Bioartificial organs in the twenty-first century: nano-biological devices. Ann NY Acad Sci. 2001;944:472–490. doi: 10.1111/j.1749-6632.2001.tb03856.x. [DOI] [PubMed] [Google Scholar]
- 2.Wilson GS, Zhang Y, Reach G, Moatti-Sirat D, Poitout V, Thévenot DR, et al. Progress toward the development of an implantable sensor for glucose. Clin Chem. 1992;38(9):1613–1617. [PubMed] [Google Scholar]
- 3.Klonoff DC. Technological advances in the treatment of diabetes mellitus: better bioengineering begets benefits in glucose measurement, the artificial pancreas, and insulin delivery. Pediatr Endocrinol Rev. 2003;1(2):94–100. [PubMed] [Google Scholar]
- 4.Koschwanez HE, Reichert WM. In vitro, in vivo and post explantation testing of glucose-detecting biosensors: current methods and recommendations. Biomaterials. 2007;28(25):3687–3703. doi: 10.1016/j.biomaterials.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailey TS, Zisser HC, Garg SK. Reduction in hemoglobin A1C with real-time continuous glucose monitoring: results from a 12-week observational study. Diabetes Technol Ther. 2007;9(3):203–210. doi: 10.1089/dia.2007.0205. [DOI] [PubMed] [Google Scholar]
- 6.Buckingham B, Caswell K, Wilson DM. Real-time continuous glucose monitoring. Curr Opin Endocrinol Diabetes Obes. 2007;14(4):288–295. doi: 10.1097/MED.0b013e32825a675e. [DOI] [PubMed] [Google Scholar]
- 7.Callahan TD, 4th, Natale A. Catheter ablation of atrial fibrillation. Med Clin North Am. 2008;92(1):179–201. doi: 10.1016/j.mcna.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Black MM, Drury PJ. Mechanical and other problems of artificial valves. Curr Top Pathol. 1994;86:127–159. doi: 10.1007/978-3-642-76846-0_4. [DOI] [PubMed] [Google Scholar]
- 9.Salgado AJ, Coutinho OP, Reis RL. Bone tissue engineering: state of the art and future trends. Macromol Biosci. 2004;4(8):743–765. doi: 10.1002/mabi.200400026. [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto T, Okazaki M, Nakahira A, Sasaki J, Egusa H, Sohmura T. Modification of apatite materials for bone tissue engineering and drug delivery carriers. Curr Med Chem. 2007;14(25):2726–2733. doi: 10.2174/092986707782023208. [DOI] [PubMed] [Google Scholar]
- 11.Kipshidze NN, Tsapenko MV, Leon MB, Stone GW, Moses JW. Update on drug-eluting coronary stents. Expert Rev Cardiovasc Ther. 2005;3(5):953–968. doi: 10.1586/14779072.3.5.953. [DOI] [PubMed] [Google Scholar]
- 12.Williams DF. Tissue–biomaterial interactions. J Mater Sci. 1987;22:3421–3445. doi: 10.1007/BF01161439. [DOI] [Google Scholar]
- 13.Laurencin CT, Elgendy H. The biocompatibility and toxicity of degradable polymeric materials: implication for drug delivery. In: Domb A, Maniar M, editors. Site specific drug delivery. New York: Wiley; 1994. pp. 27–46. [Google Scholar]
- 14.Anderson JM. Biological responses to materials. Annu Rev Mater Res. 2001;31:81–110. doi: 10.1146/annurev.matsci.31.1.81. [DOI] [Google Scholar]
- 15.Fournier E, Passirani C, Montero-Menei CN, Benoit JP. Biocompatibility of implantable synthetic polymeric drug carriers: focus on brain biocompatibility. Biomaterials. 2003;24(19):3311–3331. doi: 10.1016/S0142-9612(03)00161-3. [DOI] [PubMed] [Google Scholar]
- 16.Arshady R. Polymeric biomaterials: chemistry, concepts, criteria. In: Arshady R, editor. Introduction to polymeric biomaterials: the polymeric biomaterials series. London: Citus Books; 2003. pp. 1–62. [Google Scholar]
- 17.Ratner BA, Horbett TA. Some background concepts. In: Ratner BD, Schoen FJ, Lemons JE, editors. Biomaterials science: an introduction to materials in medicine. 2. San Diego: Elsevier; 2004. p. 237. [Google Scholar]
- 18.Anderson JM. Inflammatory response to implants. ASAIO Trans. 1988;34(2):101–107. doi: 10.1097/00002480-198804000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Ziats NP, Miller KM, Anderson JM. In vitro and in vivo interactions of cells with biomaterials. Biomaterials. 1988;9(1):5–13. doi: 10.1016/0142-9612(88)90063-4. [DOI] [PubMed] [Google Scholar]
- 20.Pappas N. An introduction to materials in medicine. Biomaterials science. New York: Academic; 1996. pp. 60–64. [Google Scholar]
- 21.Schoen FJ, Anderson JM. Host response to biomaterials and their evaluation. In: Ratner BD, Schoen FJ, Lemons JE, editors. Biomaterials science: an introduction to materials in medicine, 2nd. San Diego: Elsevier; 2004. pp. 293–296. [Google Scholar]
- 22.Sieminski AL, Gooch KJ. Biomaterial–microvasculature interactions. Biomaterials. 2000;21(22):2232–2241. doi: 10.1016/S0142-9612(00)00149-6. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell RN, Cotran RS. Acute and chronic inflammation in Robbins basic pathology. Philadelphia: Saunders; 2002. pp. 33–60. [Google Scholar]
- 24.Ratner BD, Bryant SJ. Biomaterials: where we have been and where we are going. Annu Rev Biomed Eng. 2004;6:41–75. doi: 10.1146/annurev.bioeng.6.040803.140027. [DOI] [PubMed] [Google Scholar]
- 25.Jutila MA. Leukocyte traffic to sites of inflammation. APMIS. 1992;100(3):191–201. doi: 10.1111/j.1699-0463.1992.tb00861.x. [DOI] [PubMed] [Google Scholar]
- 26.Pober JS, Cotran RS. The role of endothelial cells in inflammation. Transplantation. 1990;50(4):537–544. doi: 10.1097/00007890-199010000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Williams GT, Williams WJ. Granulomatous inflammation—a review. J Clin Pathol. 1983;36(7):723–773. doi: 10.1136/jcp.36.7.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wahl SM, Wong H, McCartney-Francis N. Role of growth factors in inflammation and repair. J Cell Biochem. 1989;40(2):193–199. doi: 10.1002/jcb.240400208. [DOI] [PubMed] [Google Scholar]
- 29.Johnston RB., Jr Current concepts: immunology, monocytes and macrophages. N Engl J Med. 1988;318(12):747–752. doi: 10.1056/NEJM198803243181205. [DOI] [PubMed] [Google Scholar]
- 30.Kovacs EJ. Fibrogenic cytokines: the role of immune mediators in the development of scar tissue. Immunol Today. 1991;12(1):17–23. doi: 10.1016/0167-5699(91)90107-5. [DOI] [PubMed] [Google Scholar]
- 31.Pierce GF, Mustoe TA, Altrock BW, Deuel TF, Thomason A. Role of platelet-derived growth factor in wound healing. J Cell Biochem. 1991;45(4):319–326. doi: 10.1002/jcb.240450403. [DOI] [PubMed] [Google Scholar]
- 32.Labat-Robert J, Bihari-Varga M, Robert L. Extracellular matrix. FEBS Lett. 1990;268(2):386–393. doi: 10.1016/0014-5793(90)81291-U. [DOI] [PubMed] [Google Scholar]
- 33.Anderson JM, Langone JJ. Issues and perspectives on the biocompatibility and immunotoxicity evaluation of implanted controlled release systems. J Control Release. 1999;57(2):107–113. doi: 10.1016/S0168-3659(98)00178-3. [DOI] [PubMed] [Google Scholar]
- 34.Mikos AG, McIntire LV, Anderson JM, Babensee JE. Host response to tissue engineered devices. Adv Drug Deliv Rev. 1998;33(1–2):111–139. doi: 10.1016/s0169-409x(98)00023-4. [DOI] [PubMed] [Google Scholar]
- 35.Saad B, Abu-Hijleh G, Suter UW. Polymer biocompatibility assessment by cell culture techniques. In: Arshady R, editor. Introduction to polymeric biomaterials: the polymeric biomaterials series. London: Citus Books; 2003. pp. 263–299. [Google Scholar]
- 36.Ratner BD, Northup SJ, Anderson JM. Biological testing of biomaterials. In: Ratner BD, Schoen FJ, Lemons JE, editors. Biomaterials science: an introduction to materials in medicine. 2. San Diego: Elsevier; 2004. pp. 355–360. [Google Scholar]
- 37.Dekker A, Panfil C, Valdor M, Richter H, Mittermayer Ch, Kirkpatrick CJ. Quantitative methods for in vitro cytotoxicity testing of biomaterials. Cells Mater. 1994;4:101–112. [Google Scholar]
- 38.Kirkpatrick CJ, Bittinger F, Wagner M, Köhler H, van Kooten TG, Klein CL, Otto M. Current trends in biocompatibility testing. Proc Inst Mech Eng [H] 1998;212(2):75–84. doi: 10.1243/0954411981533845. [DOI] [PubMed] [Google Scholar]
- 39.Royals MA, Fujita SM, Yewey GL, Rodriguez J, Schultheiss PC, Dunn RL. Biocompatibility of a biodegradable in situ forming implant system in rhesus monkeys. J Biomed Mater Res. 1999;45(3):231–239. doi: 10.1002/(SICI)1097-4636(19990605)45:3<231::AID-JBM11>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 40.ISO 10,933 . Biological evaluation of medical devices. Geneva: International Standards Organizations; 1992. [Google Scholar]
- 41.Association for the Advancement of Medical Instrumentation . AAMI standards and recommended practices. Biological evaluation of medical devices, v.4., Suppl. Arlington: AAMI; 1997. [Google Scholar]
- 42.FDA . Blue Book Memorandum G95–1: FDA-modified version of ISO10, 933—part 1, biological evaluation of medical devices. Silver Spring: FDA; 1996. [Google Scholar]
- 43.US Pharmacopeia . Biological reactivity tests in vitro. In: US Pharmacopeia XXIII, editor. United States Pharmacopeial Convention, Inc., Rockville, MD, v.27. Rockville: US Pharmacopeia; 1995. pp. 2173–2175. [Google Scholar]
- 44.Hoffman AS, Cooper SL, Visser SA, Hergenrother RW, Lamba NMK, Peppas NA, et al. Classes of materials used in medicine. In: Ratner BD, Schoen FJ, Lemons JE, et al., editors. Biomaterials science: an introduction to materials in medicine. 2. San Diego: Elsevier; 2004. pp. 67–127. [Google Scholar]
- 45.Göpferich A. Polymer degradation and erosion: mechanisms and applications. Eur J Pharm Biopharm. 1996;42(1):1–11. [Google Scholar]
- 46.Shastri VP. Non-degradable biocompatible polymers in medicine: past, present and future. Current Pharm Biotech. 2003;4:331–337. doi: 10.2174/1389201033489694. [DOI] [PubMed] [Google Scholar]
- 47.Wisniewski N, Reichert M. Methods for reducing biosensor membrane biofouling. Colloids Surf B Biointerfaces. 2000;18(3–4):197–219. doi: 10.1016/S0927-7765(99)00148-4. [DOI] [PubMed] [Google Scholar]
- 48.Shen M, Horbett TA. The effects of surface chemistry and adsorbed proteins on monocyte /macrophage adhesion to chemically modified polystyrene surfaces. J Biomed Mater Res. 2001;57(3):336–345. doi: 10.1002/1097-4636(20011205)57:3<336::AID-JBM1176>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 49.Dalsin JL, Hu BH, Lee BP, Messersmith PB. Mussel adhesive protein mimetic polymers for the preparation of nonfouling surfaces. J Am Chem Soc. 2003;125(14):4253–4258. doi: 10.1021/ja0284963. [DOI] [PubMed] [Google Scholar]
- 50.de Vos P, Hoogmoed CG, Busscher HJ. Chemistry and biocompatibility of alginate-PLL capsules for immunoprotection of mammalian cells. J Biomed Mater Res. 2002;60(2):252–259. doi: 10.1002/jbm.10060. [DOI] [PubMed] [Google Scholar]
- 51.Uchegbu IF, Schätzlein AG, Tetley L, Gray AI, Sludden J, Siddique S, et al. Polymeric chitosan-based vesicles for drug delivery. J Pharm Pharmacol. 1998;50(5):453–458. doi: 10.1111/j.2042-7158.1998.tb06185.x. [DOI] [PubMed] [Google Scholar]
- 52.Borchard G, Junginger HE. Modern drug delivery applications of chitosan. Adv Drug Deliv Rev. 2001;52(2):103. doi: 10.1016/S0169-409X(01)00198-3. [DOI] [PubMed] [Google Scholar]
- 53.Sano A, Hojo T, Maeda M, Fujioka K. Protein release from collagen matrices. Adv Drug Deliv Rev. 1998;31(3):247–266. doi: 10.1016/S0169-409X(97)00119-1. [DOI] [PubMed] [Google Scholar]
- 54.Geiger M, Li RH, Friess W. Collagen sponges for bone regeneration with rhBMP-2. Adv Drug Deliv Rev. 2003;55(12):1613–1629. doi: 10.1016/j.addr.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 55.Draye JP, Delaey B, van de Voorde A, van Den Bulcke A, de Reu B, Schacht E. In vitro and in vivo biocompatibility of dextran dialdehyde cross-linked gelatin hydrogel films. Biomaterials. 1998;19(18):1677–1687. doi: 10.1016/S0142-9612(98)00049-0. [DOI] [PubMed] [Google Scholar]
- 56.Vercruysse KP, Prestwich GD. Hyaluronate derivatives in drug delivery. Crit Rev Ther Drug Carrier Syst. 1998;15(5):513–555. [PubMed] [Google Scholar]
- 57.Athanasiou KA, Niederauer GG, Agrawal CM. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials. 1996;17(2):93–102. doi: 10.1016/0142-9612(96)85754-1. [DOI] [PubMed] [Google Scholar]
- 58.Shive MS, Anderson JM. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Delivery Rev. 1997;28(1):5–24. doi: 10.1016/S0169-409X(97)00048-3. [DOI] [PubMed] [Google Scholar]
- 59.Quinn CP, Pathak CP, Heller A, Hubbell JA. Photo-crosslinked copolymers of 2-hydroxyethyl methacrylate, poly(ethylene glycol) tetra-acrylate and ethylene dimethacrylate for improving biocompatibility of biosensors. Biomaterials. 1995;16(5):389–396. doi: 10.1016/0142-9612(95)98856-9. [DOI] [PubMed] [Google Scholar]
- 60.Espadas-Torre C, Meyerhoff ME. Thrombogenic properties of untreated and poly(ethylene oxide)-modified polymeric matrices useful for preparing intra-arterial ion-selective electrodes. Anal Chem. 1995;67(18):3108–3114. doi: 10.1021/ac00114a003. [DOI] [PubMed] [Google Scholar]
- 61.Paradossi G, Cavalieri F, Chiessi E, Spagnoli C, Cowman MK. Poly(vinyl alcohol) as versatile biomaterial for potential biomedical applications. J Mater Sci Mater Med. 2003;14(8):687–691. doi: 10.1023/A:1024907615244. [DOI] [PubMed] [Google Scholar]
- 62.Maruoka S, Matsuura T, Kawasaki K, Okamoto M, Yoshiaki H, Kodama M, et al. Biocompatibility of poly-vinyl-alcohol gel as a vitreous substitute. Curr Eye Res. 2006;31(7–8):599–606. doi: 10.1080/02713680600813854. [DOI] [PubMed] [Google Scholar]
- 63.Kost J, Langer R. Equilibrium swollen hydrogels in controlled release applications. In: Peppas NA, editor. Hydrogels in medicine and pharmacy, v.3. Boca Raton: CRC; 1997. pp. 95–108. [Google Scholar]
- 64.Ravin AG, Olbrich KC, Levin LS, Usala AL, Klizman B. Long-and short-term effects of biological hydrogels on capsule microvascular density around implants in rats. J Biomed Mater Res. 2001;58:313–318. doi: 10.1002/1097-4636(2001)58:3<313::AID-JBM1023>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 65.Quinn CA, Connor RE, Heller A. Biocompatible, glucose-permeable hydrogel for in situ coating of implantable biosensors. Biomaterials. 1997;18(24):1665–1670. doi: 10.1016/S0142-9612(97)00125-7. [DOI] [PubMed] [Google Scholar]
- 66.Hickey T, Kreutzer D, Burgess DJ, Moussy F. In vivo evaluation of a dexamethasone/PLGA microsphere system designed to suppress the inflammatory tissue response to implantable medical devices. J Biomed Mater Res. 2002;61(2):180–187. doi: 10.1002/jbm.10016. [DOI] [PubMed] [Google Scholar]
- 67.Patil SD, Papadimitrakopoulos F, Burgess DJ. Dexamethasone-loaded poly(lactic-co-glycolic) acid microspheres/poly(vinyl alcohol) hydrogel composite coatings for inflammation control. Diabetes Technol Ther. 2004;6(6):887–897. doi: 10.1089/dia.2004.6.887. [DOI] [PubMed] [Google Scholar]
- 68.Galeska I, Kim TK, Patil SD, Bhardwaj U, Chatttopadhyay D, Papadimitrakopoulos F, et al. Controlled release of dexamethasone from PLGA microspheres embedded within polyacid-containing PVA hydrogels. AAPS J. 2005;7(1):E231–E240. doi: 10.1208/aapsj070122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Norton LW, Tegnell E, Topored SS, Reichert WM. In vitro characterization of vascular endothelial growth factor and dexamethasone releasing hydrogels for implantable probe coatings. Biomaterials. 2005;26(16):3285–3297. doi: 10.1016/j.biomaterials.2004.07.069. [DOI] [PubMed] [Google Scholar]
- 70.Norton LW, Koschwanez HE, Wisniewski NA, Klitzman B, Reichert WM. Vascular endothelial grown factor and dexamethasone release from non-fouling sensor coating affect the foreign body response. J Bio Mater Res A. 2007;81(4):858–869. doi: 10.1002/jbm.a.31088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Patil SD, Papadimitrakopouos F, Burgess DJ. Concurrent delivery of dexamethasone and VEGF for localized inflammation control and angiogenesis. J Control. Release. 2007;117:68–79. doi: 10.1016/j.jconrel.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 72.Bhardwaj U, Sura R, Papadimitrakopoulos F, Burgess DJ. Controlling acute inflammation with fast releasing dexamethasone-PLGA microsphere/PVA hydrogel composites for implantable devices. Diabetes Sci Technol. 2007;1(1):8–17. doi: 10.1177/193229680700100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bhardwaj U, Radhacirsshana S, Papadimitrakopoulos F, Burgess DJ. PLGA/PVA hydrogel composites for long-term inflammation control following s.c. implantation. Int J Pham. 2010;484:78–86. doi: 10.1016/j.ijpharm.2009.09.046. [DOI] [PubMed] [Google Scholar]
- 74.Perreti M, Ahluwalia A. The microcirculation and inflammation: site of action for glucocorticoids. Microcirculation. 2000;7:147–161. [PubMed] [Google Scholar]
- 75.Strecker EP, Gabelmann A, Boos I, Lucas C, Xu Z, Haberstroh J, et al. Effect on intimal hyperplasia of dexamethasone released from coated metal stents compared with non-coated stents in canine femoral arteries. Cardiovasc Intervent Radiol. 1998;21(6):487–496. doi: 10.1007/s002709900309. [DOI] [PubMed] [Google Scholar]
- 76.Ronneberger B, Kissel T, Anderson JM. Biocompatibility of ABA triblock copolymer microparticles consisting of poly(L-lactic-co-glycolic-acid) A-blocks attached to central poly(oxyethylene) B-blocks in rats after intramuscular injection. Eur J Pharm Biopharm. 1997;43:19–28. doi: 10.1016/S0939-6411(96)00006-9. [DOI] [Google Scholar]
- 77.Daugherty AL, Cleland JL, Duenas EM, Mrsny RJ. Pharmacological modulation of the tissue response to implanted polylactic-co-glycolic acid microspheres. Eur J Pharm Biopharm. 1997;44(1):89–102. doi: 10.1016/S0939-6411(97)00065-9. [DOI] [Google Scholar]
- 78.Lunsford L, McKeever U, Eckstein V, Hedley ML. Tissue distribution and persistence in mice of plasmid DNA encapsulated in a PLGA-based microsphere delivery vehicle. J Drug Target. 2000;8(1):39–50. doi: 10.3109/10611860009009208. [DOI] [PubMed] [Google Scholar]
- 79.Hickey T, Kreutzer D, Burgess DJ, Moussy F. Dexamethasone/PLGA microspheres for continuous delivery of an anti-inflammatory drug for implantable medical devices. Biomaterials. 2002;23:1649–1656. doi: 10.1016/S0142-9612(01)00291-5. [DOI] [PubMed] [Google Scholar]
- 80.Kyrolainen M, Rigsby P, Eddy S, Vadgama P. Bio-/hemocompatibility: implications and outcomes for sensors? Acta Anaesthesiol Scand Suppl. 1995;104:55–60. doi: 10.1111/j.1399-6576.1995.tb04255.x. [DOI] [PubMed] [Google Scholar]
- 81.Gerritsen M, Jansen JA, Kros A, Vriezema DM, Sommerdijk NA, Nolte RJ, et al. Influence of inflammatory cells and serum on the performance of implantable glucose sensors. J Biomed Mater Res. 2001;54(1):69–75. doi: 10.1002/1097-4636(200101)54:1<69::AID-JBM8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 82.Tanihara M, Suzuki Y, Yamamoto E, Noguchi A, Mizushima Y. Sustained release of basic fibroblast growth factor and angiogenesis in a novel covalently cross-linked gel of heparin and alginate. J Biomed Mater Res. 2001;56:216–221. doi: 10.1002/1097-4636(200108)56:2<216::AID-JBM1086>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 83.Ward WK, Quinn MJ, Wood MD, Tiekotter KL, Pidikiti S, Gallagher JA. Vascularizing the tissue surrounding a model biosensor: how localized is the effect of a subcutaneous infusion of vascular endothelial growth factor (VEGF)? Biosens Bioelectron. 2003;19:155–163. doi: 10.1016/S0956-5663(03)00180-5. [DOI] [PubMed] [Google Scholar]
- 84.Kedem A, Perets A, Gamlieli-Bonshtein I, Dvir-Ginzberg M, Mizrahi S, Cohen S. Vascular endothelial growth factor-releasing enhance vascularization and engraftment of hepatocytes transplanted on liver lobes. Tissue Eng. 2005;11:715–722. doi: 10.1089/ten.2005.11.715. [DOI] [PubMed] [Google Scholar]
- 85.Gaytan F, Morales C, Bellido C, Sanchez-Criado JE. Selective apoptosis of luteal endothelial cells in dexamethasone-treated rats leads to ischemic necrosis of luteal tissue. Biol Reprod. 2002;66:232–240. doi: 10.1095/biolreprod66.1.232. [DOI] [PubMed] [Google Scholar]
- 86.Halaby IA, Lyden SP, Davies MG, Roztocil E, Salamone LJ, Brooks AI. Glucocorticoid-regulated VEGF expression in ischemic skeletal muscle. Molec Ther. 2002;5(3):300–306. doi: 10.1006/mthe.2002.0539. [DOI] [PubMed] [Google Scholar]
- 87.Reach G, Wilson GS. Can continuous glucose monitoring be used for the treatment of diabetes? Anal Chem. 1992;64(6):381A–386A. doi: 10.1021/ac00030a001. [DOI] [PubMed] [Google Scholar]
- 88.Rebrin K, Fischer U, Hahn von Dorsche H, von Woetke T, Abel P, Brunstein E. Subcutaneous glucose monitoring by means of electrochemical sensors: fiction or reality. J Biomed Eng. 1992;14(1):33–40. doi: 10.1016/0141-5425(92)90033-H. [DOI] [PubMed] [Google Scholar]
- 89.Bobbioni-Harsch E, Rohner-Jeanrenaud F, Koudelka M, de Rooij N, Jeanrenaud B. Lifespan of subcutaneous glucose sensors and their performances during dynamic glycemia changes in rats. J Biomed Eng. 1993;15(6):457–463. doi: 10.1016/0141-5425(93)90058-7. [DOI] [PubMed] [Google Scholar]
- 90.Gilligan BJ, Shults MC, Rhodes RK, Updike SJ. Evaluation of a subcutaneous glucose sensor out to 3 months in a dog model. Diabetes Care. 1994;17(8):882–887. doi: 10.2337/diacare.17.8.882. [DOI] [PubMed] [Google Scholar]
- 91.Gerritsen M, Kros A, Srpakel V, Lutterman JA, Notle RJM, Jansen JA. Biocompatibility evaluation of sol–gel coating for subcutaneously implantable glucose sensors. Biomaterials. 2000;21:71–78. doi: 10.1016/S0142-9612(99)00136-2. [DOI] [PubMed] [Google Scholar]
- 92.Yu B, Wang C, Ju YM, West L, Harmon J, Moussy Y, et al. Use of hydrogel coating to improve the performance of implanted glucose sensors. Biosens Bioelectron. 2008;23(8):1278–1284. doi: 10.1016/j.bios.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 93.Wilson GS, Gifford R. Biosensors for real-time measurements in vivo. Biosens Bioelectron. 2005;20:2388–2403. doi: 10.1016/j.bios.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 94.Rigby G, Ahmed S, Horseman G, Vadgama P. In vivo glucose monitoring with open microflow—influences of fluid composition and preliminary evaluation in man. Anal Chim Acta. 1999;385:23–32. doi: 10.1016/S0003-2670(98)00665-5. [DOI] [Google Scholar]
- 95.Bhardwaj U, Papadimitrakopoulos F, Burgess DJ. A review of the development of a vehicle for localized and controlled drug delivery for implantable biosensors. J Diabetes Sci Tech. 2008;2(6):1016–1029. doi: 10.1177/193229680800200611. [DOI] [PMC free article] [PubMed] [Google Scholar]