Abstract
In this study, hepatitis B surface antigen (HBsAg) loaded poly(lactic-co-glycolic acid) (PLGA) microparticles were prepared and coated with chitosan and trimethyl chitosan (TMC) to evaluate the effect of coating material for nasal vaccine delivery. The developed formulations were characterized for size, zeta potential, entrapment efficiency, and mucin adsorption ability. Plain PLGA microparticles demonstrated negative zeta potential. However, coated microparticles showed higher positive zeta potential. Results indicated that TMC microparticles demonstrated substantially higher mucin adsorption when compared to chitosan-coated microparticles and plain PLGA microparticles. The coated and uncoated microparticles showed deposition in nasal-associated lymphoid tissue under fluorescence microscopy. The coated and uncoated microparticles were then administered intranasally to mice. Immune-adjuvant effect was determined on the basis of specific antibody titer observed in serum and secretions using enzyme-linked immunosorbent assay. It was observed that coated particles showed a markedly increased anti-HBsAg titer as compared to plain PLGA microparticles, but the results were more pronounced with the TMC-coated PLGA microparticles.
Key words: chitosan, microparticles, mucosal vaccination, PLGA, vaccine delivery
INTRODUCTION
Hepatitis B virus (HBV) infection is one of the most prevalent chronic viral infections worldwide, with approximately 60% of the world’s population living in areas where HBV is highly endemic (1). The hepatitis B virus may be transmitted through contact with infected blood or other body fluids (viz., semen, vaginal fluid, etc.). Chronic HBV infection leads to the development of cirrhosis and/or liver cancer in approximately 20% of infected individuals, and immunization with hepatitis B vaccine is the most effective prevention measure. The currently available hepatitis B vaccine comprises of recombinant hepatitis B surface antigen (HBsAg) adsorbed to aluminum-based adjuvant (aluminum hydroxide or aluminum phosphate) and is recommended to be administered through intramuscular route, but it does not induce mucosal antibodies efficiently (2).
Mucosal immunization via nasal route is an attractive alternative to parenteral immunization as it does not require needles, avoiding the pain and discomfort associated with the parenteral administration (3). Moreover, mucosal immunization accompanies the induction of both mucosal and systemic immune responses (4,5). Biodegradable polymeric particles such as microparticles (6,7) and nanoparticles (8,9) have emerged as promising candidates due to their inherent immune-adjuvant property and ability to provide prolonged release characteristics. These particulate carriers in association with antigen induce strong immune response as compared to soluble antigen (6–9). Several studies have shown microparticles to offer many advantages over other nasal dosage forms (8,10). It has been shown that these particles are taken up by nasal epithelia and nasal-associated lymphoid tissue (NALT) (11,12).
Poly(lactic-co-glycolic acid) (PLGA), a biocompatible and biodegradable polymer with sustained release property, is extensively used for the therapeutic delivery of proteins and peptides including vaccines. PLGA has however limited use in mucosal vaccination due to its poor mucoadhesiveness and immunoenhancing ability. The half time of clearance of non-mucoadhesive formulations from the human nasal cavity is only about 20 min (13). Such a rapid clearance time may not allow sufficient retention for antigen to be taken up by antigen-presenting cells in the NALT.
Incorporation of mucoadhesive polymers such as chitosan to the delivery system can overcome such limitations and increases absorption of protein and peptides across the mucosal barrier (14,15) by prolonging their residence time in the nasal cavity (16,17). In case of vaccine delivery, such polymers enhance uptake by microfold (M)-cells, allowing antigens to be taken up specifically by antigen-presenting cells (18–20). Several studies have employed chitosan as coating material for its penetration-enhancing properties (21,22). It has been postulated that positive charge of chitosan, imparted by amine groups, interact with apical cell membrane by the mechanism of direct electrostatic interaction and leads to transient opening of tight junctions, subsequently increasing particle permeability (14,23). However, at physiological pH, native chitosan and its salts fail to act as permeability enhancer, due to reduced solubility and low positive charge (24). Therefore, there is a need for chitosan derivatives with increased solubility and high positive charge at neutral or basic pH, such as quaternized derivatives of chitosan with polyampholytic properties. These derivatives, e.g., trimethyl chitosan (TMC) can increase the solubility without affecting their cationic character (23,25–27). Because of these properties, TMC may be an attractive alternative to chitosan for the design of mucosal delivery purposes. To date, several studies have used chitosan as coating material, but the use of TMC as a coating material has been overlooked.
In a previous study, we have shown that coating of chitosan over PLGA microparticles can significantly enhance the immune response as compared to PLGA microparticles (21). The specific intent of the present study was to compare the efficacy of chitosan- and TMC-coated PLGA microparticles for nasal immunization. Thus, PLGA microparticles were prepared and coated with chitosan and TMC. The antigen-loaded coated and uncoated microparticles were administered intranasally to mice, and the immune response was determined using enzyme-linked immunosorbent assay (ELISA).
MATERIALS AND METHODS
Materials
PLGA with a lactide to glycolide ratio of 50:50 (MW 40–75 kDa) was kindly gifted by the National Institute of Immunology (New Delhi, India). Chitosan was purchased from Fluka with the deacetylation value 80% (according to provider’s specifications). Recombinant HBsAg (MW 24 kDa) was kindly gifted by Serum Institute of India Ltd. (Pune, India). BCA protein estimation kit and protein molecular weight markers were purchased from Genei, Bangalore, India. AUSAB® monoclonal antibody kit was procured from Abbott Laboratories, USA. All other chemicals and reagents were of analytical grade. TMC was synthesized by the method previously reported by Sieval et al. with minor modifications (28).
Preparation of Surface-modified PLGA Microparticles
Surface-modified PLGA microparticles were prepared by a modified double emulsion solvent evaporation process (21). Briefly, a primary emulsion (water-in-oil) was formulated by emulsifying HBsAg aqueous phase containing 1.5% (w/v) trehalose and 2% (w/v) Mg(OH)2 with 4% (w/v) PLGA in methylene chloride using a probe sonicator (Soniweld, India) for 1 min. The coating polymers were dissolved in different concentrations (0.1%, 0.25%, 0.5%, and 0.75%, w/v) in 1% polyvinyl alcohol (PVA) solution. Chitosan was dissolved in acetate buffer (pH 4.4), whereas TMC was dissolved in distilled water. The secondary emulsion (water-in-oil-in-water) was obtained by adding the primary emulsion dropwise to the PVA solution containing different concentrations of coating polymers, followed by probe sonication for 3 min. The resultant emulsion was stirred vigorously for 3 h to evaporate the organic phase and to obtain the microparticles, which were collected by centrifugation at 22,000 g and washed twice with distilled water to remove PVA. The microparticles were then subjected to lyophilization. Uncoated PLGA microparticles were also prepared with 1% PVA solution.
Characterization
Surface Morphology by Scanning Electron Microscopy
The morphology and surface appearance of the particles were examined by scanning electron microscopy (SEM). One drop of the particles suspension was placed on a gold-coated plate and maintained at least 12 h at room temperature in desiccators for complete dryness of the sample. The stub was then coated with gold using sputter coater. The sample was randomly scanned using SEM (LEO-435 VP, Cambridge, UK), and photomicrographs were taken.
Particle Size and Zeta Potential
Malvern zetasizer Nano ZS 90 (Malvern apparatus, UK) was used to evaluate the mean diameter and size distribution profiles of the microparticles by dynamic light scattering. The same instrument was used to determine the zeta potential of the formulations, based on electrophoretic mobility of the microparticles in diluted aqueous suspensions. For the determination of zeta potential, microparticles were suspended in 1 mM HEPES buffer (Sigma, USA), and the pH was adjusted to 7.4.
Protein Loading Efficiency
The loading efficiency of the antigen in microparticles was determined by dissolving 20 mg the microparticles in 2 ml of 5% (w/v) sodium dodecyl sulfate (SDS) in 0.1 M sodium hydroxide solution. The amount of the antigen was determined by the bicinchoninic acid assay using the BCA protein estimation kit (KT-31).
Assessment of Structural Integrity of HBsAg
The structural integrity of HBsAg extracted from the microparticles was detected by SDS polyacrylamide gel electrophoresis (PAGE) and compared with the native HBsAg and reference markers. HBsAg was extracted by dissolving the microparticles in 2 ml of 5% (w/v) SDS in 0.1 M sodium hydroxide solution (29). The extracted antigen was concentrated and loaded onto 3.5% stacking gel and subjected to electrophoresis on a 12% separation gel at 200 V (Bio-Rad, USA) until the dye band reached the gel bottom. After migration, the gel was stained with Coomassie blue to reveal the antigen, which was then destained and dried.
Adsorption of Mucin on Microparticles
Adsorption of mucin on the plain and coated PLGA microparticles was studied by following the procedure previously used in our laboratory (30). Briefly, equal volumes of microparticles (2 mg/ml) and an aqueous solution of mucin (0.5 mg/ml) were mixed, vortexed, and shaken at room temperature for 60 min. The suspension was then centrifuged, and the supernatant was used to determine the free mucin content. A colorimetric assay for glycoproteins based on the periodic acid/Schiff staining was used for the determination of mucin concentration. The mucin adsorbed on the surface of the microparticles was calculated from the total and free mucin.
In Vitro Release of HBsAg
An amount of 40 mg of microparticles was suspended in 5 ml of phosphate buffered saline (PBS; pH 7.4) and kept on a shaking water bath for incubation at 37°C. Tween-80 (0.02%, w/v) was added to the release media to reduce the adsorption of the released protein on to the microparticles and to prevent the particles from clumping. At appropriate time intervals, 1.0 ml of release medium was collected and centrifuged at 22,000 g for 30 min, and 1.0 ml of fresh PBS (pH 7.4) was again added to maintain the sink conditions.
Fluorescence Microscopy
Fluorescence microscopy was performed to confirm deposition of microparticles in NALT. Fluorescent isothiocyanate-conjugated bovine serum albumin (FITC-BSA) was used as a fluorescence marker and was loaded into microparticles. FITC-BSA microparticles were prepared according to the optimized double emulsion solvent evaporation method, described elsewhere in the text, using a 0.05% FITC-BSA solution in PBS as internal aqueous phase. FITC-BSA-loaded formulation was administered to mice through the nostrils, and the mice were sacrificed after 30 min. The nasal cavity containing nasal mucosa was cut into pieces, and microtomy was performed. Sections of around 5 μm thickness were examined under fluorescence microscope (Nikon Eclipse, E-600). Control animals were administered intranasally with the equivalent amount of free FITC-BSA solution (5 mg in 500 μl PBS, pH 7.4), and microtomy was performed.
In Vivo Immunological Response
Female BALB/c mice of 7–9 weeks of age were used in all experiments as mice NALT is comparable to the Waldeyer’s rings in humans (31). Animals were housed in groups of six (n = 6) with free access to food and water, and were fasted for 3 h before immunization. The study protocol was approved by Institutional Animals Ethical Committee of Dr. Hari Singh Gour University. The studies were carried out according to the guidelines of Council for the Purpose of Control and Supervision of Experiments on Animals, Ministry of Environment and Forestry, Government of India. There were five groups of mice in this study, three of which received a single immunization regimen of HBsAg loaded plain PLGA, chitosan, and TMC-coated PLGA microparticles. The remaining two groups were immunized with alum adsorbed HBsAg (intramuscular) and soluble HBsAg (intranasal) and received a booster dose on day 28.
A dose of the formulations equivalent to 10 µg antigen was inoculated intranasally in small drops (10 µl). Nasal dosing was performed by inserting a small piece of sterile polyethylene tubing, attached to a Hamilton syringe, 0.2 cm into the nostril. A volume of 10 µl microparticles formulation/nostril was injected into the nasal cavity of each non-anesthetized animal held in a supine position. A new drop was given only when the former had been entirely inspired.
Sample Collection
Blood was collected by retro-orbital puncture under mild ether anesthesia after 2, 4, 6, and 8 weeks of booster injections, and sera were stored at −40°C until tested by ELISA for anti-HBsAg antibody. Nasal, vaginal, and salivary secretions were collected on day 42 of primary immunization. Vaginal wash was obtained according to the method reported by Debin et al. (32). Briefly, 50 µl of PBS containing 1% (w/v) BSA (1% BSA-PBS) was introduced into the vaginal tract of non-anesthetized mice using a Gilson pipette. Aliquots of 50 µl were withdrawn and reintroduced nine times. The nasal wash was collected by cannulation of the trachea of sacrificed mice. The nasal cavity was then flushed three times with 0.5 ml of 1% BSA/PBS (pH 7.4). Salivation was induced by injecting 0.2 ml sterile pilocarpine solution (10 mg/ml) intraperitoneally (33). The saliva from mice after 20 min was collected using capillary tube. These fluids were stored with 100 mM phenylmethyl sulfonyl fluoride as a protease inhibitor at −40°C until tested by ELISA for secretory antibody (sIgA) levels.
Specific IgG and IgA Titer
Anti-HBsAg antibodies in blood samples were determined by an enzyme-linked immunoassay. Briefly, microtiter plates (Nunc-Immuno Plate® Fb 96 Mexisorp, NUNC) were coated with 100 μl/well of 2 μg/ml HBsAg in carbonate buffer (pH 9.6) and incubated overnight at 4°C. The plates were washed three times with PBS-Tween 20 (0.05%, v/v) (PBS-T) and blocked with PBS-BSA (3% w/v) for 2 h at 37°C, followed by washing with PBS-T. The serum/secretion samples were serially diluted with PBS. One hundred microliters of these serially diluted serum and secretion samples were added to the wells of coated ELISA plates. The plates were incubated for 1 h at room temperature and washed three times with PBS-T. One hundred microliters of horse reddish peroxidase labeled goat anti-mouse IgG and IgA (1:1,000 dilution, Sigma, USA) antibodies were added to well for the determination of IgG and IgA titer, respectively. The plates were kept for 1 h at room temperature and then washing was repeated. One hundred microliters of tetramethyl benzidine (TMB-H2O2) solution was added to each well. Color development was stopped after 30 min by adding 50 μl of 1 N H2SO4 to each well, and absorbance was taken at 490 nm using a plate reader (Bio-Rad, USA). The end-point titers were expressed as the log reciprocal of the last dilution, which gave the absorbance value above the absorbance of negative control at a wavelength of 490 nm.
Statistical Analysis
All data were expressed as mean ± standard deviation. Comparisons among three or more groups were performed by analysis of variance followed by post hoc Tukey–Kramer test. For comparison between two groups, Student’s t test was applied. A p value less than 0.05 was considered statistically significant.
RESULTS
Formulation and Characterization
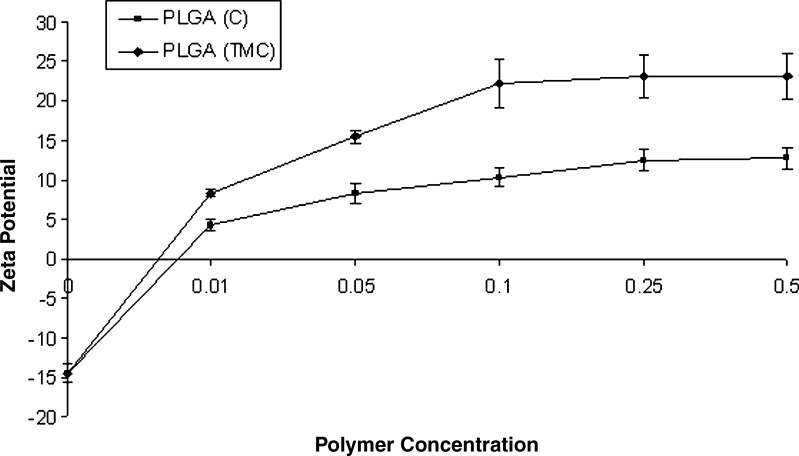
In order to achieve complete coating, various concentration of chitosan and TMC were used, and zeta potential was determined (Fig. 1). It was observed that unmodified PLGA microparticles indicated negative zeta potential (−14.4 ± 1.2). However, chitosan and TMC-coated microparticles (PLGA-C and PLGA-TMC, respectively) demonstrated positive zeta potential. The charge of the coated particles increased with the concentration of the coating polymer, reaching a plateau at 0.25% w/v of chitosan and TMC, possibly indicating the complete coating over the microparticles. This concentration of the polymer is designated as optimum, and microparticles formulated using 0.25% of chitosan and TMC were used for further studies. PLGA-TMC microparticles demonstrated a sharper increase in zeta potential as a function of polymer concentration when compared to PLGA-C microparticles.
Fig. 1.
Zeta potential analysis: graph showing change in zeta potential with change in coating polymer concentration
The external morphology of the microparticles was studied by SEM. The study revealed that most of the microparticles were approximately spherical in shape having a smooth surface. The particle characteristics of plain PLGA, PLGA-C, and PLGA-TMC microparticles were shown in Table I. The antigen loading efficiency was comparable in both coated and uncoated PLGA microparticles (Table I).
Table I.
Particle Characteristics of Prepared Microparticles
| Microparticles | Average particle size (nm) | PDI | % EE |
|---|---|---|---|
| PLGA | 421 ± 34 | 0.083 ± 0.003 | 54.2 ± 5.2 |
| PLGA-C | 485 ± 58 | 0.121 ± 0.006 | 42.4 ± 4.6 |
| PLGA-TMC | 467 ± 65 | 0.144 ± 0.009 | 42.4 ± 6.3 |
Values are expressed as mean ± SD
% EE percentage entrapment efficiency, PDI polydispersity index
In Vitro Release
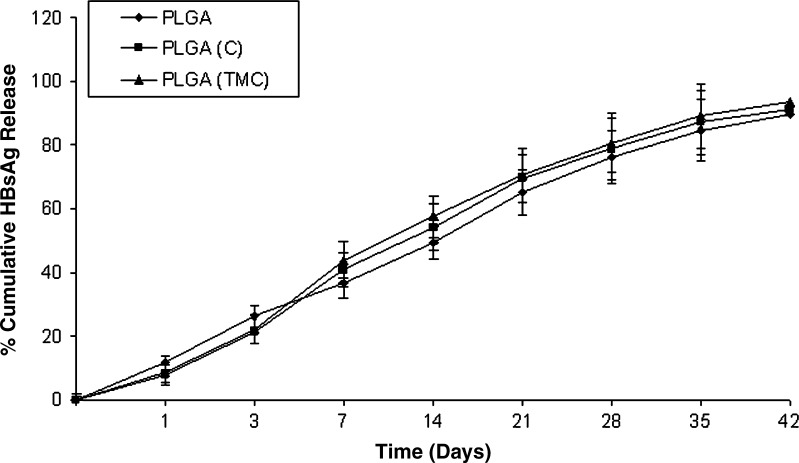
In vitro release of HBsAg from the uncoated PLGA, PLGA-C, and PLGA-TMC microparticles was determined in PBS, pH 7.4 (Fig. 2). Both coated and uncoated microparticles exhibited an initial burst release followed by a sustained release of HBsAg. The initial burst release observed may be attributed to the release of antigen loosely attached to the surface of the particles. However, the sustained release observed may be attributed to the diffusion of HBsAg from microparticles and gradual erosion of the polymers. It was observed that antigen released from the microparticles was approximately 70% on day 42 in both coated and uncoated microparticles. This result indicated that PLGA microparticles can offer prolonged delivery of the antigen for development of single shot vaccine.
Fig. 2.
In vitro release study: graph showing percentage of cumulative hepatitis B surface antigen release from coated and uncoated poly(lactic-co-glycolic acid) microparticles in PBS (pH 7.4)
Confirmation of the Structural Integrity of the Antigen
The encapsulation of protein and peptides in PLGA microparticles involve the use of organic solvents and harsh shearing conditions, which may cause the alteration in the native form of such susceptible moieties. In addition, release of lactic acid and glycolic acid may causes aggregation of protein and antigen. We used trehalose as stabilizer and Mg(OH)2 as acid neutralizing agent to impart the stability to the antigen. In-process stability and integrity of the entrapped antigen was assessed using SDS-PAGE (data not shown). The SDS-PAGE analysis revealed that the native antigen and antigen released from the formulation demonstrated the bands at identical positions. This confirmed that no aggregation and fragmentation of the antigen occur during the process of antigen encapsulation and release.
Adsorption of Mucin on Microparticles
Coated and uncoated PLGA microparticles were evaluated for their mucin adhesion ability as a measure of their mucoadhesiveness. Mucin adsorption (milligram/milligram) of particles were 0.012 ± 0.003, 0.141 ± 0.009, and 0.264 ± 0.020 for PLGA, PLGA-C, and PLGA-TMC microparticles, respectively. These results indicated that PLGA microparticles demonstrated negligible mucin retention, while PLGA-C and PLGA-TMC microparticles demonstrated better mucin retention ability as compared to uncoated PLGA microparticles (p < 0.05). It was observed that TMC-coated microparticles demonstrated substantially high mucin adsorption as compared to chitosan-coated PLGA microparticles (p < 0.05).
Fluorescence Microscopy
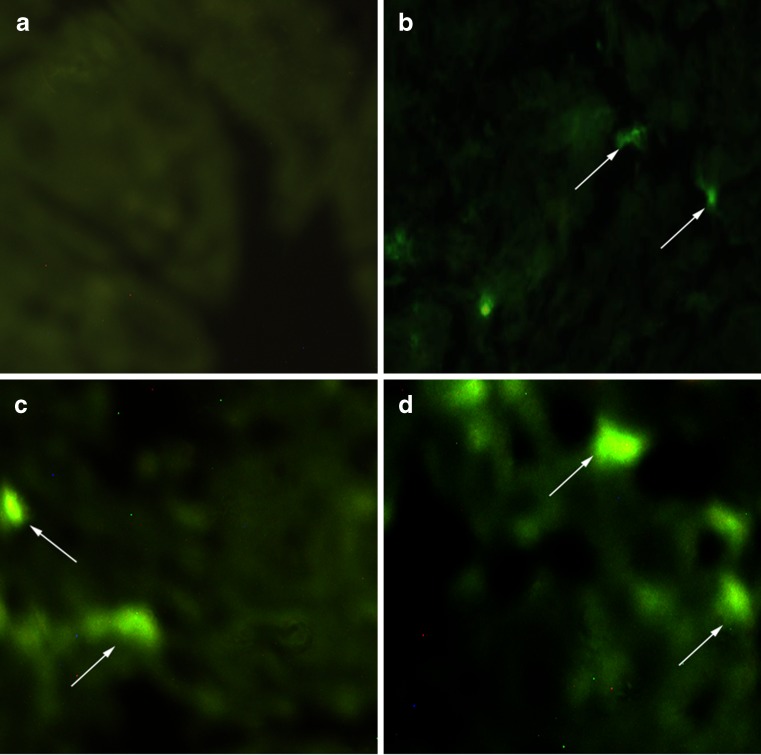
It has been reported that microparticles are selectively taken up by M cells (34–36). These M cells are mainly responsible for antigen delivery to the NALT for induction of specific systemic and mucosal immune response (37–39). The uptake of coated and uncoated microparticles into the NALT was investigated using FITC-BSA as a fluorescent marker (Fig. 3). Fluorescence microscopy confirmed that FITC-BSA solution (PBS, pH 7.4) could not produce any fluorescence under fluorescent microscope. However, fluorescent microscopy image of mice treated nasally with dye loaded microparticles demonstrated uptake of microparticles in nasal mucosa.
Fig. 3.
Fluorescence microscopy images showing the uptake of FITC-BSA loaded microparticles in NALT. a Soluble FITC-BSA (PBS, pH 7.4). b Plain poly(lactic-co-glycolic acid) (PLGA) microparticles. c PLGA-C microparticles. d PLGA-TMC microparticles. Several fluorescence spots were indicated by arrows showing the uptake of microparticles
Immunological Response
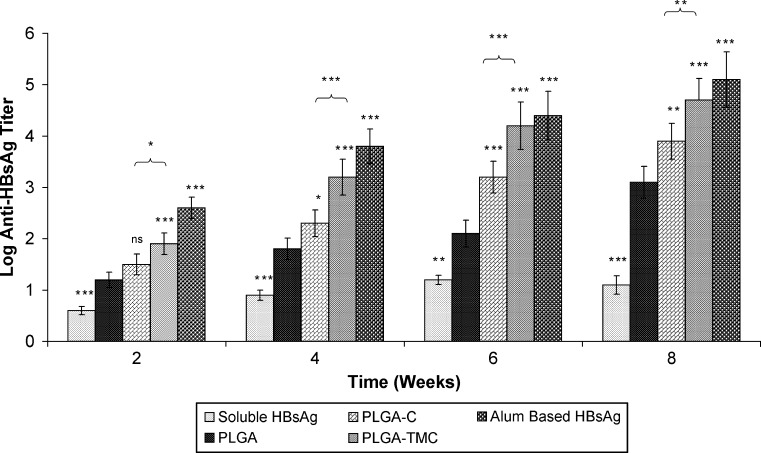
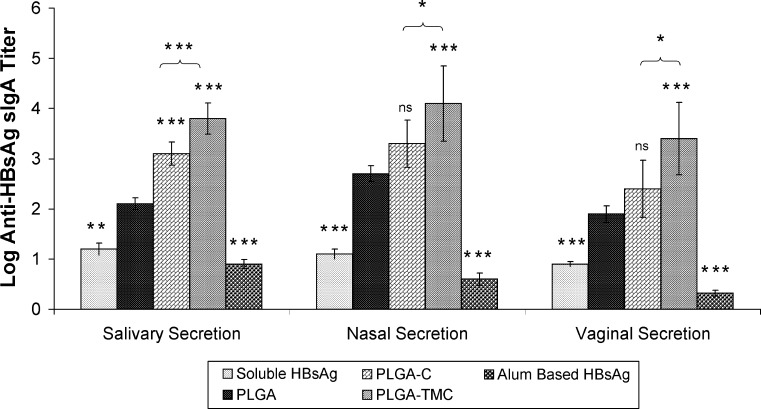
The specific antibody titer (anti-HBsAg) in serum and secretions is shown in Figs. 4 and 5, respectively. Our results indicated that all mice immunized intranasally with microparticles-loaded HBsAg were seropositive after 2 weeks. It was observed that intramuscular injection of alum adsorbed HBsAg induces high anti-HBsAg antibody titer as compared to both coated and uncoated PLGA microparticles following second week of immunization, and the coated microparticles could induce strong antibody titer as compared to uncoated PLGA microparticles. Results also indicated that PLGA-TMC microparticles could induce a substantially higher IgG titer as compared to PLGA-C microparticles (p < 0.05) throughout the study. A major advantage of intranasal vaccination is the potential induction of sIgA antibodies at the mucosal epithelium. sIgA not only has an important role as the first defense line against viruses at the portal of virus entry in the mucosal tract but also has been proven to elicit cross protective immunity more effectively than serum IgG (39). Specific sIgA was determined in local (nasal) and distal (vaginal and salivary) secretions (Fig. 5). Results indicated that nasal immunization with microparticles-based HBsAg could induce substantially high antibody titer in local and distal secretions as compared to soluble or alum adsorbed HBsAg. Amongst these microparticles, PLGA-TMC microparticles were found to be most impressive as they showed substantially higher antibody titer (p < 0.001) in all secretions as compared to PLGA microparticles, whereas PLGA-C showed significantly higher sIgA titer only in salivary (p < 0.001) secretions as compare to PLGA microparticles.
Fig. 4.
Anti-hepatitis B surface antigen IgG titer in serum after the indicated treatments. Asterisk over bars indicated degree of significance as compare to poly(lactic-co-glycolic acid) microparticles (unless indicated), where *p < 0.05, **p < 0.01, ***p < 0.001; ns not significant
Fig. 5.
Anti-hepatitis B surface antigen sIgA titer in nasal, salivary, and vaginal secretions after the indicated treatments. Asterisk over bars indicated degree of significance as compare to poly(lactic-co-glycolic acid) microparticles (unless indicated), where *p < 0.05, **p < 0.01, ***p < 0.001; ns not significant
DISCUSSION
In this study, we explored the mucoadhesive property of chitosan and TMC and sustained release property of PLGA to develop effective vaccine against hepatitis B. The uptake of microparticles by nasal epithelial and NALT cells depends in particular on their size and charge (40,41). It was observed that PLGA microparticles demonstrated negative zeta potential, which was found to be inverted following coating with chitosan and TMC. The zeta potential of TMC-coated PLGA microparticles was substantially higher as compared to chitosan-coated PLGA microparticles. Interestingly, despite its negative charge, PLGA microparticles showed deposition in NALT under fluorescent microscopy. This may be attributed to the size-dependent uptake of microparticles in NALT as it has been a widely documented fact that microparticles are taken up by both M cells and epithelial cells (40,42–44). It was also observed that plain PLGA microparticles showed minimal mucin adhesion. Therefore, it can be postulated that although the PLGA microparticles can be taken up by NALT, the residence time of microparticles in the nasal cavity is low due to lack of mucoadhesiveness. In view of the fact that chitosan demonstrated low positively charged at physiological pH, such as in the mucus, we can suggest that the better immune-adjuvant effect of TMC over chitosan may be attributed to the high positive charge of the TMC-coated particles. It has been reported that mucin is a negatively charged molecule, and the particles with high charge density shows better interaction with mucus glycoproteins and consequently result into the better mucoadhesiveness (45). Hence, TMC could substantially reduce the rate of clearance of PLGA microparticles from the nasal cavity and increase their residence time, thereby promoting its entry into epithelial cells.
The in vivo data obtained indicated that the PLGA microparticles induce low antibody titer as compared to chitosan and TMC-coated microparticles in serum and secretions. It can be suggested that coating of PLGA microparticles with mucoadhesive polymers such as chitosan and TMC enhances their residence time in the nasal cavity. Therefore, coated particles are expected to remain homogeneously dispersed in the mucus and in good contact with nasal mucosa. This could likely be one possible explanation why the chitosan and TMC-coated PLGA microparticles have shown higher antibody titer following IN administration as compared with plain PLGA microparticles. It has been suggested that due to better solubility and penetration-enhancing ability at physiological pH, TMC can act as a good carrier for mucosal drug delivery. It was also found that the PLGA-TMC microparticles demonstrated much stronger immune-adjuvant property as compared to PLGA-C microparticles (p < 0.05 throughout the study). The reasons for these observations are likely due to higher charge density observed in case of TMC-coated PLGA microparticles. In addition, it is known that chitosan is insoluble and precipitates at physiological pH, while TMC is soluble and demonstrate the absorption enhancing ability at wide variety of pH.
CONCLUSION
Our results provide evidence that the immunogenicity after intranasal immunization of HBsAg could be substantially improved by loading the antigen into chitosan and TMC-coated PLGA microparticles. Our study clearly indicated that TMC is a promising coating material for PLGA microparticles and demonstrate strong immuno-adjuvant activity as compared to chitosan for nasal immunization. More specifically, PLGA microparticles coated with positively charged, hydrophilic polymer such as TMC have shown an improved ability to deliver vaccines across the nasal mucosa for induction of strong immune response in systemic and mucosal compartments. Finally, we speculate that TMC-coated microparticles represent a new generation intranasal vaccine delivery system. However, further in vitro and in vivo toxicity studies should be performed to check the safety of the developed formulations.
Acknowledgements
We are grateful to Dr. Umesh Shaligram (Serum Institute of India Ltd, Pune) for providing recombinant hepatitis B surface antigen (HBsAg). We are thankful to All India Institute of Medical Sciences and Punjab University for providing SAIF facility. We would also like to acknowledge All India Council of Technical Education (AICTE) for providing Junior Research Fellowship to carry out the research work.
References
- 1.Shepard CW, Simard EP, Finelli L, Fiore AE, Bell BP. Hepatitis B virus infection: epidemiology and vaccination. Epidemiol Rev. 2006;28:112–125. doi: 10.1093/epirev/mxj009. [DOI] [PubMed] [Google Scholar]
- 2.Gupta RK, Siber GR. Adjuvants for human vaccines—current status, problems and future prospects. Vaccine. 1995;13:1263–1276. doi: 10.1016/0264-410X(95)00011-O. [DOI] [PubMed] [Google Scholar]
- 3.Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med. 2005;11(4):45–53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- 4.Illum L, Davis SS. Nasal vaccination: a non-invasive vaccine delivery method that holds great promise for the future. Adv Drug Deliv Rev. 2001;51:1–3. doi: 10.1016/S0169-409X(01)00171-5. [DOI] [PubMed] [Google Scholar]
- 5.Iqbal M, Lin W, Jabbal-Gill I, Davis SS, Steward MW, Illum L. Nasal delivery of chitosan-DNA plasmid expressing epitopes of respiratory syncytial virus (RSV) induces protective CTL response in BALB/c mice. Vaccine. 2003;21:1478–1485. doi: 10.1016/S0264-410X(02)00662-X. [DOI] [PubMed] [Google Scholar]
- 6.Moynihan JS, Jones DH, Farrar GH, Howard CR. A novel microencapsulated peptide vaccine against hepatitis B. Vaccine. 2001;19:3292–3300. doi: 10.1016/S0264-410X(00)00540-5. [DOI] [PubMed] [Google Scholar]
- 7.Jaganathan KS, Singh P, Prabakaran D, Mishra V, Vyas SP. Development of a single dose stabilized poly(D, L-lactic-co-glycolic acid) microspheres-based vaccine against hepatitis B. J Pharm Pharmacol. 2004;56:1243–1250. doi: 10.1211/0022357044418. [DOI] [PubMed] [Google Scholar]
- 8.Vila A, Sanchez A, Tobio M, Calvo P, Alonso MJ. Design of biodegradable particles for protein delivery. J Control Release. 2002;78:15–24. doi: 10.1016/S0168-3659(01)00486-2. [DOI] [PubMed] [Google Scholar]
- 9.Koping-Hoggard M, Sanchez A, Alonso MJ. Nanoparticles as carriers for nasal vaccine delivery. Expert Rev Vaccines. 2005;4:185–196. doi: 10.1586/14760584.4.2.185. [DOI] [PubMed] [Google Scholar]
- 10.Soane RJ, Ferier M, Perkins AC, Jones NS, Davis SS, Illum L. Evaluation of the clearance characteristics of bioadhesive system in humans. Int J Pharm. 1999;178:55–65. doi: 10.1016/S0378-5173(98)00367-6. [DOI] [PubMed] [Google Scholar]
- 11.Almeida AJ, Alpar HO, Brown MRW. Immune response to nasal delivery of antigenically intact tetanus toxoid associated with poly (l-lactic acid) microspheres in rats, rabbits and guinea-pigs. J Pharm Pharmacol. 1993;45:198–203. doi: 10.1111/j.2042-7158.1993.tb05532.x. [DOI] [PubMed] [Google Scholar]
- 12.Alpar HO, Almeida AJ, Brown MRW. Microsphere absorption by the nasal mucosa of the rat. J Drug Target. 1994;2:147–149. doi: 10.3109/10611869409015903. [DOI] [PubMed] [Google Scholar]
- 13.Illum L. Nasal drug delivery-possibilities, problems and solutions. J Control Release. 2003;87:187–198. doi: 10.1016/S0168-3659(02)00363-2. [DOI] [PubMed] [Google Scholar]
- 14.Artursson P, Lindmark T, Davis SS, Illum L. Effect of chitosan on the permeability of monolayer of intestinal epithelial cells (Caco-2) Pharm Res. 1994;11:1358–1361. doi: 10.1023/A:1018967116988. [DOI] [PubMed] [Google Scholar]
- 15.Borchard G, Lueßen HL, de Boer AG, Verhoef JC, Lehr CM, Junginger HE. The potential of mucoadhesive polymers in enhancing intestinal peptide drug absorption. III: effects of chitosan-glutamate and carbomer on epithelial tight junctions in vitro. J Control Release. 1996;39:131–138. doi: 10.1016/0168-3659(95)00146-8. [DOI] [Google Scholar]
- 16.Soane RJ, Hinchcliffe M, Davis SS, Illum L. Clearance characteristics of chitosan based formulation in the sheep nasal cavity. Int J Pharm. 2001;217:183–191. doi: 10.1016/S0378-5173(01)00602-0. [DOI] [PubMed] [Google Scholar]
- 17.Bivas-Benita M, Laloup M, Versteyhe S, Dewit J, De Braekeleer J, Jongert E, et al. Generation of Toxoplasma gondii GRA1 protein and DNA vaccine loaded chitosan particles: preparation, characterization, and preliminary in vivo studies. Int J Pharm. 2003;266:17–27. doi: 10.1016/S0378-5173(03)00377-6. [DOI] [PubMed] [Google Scholar]
- 18.van der Lubben IM, Kersten G, Fretz MM, Beuvery C, Verhoef JC, Junginger HE. Chitosan microparticles for mucosal vaccination against diphtheria: oral and nasal efficacy studies in mice. Vaccine. 2003;28:1400–1408. doi: 10.1016/s0264-410x(02)00686-2. [DOI] [PubMed] [Google Scholar]
- 19.Vila A, Sanchez A, Janes K, Behrens I, Kissel T, Vila Jato JL, et al. Low molecular weight chitosan nanoparticles as new carriers for nasal vaccine delivery in mice. Eur J Pharm Biopharm. 2004;57:123–131. doi: 10.1016/j.ejpb.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Atyabia F, Moghaddama FA, Dinarvanda R, Zohuriaan-Mehrd MJ, Ponchele G. Thiolated chitosan coated poly hydroxyethyl methacrylate nanoparticles: synthesis and characterization. Carbohydr Polym. 2008;74(1):59–67. doi: 10.1016/j.carbpol.2008.01.015. [DOI] [Google Scholar]
- 21.Jaganathan KS, Vyas SP. Strong systemic and mucosal immune responses to surface-modified PLGA microspheres containing recombinant hepatitis B antigen administered intranasally. Vaccine. 2006;24:4201–4211. doi: 10.1016/j.vaccine.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 22.Ravi Kumar MNV, Bakowsky U, Lehr CM. Preparation and characterization of cationic PLGA nanospheres as DNA carriers. Biomaterials. 2004;25:1771–1777. doi: 10.1016/j.biomaterials.2003.08.069. [DOI] [PubMed] [Google Scholar]
- 23.Schipper NG, Olsson S, Hoogstraate JA. deBoer AG, Varum KM, Artursson P. Chitosan as absorption enhancers for poorly absorbable drugs 2: mechanism of absorption enhancement. Pharm Res. 1997;14:923–929. doi: 10.1023/A:1012160102740. [DOI] [PubMed] [Google Scholar]
- 24.Kotzé AF, Luessen HL, de Leeuw BJ, de Boer AG, Verhoef JC, Junginger HE. Comparison of the effect of different chitosan salts and N-trimethyl chitosan chloride on the permeability of intestinal epithelial cells (Caco-2) J Control Release. 1998;51:35–46. doi: 10.1016/S0168-3659(97)00154-5. [DOI] [PubMed] [Google Scholar]
- 25.Thanou MM, Kotze AF, Scharringhausen T, Luessen HL, de Boer AG, Verhoef JC, et al. Effect of degree of quaternization of N-trimethyl chitosan chloride for enhanced transport of hydrophilic compounds across intestinal caco-2 cell monolayers. J Control Release. 2000;64:15–25. doi: 10.1016/S0168-3659(99)00131-5. [DOI] [PubMed] [Google Scholar]
- 26.Thanou M, Verhoef JC, Junginger HE. Oral drug absorption enhancement by chitosan and its derivatives. Adv Drug Deliv Rev. 2001;52:117–126. doi: 10.1016/S0169-409X(01)00231-9. [DOI] [PubMed] [Google Scholar]
- 27.Sadeghi AMM, Dorkoosh FA, Avadi MR, Weinhold M, Bayat A, Delie F, Gurny R, Larijani B, Rafiee-Tehrani M, Junginger HE. Permeation enhancer effect of chitosan and chitosan derivatives: comparison of formulations as soluble polymers and nanoparticulate systems on insulin absorption in Caco-2 cells. Eur J Pharm Biopharm. 2008;70:270–278. doi: 10.1016/j.ejpb.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Sieval AB, Thanou M, Kotze AF, Verhoef JC, Brussee J, Junginger HE. Preparation and NMR characterization of highly substituted N-trimethyl chitosan chloride. Carbohydr Polym. 1998;36:157–165. doi: 10.1016/S0144-8617(98)00009-5. [DOI] [Google Scholar]
- 29.Eldridge JH, Hammond CJ, Meulbroek JA, Staas JK, Gilley RM, Rice TR. Controlled vaccine release in the gut associated lymphoid tissues. Orally administered biodegradable microspheres target the Peyer’s patches. J Control Release. 1990;11:205–214. doi: 10.1016/0168-3659(90)90133-E. [DOI] [Google Scholar]
- 30.Khatri K, Goyal AK, Gupta PN, Mishra N, Mehta A, Vyas SP. Surface modified liposomes for nasal delivery of DNA vaccine. Vaccine. 2008;26:225–2233. doi: 10.1016/j.vaccine.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 31.Vajdy M, O’Hagan DT. Microparticles for intranasal immunization. Adv Drug Deliv Rev. 2001;51:127–141. doi: 10.1016/S0169-409X(01)00167-3. [DOI] [PubMed] [Google Scholar]
- 32.Debin A, Kravtzoff R, Santiago JV, Cazales L, Sperandio S, Melber K, et al. Intranasal immunization with recombinant antigens associated with new cationic particles induces strong mucosal as well as systemic antibody and CTL responses. Vaccine. 2002;20:2752–2763. doi: 10.1016/S0264-410X(02)00191-3. [DOI] [PubMed] [Google Scholar]
- 33.Jain S, Singh P, Mishra V, Vyas SP. Mannosylated niosomes as adjuvant-carrier system for oral genetic immunization against hepatitis B. Immunol Lett. 2005;101(1):41–49. doi: 10.1016/j.imlet.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Smith MW, Thomas NW, Jenkins NW, Miller PG, Cremaschi NGA, Porta C. Selective transport of microparticles across Peyer’s patches follicle associated M cells from mice and rats. Exp Physiol. 1995;80:735–743. doi: 10.1113/expphysiol.1995.sp003882. [DOI] [PubMed] [Google Scholar]
- 35.Jani PU, McCarthy DE, Florence AT. Nanosphere and microsphere uptake via Peyer’s patches: observation of the rate of uptake after a single oral dose. Int J Pharm. 1992;86:239–246. doi: 10.1016/0378-5173(92)90202-D. [DOI] [Google Scholar]
- 36.Almeida AJ, Alpar HO. Nasal delivery of vaccines. J Drug Target. 1996;3:455–467. doi: 10.3109/10611869609015965. [DOI] [PubMed] [Google Scholar]
- 37.Kuper CF, Koornstra PJ, Hameleers DM, Biewenga J, Spit BJ, Duijvestijn AM, et al. The role of nasopharyngeal lymphoid tissue. Immunol Today. 1992;13:219–224. doi: 10.1016/0167-5699(92)90158-4. [DOI] [PubMed] [Google Scholar]
- 38.Kiyono H, Fukuyama S. NALT versus Peyer’s-patch-mediated mucosal immunity. Nat Rev Immunol. 2004;4:699–710. doi: 10.1038/nri1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ito R, Ozaki YA, Yoshikawa T, Hasegawa H, Sato Y, Suzuki Y, et al. Roles of antihemagglutinin IgA and IgG antibodies in different sites of the respiratory tract of vaccinated mice in preventing lethal influenza pneumonia. Vaccine. 2003;21:2362–2371. doi: 10.1016/S0264-410X(03)00078-1. [DOI] [PubMed] [Google Scholar]
- 40.Huang Y, Donovan MD. Large molecule and particulate uptake in the nasal cavity: the effect of size on nasal absorption. Adv Drug Deliv Rev. 1998;29:147–155. doi: 10.1016/S0169-409X(97)00066-5. [DOI] [PubMed] [Google Scholar]
- 41.Desai M, Labhasetwar V, Amidon G, Levy R. Gastrointestinal uptake of biodegradable microparticles: effect of particle size. Pharm Res. 1996;13:1838–1845. doi: 10.1023/A:1016085108889. [DOI] [PubMed] [Google Scholar]
- 42.Delie F. Evaluation of nano- and microparticle uptake by the gastrointestinal tract. Adv Drug Deliv Rev. 1998;34:221–233. doi: 10.1016/S0169-409X(98)00041-6. [DOI] [PubMed] [Google Scholar]
- 43.Brooking J, Davis SS, Illum L. Transport of nanoparticles across the rat nasal mucosa. J Drug Target. 2001;9:267–279. doi: 10.3109/10611860108997935. [DOI] [PubMed] [Google Scholar]
- 44.Smart JD. The basics and underlying mechanisms of mucoadhesion. Adv Drug Deliv Rev. 2005;57(11):1556–1568. doi: 10.1016/j.addr.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 45.Peppas NA, Buri PA. Surface, interfacial and molecular aspects of polymer bioadhesion on soft tissues. J Control Release. 1985;2:257–275. doi: 10.1016/0168-3659(85)90050-1. [DOI] [Google Scholar]