Abstract
We examined the cross-sectional relationships between malaria parasitemia and CD4 T cell count and viral load among human immunodeficiency virus (HIV)-infected pregnant women. We then followed women to investigate whether or not baseline parasitemia predicted CD4 T cell counts or viral loads > 90 days post-baseline or predicted time to HIV disease stage 3 or 4 or acquired immune deficiency syndrome (AIDS)-related death (ARD). Parasitemia level was nonlinearly associated with viral load at baseline and among measurements taken > 90 days post-baseline; women with low baseline parasitemia, versus none, had higher viral loads at both time points. Any baseline parasitemia predicted an increased rate of ARD among women with baseline CD4 T cell counts ≥ 500 cells/µL (ratio rate [RR] = 2.6; 95% confidence interval [CI] = 1.1–6.0; P test for heterogeneity = 0.05). Further study is warranted to determine whether or not parasitemia is especially detrimental to individuals with lower levels of immunosuppression or chronic low parasitemia.
Introduction
Since 2003, great strides have been made in the provision of life-saving antiretroviral therapy (ART) to individuals who are infected with human immunodeficiency virus (HIV) in sub-Saharan Africa. In spite of these efforts, recent estimates suggest that approximately two-thirds of HIV-infected individuals in need of ART do not receive it.1 Furthermore, the majority of adults living with HIV has not reached advanced stages of disease and therefore, may not be eligible for ART according to current treatment guidelines.2 The identification of modifiable risk factors for HIV disease progression and death will inform interventions to reduce the incidence of these events and prolong the time before ART is needed.
Worldwide, millions of individuals living with HIV reside in areas where malaria is endemic, and malaria is a leading cause of morbidity among individuals infected with HIV in sub-Saharan Africa.3 Growing evidence suggests a detrimental synergy between these two infectious diseases. Among adults living in malaria-endemic areas, HIV-related immune suppression seems to increase vulnerability to malaria parasitemia (henceforth, parasitemia) or clinical malaria.4–9 Malaria may also facilitate HIV replication through incitement of cytokine production and immune-cell activation.10–12 An increased rate of HIV replication that leads to a lasting elevation in viral load could, in turn, hasten immune system decline and HIV disease progression.13,14 Previous studies among HIV-infected individuals have reported an association between elevated viral load and malaria infection or clinical malaria.15–17 At present, there is insufficient evidence to determine whether or not malaria-induced changes in CD4 T cell counts or viral loads translate to an increased rate of HIV disease progression or death in areas of stable malaria transmission.18
In this paper, we examined the relationship between baseline parasitemia and HIV-related outcomes in the context of a randomized micronutrient supplementation trial conducted among HIV-infected pregnant women in Dar es Salaam, Tanzania. First, we studied whether or not malaria parasitemia was associated with CD4 T cell count or viral load in cross-sectional analysis. We then followed women to investigate whether or not baseline parasitemia predicted CD4 T cell counts or viral loads > 90 days post-baseline or predicted time to HIV disease stage 3 or 4 or acquired immune deficiency syndrome (AIDS)-related death (ARD). We also examined if the relationship between malaria parasitemia and these outcomes varied according to the baseline level of host immunosuppression.
Methods
Study population and location.
The study population consisted of 1,078 HIV-infected pregnant women who enrolled in a double-blind, placebo-controlled randomized trial at a participating antenatal clinic in Dar es Salaam, Tanzania, to examine the effects of daily micronutrient supplements on HIV disease progression and mortality. Trial results and a detailed description of the trial design have been published elsewhere.19,20 Ethical approvals for the trial were obtained from Research and Publications Committee of Muhimbili University College of Health Sciences, the Ethical Committee of the Tanzania National AIDS Control Program, and the Institutional Review Board of the Harvard School of Public Health. Informed consent was obtained from all women. The study enrollment took place from April 1995 through July 1997, a time during which women did not have access to ART in Tanzania. Women were followed until August 2003. Malaria is endemic in Dar es Salaam, and stable transmission occurs all year. The national annual incidence of malaria disease was estimated to be 400–500 per 1,000 in the general population in 2003.21 Bed nets were reported to have been used by all or some children in an estimated 52% of urban Tanzanian households in 1999; however, only a small percentage of these bed nets (10%) had been previously treated with insecticides.22
Exposure and covariate assessment.
After randomization, women completed baseline interviews regarding socio-demographic characteristics and medical history, and they were asked to provide blood, stool, and vaginal-fluid specimens for detection of malaria parasites, intestinal parasites, and sexually transmitted infections. Follow-up consisted of monthly clinic visits throughout pregnancy and thereafter for a minimum of 2 years. To diagnose malaria, thick and thin blood films were air-dried and stained with 5% Giemsa at pH 7.2 for 20 minutes. Presence of asexual Plasmodium falciparum malaria parasites was determined by microscopic examination of stained slides.23 A slide was considered negative when no parasites were detected in the process of counting 200 leukocytes on a blood film. Quality control for smear microscopy was ensured through multiple mechanisms. First, known negative and positive control slides were included in every microscopic examination of stained slides. Second, all results from microscopic examination of stained slides were verified by a second testing laboratory technologist, and any discrepant results were resolved by a third senior laboratory technologist. The study laboratory also participated in the World Health Organization (WHO)/National Institute for Communicable Diseases (NICD) proficiency testing program. Women with malaria parasites and other infections received treatment, free-of-charge, in accordance with the Tanzania Ministry of Health treatment guidelines. Chloroquine was the first-line drug for treatment of uncomplicated malaria until August 2001, when it was changed to sulfadoxine-pyrimethamine because of high levels of treatment failure with chloroquine.21,24
Outcomes assessments.
A blood specimen was requested at baseline and every 6 months thereafter for the enumeration of CD4 T cell counts using the FACSCount system (Becton Dickinson, San Jose, CA). For a random sample of 415 women, plasma viral load was quantified at a minimum of one time point using the Roche Amplicor HIV-1 monitor version 1.5 assay (Roche Diagnostics Corp., Indianapolis, IN), which has a detection limit of 400 copies/mL. For these analyses, results below this limit (1.6% of all viral load results) were assigned a value of 399. A positive association between parasitemia and viral load has been previously reported in a subset of these women.15
At each follow-up visit, clinicians provided routine clinical care and updated data regarding HIV disease stage, death, and cause of death. HIV disease stage was evaluated in accordance with the WHO criteria on the basis of the woman's history and physical examination.25 Verbal autopsy techniques were used to approximate the cause of death by conducting standardized interviews with relatives, reviewing medical records, or both. Deaths caused by the following conditions were considered to be because of or related to AIDS: AIDS, tuberculosis (pulmonary or extrapulmonary), anemia, meningitis, stroke, pneumonia, diarrhea, and fever. For women who did not attend the clinic or who traveled out of Dar es Salaam, a home visit was made, and neighbors or relatives were asked about the woman's vital status.
Statistical analyses.
Data analysis consisted of three main parts. The first part consisted of cross-sectional analyses of baseline data to investigate the relationship between parasitemia and continuous CD4 T cell counts and viral loads using generalized linear regression models. Next, we used repeated-measures generalized linear models with an exchangeable correlation structure to investigate the relationship between baseline parasitemia and CD4 T cell count and viral load measurements taken > 90 days after baseline. Stepwise splines were used to model the time from baseline to the CD4 T cell count or viral-load measurement in a nonlinear fashion.26 The goal of this analysis was to examine if any relationship between parasitemia and CD4 T cell count or viral load persisted among measurements taken at least several months after antimalarial treatment. Last, we used Cox proportional-hazards regression models to examine the association between baseline parasitemia and time to progression to HIV disease stage 3 or 4 and time to AIDS-related death.27 Women with a baseline HIV disease stage 3 or 4 were excluded from the time to HIV disease stage 3 or 4 analysis, because they had already experienced the outcome of interest. Women for whom the cause of death was not determined (N = 54; 15.9% of all deaths) or for whom death was deemed unrelated to AIDS (N = 44; 12.9% of all deaths) were censored at the time of death.
For all analyses, we examined baseline parasitemia as a binary variable (the presence of any versus none) and as a categorical variable (none; low = 1–999 parasites/µL; medium = 1,000–10,000 parasites/µL; or high > 10,000 parasites/µL). The categorical variable was tested for linearity using a likelihood ratio test with two degrees of freedom. We considered the following baseline characteristics to be potential confounders if they predicted the outcome in a univariable regression model at a P value ≤ 0.20: maternal age, gestational age, body mass index (BMI; kg/m2), mid-upper arm circumference, year of recruitment, WHO HIV disease stage, primiparity, medical antecedents, presence of coprevalent parasitic and sexually transmitted infections, and socio-demographic characteristics (education level, marital status, per person daily food expenditure, and reliance on others for financial support). We did not consider multivitamin use as a potential confounder in this study, because we expected that randomization would have yielded comparable exposure to the multivitamin interventions for women with and without baseline parasitemia. Final multivariable models included all potential confounders that changed the effect estimate by ≥ 10% in either direction as well as other established risk factors for the outcomes. Viral loads were transformed to the log10 scale. The missing-indicator method was used to account for missing covariate data. We excluded extreme outlying values for log10 viral load and CD4 T cell counts as well as values for individuals with outlying within-person standard deviations for these outcomes. Because we hypothesized that the effect of parasitemia on all outcomes could differ according to host immunological status, we stratified each final model by baseline CD4 T cell count group (< 200 cells/µL, 200–499 cells/µL, ≥ 500 cells/µL) and tested for heterogeneity across strata using Cochran's Q test.28
Results
Because they lacked a baseline malaria slide result, 12 of 1,078 women who enrolled in the study (1.1%) were excluded from these analyses. Of the remaining 1,066 women, 199 (18.7%) had slide-confirmed parasitemia at the time of study enrollment. Table 1 displays baseline characteristics of the study participants. Women with and without parasitemia were similar; however, women with parasitemia tended to be primiparous (prevalence ratio [PR] = 1.3; 95% confidence interval [CI] = 1.1–1.6; P value = 0.01), depend entirely on others for financial support (PR = 1.1; CI = 1.02–1.2; P = 0.02), and have hookworm infection (PR = 1.6; CI = 1.1–2.3; P = 0.01).
Table 1.
Baseline characteristics of the study population
| Variable | Malaria parasitemia (N = 199) | No malaria parasitemia (N = 867) | Total n with data |
|---|---|---|---|
| Continuous variables (median IQR) | |||
| Maternal age (years) | 24 (21–27) | 24 (21–28) | 1,066 |
| Gestational age (weeks) | 20.6 (18–23) | 21 (18–23) | 1,066 |
| Mid-upper arm circumference (cm) | 25.0 (23.5–27.0) | 25.1 (23.5–27.5) | 1,051 |
| Binary and categorical variables | |||
| Literate | 92.9 | 91.4 | 1,061 |
| Low daily per capita expenditure on food* | 44.1 | 40.1 | 954 |
| Completely dependent on others for financial support | 81.4 | 73.8 | 1,065 |
| Primiparous | 41.6 | 31.9 | 962 |
| Any sexually transmitted infection | 26.1 | 29.3 | 1,063 |
| Previous history of tuberculosis | 6.7 | 4.3 | 962 |
| Pathological protozoa infection | 4.5 | 7.3 | 870 |
| Hookworm infection | 18.1 | 10.9 | 868 |
| WHO HIV disease stage | 1,065 | ||
| 1 | 82.9 | 84.8 | |
| 2 | 15.6 | 13.9 | |
| 3 | 1.5 | 1.3 | |
| 4 | 0 | 0.12 |
Low daily per capita expenditure on food defined as expenditure below the median value of 500 Tanzanian shillings per person per day.
Cross-sectional analyses.
Among 408 women with baseline viral load and parasitemia measurements, the presence of any parasitemia was associated with a 0.34 greater log10 viral load (CI = 0.17–0.52; P < 0.0001; Table 2). Baseline CD4 T cell count results were available for 1,008 women; however, two measurements with outlying values were excluded. The association between the presence of any parasitemia and CD4 T cell count was statistically significant only for the subset of women with a baseline value ≥ 500 cells/µL (Table 2). Among this group, parasitemia was associated with a lower CD4 T cell count by 51 cells/µL (CI = −86.6–−14.3; P = 0.006; P test for heterogeneity = 0.03). Parasitemia category was not associated with baseline CD4 T cell count (P = 0.24) or baseline CD4 T cell count group (P = 0.95).
Table 2.
Cross-sectional analyses of baseline malaria parasitemia and baseline viral loads and CD4 T cell counts
| Variable | Log10 viral load* | CD4 T cell count* | ||||
|---|---|---|---|---|---|---|
| n/N† | Difference (95% CI)‡ | P value | n/N | Difference (95% CI) | P value | |
| No parasitemia | 330/408 | Referent | 821/1,006 | Referent | ||
| Any malaria parasitemia | 78/408 | 0.34 (0.17–0.52) | < 0.0001 | 185/1,006 | −16.6 (−45.3–12.1) | 0.26 |
| No parasitemia | 330/408 | Referent | 821/1,006 | Referent | ||
| Low parasitemia§ | 23/408 | 0.50 (0.23–0.77) | 52/1,006 | −12.9 (−62.9–37.0) | ||
| Medium parasitemia | 43/408 | 0.18 (−0.05–0.40) | 0.001¶ | 109/1,006 | −11.7 (−47.0–23.5) | 0.24‖ |
| High parasitemia | 12/408 | 0.64 ( 0.25–1.03) | 24/1,006 | −48.1 (−116.9–20.8) | ||
| Malaria parasitemia versus none stratified by baseline CD4 T cell count | ||||||
| < 200 cells/µL | 9/50 | −0.01 (−0.50–0.48) | 21/128 | 5.5 (−14.5–25.4) | ||
| 200–499 cells/µL | 42/230 | 0.35 (0.10–0.59) | 0.37** | 109/569 | −8.8 (−26.0–8.5) | 0.03** |
| ≥ 500 cells/µL | 18/103 | 0.39 (0.08–0.71) | 55/309 | −50.5 (−86.6––14.3) | ||
Models include the following baseline variables: age in years (continuous), previous history of tuberculosis (yes/no), completely dependent on others for financial support (yes/no), gestational age in weeks at study entry (continuous), primiparous (yes/no), low daily per capita food expenditure (yes/no), WHO HIV disease stage (continuous categories: stage 1, 2, or ≥ 3), mid-upper arm circumference (continuous), and missing indicator variables when needed.
Number of women with corresponding parasitemia status/total number of women.
CI = confidence interval.
Low parasitemia: < 1,000 parasites/µL; medium: 1,000–10,000 parasites/µL; high: > 10,000 parasites/µL.
P value for likelihood ratio test.
P value for linear association.
P value for test of heterogeneity across strata of CD4 T cell count.
CD4 T cell count and viral-load measurements taken > 90 days after baseline.
A total of 674 viral-load measurements taken > 90 days after baseline (median = 386 days; interquartile range [IQR] = 182–828) were available for 289 women (median number of measurements per person = 3; range = 1–5). The association between any baseline parasitemia and viral loads after 90 days was not statistically significant (0.19; CI= −0.03–0.40; P = 0.09; Table 3); however, we observed a nonlinear relationship between parasitemia category and viral loads after 90 days (P likelihood ratio test < 0.0001). Women with a low baseline parasitemia showed log10 viral loads that were, on average, 0.67 higher (CI = 0.34–0.99) compared with those without parasitemia. A similar finding emerged for CD4 T cell counts taken > 90 days after baseline; women with low baseline parasitemia tended to have lower CD4 T cell counts compared with those without parasitemia, whereas women with higher levels of baseline parasitemia showed CD4 T cell counts that were comparable or slightly higher compared with women without parasitemia (P likelihood ratio test < 0.0001). Figure 1 shows mean log10 viral load over time among women with low and no baseline parasitemia.
Table 3.
Baseline parasitemia and CD4 T cell counts and viral loads > 90 days after baseline
| Variable | Log10 viral loads* | CD4 T cell counts* | ||||||
|---|---|---|---|---|---|---|---|---|
| n women | n meas† | Difference (95% CI)‡ | P value | n women | N meas | Difference (95% CI) | P value | |
| No parasitemia | 233 | 537 | Referent | 728 | 5,796 | Referent | ||
| Any malaria parasitemia | 56 | 137 | 0.19 (−0.03–0.40) | 0.09 | 164 | 1,239 | −1.5 (−41.2–38.1) | 0.94 |
| No parasitemia | 233 | 537 | Referent | 728 | 5,796 | Referent | ||
| Low parasitemia§ | 14 | 36 | 0.67 (0.34–0.99) | 48 | 377 | −54.4 (−119.1–10.3) | ||
| Medium parasitemia | 34 | 83 | 0.04 (−0.21–0.30) | < 0.0001¶ | 95 | 698 | 25.8 (−24.5–76.1) | < 0.0001¶ |
| High parasitemia | 8 | 18 | −0.07 (−0.55–0.42) | 21 | 164 | −4.9 (−103.7–94.0) | ||
| Malaria parasitemia versus none stratified by baseline CD4 T cell count | ||||||||
| < 200 cells/µL | 26 | 59 | −0.08 (−0.47–0.32) | 99 | 635 | 25.5 (−33.7–84.6) | ||
| 200–499 cells/µL | 169 | 400 | 0.09 (−0.18–0.37) | 0.27‖ | 487 | 3,901 | 20.7 (−17.4–58.8) | 0.41‖ |
| ≥ 500 cells/µL | 75 | 163 | 0.40 (−0.04–0.83) | 262 | 2,197 | −30.3 (−100.9–40.2) | ||
Models include the following baseline variables: age in years (continuous), previous history of tuberculosis (yes/no), completely dependent on others for financial support (yes/no), gestational age in weeks at study entry (continuous), primiparous (yes/no), low daily per capita food expenditure (yes/no), WHO HIV disease stage (continuous categories: stage 1, 2, or ≥ 3), mid-upper arm circumference (continuous), time since study enrollment, and missing indicator variables when needed.
Meas = measurement.
CI = confidence interval.
Low parasitemia: < 1,000 parasites/µL; medium: 1,000–10,000 parasites/µL; high: > 10,000 parasites/µL.
P value for likelihood ratio test.
P value for test of heterogeneity across strata of CD4 T cell count.
Figure 1.
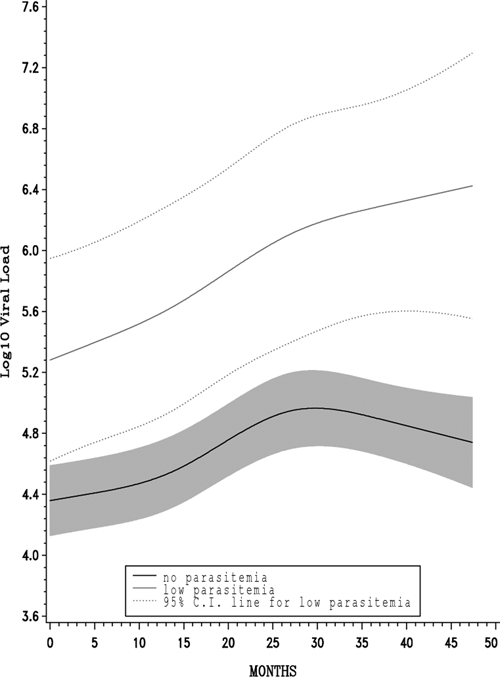
Adjusted mean log10 viral load over time. Low versus no baseline parasitemia.
Time to progression to stage 3 or 4 or ARD.
Fifteen women who had already reached HIV disease stage 3 or 4 at the time of study enrollment and 10 who were missing HIV disease stage data were excluded from the analyses of progression to HIV disease stage 3 or 4. The median durations of follow-up with respect to HIV disease progression and ARD were 16.9 months (IQR = 7.0–39.8) and 70.8 months (IQR = 45.8–80.1), respectively. We found no association between baseline parasitemia and progression to HIV disease stage 3 or 4 (Table 4). Overall, parasitemia category did not predict the rate of ARD (P likelihood ratio test = 0.87); however, we observed an elevated mortality rate of borderline statistical significance among individuals with low baseline parasitemia compared with those with no parasitemia (rate ratio [RR] = 1.6; CI = 0.99–2.6; P = 0.05). A statistically significant association between parasitemia and ARD was also observed among women with a baseline CD4 T cell count ≥ 500 cells/µL (RR = 2.6; CI = 1.1–6.0; P = 0.03; P test for heterogeneity = 0.05). Baseline parasitemia did not predict non-ARD among this group (RR = 0.74; CI = 0.08–6.5; P = 0.78).
Table 4.
Baseline parasitemia and time to HIV disease progression and AIDS-related death
| Variable | Progression to HIV stage 3 or 4 (N = 1,041)* | AIDS-related death (N = 1,066)* | ||||||
|---|---|---|---|---|---|---|---|---|
| n women | n events per total person- months | RR (95% CI)† | P value | n women | n events per total person-months | RR (95% CI) | P value | |
| No parasitemia | 848 | 536/22,421 | Referent | – | 867 | 194/53,764 | Referent | – |
| Any malaria parasitemia | 193 | 113/5,072 | 0.95 (0.77–1.2) | 0.61 | 199 | 48/11,547 | 1.1 (0.82–1.6) | 0.45 |
| No parasitemia | 848 | 536/22,421 | Referent | – | 867 | 194/53,764 | Referent | – |
| Low parasitemia‡ | 55 | 33/1,410 | 1.04 (0.73–1.5) | 56 | 18/3,226 | 1.6 (0.99–2.6) | ||
| Medium parasitemia | 114 | 62/2,981 | 0.88 (0.67–1.1) | 0.61§ | 117 | 24/6,849 | 0.96 (0.62–1.5) | 0.87§ |
| High parasitemia | 24 | 18/681 | 1.08 (0.67–1.7) | 26 | 6/1,472 | 0.95 (0.41–2.2) | ||
| Any malaria parasitemia versus none stratified by baseline CD4 T-cell count | ||||||||
| < 200 cells/µL | 121 | 85/2,151 | 1.2 (0.66–2.3) | 128 | 63/5,658 | 0.65 (0.29–1.5) | ||
| 200–499 cells/µL | 556 | 367/14,855 | 0.80 (0.60–1.1) | 0.35¶ | 569 | 133/35,364 | 0.93 (0.59–1.5) | 0.05¶ |
| ≥ 500 cells/µL | 305 | 164/9,450 | 1.04 (0.66–1.6) | 309 | 29/20,900 | 2.6 (1.1–6.0) | ||
Model includes the following baseline variables: age in years (continuous), previous history of tuberculosis (yes/no), completely dependent on others for financial support (yes/no), gestational age in weeks at study entry (continuous), primiparous (yes/no), low daily per capita food expenditure (yes/no), WHO HIV disease stage (continuous categories: stage 1, 2, ≥ 3), mid-upper arm circumference (continuous), and missing indicator variables when needed.
CI = confidence interval.
Low parasitemia: < 1,000 parasites/µL; medium: 1,000–10,000 parasites/µL; high: > 10,000 parasites/µL.
P value for linear association.
P value for test of heterogeneity across strata of CD4 T cell count.
Discussion
Consistent with previous reports, we observed a positive cross-sectional association between parasitemia and viral load among HIV-infected pregnant women. Although we did not find strong evidence in support of an overall association between parasitemia and progression to HIV disease stage 3 or 4 or ARD, the rate of ARD was elevated among two subgroups: HIV-infected individuals with lower levels of immunosuppression and those with low parasitemia. The association between parasitemia and ARD in women with baseline CD4 T cell counts ≥ 500 cells/µL was statistically significant, whereas the relationship between low parasitemia, versus none, and ARD was of borderline statistical significance.
This paper is not the first to report that malaria may be especially detrimental to individuals with higher CD4 T cell counts. In a cohort study in Malawi, Kublin and others17 found that the harmful association between malaria episodes and viral load was exacerbated for individuals with CD4 T cell counts > 300 cells/µL. The authors proposed that this was a function of relatively more T cells available for HIV viral replication after cytokine simulation by malaria parasites among individuals with higher CD4 T cell counts.17 Consistent with these findings, in Uganda, Whalen and others29,30 observed that the influence of tuberculosis on viral load and mortality was greatest among HIV-infected adults with higher CD4 T cell counts.29,30 In this study, we observed statistically significant heterogeneity in the associations between parasitemia and baseline CD4 T cell count and ARD according to CD4 T cell group. For both outcomes, parasitemia seemed most detrimental to women with T cell counts ≥ 500 cells/µL. Similar trends were observed for the associations between baseline parasitemia and viral load and CD4 T cell counts > 90 days post-baseline; however, the heterogeneity was not statistically significant.
We also noted an elevated rate of death of borderline statistical significance among individuals with low baseline parasitemia. It is possible that this is a chance finding; however, it may be that a low baseline parasitemia level, one consequence of acquired malaria immunity that results from repeated malaria infections in endemic settings,31–34 is a proxy for a high level of exposure to malaria parasites. Women with frequent exposure to malaria parasites may be at greater risk for maintaining chronic parasitemia and consequently, an elevated viral load. This would be especially true if malaria infection in this group tended to be asymptomatic and left untreated. Asymptomatic infection has been shown to be associated with lower parasitemia levels.4,6 This hypothesis is consistent with the harmful cross-sectional relationship that we observed between low parasitemia and viral load at baseline and the fact that it persisted even when viral-load measurements taken > 90 days post-baseline were considered. Individuals with medium and high baseline parasitemia had elevated viral loads at baseline but values similar to those with no baseline parasitemia when measurements taken > 90 days post-baseline were considered. This latter finding corroborates previous reports that have found that malaria-associated increases in viral load may be at least partially reversible with antimalarial treatment.16,17 Persistently higher viral loads among those with low baseline parasitemia could explain the elevated rate of ARD in this group.
It is unclear whether or not parasitemia exacerbates HIV infection in the absence of clinical malaria. One study reported a weaker association between malaria and viral load when any parasitemia, rather than symptomatic malaria illness, was considered.17 In contrast, findings by Van Geertruyden and others35 suggest that parasitemia may be important. In their study, individuals who were cured of malaria 45 days after a clinical episode showed significant improvements in CD4 T cell counts; however, people with recurrent, primarily asymptomatic, parasitemia at 45 days showed CD4 T cell counts that were similar to values during the clinical episode.35 If parasitemia alone influences viral load or CD4 T cell count, interventions to prevent and interrupt chronic parasitemia, which may not be clinically evident, may improve HIV-related health outcomes among HIV-infected individuals. Such interventions would include those already recommended by the WHO, including the habitual use of insecticide-treated bed nets36; however, additional interventions, such as intermittent malaria prophylaxis for HIV-infected individuals, should be evaluated.
Longitudinal analyses were limited by the lack of routinely conducted post-baseline malaria blood smears. Lack of measurement of the duration of parasitemia during the follow-up period may have attenuated effect estimates for the relationship between parasitemia and the study outcomes. We, therefore, cannot discount an association between parasitemia and the prospective outcomes for which we observed null effect estimates or effect estimates that lacked statistical significance. A second potential limitation relates to the stratification of analyses by the baseline CD4 T cell count group. A transient influence of parasitemia on baseline CD4 T cell counts among individuals with higher baseline CD4 T cell counts may have led to some misclassification of baseline immunological status for stratified analyses. The result of this misclassification would be the addition of relatively healthier people (i.e., less immunosuppressed in the absence of malaria) to the strata of individuals with CD4 T cell counts ≥ 200 and < 500 cells/µL and a bias of these strata-specific estimates in the direction of the estimate corresponding to individuals with CD4 T cell counts ≥ 500 cells/µL. Because the RRs for parasitemia and ARD among individuals with lower CD4 T cell counts (< 200 and 200–499 cells/µL) were similar and smaller than the RRs for individuals with CD4 T cell counts ≥ 500 cells/µL, such misclassification is unlikely to explain the association between baseline parasitemia and ARD among individuals with CD4 T cell counts ≥ 500 cells/µL.
Cause of death was ascertained through verbal autopsy, which may have imperfectly classified deaths as AIDS-related or unrelated. Providing that deaths among women with baseline parasitemia were not more likely to be misclassified as AIDS-related, outcome misclassification is an unlikely explanation for the observed association between baseline parasitemia and ARD among women with baseline CD4 T cell counts ≥ 500 cells/µL. This study was conducted in an era when HIV-infected women did not have access to ART in Tanzania; therefore, these results are not able to be generalized to individuals receiving ART. Although women were pregnant during baseline measurements, follow-up extended beyond pregnancy and therefore, results of prospective analyses should be generalized to parous HIV-infected, ART naïve women living in areas with endemic malaria.
In summary, these findings are consistent with a detrimental relationship between malaria and CD4 T cell count and viral load. Further study is warranted to confirm these findings and elucidate the relationship between chronic asymptomatic parasitemia and long-term adverse HIV-related outcomes, such as disease progression and ARD, particularly among HIV-infected individuals with lower levels of immunosuppression.
Acknowledgments
We acknowledge and thank the women who participated in this study.
Footnotes
Financial support: The randomized trial of multivitamin supplementation was funded by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (5R01HD032257-13). M.F.F. received support from the National Institute of Allergy and Infectious Diseases Pre-Doctoral Training Program in the Epidemiology of Infectious Diseases and Biodefense (T32 AI007535).
Authors' addresses: Molly F. Franke, Department of Epidemiology, Harvard School of Public Health, Boston, MA 02115, E-mail: mfranke@hsph.harvard.edu. Donna Spiegelman, Departments of Epidemiology and Biostatistics, Harvard School of Public Health, Boston, MA 02115, E-mail: stdls@channing.harvard.edu. Amara Ezeamama, Department of Nutrition, Harvard School of Public Health, Boston, MA 02115, E-mail: aezeamam@hsph.harvard.edu. Said Aboud Department of Microbiology and Immunology, Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania, E-mail: aboudsaid@yahoo.com. Gernard I. Msamanga, Department of Community Health, Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania, E-mail: gmsamanga@muhas.ac.tz. Saurabh Mehta, Department of Nutrition, Harvard School of Public Health, Boston, MA 02115, E-mail: smehta@hsph.harvard.edu. Wafaie W. Fawzi, Departments of Epidemiology, Nutrition, and Global Health and Population, Harvard School of Public Health, Boston, MA 02115, E-mail: mina@hsph.harvard.edu.
References
- 1.World Health Organization, UNAIDS 2008Towards Universal Access: Scaling Up Priority HIV/AIDS Interventions in the Health Sector, Progress Report Available at: http://www.unaidsrstesa.org/userfiles/file/towards_universal_access_report_2008%5B1%5D(1).pdf Accessed March 7, 2009
- 2.World Health Organization 2008Antiretroviral Therapy for HIV Infection in Adults and Adolescents: Recommendations for a Public Health Approach, 2006 Revision Available at: http://www.who.int/hiv/pub/guidelines/artadultguidelines.pdf Accessed March 7, 2009 [PubMed]
- 3.Holmes CB, Losina E, Walensky RP, Yazdanpanah Y, Freedberg KA. Review of human immunodeficiency virus type 1-related opportunistic infections in sub-Saharan Africa. Clin Infect Dis. 2003;36:652–662. doi: 10.1086/367655. [DOI] [PubMed] [Google Scholar]
- 4.French N, Nakiyingi J, Lugada E, Watera C, Whitworth JA, Gilks CF. Increasing rates of malarial fever with deteriorating immune status in HIV-1-infected Ugandan adults. AIDS. 2001;15:899–906. doi: 10.1097/00002030-200105040-00010. [DOI] [PubMed] [Google Scholar]
- 5.Ladner J, Leroy V, Simonon A, Karita E, Bogaerts J, De Clercq A, Van De Perre P, Dabis F. HIV infection, malaria, and pregnancy: a prospective cohort study in Kigali, Rwanda. Am J Trop Med Hyg. 2002;66:56–60. doi: 10.4269/ajtmh.2002.66.56. [DOI] [PubMed] [Google Scholar]
- 6.Laufer MK, van Oosterhout JJ, Thesing PC, Thumba F, Zijlstra EE, Graham SM, Taylor TE, Plowe CV. Impact of HIV-associated immunosuppression on malaria infection and disease in Malawi. J Infect Dis. 2006;193:872–878. doi: 10.1086/500245. [DOI] [PubMed] [Google Scholar]
- 7.Patnaik P, Jere CS, Miller WC, Hoffman IF, Wirima J, Pendame R, Meshnick SR, Taylor TE, Molyneux ME, Kublin JG. Effects of HIV-1 serostatus, HIV-1 RNA concentration, and CD4 cell count on the incidence of malaria infection in a cohort of adults in rural Malawi. J Infect Dis. 2005;192:984–991. doi: 10.1086/432730. [DOI] [PubMed] [Google Scholar]
- 8.Kamya MR, Gasasira AF, Yeka A, Bakyaita N, Nsobya SL, Francis D, Rosenthal PJ, Dorsey G, Havlir D. Effect of HIV-1 infection on antimalarial treatment outcomes in Uganda: a population-based study. J Infect Dis. 2006;193:9–15. doi: 10.1086/498577. [DOI] [PubMed] [Google Scholar]
- 9.Whitworth J, Morgan D, Quigley M, Smith A, Mayanja B, Eotu H, Omoding N, Okongo M, Malamba S, Ojwiya A. Effect of HIV-1 and increasing immunosuppression on malaria parasitaemia and clinical episodes in adults in rural Uganda: a cohort study. Lancet. 2000;356:1051–1056. doi: 10.1016/S0140-6736(00)02727-6. [DOI] [PubMed] [Google Scholar]
- 10.Froebel K, Howard W, Schafer JR, Howie F, Whitworth J, Kaleebu P, Brown AL, Riley E. Activation by malaria antigens renders mononuclear cells susceptible to HIV infection and re-activates replication of endogenous HIV in cells from HIV-infected adults. Parasite Immunol. 2004;26:213–217. doi: 10.1111/j.0141-9838.2004.00701.x. [DOI] [PubMed] [Google Scholar]
- 11.Pisell TL, Hoffman IF, Jere CS, Ballard SB, Molyneux ME, Butera ST, Lawn SD. Immune activation and induction of HIV-1 replication within CD14 macrophages during acute Plasmodium falciparum malaria coinfection. AIDS. 2002;16:1503–1509. doi: 10.1097/00002030-200207260-00007. [DOI] [PubMed] [Google Scholar]
- 12.Xiao L, Owen SM, Rudolph DL, Lal RB, Lal AA. Plasmodium falciparum antigen-induced human immunodeficiency virus type 1 replication is mediated through induction of tumor necrosis factor-alpha. J Infect Dis. 1998;177:437–445. doi: 10.1086/514212. [DOI] [PubMed] [Google Scholar]
- 13.O'Brien WA, Hartigan PM, Martin D, Esinhart J, Hill A, Benoit S, Rubin M, Simberkoff MS, Hamilton JD. Changes in plasma HIV-1 RNA and CD4+ lymphocyte counts and the risk of progression to AIDS. Veterans Affairs Cooperative Study Group on AIDS. N Engl J Med. 1996;334:426–431. doi: 10.1056/NEJM199602153340703. [DOI] [PubMed] [Google Scholar]
- 14.Sterling TR, Vlahov D, Astemborski J, Hoover DR, Margolick JB, Quinn TC. Initial plasma HIV-1 RNA levels and progression to AIDS in women and men. N Engl J Med. 2001;344:720–725. doi: 10.1056/NEJM200103083441003. [DOI] [PubMed] [Google Scholar]
- 15.Kapiga SH, Bang H, Spiegelman D, Msamanga GI, Coley J, Hunter DJ, Fawzi WW. Correlates of plasma HIV-1 RNA viral load among HIV-1-seropositive women in Dar es Salaam, Tanzania. J Acquir Immune Defic Syndr. 2002;30:316–323. doi: 10.1097/00126334-200207010-00008. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman IF, Jere CS, Taylor TE, Munthali P, Dyer JR, Wirima JJ, Rogerson SJ, Kumwenda N, Eron JJ, Fiscus SA, Chakraborty H, Taha TE, Cohen MS, Molyneux ME. The effect of Plasmodium falciparum malaria on HIV-1 RNA blood plasma concentration. AIDS. 1999;13:487–494. doi: 10.1097/00002030-199903110-00007. [DOI] [PubMed] [Google Scholar]
- 17.Kublin JG, Patnaik P, Jere CS, Miller WC, Hoffman IF, Chimbiya N, Pendame R, Taylor TE, Molyneux ME. Effect of Plasmodium falciparum malaria on concentration of HIV-1-RNA in the blood of adults in rural Malawi: a prospective cohort study. Lancet. 2005;365:233–240. doi: 10.1016/S0140-6736(05)17743-5. [DOI] [PubMed] [Google Scholar]
- 18.Quigley MA, Hewitt K, Mayanja B, Morgan D, Eotu H, Ojwiya A, Whitworth JA. The effect of malaria on mortality in a cohort of HIV-infected Ugandan adults. Trop Med Int Health. 2005;10:894–900. doi: 10.1111/j.1365-3156.2005.01461.x. [DOI] [PubMed] [Google Scholar]
- 19.Fawzi WW, Msamanga GI, Spiegelman D, Urassa EJ, Hunter DJ. Rationale and design of the Tanzania vitamin and HIV infection trial. Control Clin Trials. 1999;20:75–90. doi: 10.1016/s0197-2456(98)00045-2. [DOI] [PubMed] [Google Scholar]
- 20.Fawzi WW, Msamanga GI, Spiegelman D, Wei R, Kapiga S, Villamor E, Mwakagile D, Mugusi F, Hertzmark E, Essex M, Hunter DJ. A randomized trial of multivitamin supplements and HIV disease progression and mortality. N Engl J Med. 2004;351:23–32. doi: 10.1056/NEJMoa040541. [DOI] [PubMed] [Google Scholar]
- 21.Tanzania Ministry of Health 2009National Medium Term Strategic Plan 2002–2007 Available at: http://www.rollbackmalaria.org/countryaction/nsp/tanzania.pdf Accessed March 7, 2009
- 22.National Bureau of Statistics (Tanzania) and Macro International Inc. Tanzania Reproductive and Child Health Survey 1999. Calverton, MD: National Bureau of Statistics and Macro International Inc.; 2000. [Google Scholar]
- 23.Trape JF. Rapid evaluation of malaria parasite density and standardization of thick smear examination for epidemiological investigations. Trans R Soc Trop Med Hyg. 1985;79:181–184. doi: 10.1016/0035-9203(85)90329-3. [DOI] [PubMed] [Google Scholar]
- 24.Mulligan JA, Mandike R, Palmer N, Williams H, Abdulla S, Bloland P, Mills A. The costs of changing national policy: lessons from malaria treatment policy guidelines in Tanzania. Trop Med Int Health. 2006;11:452–461. doi: 10.1111/j.1365-3156.2006.01590.x. [DOI] [PubMed] [Google Scholar]
- 25.Interim proposal for a WHO staging system for HIV infection and disease. Wkly Epidemiol Rec. 1990;65:221–224. [PubMed] [Google Scholar]
- 26.Govindarajulu US, Spiegelman D, Thurston SW, Ganguli B, Eisen EA. Comparing smoothing techniques in Cox models for exposure-response relationships. Stat Med. 2007;26:3735–3752. doi: 10.1002/sim.2848. [DOI] [PubMed] [Google Scholar]
- 27.Cox DR. Regression models and life-tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 28.Cochran WG. The comparison of percentages in matched samples. Biometrika. 1950;37:256–266. [PubMed] [Google Scholar]
- 29.Toossi Z, Mayanja-Kizza H, Hirsch CS, Edmonds KL, Spahlinger T, Hom DL, Aung H, Mugyenyi P, Ellner JJ, Whalen CW. Impact of tuberculosis (TB) on HIV-1 activity in dually infected patients. Clin Exp Immunol. 2001;123:233–238. doi: 10.1046/j.1365-2249.2001.01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whalen CC, Nsubuga P, Okwera A, Johnson JL, Hom DL, Michael NL, Mugerwa RD, Ellner JJ. Impact of pulmonary tuberculosis on survival of HIV-infected adults: a prospective epidemiologic study in Uganda. AIDS. 2000;14:1219–1228. doi: 10.1097/00002030-200006160-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bodker R, Msangeni HA, Kisinza W, Lindsay SW. Relationship between the intensity of exposure to malaria parasites and infection in the Usambara Mountains, Tanzania. Am J Trop Med Hyg. 2006;74:716–723. [PubMed] [Google Scholar]
- 32.Ladeia-Andrade S, Ferreira MU, de Carvalho ME, Curado I, Coura JR. Age-dependent acquisition of protective immunity to malaria in riverine populations of the Amazon Basin of Brazil. Am J Trop Med Hyg. 2009;80:452–459. [PubMed] [Google Scholar]
- 33.Trape JF, Rogier C, Konate L, Diagne N, Bouganali H, Canque B, Legros F, Badji A, Ndiaye G, Ndiaye P, Brahimi K, Faye O, Druilhe P, Pereira Da Silva L. The Dielmo project: a longitudinal study of natural malaria infection and the mechanisms of protective immunity in a community living in a holoendemic area of Senegal. Am J Trop Med Hyg. 1994;51:123–137. doi: 10.4269/ajtmh.1994.51.123. [DOI] [PubMed] [Google Scholar]
- 34.Mayor A, Aponte JJ, Fogg C, Saute F, Greenwood B, Dgedge M, Menendez C, Alonso PL. The epidemiology of malaria in adults in a rural area of southern Mozambique. Malar J. 2007;6:3.. doi: 10.1186/1475-2875-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Geertruyden JP, Mulenga M, Kasongo W, Polman K, Colebunders R, Kestens L, D'Alessandro U. CD4 T-cell count and HIV-1 infection in adults with uncomplicated malaria. J Acquir Immune Defic Syndr. 2006;43:363–367. doi: 10.1097/01.qai.0000243125.98024.da. [DOI] [PubMed] [Google Scholar]
- 36.World Health Organization 2009Malaria and HIV Interactions and Their Implications for Public Health Policy Available at: http://www.who.int/malaria/malaria_HIV/MalariaHIVinteractions_report.pdf Accessed March 21, 2009


