Abstract
A recent drug efficacy trial reported Coartem (artemether-lumefantrine) to be highly effective against Plasmodium falciparum in children less than 5 years of age in Papua New Guinea (PNG). In contrast, we have observed high levels of treatment failures in non-trial conditions in a longitudinal cohort study in the same age group in PNG. Recrudescences were confirmed by genotyping of three different marker genes to provide optimal discrimination power between parasite clones. After excluding genetic host factors by genotyping potentially relevant cytochrome P450 loci, the high number of treatment failures in our study is best explained by poor adherence to complex dosing regimens in combination with insufficient fat supplementation, which are both crucial parameters for the outcome of Coartem treatment. In contrast to the situation in classic drug trials with ideal treatment conditions, our field survey highlights potential problems with unsupervised usage of Coartem in routine clinical practice and under program conditions.
Introduction
With respect to increasing drug resistance, the World Health Organization (WHO) currently recommends a switch of first-line treatment against uncomplicated malaria to artemisinin-based combination therapies (ACTs) for countries where conventional antimalarial treatments such as chloroquine (CQ), amodiaquine (AQ), or sulphadoxine-pyrimethamine (SP) have become ineffective.1
First-line treatment of uncomplicated malaria in Papua New Guinea (PNG) has been chloroquine or amodiaquine plus sulphadoxine-pyrimethamine since the year 2000. However, the efficacy of this treatment regimen was found to be low only 3 years after its implementation,2 which gives a strong argument for the introduction of artemisinin-based combination therapies. A recent study conducted in PNG3 has tested the efficacy of different artemisinin-combination therapies in children < 5 years of age with Plasmodium falciparum or Plasmodium vivax malaria. Artemether-lumefantrine was found to be the most effective combination therapy against P. falciparum with an adequate clinical and parasitological response of 97.3% at Day 28 and 95.2% at Day 42 after treatment.
Although the effectiveness of Coartem (artemether-lumefantrine) under ideal trial conditions was very promising, the situation may look different in less controlled conditions in routine practice. It is likely that adherence to the recommended treatment is sub-optimal under non-study conditions where on one hand, the rapid relief of symptoms and on the other hand, concerns about access to and availability of drugs might tempt patients to early interrupt treatment.
We undertook a longitudinal study in PNG during which children between 1 and 5 years of age were followed up in 8-weekly intervals with the aim to study the infection dynamics of P. falciparum clones. All children presenting with a clinical malaria episode were treated with Coartem. In our study, we observed an unexpected high number of P. falciparum recurrent infections, which were confirmed by molecular genotyping of three different markers. Our observations are in contrast to recently published results of a drug efficacy trial in the study area.3 We discuss our findings with respect to the forthcoming introduction of Coartem as a first-line treatment in the country.4
Materials and Methods
Study design and site.
The study was carried out in an area near Maprik, East Sepik Province in Papua New Guinea, which is endemic for malaria. There is little seasonal variation in temperature or rainfall and malaria transmission is perennial.5 A detailed description of the study design is given elsewhere (Lin and others, unpublished data). Briefly, 264 children 1–3 years of age at enrollment were followed up in 8-weekly intervals from March 2006 until July 2007. At each visit the children were clinically assessed and a 250 µL blood sample was collected from each participant by finger prick. In between these regular follow-up bleeds, each child was clinically examined every 2 weeks and in case of malaria symptoms a 250 µL blood sample was collected. Furthermore, a blood sample was collected at the local health center whenever a study participant presented with malaria symptoms. Symptomatic children that were confirmed to carry malaria parasites by rapid diagnostic test and/or microscopy were treated with a 6-dose regimen of Coartem (Novartis Pharma, Switzerland). During the initiation phase of the study, intake of the first dose was supervised by a study nurse, and treatment was mainly administered unsupervised during the progress of the study. Caretakers were advised to complete the full treatment regimen and to supplement the drugs with a fatty diet.
Scientific approval and ethical clearance for the study was obtained from the Medical Research and Advisory Committee (MRAC) of the Ministry of Health in PNG (MRAC no. 05/21) and from the Ethikkommission beider Basel (EKBB) in Switzerland (EKBB no. 03/06). Informed consent was sought from all parents or guardians before recruitment of each child.
DNA isolation and msp2 genotyping.
All finger-prick blood samples were separated into plasma and cells. The DNA was extracted from cell pellets using QIAamp 96 DNA Blood Kit (Qiagen, Australia), according to the manufacturer's instructions. All samples were genotyped for the highly polymorphic marker gene merozoite surface protein 2 (msp2) by capillary electrophoresis, as previously described by Falk and others,6 with some minor changes and adaptations of polymerase chain reaction (PCR) conditions for highly purified DNA.7
Determination of treatment failures and msp1 genotyping.
The current protocol of the WHO to assess drug efficacy recommends a follow-up of 28 days for most antimalarial drugs and an extended follow-up to 42 days for Coartem.8 In consideration of these guidelines, the genotype dataset was screened for msp2 alleles that reappeared in the same patient within 7 to 42 days after treatment with Coartem. Because most of the study participants were treated several times throughout the course of the study, there were a total of 89 individuals that accounted for more than one entry in the database, but from different treatment time points during the survey. An outcome was defined as recrudescence if the first P. falciparum positive sample after treatment contained at least one msp2 allele that was identical to any of the alleles present in the treatment sample. An infection was defined as new infection when the first positive sample after treatment only contained new msp2 alleles. If the first positive sample contained both, alleles present in the treatment sample and new alleles, the outcome was considered recrudescent. As suggested by Mugittu and others,9 we used a stepwise approach to discriminate recrudescent from new infections. All msp2 treatment failures were further genotyped for the polymorphic marker gene, merozoite surface protein 1 (msp1). For the amplification of the msp1 block 2,10 a nested PCR approach followed by capillary gel electrophoresis was used (for details see Schoepflin and others, 2009; in preparation). Classification as recrudescent or new infection was done in the same way as for msp2.
Genotyping of microsatellite TA81.
Samples identified as treatment failure by msp2 and msp1 and those samples for which msp1 PCR had failed were further genotyped for the polymorphic microsatellite TA81. Genotyping of microsatellite TA81 was only performed for follow-up samples collected within 28 days after treatment. Samples between Day 28 and Day 42 were not further genotyped for TA81. Amplification of the TA81 locus was performed using primers and conditions previously described.11 One of the amplification primers was 5¢ end-labeled with Cy5. The PCR products were mixed 3:1 (vol/vol) with denaturing loading dye buffer and analyzed on a 6% denaturing polyacrylamide gel as previously described by DaRe and others.12
Sequencing of cytochrome P450 and UDP-glucuronosyltransferase.
Amplification of CYP3A4 was performed in a total volume of 50 µL containing 5 µL of 10× BufferB (0.8M Tris-HCl, 0.2M [NH4]2SO4, 0.2% w/v Tween-20), 2 mM MgCl2, 200 µM dNTPs, 2.5U Taq DNA polymerase (FirePol, Solis BioDyne, Tartu, Estonia). The PCR primers (forward: 5¢CTCACCTCTGTTCAGGGAAAC 3¢; reverse: 5¢ATGGCCAAGTCTGGGATGAG 3¢) were used at a final concentration of 1 µM each. The 2.5 µL of purified DNA was used as template for this reaction. An initial denaturation step of 96°C for 3 min was followed by 40 amplification cycles of 30 s at 96°C, 1 min at 64°C, 1 min at 72°C, and a final extension for 10 min at 72°C. Polymerase chain reaction products were purified using NucleoSpin Extract II Kit (Macherey-Nagel GmbH, Düren, Germany) and sequenced. Sequences were analyzed using the ABI Prism AutoAssembler (version 1.4.0, Applied Biosystems, Foster City, CA) for assembly. Amplification and single nucleotide polymorphism (SNP)-genotyping of CYP2B6 516G>T and UGT2B7 802C>T was performed as previously described.13,14
Results
During the 16 months follow-up of our field survey, a total of 793 Coartem treatment regimens were administered to symptomatic children infected with P. falciparum, as diagnosed by msp2 PCR. Six hundred seventy-one (671) of these children were also positive by microscopy for P. falciparum and 188 carried mixed species infections at the time of treatment. Clinical and parasitological failure rates were determined in samples selected according to the inclusion criteria depicted in Figure 1. For determination of the parasitological treatment failure rate, only treated episodes occurring within 42 days before a regular follow-up visit were taken into account (Figure 1A). For clinical failure rate, two consecutive P. falciparum episodes of an individual were taken into account that both had occurred within an interval of 7 to 42 days (Figure 1B). The recurrent episode was detected either at regular follow-up bleed or by active or passive case detection.
Figure 1.
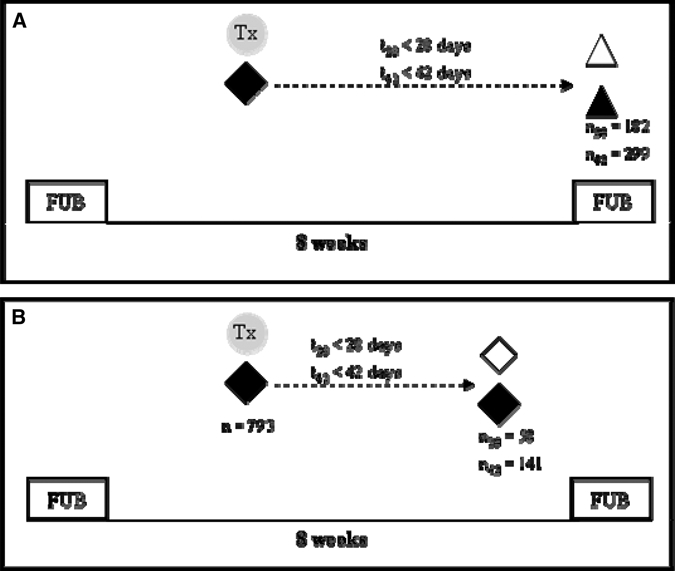
Schematic representation of inclusion criteria for the analysis of parasitological treatment failures (A) and clinical failures (B). regular 8-weekly follow-up bleed; ◆ clinical episode sample;  treatment with Coartem®; ▲ FUB sample PCR positive for recrudescent allele; Δ FUB sample PCR negative for recrudescent allele.
treatment with Coartem®; ▲ FUB sample PCR positive for recrudescent allele; Δ FUB sample PCR negative for recrudescent allele.
Figure 2 summarizes genotyping results generated with three marker genes. Out of the 793 Coartem-treated children, only 182 had a consecutive sample collected within the next 28 days after treatment and 299 within the next 42 days. To estimate the parasitological treatment failure rate, all sample pairs were genotyped sequentially for three markers starting with the most polymorphic marker, msp2. For the remaining 494 Coartem-treated children, no consecutive blood sample was available within 42 days, and these samples were excluded from further analysis.
Figure 2.
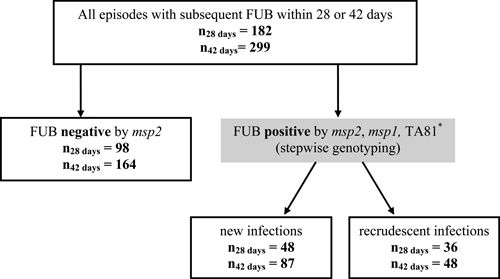
Summary of final treatment outcome based on msp2, msp1, and TA81 genotyping. Follow-up bleed (FUB). * Genotyping of the microsatellite TA81 was only performed for infections occurring within 28 days after Coartem treatment. Samples collected between Day 28 and Day 42 after treatment was genotyped only for msp2 and msp1.
The PCR-corrected parasitological treatment failure rates were 36/182 (19.78%) and 48/299 (16.05%) at Day 28 and Day 42, respectively. Samples collected between Day 28 and Day 42 after treatment were only genotyped for msp2 and msp1, and final classification of infections occurring during this time period were therefore only based on these two marker genes.
Out of the 793 P. falciparum episodes treated with Coartem, 141 cases experienced a further episode within 7–42 days after treatment. The PCR-corrected clinical failure rates were 26/793 (3.27%) and 42/793 (5.3%) at Day 28 and Day 42, respectively.
Cytochrome P450 genotypes.
Sixty-four (64) different individuals representing persistent or new infections were genotyped for the cytochrome P450 3A4 392A>G and 2B6 516G>T and for the UDP-glucuronosyltransferase UGT2B7 802C>T, which are all involved in the metabolism of artemisinin drugs and/or lumefantrine.15–18 Sequencing of cytochrome P450 3A4 was successfully performed for 56/64 participants. Results from sequencing of cytochrome P450 3A4 showed homozygote wild-type carriers at position 392, i.e., adenosine, for all 56 individuals. The P450 loci CYP2B6 516G>T and UGT2B7 802C>T were found to be polymorphic in the 64 individuals from our study population, which was in agreement with previous results from the same population in PNG.13,14 It has been proposed by these authors that the outcome of treatment with artemisinin drugs might be associated with a combined effect of polymorphisms in both CYP2B6 and UGT2B7 genes. In our study population, the combination of CYP2B6 516T and UGT2B7 802T was observed in individuals carrying new infections and in individuals showing treatment failures. There was a trend toward increased frequency of double mutants in individuals carrying recrudescent infections (30.3% of all CYP2B6-UGT2B7 double mutants were observed in individuals carrying new infections and 69.7% in treatment failures), however, the association between treatment outcome and the presence of double mutants in CYP2B6 and UGT2B7 was not statistically significant (P = 0.79).
Discussion
A number of different drug trials were conducted worldwide to test the efficacy of artemether-lumefantrine. This combination treatment has proven highly efficacious in studies conducted in Ghana,19 Tanzania,20,21 Thailand,22,23 or Papua New Guinea3 with > 97% PCR adjusted cure rates at Day 28. In contrast, we observed an unexpected high number of recurrent infections after treatment with Coartem in our longitudinal survey in children less than 5 years of age in Papua New Guinea. Treatment failures were confirmed by molecular typing of three different marker genes using a high-resolution technique with optimal discrimination power between genotypes. Using three different marker genes for discrimination of recrudescent from new infections provides confident identification of treatment failures.
Our study was not designed as a classic drug efficacy trial, but as a cohort study with longitudinal follow-up in 8-weekly intervals. This study design does not allow estimating in a standardized way the true rate of parasitological treatment failures, because recrudescent infections were only detected when the treated episode occurred within 28 or 42 days before a regular follow-up visit. If the interval between the episode and the next regular follow-up bleed was longer than 42 days, any recrudescent or newly occurring parasitemia remained undetected. Thus, with our study design we certainly underestimated the number of both, recrudescences and new infections. However, the specific advantage of our study consists in the fact that treatment success was viewed outside a clinical trial setting with outcomes reflecting the local situation in village health care. Our finding of a substantial number of recrudescences in non-trial conditions highlights potential problems of unsupervised Coartem usage.
In principle, several factors could have reduced the effectiveness of Coartem treatment in our study: 1) reduced sensitivity of parasites to Coartem, 2) host factors accounting for differences in metabolism of the drug, 3) inadequate adherence to treatment regimen, and 4) suboptimal absorption of drugs.
A reduced sensitivity to artemether-lumefantrine has been associated with an increased copy number of pfmdr1 in vitro and in vivo.23 Similar findings of a reduced sensitivity to lumefantrine in vitro were described by Lim and others,24 however, in that study the correlation could not be confirmed in vivo. Recently, it has been shown that pfmdr1 gene amplifications were absent from different study sites in PNG, including an area adjacent to our study site.25 These results, together with the fact that an efficacy trial3 had confirmed Coartem to be highly effective in our study area indicate that reasons other than reduced drug sensitivity caused by an increase in pfmdr1 copy numbers are more likely to account for the high frequency of recrudescent infections.
Artemisinin derivatives are mainly metabolized by the human cytochrome P450 3A416 and 2B618 and also by the metabolic enzyme UDP-glucuronosyltransferase UGT2B7.17 The lumefantrine component of Coartem is also metabolized by CYP3A4.15 So far, functional significance of polymorphisms in CYP2B6 and UGT2B7 on the treatment outcome of artemisinin drugs is lacking. However, previous in vitro studies have shown that these polymorphisms caused significant reduction in enzyme activity or expression,13,14 which generally elevated the plasma drug concentration of a antiretroviral drugs.13 It is likely that these polymorphisms act in a similar way on artemisinin plasma levels, and it has been proposed by Mehlotra and others,13,14 that the large inter-individual variability in the pharmacokinetics of artemisinin drugs, which has frequently been observed might partly be a result of a joint contribution of both polymorphisms in CYP2B6 and UGT2B7. To exclude the possibility that host genetic factors account for the frequent treatment failures in our study population, polymorphisms in the human cytochrome 3A4, 2B6, and in UGT2B7 were studied. For the CYP2B6 and UGT2B7, we only focused on the two polymorphisms CYP2B6 516G>T and UGT2B7 802C>T, which have been observed at high frequency in a previous study in PNG.13,14 We found mutant alleles in both, patients carrying new infections and in recrudescent infections, but treatment outcome and the presence of double mutants were not significantly associated. Thus, the double mutant CYP2B6/UGT2B7 seems not to influence the outcome of Coartem treatment in this population. However, the trend toward increased proportion of double mutants in patients with treatment failures suggest that the correlation between treatment outcome and presence of double mutant should be further studied in a larger study population under standard drug trial conditions. The mutant allele CYP3A4 392A>G has previously been associated with a significant decrease in CYP3A4 activity26 and therefore leads to an increased exposure to lumefantrine. Our sequencing results did not reveal any polymorphisms at position 392. Homozygous carriers of the wild-type SNP were found in individuals harboring recrudescent or new infections. These results indicate that differences in metabolism of lumefantrine caused by mutations in CYP3A4 do not seem to account for the frequent treatment failures observed.
In most drug efficacy studies, treatment regimens are administered under supervision of a team nurse and therefore optimal compliance is mostly guaranteed or at least information on the adherence to the recommended treatment intervals or early interruption of treatment are available and can be accounted for in the analysis. In our study, treatment was mostly administered unsupervised. Parents were encouraged to complete all treatment doses, but in fact, no information is available from our study participants whether the complete treatment regimen was taken. Coartem rapidly alleviates malaria symptoms, which might frequently tempt patients to stop treatment earlier and keep the remaining tablets for a later malaria attack. Incomplete adherence to the full 6-dose regimen might have contributed to the observed frequency of treatment failures.
After initial reduction of the parasite biomass by the fast acting artemether, clearance of the residual parasites greatly depends on the longer lasting partner drug lumefantrine. Previous studies have shown that the plasma level of lumefantrine is a critical factor for treatment success.23,27 Food has a significant effect on the bioavailability of both components of the drug with an increased absorption when supplemented with fatty food.28 An increase in treatment success by 15% was observed in a study in Thailand when a fatty diet was co-administered with Coartem.29 In this study, parents or guardians of the study participants were advised to administer treatment with a fatty diet, however, no information is available to what extent these recommendations were followed. Inadequate plasma concentrations of lumefantrine might therefore have contributed to the high rate of treatment failures.
The high frequency of parasitological and clinical treatment failures in our study are contrary to findings from a recent drug efficacy trial, which was conducted in an area adjacent to our study site. In this previous study Coartem was reported to be highly efficacious in clearing P. falciparum in children less than 5 years of age.3 An important difference between the two studies was that in the drug efficacy trial at least half of the doses were dispensed under supervision and supplemented with milk. This implies that incomplete adherence to the treatment regimen in combination with a lack of fatty diet might have contributed to the observed differences in treatment outcome. Furthermore, in our study Coartem was also given to P. falciparum-infected children who presented with anemia (Hb < 7.5 g/dL), but did not show any other sign of symptoms. It is likely that these children were even less adherent to treatment, because they were without noticeable signs of disease. In the drug trial by Karunajeewa and others3 only microscopy positive recurrent infections were genotyped. In our analysis, all samples were genotyped regardless of microscopy results. Because sensitivity of PCR is considerably higher than that of microscopy, we most likely have detected recrudescences of low parasite density that would have remained undetected by microscopy. This might have further contributed to the higher treatment failure rate in our study.
Rather low levels of drug efficacy had also been reported from two studies in Ghana30,31 where PCR corrected cure rates at Day 28 were only 86.2% and 88.3%, respectively. In one of these studies only the first dose was given under supervision, whereas in the other study the administration of all 6 tablets was supervised. In both studies, no fatty diet was provided by the study team, but caretakers were encouraged to give fatty food at the time of drug administration. It was not checked by the study team whether these recommendations were followed. In a study in Uganda, no difference in treatment efficacy was observed between supervised and unsupervised administration of Coartem.32 In the latter study detailed explanations on intake of tablets and treatment schedule were provided to the patients. Such information may have resulted in adequate adherence. In a study conducted in Tanzania the issue of poor quality of malaria case management was demonstrated.33 Despite the high frequency of health facility attendance observed in this study, the proportion of patients receiving the appropriate antimalarial timely and correctly dosed was very low. Poor compliance to the prescribed treatment regimen leads to sub-curative doses and therefore does not only increase the rate of treatment failures, but also contributes to the emergence of antimalarial drug resistance.34 Adherence problems in home treatment could therefore lead to a situation similar to Cambodia, where a reduced in vivo susceptibility of parasites to artemisinin has been reported.35,36 Further education on how, when, and where to treat febrile illness could therefore help to translate the high efficacy of new antimalarials such as ACT into effective community use and reduce the risk of emergence of resistance against these drugs. Recognizing the importance of good adherence to the Coartem treatment schedule, the PNG health authorities will provide extensive training to all health workers and conduct behavior change communication campaigns aimed at improving patient compliance as part of the upcoming introduction of Coartem as the national first-line treatment.
In summary, our observations highlight the importance of strict adherence to the complex dosing regimens of Coartem and the need to supplement the treatment with a fatty diet. The study by Kuranajeewa and others3 had shown that under optimal treatment conditions Coartem was highly effective in Papua New Guinea, whereas our results indicate that these high success rates might be difficult to achieve under routine clinical practice. Thus, it is of great importance that the introduction of Coartem as first-line treatment in PNG is accompanied by a provision of training and education for health workers to guarantee accurate treatment and compliance to the recommended guidelines. As pointed out by Piola and others,32 a great effort has to be made to convince patients and caretakers to complete the full 6-dose regimen despite the fast relief of symptoms.
Acknowledgments
We are grateful to the study participants and their parents or guardians, and to the IMR field team and microscopists. We thank Serej Ley and Eva Maria Hodel for technical help. We also thank the unconditional provision of the drug, Coartem, by Novartis Pharma Inc., Sitzerland.
Footnotes
Financial support: The study was supported by the Swiss National Science Foundation (grant no: 31003A-112196) and the National Institute of Health. S. Schoepflin was supported by the Forlen Foundation.
Authors' addresses: Sonja Schoepflin and Ingrid Felger, Swiss Tropical Institute, Basel, Switzerland, E-mail: engrid.felger@unibas.ch. Enmoore Lin, Benson Kiniboro, and Ivo Mueller, Papua New Guinea Institute of Medical Research, Goroka, Eastern Highland Province 441, Papua New Guinea. Jeana T. DaRe, Rajeev K. Mehlotra, and Peter A. Zimmerman, Center for Global Health and Diseases, Case Western Reserve University, Wolstein Research Building, Cleveland, OH.
Reprint requests: Ingrid Felger, Swiss Tropical Institute, Socinstr. 57, 4002-Basel, Switzerland, Tel: +41-61-2848-117, Fax: +41-61-2848-101, E-mail: ingrid.felger@unibas.ch.
References
- 1.World Health Organization . Guidelines for the Treatment of Malaria. Geneva, Switzerland: WHO; 2006. [Google Scholar]
- 2.Marfurt J, Mueller I, Sie A, Maku P, Goroti M, Reeder JC, Beck HP, Genton B. Low efficacy of amodiaquine or chloroquine plus sulfadoxine-pyrimethamine against Plasmodium falciparum and P. vivax malaria in Papua New Guinea. Am J Trop Med Hyg. 2007;77:947–954. [PubMed] [Google Scholar]
- 3.Karunajeewa HA, Mueller I, Senn M, Lin E, Law I, Gomorrai PS, Oa O, Griffin S, Kotab K, Suano P, Tarongka N, Ura A, Lautu D, Page-Sharp M, Wong R, Salman S, Siba P, Ilett KF, Davis TM. A trial of combination antimalarial therapies in children from Papua New Guinea. N Engl J Med. 2008;359:2545–2557. doi: 10.1056/NEJMoa0804915. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization . Global AMDP Database - WPRO. 2007. [Google Scholar]
- 5.Genton B, al-Yaman F, Beck HP, Hii J, Mellor S, Narara A, Gibson N, Smith T, Alpers MP. The epidemiology of malaria in the Wosera area, East Sepik Province, Papua New Guinea, in preparation for vaccine trials. I. Malariometric indices and immunity. Ann Trop Med Parasitol. 1995;89:359–376. doi: 10.1080/00034983.1995.11812965. [DOI] [PubMed] [Google Scholar]
- 6.Falk N, Maire N, Sama W, Owusu-Agyei S, Smith T, Beck HP, Felger I. Comparison of PCR-RFLP and Genescan-based genotyping for analyzing infection dynamics of Plasmodium falciparum. Am J Trop Med Hyg. 2006;74:944–950. [PubMed] [Google Scholar]
- 7.Schoepflin S, Valsangiacomo F, Lin E, Kiniboro B, Mueller I, Felger I. Comparison of Plasmodium falciparum allelic frequency distribution in different endemic settings by high-resolution genotyping. Malar J. 2009;30:250. doi: 10.1186/1475-2875-8-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization . Assessment and monitoring of antimalarial drug efficacy for the treatment of uncomplicated falciparum malaria. Geneva: WHO; 2003. [Google Scholar]
- 9.Mugittu K, Adjuik M, Snounou G, Ntoumi F, Taylor W, Mshinda H, Olliaro P, Beck HP. Molecular genotyping to distinguish between recrudescents and new infections in treatment trials of Plasmodium falciparum malaria conducted in Sub-Saharan Africa: adjustment of parasitological outcomes and assessment of genotyping effectiveness. Trop Med Int Health. 2006;11:1350–1359. doi: 10.1111/j.1365-3156.2006.01688.x. [DOI] [PubMed] [Google Scholar]
- 10.Miller LH, Roberts T, Shahabuddin M, McCutchan TF. Analysis of sequence diversity in the Plasmodium falciparum merozoite surface protein-1 (MSP-1) Mol Biochem Parasitol. 1993;59:1–14. doi: 10.1016/0166-6851(93)90002-f. [DOI] [PubMed] [Google Scholar]
- 11.Greenhouse B, Myrick A, Dokomajilar C, Woo JM, Carlson EJ, Rosenthal PJ, Dorsey G. Validation of microsatellite markers for use in genotyping polyclonal Plasmodium falciparum infections. Am J Trop Med Hyg. 2006;75:836–842. [PMC free article] [PubMed] [Google Scholar]
- 12.DaRe JT, Mehlotra RK, Michon P, Mueller I, Reeder J, Sharma YD, Stoneking M, Zimmerman PA. Microsatellite polymorphism within pfcrt provides evidence of continuing evolution of chloroquine-resistant alleles in Papua New Guinea. Malar J. 2007;6:34. doi: 10.1186/1475-2875-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehlotra RK, Ziats MN, Bockarie MJ, Zimmerman PA. Prevalence of CYP2B6 alleles in malaria-endemic populations of West Africa and Papua New Guinea. Eur J Clin Pharmacol. 2006;62:267–275. doi: 10.1007/s00228-005-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehlotra RK, Bockarie MJ, Zimmerman PA. Prevalence of UGT1A9 and UGT2B7 nonsynonymous single nucleotide polymorphisms in West African, Papua New Guinean, and North American populations. Eur J Clin Pharmacol. 2007;63:1–8. doi: 10.1007/s00228-006-0206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giao PT, de Vries PJ. Pharmacokinetic interactions of antimalarial agents. Clin Pharmacokinet. 2001;40:343–373. doi: 10.2165/00003088-200140050-00003. [DOI] [PubMed] [Google Scholar]
- 16.Grace JM, Aguilar AJ, Trotman KM, Peggins JO, Brewer TG. Metabolism of beta-arteether to dihydroqinghaosu by human liver microsomes and recombinant cytochrome P450. Drug Metab Dispos. 1998;26:313–317. [PubMed] [Google Scholar]
- 17.Ilett KF, Ethell BT, Maggs JL, Davis TM, Batty KT, Burchell B, Binh TQ, Thu le TA, Hung NC, Pirmohamed M, Park BK, Edwards G. Glucuronidation of dihydroartemisinin in vivo and by human liver microsomes and expressed UDP-glucuronosyltransferases. Drug Metab Dispos. 2002;30:1005–1012. doi: 10.1124/dmd.30.9.1005. [DOI] [PubMed] [Google Scholar]
- 18.Svensson US, Ashton M. Identification of the human cytochrome P450 enzymes involved in the in vitro metabolism of artemisinin. Br J Clin Pharmacol. 1999;48:528–535. doi: 10.1046/j.1365-2125.1999.00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koram KA, Abuaku B, Duah N, Quashie N. Comparative efficacy of antimalarial drugs including ACTs in the treatment of uncomplicated malaria among children under 5 years in Ghana. Acta Trop. 2005;95:194–203. doi: 10.1016/j.actatropica.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 20.Martensson A, Stromberg J, Sisowath C, Msellem MI, Gil JP, Montgomery SM, Olliaro P, Ali AS, Bjorkman A. Efficacy of artesunate plus amodiaquine versus that of artemether-lumefantrine for the treatment of uncomplicated childhood Plasmodium falciparum malaria in Zanzibar, Tanzania. Clin Infect Dis. 2005;41:1079–1086. doi: 10.1086/444460. [DOI] [PubMed] [Google Scholar]
- 21.Mutabingwa TK, Anthony D, Heller A, Hallett R, Ahmed J, Drakeley C, Greenwood BM, Whitty CJ. Amodiaquine alone, amodiaquine+sulfadoxine-pyrimethamine, amodiaquine+artesunate, and artemether-lumefantrine for outpatient treatment of malaria in Tanzanian children: a four-arm randomised effectiveness trial. Lancet. 2005;365:1474–1480. doi: 10.1016/S0140-6736(05)66417-3. [DOI] [PubMed] [Google Scholar]
- 22.Vugt MV, Wilairatana P, Gemperli B, Gathmann I, Phaipun L, Brockman A, Luxemburger C, White NJ, Nosten F, Looareesuwan S. Efficacy of six doses of artemether-lumefantrine (benflumetol) in multidrug-resistant Plasmodium falciparum malaria. Am J Trop Med Hyg. 1999;60:936–942. doi: 10.4269/ajtmh.1999.60.936. [DOI] [PubMed] [Google Scholar]
- 23.Price RN, Uhlemann AC, van Vugt M, Brockman A, Hutagalung R, Nair S, Nash D, Singhasivanon P, Anderson TJ, Krishna S, White NJ, Nosten F. Molecular and pharmacological determinants of the therapeutic response to artemether-lumefantrine in multidrug-resistant Plasmodium falciparum malaria. Clin Infect Dis. 2006;42:1570–1577. doi: 10.1086/503423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim P, Alker AP, Khim N, Shah NK, Incardona S, Doung S, Yi P, Bouth DM, Bouchier C, Puijalon OM, Meshnick SR, Wongsrichanalai C, Fandeur T, Le Bras J, Ringwald P, Ariey F. Pfmdr1 copy number and arteminisin derivatives combination therapy failure in falciparum malaria in Cambodia. Malar J. 2009;8:11. doi: 10.1186/1475-2875-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hodel EM, Marfurt J, Muller D, Rippert A, Borrmann S, Muller I, Reeder JC, Siba P, Genton B, Beck HP. Lack of multiple copies of pfmdr1 gene in Papua New Guinea. Trans R Soc Trop Med Hyg. 2008;102:1151–1153. doi: 10.1016/j.trstmh.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez-Antona C, Sayi JG, Gustafsson LL, Bertilsson L, Ingelman-Sundberg M. Phenotype-genotype variability in the human CYP3A locus as assessed by the probe drug quinine and analyses of variant CYP3A4 alleles. Biochem Biophys Res Commun. 2005;338:299–305. doi: 10.1016/j.bbrc.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 27.Ezzet F, Mull R, Karbwang J. Population pharmacokinetics and therapeutic response of CGP 56697 (artemether + benflumetol) in malaria patients. Br J Clin Pharmacol. 1998;46:553–561. doi: 10.1046/j.1365-2125.1998.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White NJ, van Vugt M, Ezzet F. Clinical pharmacokinetics and pharmacodynamics and pharmacodynamics of artemether-lumefantrine. Clin Pharmacokinet. 1999;37:105–125. doi: 10.2165/00003088-199937020-00002. [DOI] [PubMed] [Google Scholar]
- 29.Denis MB, Tsuyuoka R, Lim P, Lindegardh N, Yi P, Top SN, Socheat D, Fandeur T, Annerberg A, Christophel EM, Ringwald P. Efficacy of artemether-lumefantrine for the treatment of uncomplicated falciparum malaria in northwest Cambodia. Trop Med Int Health. 2006;11:1800–1807. doi: 10.1111/j.1365-3156.2006.01739.x. [DOI] [PubMed] [Google Scholar]
- 30.Owusu-Agyei S, Asante KP, Owusu R, Adjuik M, menga-Etego S, Dosoo DK, Gyapong J, Greenwood B, Chandramohan D. An open label, randomised trial of artesunate+amodiaquine, artesunate+chlorproguanil-dapsone and artemether-lumefantrine for the treatment of uncomplicated malaria. PLoS One. 2008;3:e2530. doi: 10.1371/journal.pone.0002530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kobbe R, Klein P, Adjei S, Amemasor S, Thompson WN, Heidemann H, Nielsen MV, Vohwinkel J, Hogan B, Kreuels B, Buhrlen M, Loag W, Ansong D, May J. A randomized trial on effectiveness of artemether-lumefantrine versus artesunate plus amodiaquine for unsupervised treatment of uncomplicated Plasmodium falciparum malaria in Ghanaian children. Malar J. 2008;7:261. doi: 10.1186/1475-2875-7-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piola P, Fogg C, Bajunirwe F, Biraro S, Grandesso F, Ruzagira E, Babigumira J, Kigozi I, Kiguli J, Kyomuhendo J, Ferradini L, Taylor W, Checchi F, Guthmann JP. Supervised versus unsupervised intake of six-dose artemether-lumefantrine for treatment of acute, uncomplicated Plasmodium falciparum malaria in Mbarara, Uganda: a randomised trial. Lancet. 2005;365:1467–1473. doi: 10.1016/S0140-6736(05)66416-1. [DOI] [PubMed] [Google Scholar]
- 33.Hetzel MW, Obrist B, Lengeler C, Msechu JJ, Nathan R, Dillip A, Makemba AM, Mshana C, Schulze A, Mshinda H. Obstacles to prompt and effective malaria treatment lead to low community-coverage in two rural districts of Tanzania. BMC Public Health. 2008;8:317. doi: 10.1186/1471-2458-8-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White NJ, Pan-Ngum W, Maude RJ, Saralamba S, Aguas R, Stepniewska K, Lee SJ, Dondorp AM, White LJ, Day NP. Hyperparasitaemia and low dosing are an important source of anti-malarial drug resistance. Malar J. 2009;8:253. doi: 10.1186/1475-2875-8-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med. 2008;359:2619–2620. doi: 10.1056/NEJMc0805011. [DOI] [PubMed] [Google Scholar]


