Abstract
The epidemiology of Chagas disease was studied in five rural communities located in the eastern region of the Panama Province. Serological tests for Trypanosoma cruzi infection revealed a prevalence of 5.88% (12/204). Hemocultures coupled with polymerase chain reaction (PCR) analysis showed a Trypanosoma rangeli infection rate of 5.88% (12/204). An overall trypanosome infection index of 11.76% (24/204) was detected in this population. A total of 121 triatomine specimens were collected in domestic and peridomestic habitats. Rhodnius pallescens was confirmed as the predominant species. Molecular analysis showed that 17.8% (13/73) of the examined insects were positive for T. cruzi, 17.8% (13/73) for T. rangeli, and 35.6% (26/73) presented mixed infections. Among 73 R. pallescens evaluated, 16.4% (12/73) contained opossum blood meals. The epidemiological implications of these findings are discussed.
Chagas disease is one of the 10 most neglected diseases that affect the poor and underserved in the Americas. With 50,000–200,000 new infections occurring per year, Chagas impacts up to 15 million people.1 The infection is caused by Trypanosoma cruzi, a parasitic protozoan mainly transmitted through contact with parasite-infected excreta from triatomine bugs. Although considerable progress has been made in disease control throughout South America,2,3 the infection in Central America remains a serious health problem, affecting an estimate of 2 million people and, hindering development in one of the poorest regions of the world.4 In Panama, the sylvatic behavior of the main Chagas disease vector, Rhodnius pallescens, complicates control measures.5 This species of vector also transmits Trypanosoma rangeli, a non-pathogenic trypanosome, through the saliva. In the blood of people from endemic regions of Central Panama, T. rangeli is more frequently found than T. cruzi.6,7
To address this health problem, the Central American Initiative for Chagas disease Control (IPCA) has established as one of its priorities, the identification of risk and transmission areas within a country. In accordance with IPCA recommendations, Panamanian health authorities have focused efforts in the detection of new endemic regions and the active detection of cases, particularly children who will benefit from specific treatment. It is on this foundation that a preliminary epidemiological and entomological study on Chagas disease was conducted in the eastern part of the Panama Province, a region with appropriate eco-epidemiological conditions for T. cruzi transmission, and from which no current data on the disease are available.
During 2006–2007, we evaluated the presence of anti-T. cruzi antibodies and blood trypanosomes in the residents of five rural communities (Union Herrerana, Jagüito, Rio La Mina, La Placita, and El Nazareno) from the Chepo and Chiman Districts located in the eastern part of Panama Province (Figure 1). This region is a remote and extremely poor area, within a 4-hour drive from Panama City, only accessible by car during the dry season. Deforestation is widespread and in the remaining secondary forest “royal palm” trees (Attalea butyracea) are common. Royal palms are considered the primary biotope of several triatomine species,8 and R. pallescens is a common inhabitant of these trees in central Panama.9 Overall, the number of houses in these locations is 82, with an estimated population of 324 people in the year 2000. A total of 204 blood samples were taken from apparently healthy volunteers, between 6 months and 82 years of age, after obtaining informed consent. Fifty-five percent (N = 113) of the participants were male and 44.6% (N = 91) were female. The mean ± SD age for both males and females was 20.87 ± 16.05 years. Serum samples were screened for the presence of antibodies against T. cruzi with three serological tests: a commercial recombinant enzyme-linked immunosorbent assay (ELISA) (ELISA Chagatest, Wiener Laboratory, Argentina), a recombinant ImmunoComb commercial test (Orgenics, Israel), and an immunoblotting technique using a crude epimastigote antigenic preparation derived from a Panamanian T. cruzi strain (Burunga).10 Samples were considered positive when they showed reactivity in at least two of the serologic tests. Hemoculture of blood samples was performed as described by Vasquez and others,11 and isolated trypanosomes were characterized by a multiplex PCR.12 Questionnaires were used to assess epidemiological data on risk factors associated with the disease.
Figure 1.
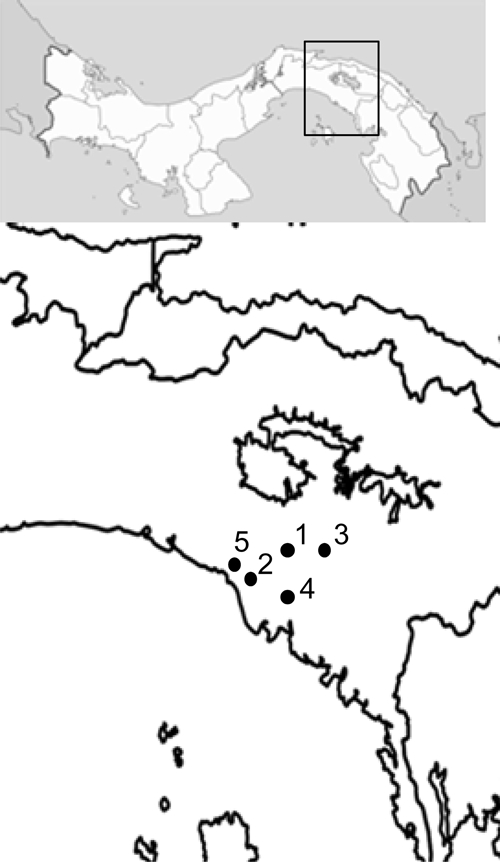
Map of Panama, displaying studied sites from the Chepo (9°10′0²N and 79°5′60²W) and Chiman Districts (8°43′1²N and 78°38′2²W) in Eastern Panama (1: Unión Herrerana, 2: El Nazareno, 3: Jaguito, 4: Río La Mina, and 5: La Placita).
Additionally, an entomological search to collect triatomines in domestic and peridomestic habitats was carried out with community participation. In peridomestic areas, insects were found on palm trees near surveyed houses (less than 100 m). Collected triatomines were analyzed for trypanosome infection using a multiplex PCR12 and for host feeding sources using a dot blot assay with antibodies against human, chicken, dog, opossum, and mouse immunoglobulins.13
Serological tests for T. cruzi infection revealed a prevalence of 5.88% (12/204) in the five rural localities evaluated in Eastern Panama. Four seropositive patients were from Union Herrerana (5.26%; 4/76), four from Nazareno (8.0%; 4/50), three from La Mina (17.64%; 3/17), and one from La Placita (2.22%; 1/45). In Jaguito, of the 16 persons evaluated, no evidence of infection was found. Among seropositives, four (33.3%; 4/12) were children less than 15 years of age. They were referred to the National Care Center for further evaluation and specific etiological treatment. Trypanosomes were isolated from 12 hemocultures (5.88%; 12/204), nine were from children less than 15 years of age. The multiplex PCR showed that all of the isolated trypanosomes were T. rangeli. None of the subjects harbored mix infections. On the basis of both blood test results, an overall trypanosome infection prevalence of 11.76% (24/204) was determined in this population.
A total of 121 triatomines were collected, all of them identified as R. pallescens (89 immature stages and 32 adults). Most of the specimens (119; 31 adults/88 nymph) were found on peridomestic palm trees, with an average density of 12 triatomines/palm. Only one adult and one nymph were collected in the domestic habitat. The host feeding profile and trypanosomes infection rate were analyzed on 73 specimens (adults and IV and V nymphs). It was only possible to identify the blood origin on 21.9% (16/73) of these triatomines, because many insects were too dry, did not contain sufficient blood or, most likely, other mammalian blood not evaluated was present. In concurrence with previous reports from other regions in Panama,14 opossums were a common blood meal source of R. pallescens in the endemic areas of this study (16.4% 12/73). Additionally, one insect was positive for mouse blood, one for dog, and two for chicken blood. Regarding the trypanosomes infection rate, 17.8% (13/73) of the triatomines were solely positive for T. cruzi, 17.8% (13/73) for T. rangeli, 35.6% (26/73) presented mixed infections, and 28.8% (21/73) were negative for both parasites (Figure 2).
Figure 2.
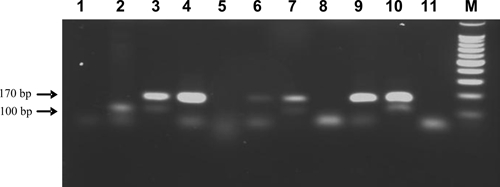
Detection of Trypanosoma cruzi and Trypanosoma rangeli infections in vector specimens by a duplex polymerase chain reaction (PCR) assay based on the amplification of telomeric sequences (Chiurrillo and others12). Samples were visualized in ethidium bromide-stained 2% agarose gels. Lane 1 and 11: Negative controls (no DNA); Lane 2: T. cruzi positive control; Lanes 3–10: DNA samples extracted from captured Rhodnius pallescens; M: molecular marker: 100-bp ladder (Promega, Madison, WI). Trypanosoma cruzi and T. rangeli amplifies a 100- and 170-bp specific product, respectively. Mixed infections produce both amplification products.
The low standard of living in each of the five communities was evidenced by the poor housing conditions. Most of the inhabitants live in typical “ranchos” constructed with natural local materials such as palm thatched roofs (90%), wood or cane walls (98.4%), and dirt floors (91.3%). Among trypanosome positive individuals, 50% stated that they or a family member had been bitten by the vector and 92% lived in houses near “royal palms”.
Although the majority of the Panamanian territory has eco-epidemiological conditions suitable for T. cruzi transmission, for decades Chagas disease research has focused on the central part of the country, in areas adjacent to the Panama Canal. Attentiveness toward this area is most likely caused by the human population density and activities related to the Panama Canal operation. The disease epidemiology in this area is well documented and consequently, most of the past and present control and prevention efforts have been focused in this part of the country. To update the extent of Chagas disease in Panama, we examined other regions with high-risk factors for transmission of this vector-borne disease. A newly discovered endemic region for Chagas disease was identified in rural communities of the eastern part of Panama Province, Districts of Chepo and Chiman (Figure 1). The serological and parasitological results show that an active trypanosome transmission is currently occurring in the human population from this area of the country. The fact that many of the trypanosome-infected individuals were children (54.2%; 13/24) further indicates a high exposure to the vector early in life and thus, this already vulnerable population is at a higher risk of suffering from Chagas disease. Epidemiologically, the situation is similar to the one recently described in rural communities from central Panama, where T. rangeli infections are also significantly high.10 This parasite, which is non-pathogenic to humans, may have epidemiological relevance. It has been suggested that a previous T. rangeli infection may confer partial protection against subsequent T. cruzi infections, at least in animal models.15,16
Our preliminary entomological results need to be complemented by further research; however, as in most of the documented Chagas disease endemic areas in Panama, R. pallescens is likely the main vector in the newly described endemic region. The fact that more than 50% of the collected triatomines were infected with trypanosomes and the sporadic findings of the insects inside domestic habitats explains the high prevalence of human trypanosome infection observed in this area. As in other recent studies done in central Panama,14 the opossum was found to be a common blood source for R. pallescens in this part of the country. Nevertheless, additional evaluations about the eco-biological characteristics of the R. pallescens populations are necessary to understand the epidemiological pattern adopted by Chagas diseases in these communities.
Several other communities with similar ecological conditions exist in this area of Panama and are deserved priorities for assistance from the National Chagas Disease Control Program. Community educational programs regarding both the disease and control measures are necessary. Our results provide baseline data on Chagas disease epidemiology in this part of the country and may serve as a reference for future studies in potentially endemic areas.
Acknowledgments
We acknowledge with appreciation the field and technical assistance of Roberto Rojas, Temistocles Bultrón, Jose Montenegro, and Juan De León. We thank Nicole Gottdenker and Philip Scheibel for the critical review of the manuscript.
Footnotes
Financial support: This investigation received financial support from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR) grant A304558, and from the Research Fund of Instituto Conmemorativo Gorgas de Estudios de la Salud (IGCES).
Disclosure: Jose E. Calzada and Azael Saldaña are members of the Sistema Nacional de Investigación (SNI), SENACYT, Panama.
Authors' addresses: José E. Calzada, Vanesa Pineda, Juan D. Garisto, Franklyn Samudio, Ana Maria Santamaria, and Azael Saldaña. Instituto Conmemorativo Gorgas de Estudios de la Salud, Panama, Republic of Panama, E-mails: jcalzada@gorgas.gob.pa, vpineda@gorgas.gob.pa, jgaristomd@yahoo.com, fsamudio@gorgas.gob.pa, asantamaria@gorgas.gob.pa, and asaldana@gorgas.gob.pa.
References
- 1.Dias JC. Chagas disease control and the natural history of human Chagas disease: a possible interaction? Mem Inst Oswaldo Cruz. 2000;95(Suppl II):14–22. [Google Scholar]
- 2.Dias JCP, Silveira AC, Schofield CJ. The impact of Chagas disease control in Latin America: a review. Mem Inst Oswaldo Cruz. 2002;97:603–612. doi: 10.1590/s0074-02762002000500002. [DOI] [PubMed] [Google Scholar]
- 3.Moncayo A. Chagas disease: current epidemiological trends after the interruption of vectorial and transfusional transmission in the Southern Cone countries. Mem Inst Oswaldo Cruz. 2003;98: 577–591. doi: 10.1590/s0074-02762003000500001. [DOI] [PubMed] [Google Scholar]
- 4.Ponce C. Current situation of Chagas disease in Central America. Mem Inst Oswaldo Cruz. 2007;102(Suppl 1):41–44. doi: 10.1590/s0074-02762007005000082. [DOI] [PubMed] [Google Scholar]
- 5.Vasquez AM, Samudio FE, Saldana A, Paz HM, Calzada JE. Eco-epidemiological aspects of Trypanosoma cruzi, Trypanosoma rangeli and their vector (Rhodnius pallescens) in Panama. Rev Inst Med Trop Sao Paulo. 2004;46:217–222. doi: 10.1590/s0036-46652004000400008. [DOI] [PubMed] [Google Scholar]
- 6.Sousa OE, Johnson CM. Frequency and distribution of Trypanosoma cruzi and Trypanosoma rangeli in the Republic of Panama. Am J Trop Med Hyg. 1971;20:405–410. doi: 10.4269/ajtmh.1971.20.405. [DOI] [PubMed] [Google Scholar]
- 7.Saldaña A, Samudio F, Miranda F, Herrera LM, Saavedra S, Cáceres L, Bayard V, Calzada JE. Predominance of Trypanosoma rangeli infection in children from a Chagas disease endemic area in the west-shore of the Panama Canal. Mem Inst Oswaldo Cruz. 2005;100:729–731. doi: 10.1590/s0074-02762005000700008. [DOI] [PubMed] [Google Scholar]
- 8.Abad-Franch F, Palomeque FS, Aguilar HM, 5th, Miles MA. Field ecology of sylvatic Rhodnius populations (Heteroptera, Triatominae): risk factors for palm tree infestation in western Ecuador. Trop Med Int Health. 2005;10:1258–1266. doi: 10.1111/j.1365-3156.2005.01511.x. [DOI] [PubMed] [Google Scholar]
- 9.Whitlaw JT, Chaniotis BN. Palm trees and Chagas' disease in Panama. Am J Trop Med Hyg. 1978;27:873–881. doi: 10.4269/ajtmh.1978.27.873. [DOI] [PubMed] [Google Scholar]
- 10.Saldana A, Sousa OE, Orn A. Immunoparasitological studies of Trypanosoma cruzi low virulence clones from Panama: humoral immune responses and antigenic cross-reactions with Trypanosoma rangeli in experimentally infected mice. Scand J Immunol. 1995;42:644–650. doi: 10.1111/j.1365-3083.1995.tb03707.x. [DOI] [PubMed] [Google Scholar]
- 11.Vasquez JE, Krusnell J, Orn A, Sousa OE, Harris RA. Serological diagnosis of Trypanosoma rangeli infected patients. A comparison of different methods and its implications for the diagnosis of Chagas' disease. Scand J Immunol. 1997;45:322–330. doi: 10.1046/j.1365-3083.1997.d01-405.x. [DOI] [PubMed] [Google Scholar]
- 12.Chiurillo MA, Crisante G, Rojas A, Peralta A, Dias M, Guevara P, Añez N, Ramírez JL. Detection of Trypanosoma cruzi and Trypanosoma rangeli infection by duplex PCR assay based on telomeric sequences. Clin Diagn Lab Immunol. 2003;10:775–779. doi: 10.1128/CDLI.10.5.775-779.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calzada JE, Pineda V, Montalvo E, Alvarez D, Santamaría AM, Samudio F, Bayard F, Cáceres L, Saldaña A. Human trypanosome infection and the presence of intradomicile Rhodnius pallescens in the western border of the Panama Canal, Panama. Am J Trop Med Hyg. 2006;74:762–765. [PubMed] [Google Scholar]
- 14.Pineda V, Montalvo E, Alvarez D, Santamaría A, Calzada JE, Saldaña A. Feeding sources and trypanosome infection index of Rhodnius pallescens in a Chagas disease endemic area of Amador County, Panama. Rev Med Trop de São Paulo. 1998;50:113–116. doi: 10.1590/s0036-46652008000200009. [DOI] [PubMed] [Google Scholar]
- 15.Basso B, Cervetta L, Moretti E, Carlier Y, Truyens C. Acute Trypanosoma cruzi infection: IL-12, IL-18, TNF, sTNFR and NO in T. rangeli-vaccinated mice. Vaccine. 2004;15-16:1868–1872. doi: 10.1016/j.vaccine.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Palau MT, Mejia AJ, Vergara U, Zuniga CA. Action of Trypanosoma rangeli in infections with virulent Trypanosoma cruzi populations. Mem Inst Oswaldo Cruz. 2003;98:543–548. doi: 10.1590/s0074-02762003000400022. [DOI] [PubMed] [Google Scholar]


