Abstract
An investigation was conducted to identify the distribution of mosquitoes and mosquito-borne arboviruses in the Qinghai-Tibet Plateau, China from July to August in 2007. A total of 8,147 mosquitoes representing six species from three genera (Aedes, Culex, and Anopheles) were collected in three locations (Geermu city, altitude of 2,780 m; Xining city, 2,200 m; Minhe county, 1,700 m). Six virus isolates were obtained including Tahyna virus (TAHV), Liaoning virus, and Culex pipiens pallens Densovirus. A serosurvey showed immunoglobulin G antibodies by immunofluorescence assay (IFA) against TAHV in residents of all three locations. The IFA-positive human samples were confirmed by 90% plaque-reduction neutralization tests (PRNT90) against TAHV with titers ranging from 1:20 to 1:10,240. In addition, TAHV seropositive cows, sheep, and swine were found in these locations. This investigation represents the first isolation of TAHV from Ae. (Och.) detritus and the first evidence of TAHV infection in residents and livestock in the Qinghai-Tibet Plateau.
Introduction
The Qinghai area is located in the western region of mainland China, and most of its area is located in the Qinghai-Tibet Plateau. Qinghai lies between latitude 31°32′ N and 39°20′ N and longitude 89°24′ E and 103°04′ E, and it covers a 721,000-km area.1 The average altitude of Qinghai is over 3,000 m, and it reaches a high of 6,880 m (Bukadaban Peak of Kunlun Mountains) and a low of 1,650 m (Xiachuankou village in Minhe county).1 Qinghai borders Gansu Province on the northeast, Xinjiang Uygur Autonomous Region (Xinjiang) on the northwest, Sichuan Province on the southeast, and Tibet Autonomous Region (Tibet) on the southwest. In recent years, communication and trade among Tibet and the other western provinces in China has intensified as a result of the construction of the Qingzang Railway that connects Xining city (capital of Qinghai) to Lhasa city (capital of Tibet).2 Consequently, Qinghai has become a crucial region that connects inland provinces of China with countries in South Asia such as Nepal, India, and Bangladesh.2 As a result, trade and travel across Qinghai are increasing rapidly, and with them, the likelihood of introduction and exportation of vectors and vector-borne diseases increases. For example, plague bacillus was detected in a woodchuck captured on the train from Geermu city in September 2000, and two human plague cases occurred in Wulan county along the Qingzang Railway in October 2004.3 Therefore, it is important to strengthen the detection and monitoring of vector-borne diseases in the region.
Previous research showed that bacterial vector-borne diseases such as plague, typhus fever, brucellosis, and Lyme disease have occurred in Qinghai.4–7 Since the 1950s, there was considered to be no Japanese encephalitis in this area,8 and as a result, no systematic investigations of mosquito-borne arboviruses have been carried out. However, seasonal fever and viral encephalitis cases of unknown etiology are frequently reported in the summer and autumn in Qinghai.9 Here, we describe a study of the distribution of mosquitoes and mosquito-borne viruses and show evidence of arbovirus infection in humans and livestock in three locations in Qinghai (Geermu city, Xining city, and Minhe county).
Materials and Methods
Collection of mosquitoes and site descriptions.
During the period from July 30 to August 14 in 2007, three collection sites were chosen in Geermu city (Xinhua village, Baoku village, and Xiatan village), Xining city (Datonghe village, Taobei village, and Jiujiawan village), and Minhe county (Caotan village, Jintian village, and Lajia village) within Qinghai (Figure 1). Geermu city is located in the western region of Qinghai with an average altitude of 2,780 m, an annual average temperature of 4.3°C, and an annual average precipitation of 42.5 mm. Xining city is located in the eastern region of Qinghai with an average altitude of 2,200 m, an annual average temperature of 7.7°C, and an annual average precipitation of 520.3 mm. Minhe county is located in the most eastern region of Qinghai with an average altitude of 1,700 m, an annual average temperature of 9°C, and an annual average precipitation of 350 mm.1
Figure 1.
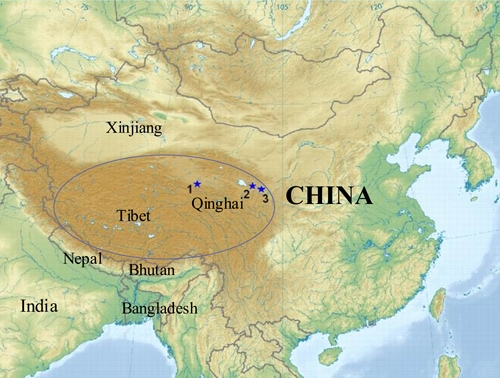
Map of China showing the locations where mosquitoes and sera were collected in Qinghai (1: Geermu city; 2: Xining city; 3: Minhe county). The dark brown area in ellipse represents the major region of the Qinghai-Tibet Plateau. This figure appears in color at www.ajtmh.org.
Each village was sampled for one night from 19:30 to 21:30 hours. Adult mosquitoes were collected using light traps baited with CO2. Three different kinds of habitat were sampled in each location: wheat field, grass river bank, and pond with heavy reed vegetation. Female mosquitoes were identified in the species using morphologic characteristics; they were pooled into groups of approximately 50–100 mosquitoes and stored in liquid nitrogen.
Isolation of viruses.
Mosquitoes were removed from the liquid nitrogen and immediately homogenized and centrifuged as reported previously.10 The supernatants were inoculated into monolayers of BHK-21 and C6/36 cells and incubated at 37°C and 28°C, respectively. Cells were observed for cytopathic effect (CPE) daily from day 1 through day 7 post-inoculation. A specimen was regarded as a positive isolate if it caused CPE in three successive cell passages.10 Suckling mice were inoculated with 0.02 ml supernatant of positive isolates intracerebrally and observed for 14 days thereafter.11,12
Indirect immunofluorescence assay.
Virus isolates in cell culture were identified initially by indirect immunofluorescence assay (IFA). Infected and uninfected cell suspensions were applied to Teflon-coated, 10-well slides; then, they were air dried and fixed in acetone. IFA was conducted with a panel of arbovirus immune ascites as described previously.12,13 The panel of antisera included broadly reactive immune ascites against Alphaviruses, specific antisera to Sindbis virus, Getah virus, Mayaro virus, Chikungunya virus, and Smiliki Forest virus, Bunyavirus broadly reactive antiserum, and antisera against the Flaviviruses Japanese Encephalitis virus, dengue virus, and West Nile virus prepared by our laboratory.14 We also used immune ascites against the prototype Tahyna virus (Bardos 92 strain) provided by the Centers for Disease Control and Prevention (CDC; Fort Collins, CO).12
RNA-polyacrylamide gel electrophoresis.
The virus RNA was extracted and processed using RNA-polyacrylamide gel electrophoresis (RNA-PAGE) as previously described.15
RNA extraction, complementary DNA synthesis, reverse transcription–polymerase chain reaction, and sequence analysis.
Extraction of viral RNA was done with cell-culture supernatants using the QIAmp Viral RNA kit (Qiagen, Inc., Valencia, CA), and first-strand complementary DNA (cDNA) was synthesized using the Ready-To-Go You Prime First Strand Beads (GE Healthcare, Uppsala, Sweden) according to the manufacturer's procedure. Reverse transcription–polymerase chain reaction (RT-PCR) was conducted with primers designed for Bunyavirus genus, Tahyna virus (TAHV), the 10th fragment of Liaoning virus, and a partial NS1 gene of Culex pipiens pallens densovirus (CppDNV) (Table 1).16–18 Amplified DNA fragments were visualized by electrophoresis in 1% agarose gels. Positive DNA fragments were extracted by a TaKaRa DNA fragment Purification Kit and sequenced by a commercial provider (Beijing Genome Institute, Beijing, China).
Table 1.
Primer information used in RT-PCR to identify virus isolates
| Primer | Amplify region | Sequence data | Site in genome | Size (base pair) |
|---|---|---|---|---|
| BUP16 | Bunyavirus genus | ATGACTGAGTTGGAGTTTGATGTCGC | S segment | 250 |
| BDW | Bunyavirus genus | TGTTCCTGTTGCCAGGAAAAT | S segment | |
| TS141f | Tahyna virus | AGGGTATGTGGACTTCTG | 141–158 | 796 |
| TS936r | Tahyna virus | CTAACTTCTATTTTTTTC | 919–936 | |
| LNVS10REV117 | Liaoning virus | GTTCCCGGACTTTCACAGCTACTTTC | 10th segment | 844 |
| LNVS10FOR1 | Liaoning virus | ATGAGTAACGTGACAGAGATTCGTGC | 10th segment | |
| DNV 3F18 | CppDNV* | TGTCTCTTTCTCTTGGTATTTCTTC | 2,096–2,120 | 903 |
| DNV 3R | CppDNV | CATACTACACATTCGTCCTCCAC | 1,176–1,198 |
Initial sequence assembly and analysis was conducted using SeqMan (DNASTAR, Madison, WI; http://www.dnastar.com). Homology and alignment analysis was carried out using the Clustal X (version 1.8; http://www.clustal.org) and MegAlign (DNASTAR, Madison, WI; http://www.dnastar.com). MEGA 3.1 (http://www.megasoftware.net) was used for phylogenetic analysis and tree construction based on neighbor-joining (N-J) assay, and bootstrap value was 1,000.
Sera collection of local residents.
Serum samples of local residents were collected from the same villages where mosquitoes were collected in Geermu city, Xining city, and Minhe county during the period from July 22 to July 27. In each site, human subjects were selected to obtain samples from several age classes by systematically visiting every other house and soliciting participants. Approximately 350–360 samples were obtained from each location. After receiving informed consent, trained personnel performed venipuncture to obtain 2-ml blood samples and recorded the age, sex, and village of residence for each subject. The separated serum samples were stored in −20°C until tested. This project was reviewed and approved by the local human subjects committee.
Sera collection of local livestock.
During the period from July 22 to July 27, serum samples were obtained from local livestock (cows, sheep, and swine) at the local abattoirs in Xining city and Minhe county. There were no local swine in Geermu city, so only cows and sheep were bled. The species, sex, and village or origin was obtained for each animal that was bled. The serum samples were stored in −20°C before tested.
Serological detection of antibodies against TAHV isolate QH07060.
After it was determined that the isolate QH07060 from mosquitoes collected in Geermu city was a TAHV strain, this isolate was used to infect BHK-21 cells for use in the IFA assay. Infected cells were applied to Teflon-coated, 10-well slides as antigen with uninfected cells as controls. The serum samples (human, cow, sheep, and swine) were tested at a dilution of 1:50 for antibodies against QH07060 by IFA as reported previously.12
Plaque-reduction neutralization tests.
Seropositive samples detected by IFA were tested for neutralizing antibodies to TAHV isolate QH07060 by 90% plaque-reduction neutralization tests (PRNT90) using standard methods. Sera were tested with serial 2-fold dilutions from 1 to 5. Diluted sera were mixed with equal volumes of culture medium containing QH07060 (100 plaque forming unit [PFU]) and incubated at 37°C for 1 hour. Six-well plates of confluent BHK-21 cells were inoculated with the serum–virus mixtures and incubated at 37°C in a 5% CO2 incubator for 1 hour. Plates were overlaid with 3 mL of the medium containing 0.8% agarose and again with 2.5 mL of second overlay medium containing neutral red vital stain (Sigma-Aldrich, Inc., CA) as described previously.19 The neutralizing antibody titer was identified as the highest serum dilution that reduced the number of virus plaques in the test by 90% or greater. The samples were considered to be positive when titers ≥ 20.19
Results
Collection of mosquitoes.
From July 30 to August 14 in 2007, a total of 8,147 mosquitoes representing six species from three genera (Aedes, Culex, and Anopheles) were collected in Geermu city, Xining city, and Minhe county (Table 2). In Geermu city, 3,520 mosquitoes representing only two species were collected (Ae. [Och.] detritus Holiday, 53.2%, 1,873/3,520; Cx. pipiens Linnaeus, 46.8%, 1,647/3,520). In Xining city, 1,329 mosquitoes representing two species were collected (Ae. vexans Meigen, 96.3%, 1,280/1,329; Cx. Pipiens, 3.7%, 49/1,329). In Minhe county, 3,298 mosquitoes in four species were collected (Cx. modestus Ficalbi, 56.0%, 1,848/3,298; Cx. Pipiens, 10.4%, 343/3,298; Ae. Vexans, 26.4%, 871/3,298). The fourth species was identified as An. nigerrimus Giles,20,21 which constituted 1.2% of the total collection in Minhe county. This represents the first detection of this species this far north in China. Of the total collected in Minhe county, 6% were Aedes that could not be identified to species.
Table 2.
Mosquito species collected in Qinghai in 2007
| Species | Collection sites | |||||||
|---|---|---|---|---|---|---|---|---|
| Aedes | Geermu city | Xining city | Minhe county | |||||
| No. | % | No. | % | No. | % | Total | ||
| Ae. (Och.) detritus | 1,873 | 53.2 | 0 | 0.0 | 0 | 0.0 | 1,873 | |
| Ae. vexans | 0 | 0.0 | 1,280 | 96.3 | 871 | 26.4 | 2,151 | |
| Ae. sp* | 0 | 0.0 | 0 | 0.0 | 197 | 6.0 | 197 | |
| Culex | ||||||||
| Cx. pipiens pallens | 1,647 | 46.8 | 49 | 3.7 | 343 | 10.4 | 2,039 | |
| Cx. modestus | 0 | 0.0 | 0 | 0.0 | 1,848 | 56.0 | 1,848 | |
| Anopheles | ||||||||
| An. nigerrimus† | 0 | 0.0 | 0 | 0.0 | 39 | 1.2 | 39 | |
| Total | 3,520 | 1,329 | 3,298 | 8,147 | ||||
Species could not be determined.
This is the first report of An. nigerrimus found in the northern region of China.
Isolation of viruses.
A total of 8,147 mosquitoes were processed in 166 pools to isolate viruses by BHK-21 and C6/36 cells. CPE was observed in six pools. The mosquito species producing the isolates and the location where the mosquitoes were collected are shown in Table 3. Isolates QH07029 and QH07060 caused CPE only in BHK-21 cells and produced no CPE on C6/36 cells. The BHK-21 cells became round by 48 hours post-infection and were lysed by 72 hours. Suckling mice inoculated with QH07029 and QH07060 intracerebrally showed signs of tremor and stiff neck at 24 hours and died within 48 hours. The other four isolates caused CPE in C6/36 cells; however, no CPE was observed in BHK-21 cells, and no illness was observed in mice. C6/36 cells infected with QH07130 became round, and they showed enlarged intercellular space and shedding by 96 hours post-infection. The CPE of QH07022, QH07092, and QH07150 resembled QH07130 except for formation of syncytia by 96 hours post-infection.
Table 3.
Viruses isolated in Qinghai in 2007
| No. | Viruses | Mosquito species | Collection date | Collection sites | Virus |
|---|---|---|---|---|---|
| 1 | QH07022 | Ae. (Och.) detritus | 8/2/2007 | Geermu city | CppDNV |
| 2 | QH07029 | Ae. (Och.) detritus | 8/2/2007 | Geermu city | TAHV |
| 3 | QH07060 | Ae. (Och.) detritus | 8/2/2007 | Geermu city | TAHV |
| 4 | QH07092 | Ae. vexans | 8/11/2007 | Xining city | CppDNV |
| 5 | QH07130 | Cx. modestus | 8/6/2007 | Minhe county | LNV |
| 6 | QH07150 | Cx. modestus | 8/7/2007 | Minhe county | CppDNV |
Identification of TAHV.
Both QH07029 and QH07060 reacted with immune ascites against Bunyaviruses and prototype TAHV (Bardos 92 strain), indicating that both isolates were TAHV or a closely related Bunyavirus. RT-PCR amplification using Bunyavirus-reactive primers amplified the nucleotide sequences of both QH07029 and QH07060. Sequence analysis showed the highest homology (90%) with TAHV Bardos 92 strain (U47142) by blast (http://blast.ncbi.nlm.nih.gov/), indicating that QH07029 and QH07060 were TAHV. The S genes of QH07029 and QH07060 were amplified, and the nucleotide sequence homology between QH07029 and QH07060 was 99.9%. The homologies of QH07029 and QH07060 with Bardos 92 strain were 90.1% and 90%, respectively, confirming that QH07029 and QH07060 were TAHV.
Phylogenetic analysis showed that both QH07029 and QH07060 were grouped in the same evolutionary branch with TAHV Bardos 92 strain and were closely related to that strain, which was first isolated in the former Czechoslovakia (Figure 2).22 This is the first time that TAHV has been isolated in the Qinghai-Tibet Plateau and represents only the second isolation in China after isolation of TAHV in Xinjiang Province in 2006.12
Figure 2.
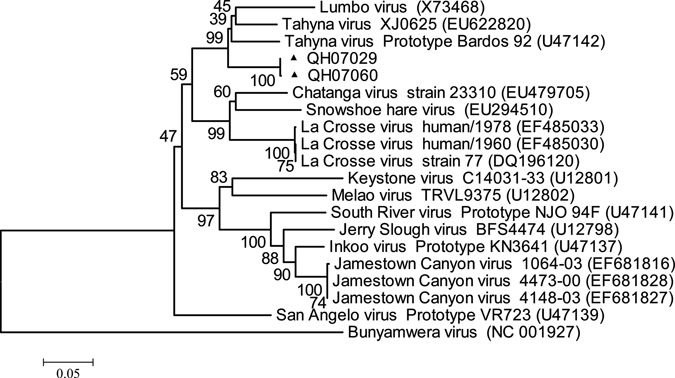
Phylogenetic analysis of TAHV QH07029 and QH07060 isolated from Qinghai, China based on the nucleotide sequence of S segment.
Identification of Liaoning virus.
RNA-PAGE showed that QH07130 was a 12-fragment double-stranded RNA virus, and the fragments could be divided into three groups, 6-5-1, which are similar to those of Liaoning virus (LNV; NE97-31 strain); this indicates that QH07130 was LNV (Figure 3A). RT-PCR was done by applying primers designed for the 10th fragment of LNV. The nucleotide sequences of QH07130 amplified showed the highest homology with LNV (NE97-12 strain, NC007745, 98%; NE97-31 strain, AY317108, 78%), and LNV was isolated in the northeast of China in 1999.23 Phylogenetic analysis showed that QH07130 distributed in the same evolutionary branch with LNV NE97-12 strain and NE97-31 strain, and it showed the closest relationship of QH07130 with the LNV NE97-12 strain, confirming that QH07130 was LNV (Figure 3B).
Figure 3.
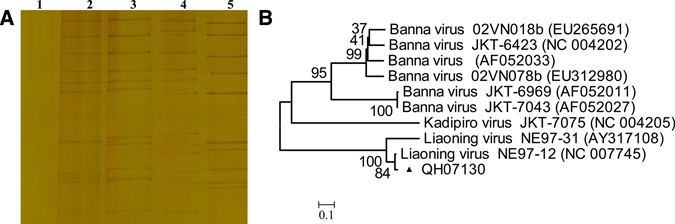
(A) RNA-PAGE of QH07130 and Seadornaviruses (1: cell control; 2: QH07130; 3: LNV, NE97-31 strain, AY317108; 4: KDV, YN0557 strain, FJ159105; 5: BAV, BJ95-75 strain, AY568289). (B) Phylogenetic analysis of LNV QH07130 isolated from Qinghai, China based on the nucleotide sequence of the 10th segment. This figure appears in color at www.ajtmh.org.
Identification of CppDNV.
Nucleotides of QH07022, QH07092, and QH07150 were extracted from supernatants and electrophoresed in 1% agarose gels. All showed a 4k genome, and then, RT-PCR was performed using primers designed for NS1 genes of CppDNV. The amplified nucleotide sequences of QH07022, QH07092, and QH07150 showed the highest homology (more than 99%) with CppDNV (such as JZ-16, EF579756; YN05217, EF579771; YN05169, EF579770; XJ0545, EF579764; GZWN1, EF579757). These were members of genus Brevidensovirus, subfamily Densovirinae, family Parvoviridae,18 confirming that QH07022, QH07092, and QH07150 were members of CppDNV.
Prevalence of TAHV antibodies in local residents.
A total of 1,078 serum samples of local residents were collected from Geermu city, Xining city, and Minhe county (Table 4). Of 1,078 serum samples, 19 (1.8%) were immunoglobulin G (IgG) positive. Among the 19 positive samples, 16 were collected from Geermu city where seroprevalence was 4.4% (16/366), 2 were from Xining city where seroprevalence was 0.6% (2/352), and 1 was from Minhe county where seroprevalence was 0.3% (1/360).
Table 4.
Prevalence of IgG antibody against TAHV by age class in the Qinghai study locations
| Age group (years) | Total | Geermu city | Xining city | Minhe county | ||||
|---|---|---|---|---|---|---|---|---|
| No. | IgG | No. | IgG | No. | IgG | No. | IgG | |
| 0–4 | 135 | 2 (1.5%) | 53 | 2 (3.8%) | 52 | 0 | 30 | 0 |
| 5–9 | 232 | 6 (2.6%) | 70 | 5 (7.1%) | 80 | 1 (1.3%) | 82 | 0 |
| 10–14 | 203 | 5 (2.5%) | 62 | 4 (6.5%) | 44 | 0 | 97 | 1 (1.0%) |
| 15–19 | 175 | 2 (1.1%) | 61 | 2 (3.3%) | 47 | 0 | 67 | 0 |
| 20–29 | 92 | 4 (4.3%) | 48 | 3 (6.3%) | 36 | 1 (2.8%) | 8 | 0 |
| 30–49 | 121 | 0 | 36 | 0 | 41 | 0 | 44 | 0 |
| > 50 | 120 | 0 | 36 | 0 | 52 | 0 | 32 | 0 |
| Total | 1,078 | 19 (1.8%) | 366 | 16 (4.4%) | 352 | 2 (0.6%) | 360 | 1 (0.3%) |
All of the IgG positive samples came from the under 30 age groups, and no TAHV IgG positives were found in the > 30 age groups in any of the locations. The highest seroprevalence was found in Geermu city in the 5–9 (7.1% positive) and the 10–14 (6.5% positive) age groups. The seroprevalence was 6.8% for the 5- to 14-year-old age group in Geermu city.
PRNTs.
Of the 19 IgG positive samples, 18 were confirmed by PRNT90 with titers of 1:20 or greater. One sample produced a titer of 1:10 (Table 5).
Table 5.
IFA and PRNT results from human serum samples tested against TAHV QH07060
| Case no. | Sex/age (years) | Collection sites | IgG (IFA) | Neutralizing antibody titer |
|---|---|---|---|---|
| 1 | F/1.5 | Geermu city | + | 1:5,120 |
| 2 | M/3 | Geermu city | + | 1:10,240 |
| 3 | F/7 | Geermu city | + | 1:1,280 |
| 4 | M/8 | Geermu city | + | 1:640 |
| 5 | M/8 | Geermu city | + | 1:160 |
| 6 | M/8 | Geermu city | + | 1:160 |
| 7 | F/9 | Geermu city | + | 1:80 |
| 8 | F/13 | Geermu city | + | 1:2,560 |
| 9 | F/13 | Geermu city | + | 1:320 |
| 10 | F/13 | Geermu city | + | 1:80 |
| 11 | F/14 | Geermu city | + | 1:2,560 |
| 12 | M/15 | Geermu city | + | 1:160 |
| 13 | M/15 | Geermu city | + | 1:20 |
| 14 | F/26 | Geermu city | + | 1:40 |
| 15 | F/28 | Geermu city | + | 1:10 |
| 16 | F/29 | Geermu city | + | 1:40 |
| 17 | M/8 | Xining city | + | 1:20 |
| 18 | F/20 | Xining city | + | 1:320 |
| 19 | M/13 | Minhe county | + | 1:20 |
Prevalence of TAHV antibodies in local livestock.
A total of 240 serum samples from local livestock were collected in Geermu city, Xining city, and Minhe county. Species included cow, sheep, and swine (Table 6). Of 90 cow sera, 3 were IgM positive, and 6 were IgG positive. Of 90 sheep sera, 7 were IgM positive, and 9 were IgG positive. Of 60 swine sera, 3 were IgM positive, and 2 were IgG positive.
Table 6.
IFA results from livestock for IgM and IgG antibodies against TAHV
| Animal | Cow | Sheep | Swine | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | IgM | IgG | No. | IgM | IgG | No. | IgM | IgG | |
| Geermu city | 30 | 2 (6.7%) | 3 (10%) | 30 | 3 (10%) | 5 (16.7%) | –* | – | – |
| Xining city | 30 | 0 | 3 (10%) | 30 | 2 (6.7%) | 2 (6.7%) | 30 | 2 (6.7%) | 2 (6.7%) |
| Minhe county | 30 | 1 (3.3%) | 0 | 30 | 2 (6.7%) | 2 (6.7%) | 30 | 1 (3.3%) | 0 |
| Total | 90 | 3 (3.3%) | 6 (6.7%) | 90 | 7 (7.8%) | 9 (10%) | 60 | 3 (5.0%) | 2 (3.3%) |
Not available.
Most of the positive livestock sera were collected from Geermu city where 13.7% (5/30) of cows and 26.7% (8/30) of sheep were positive. There were also positive sera collected from Xining city and Minhe county, but positive rates in both locations were lower than in Geermu city.
Discussion
Although mosquito sampling was limited to one collection conducted during the summer season in each site in the Qinghai-Tibet Plateau, the results were consistent with previously published observations describing species distribution and relative abundance.24 A relatively low species richness is not unexpected in this area, which is characterized by a high-altitude arid steppe interspersed with mountain ranges. Our results suggest that the predominant species varied by location with Ae. (Och.) detritus being the most abundant species in sites we sampled in Geermu city, Ae. vexans being the most abundant species in sites we sampled in Xining City, and Cx. modestus being the most abundant species in sites we sampled in Minhe county. The difference in mosquito-species distribution is associated with the local physical geography. In Geermu city, there are numerous salt lakes, alkali flats, and marshes, including Qarham Salt Lake, the biggest salt lake in China.25 Ae. (Och.) detritus larvae can develop in high salinity,26 leading to the predominance of this species in this area. It is important to note that no Cx. tritaeniorhynchus Giles were collected in this study. This species is the major vector of Japanese Encephalitis virus in China. The absence of Cx. tritaeniorhynchus is consistent with the absence of Japanese Encephalitis cases reported from Qinghai.8
The virus isolates obtained from mosquitoes collected in Qinghai were strains of TAHV, LNV, and CppDNV. Previous reports indicated that few arboviruses have been isolated at elevations above 2,500 m.10,27 However, we identified TAHV, LNV, and CppDNV from Geermu city, Xining City, and Minhe County, indicating that multiple arboviruses could be isolated in Qinghai up to altitudes of 2,780 m.
TAHV, a member of California serogroup, genus Orthobunyavirus, family Bunyaviridae, was first isolated in the former Czechoslovakia in 1958.22 TAHV is widely distributed in central Europe, and human illness from infection with TAHV has been reported as manifesting undifferentiated fever and influenza-like symptoms as well as occasionally pneumonia and central nervous system involvement.12,28 In 2006, TAHV was isolated from Cx. sp. mosquitoes collected in Xinjiang, and this was the first report of TAHV isolation in China.12 In this study, TAHV was isolated from Ae. (Och.) detritus collected in Qinghai, which is the second report of TAHV isolation in China, suggesting that TAHV is widely distributed in the western regions of China. TAHV has been isolated from 12 mosquito species in four genera: Ae. vexans Meigen, Ae. cinereus Meigen, Ae. caspius Pallas, Ae. cantans Meigen, Ae. punctor Kirby, Ae. communis De Geer, Ae. flavescens Mueller, Ae. excrucians Walker, Cs. annulata Schrank, Cx. modestus Ficalbi, Cx. pipiens Linnaeus, and An. hyrcanus Pallas.28 This is the first time that TAHV has been isolated from Ae. (Och.) detritus, suggesting that this species could serve as a vector for TAHV. In this study, the minimum infection rate of TAHV in Ae. (Och.) detritus in Geermu city was 1.07/1,000 (2 positive pools/1,873 total Ae. [Och.] detritus tested).
LNV, a member of genus Seadornavirus, family Reoviridae, was first isolated from Ae. dorsalis mosquitoes in China,23 and subsequent isolations from mosquitoes have been made in Xinjiang and Shanxi Provinces.29,30 Our results show the first isolation of LNV from Cx. modestus and the first isolation of LNV in Qinghai. This represents not only expansion of the vector associations of this virus but also suggests widespread distribution of LNV in China. Although experimental infections show that LNV-infected adult mice die with signs of hemorrhage, there is currently no evidence of human infection with this virus, and more investigation is needed to determine if LNV is an etiology of human disease.31
CppDNV, a member of genus Brevidensovirus, subfamily Densovirinae, family Parvoviridae, was first isolated from multiple mosquito species in China.18 The host range of CppDNV is limited to a few closely related invertebrates. There is no evidence suggesting a relationship of CppDNV with human infection or disease. In this study, three strains of CppDNV were isolated from Ae. (Och.) detritus, Ae. Vexans, and Cx. modestus that were collected in Geermu city, Xining city, and Minhe county, suggesting that CppDNV is widely distributed in Qinghai in multiple mosquito species.
The results of serological testing showed that TAHV infection in humans occurs in several areas within Qinghai and is relatively common in Geermu city, particularly Xinhua village where both of the TAHV isolates were found and where human seroprevalence was 11.6% (11/95). The investigation of local livestock indicated that TAHV antibodies (IgM and IgG) were detected in serum samples of cows and sheep with high seroprevalence in Geermu city.
The investigation also showed that IgG positive residents were all under the age of 30, and the high seroprevalence found in the 5–9 and 10–14 age groups indicates that TAHV readily infects children in Geermu city. A serosurvey in the Czech Republic showed that older residents had higher seroprevalence for TAHV antibodies, a pattern that was also seen in wild boars in the area.32,33 Such a pattern is typical for humans and animals living in a long-term enzootic focus, and the older age classes experience a higher probability of infection and a higher rate of antibodies. Our observation that seroprevalence was higher in young age groups may indicate that TAHV was recently imported into the Qinghai-Tibet Plateau, although this could not explain the lack of antibodies in older age classes. Larger sample sizes are required to resolve this question.
This investigation showed that TAHV is present in Geermu city at an altitude of 2,780 m, indicating that TAHV transmission cycles can be maintained in areas of high altitude in the Qinghai-Tibet Plateau. In addition, this represents the first time that TAHV has been isolated from Ae. (Och.) detritus. This shows that TAHV cycles naturally and infects people in the Qinghai-Tibet Plateau, and further investigations of the distribution and public health impact of TAHV and other arboviruses are warranted in the Qinghai-Tibet Plateau.
Acknowledgments
The work was processed in the State Key Laboratory for Infectious Disease Prevention and Control, Institute for Viral Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China. The authors also thank staff members in Geermu Center for Disease Control and Prevention, Geermu, China and Minhe Center for Disease Control and Prevention, Minhe, China for the assistance in collection of mosquito and serum samples.
Footnotes
Financial support: This work was supported by Grant 2003BA712A08-01 from the Ministry of Science and Technology of China, Grant 2008SKLID105 from the Development Grant of State Key Laboratory for Infectious Disease Prevention and Control, and Grant U19-GH000004 from the China CDC–United States CDC Cooperative Agreement.
Authors' addresses: Wen-Juan Li, Jing-Lin Wang, Ming-Hua Li, Shi-Hong Fu, Huan-Yu Wang, Qing Tang, and Guo-Dong Liang, State Key Laboratory for Infectious Disease Prevention and Control, Institute for Viral Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China, E-mails: liwenjuan_sdu@163.com, wangjinglin107@yahoo.com.cn, minghua-li@hotmail.com, fushihong_fsh@hotmail.com, rainoffall@yahoo.com, qtang04@sina.com, and gdliang@hotmail.com. Wen-Juan Li and Zhi-Yu Wang, Department of Virology, School of Public Health, Shandong University, The Key Laboratory of Experimental Teratology, Ministry of Education, China, E-mails: liwenjuan_sdu@163.com and Zhiyu.wang@sdu.edu.cn. Shuang-Ying Jiang, Xue-Wen Wang, Peng Guo, Sheng-Cang Zhao, Yan Shi, and Nan-Nan Lu, Health Inspection and Testing Center, Qinghai Center for Disease Control and Prevention, China, E-mails: ShuangYing_Jiang@163.com, XueWen_Wang_qh@163.com, Peng_Guo_qh@163.com, Shengcang_zhao@163.com, qhcdcfyk@163.com, and qhlunannan@163.com. Roger S. Nasci, Division of Vector-Borne Infectious Diseases, Centers for Disease Control and Prevention, Fort Collins, CO, E-mail: rsn0@cdc.gov.
Reprint requests: Guo-Dong Liang, State Key Laboratory for Infectious Disease Prevention and Control, Institute for Viral Disease Control and Prevention, Chinese Center for Disease Control and Prevention, 100# Yingxin Street, Xuanwu District, Beijing 100052, China, E-mail: gdliang@hotmail.com.
References
- 1.Government of Qinghai Province 2008Overview of geography in Qinghai province Available at: http://www.qh.gov.cn/html/268/41682.html Accessed March 28, 2008
- 2.Wen L. Qinghai-Tibet railway: link to peace. Zhong Guo Guo Jia Di Li Za Zhi. 2006;6:112. [Google Scholar]
- 3.Hongmei G. Discussion on control strategy about plague hazard along Qinghai-Tibet railway. Zhong Guo Gong Gong Wei Sheng Za Zhi. 2007;23:1508–1509. [Google Scholar]
- 4.Hu W, Guojun W, Zuyun W, Chao L, Min L. Retrospection and present state of plague prevention and control in Qinghai province about 50 years. Zhong Guo Di Fang Bing Xue Za Zhi. 2004;23:610–613. [Google Scholar]
- 5.Shende L, Yan S, Zhongsheng F, , Shaohui M. The report of investigation and handling on epidemic typhus in Tajia Town, Hualong County. Xian Dai Yu Fang Yi Xue Za Zhi. 2007;34:3768. [Google Scholar]
- 6.Liqing X, Jiquan L, Jungui X, Jianqiang L. The investigation and analysis two cases of new infection of brucellosis in Qinghai. Zhong Guo Di Fang Bing Xue Za Zhi. 2008;27:348. [Google Scholar]
- 7.Hua G, Jianning Y, Yuqing Q, Yan S. Investigation of Lyme disease in some regions of Qinghai. Zhong Guo Mei Jie Sheng Wu Xue Ji Kong Zhi Za Zhi. 2008;19:120. [Google Scholar]
- 8.Yixing L, Zundong Y, Junhong L, Xia N, Xuxia W, Li L, Lxiaofeng L. Epidemiological analysis of the Japanese B encephalitis in China during 2004–2006. Zhong Guo Ji Hua Mian Yi Za Zhi. 2007;13:528–532. [Google Scholar]
- 9.Han L. Clinical analysis of 21 cases of viral encephalitis with psychiatric symptoms as the first performance in the highland. Qing Hai Yi Yao Za Zhi. 2008;38:33–34. [Google Scholar]
- 10.Sun X, Fu S, Gong Z, Ge J, Meng W, Feng Y, Wang J, Zhai Y, Wang H, Nasci R, Wang H, Tang Q, Liang G. Distribution of arboviruses and mosquitoes in Northwestern Yunnan Province, China. Vector Borne Zoonotic Dis. 2009;9:623–630. doi: 10.1089/vbz.2008.0145. [DOI] [PubMed] [Google Scholar]
- 11.Kim SY, Yun SM, Han MG, Lee IY, Lee NY, Jeong YE, Lee BC, Ju YR. Isolation of tick-borne encephalitis viruses from wild rodents, South Korea. Vector Borne Zoonotic Dis. 2008;8:7–13. doi: 10.1089/vbz.2006.0634. [DOI] [PubMed] [Google Scholar]
- 12.Lv Z, Lv XJ, Fu SH, Zhang S, Li ZX, Yao XH, Feng YP, Lambert AJ, Nida X, Wang FT, Tong SX, Nasci RS, Feng Y, Dong Q, Zhai YG, Gao XY, Wang HY, Tang Q, Liang GD. Tahyna virus and human infection, China. Emerg Infect Dis. 2009;15:306–309. doi: 10.3201/eid1502.080722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bryant JE, Crabtree MB, Nam VS, Yen NT, Duc HM, Miller BR. Isolation of arboviruses from mosquitoes collected in northern Vietnam. Am J Trop Med Hyg. 2005;73:470–473. [PubMed] [Google Scholar]
- 14.Guodong L, Ying H, Boquan C, Zijiang Z, Yijun H, Li C. Preparation of arbovirus group-specific PcAb and their use to identify newly isolated viruses. Zhong Hua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 1993;7:374–376. [Google Scholar]
- 15.Jones RW, Ross J, Hoshino Y. Identification of parental origin of cognate dsRNA genome segment(s) of rotavirus reassortants by constant denaturant gel electrophoresis. J Clin Virol. 2003;26:347–354. doi: 10.1016/s1386-6532(02)00086-0. [DOI] [PubMed] [Google Scholar]
- 16.Kuno G, Mitchell CJ, Chang GJ, Smith GC. Detecting bunyaviruses of the Bunyamwera and California serogroups by a PCR technique. J Clin Microbiol. 1996;34:1184–1188. doi: 10.1128/jcm.34.5.1184-1188.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Billoir F, Attoui H, Simon S, Gallian P, de Micco P, de Lamballerie X. Molecular diagnosis of group B coltiviruses infections. J Virol Methods. 1999;81:39–45. doi: 10.1016/s0166-0934(99)00055-5. [DOI] [PubMed] [Google Scholar]
- 18.Zhai YG, Lv XJ, Sun XH, Fu SH, Gong ZD, Fen Y, Tong SX, Wang ZX, Tang Q, Attoui H, Liang GD. Isolation and characterization of the full coding sequence of a novel densovirus from the mosquito Culex pipiens pallens. J Gen Virol. 2008;89:195–199. doi: 10.1099/vir.0.83221-0. [DOI] [PubMed] [Google Scholar]
- 19.Kuniholm MH, Wolfe ND, Huang CY, Mpoudi-Ngole E, Tamoufe U, LeBreton M, Burke DS, Gubler DJ. Seroprevalence and distribution of Flaviviridae, Togaviridae, and Bunyaviridae arboviral infections in rural Cameroonian adults. Am J Trop Med Hyg. 2006;74:1078–1083. [PubMed] [Google Scholar]
- 20.Baolin L, Houyong W. Classification and Identification of Important Medical Insects of China. Zhengzhou, China: Henan Science and Technology Publishing House; 2003. pp. 17–19. [Google Scholar]
- 21.Walter Reed Biosystematics Unit 2009Anopheles nigerrimus Available at: http://wrbu.si.edu/SpeciesPages_ANO/ANO_A-hab/ANnig_hab.html Accessed July 12, 2009
- 22.Bardos V, Danielova V. The Tahyna virus—a virus isolated from mosquitoes in Czechoslovakia. J Hyg Epidemiol Microbiol Immunol. 1959;3:264–276. [PubMed] [Google Scholar]
- 23.Sanju T, Zenglin C, Dongrong Y. New subtype of coltivirus isolated from mosquitoes in the northeast part of China. Zhong Hua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 1999;13:228–231. [PubMed] [Google Scholar]
- 24.Jinxue DW, Shuanyou H, Fuxiang Q, Changming Z, Dongping Z. Preliminary investigation of mosquito composition and ecology in Geermu city. Zhong Guo Mei Jie Sheng Wu Xue Ji Kong Zhi Za Zhi. 2000;11:69–70. [Google Scholar]
- 25.Lingqin L, Haizhou M. On center role of Geermu City in development Western China. Qing Hai Shi Fan Da Xue Xue Bao. 2003;99:17–19. [Google Scholar]
- 26.Metge G, Hassaïne K. Study of the environmental factors associated with oviposition by Aedes caspius and Aedes detritus along a transect in Algeria. J Am Mosq Control Assoc. 1998;14:283–288. [PubMed] [Google Scholar]
- 27.Barnett ED. Yellow fever: epidemiology and prevention. Clin Infect Dis. 2007;44:850–856. doi: 10.1086/511869. [DOI] [PubMed] [Google Scholar]
- 28.Hubálek Z. Mosquito-borne viruses in Europe. Parasitol Res. 2008;103:S29–S43. doi: 10.1007/s00436-008-1064-7. [DOI] [PubMed] [Google Scholar]
- 29.Lv XJ, Lv Z, Sun XH, Fu SH, Wang HQ, Tong SX, Zhang S, Attoui H, Liang GD. 0507JS60 virus isolated in Xinjiang was identified as Liaoning Virus. Chin J Virol. 2008;24:438–442. [PubMed] [Google Scholar]
- 30.Minghua L, Weishan M, Shihong F, Jingxia C, Junying Z, Xiangsheng K, Peifang D, Guodong L. Arbovirus investigation in some regions of Shanxi province in 2007. Zhong Hua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2009;23:32–34. [PubMed] [Google Scholar]
- 31.Attoui H, Mohd Jaafar F, Belhouchet M, Tao S, Chen B, Liang G, Tesh RB, de Micco P, de Lamballerie X. Liao ning virus, a new Chinese seadornavirus that replicates in transformed an embryonic mammalian cells. J Gen Virol. 2005;87:199–208. doi: 10.1099/vir.0.81294-0. [DOI] [PubMed] [Google Scholar]
- 32.Zelená H, Januska J, Raszka J. Micromodification of virus-neutralisation assay with vital staining in 96-well plate and its use in diagnostics of Tahyna virus infections. Epidemiol Mikrobiol Imunol. 2008;57:106–110. [PubMed] [Google Scholar]
- 33.Halouzka J, Juricova Z, Jankova J, Hubalek Z. Serologic survey of wild boars for mosquito-borne viruses in South Moravia (Czech Republic) Vet Med (Praha) 2008;53:266–271. [Google Scholar]


