Abstract
Taiwan's dengue outbreaks have a unique type of transmission: starting by import from abroad in early summer, spreading out locally, and ending in the winter. This pattern repeats every year. Most of the dengue patients are adults, with dengue fever peaking in the 50–54 year age range, and dengue hemorrhagic fever in the 60–64 year age range. Two patterns of dengue infection were found: DENV-2 in 2002 with 74% of secondary infection in contrast to non-DENV-2 (DENV-1 or DENV-3) in 2004–2007 with ~70% of primary infection. Secondary dengue virus infection increases disease morbidity, but not mortality in adults. The active serological surveillance shows two-thirds of the dengue-infected adults are symptomatic post infection. The Taiwanese experience of adult dengue should be valuable for countries or areas where, although dengue is not endemic, the Aedes aegypti vector exists and dengue virus can be introduced by travelers.
Introduction
Dengue disease is an acute infectious disease caused by four serotypes of dengue virus, and is the most important arboviral disease of humans, occurring in tropical and sub-tropical countries of the world where over 2.5 billion people are at risk of infection. The World Health Organization (WHO) has estimated that between 50 and 100 million cases of dengue fever (DF) and several hundred thousand cases of dengue hemorrhagic fever (DHF) occur each year, depending on the epidemic activity.1,2 For the disease manifestation, DF is self-limited, the patient will recover without any sequelae after the fever subsides. On the other hand, DHF is a severe febrile disease characterized by abnormalities of hemostasis and increased vascular permeability, and severe progression may result in dengue shock syndrome (DSS). Dengue hemorrhagic fever is classified into four grades, of which the third and fourth grades are called DSS. Dengue shock syndrome is a form of hypovolemic shock that is associated clinically with hemoconcentration and hemorrhage, which will lead to death if appropriate fluid supplement is not given immediately.3,4 Like other viral infectious diseases, it displays iceberg characteristics. In Southeast Asia, most cases are symptomless, followed, in order of increasing rarity, by undifferentiated fever, DF, and life-threatening DHF/DSS. It is estimated that 76% of infected persons are asymptomatic, whereas 24% of them show clinical symptoms. Among the symptomatic patients, 94% are categorized as DF, whereas 6% will progress to DHF/DSS, and only < 1% are fatal.5 Most of the severe manifestations of DHF/DSS occur in children experiencing a secondary dengue virus infection that has a different serotype from the previous one. Antibody-dependent enhancement (ADE) theory has been proposed to explain this unique epidemiological finding.6
In endemic areas such as Southeast Asia or Latin America, DHF is the leading cause of hospitalization and death among children with secondary infection. In different areas with a recent introduction of the virus or with no endemicity, the age distribution of DHF cases is different with an increasing number of adults with DHF.7 Although several outbreaks of dengue fever were recorded in Taiwan even before World War II, no dengue case was documented from 1945 until 1981, when a DENV-2 outbreak occurred on the small island Hsiao-Liu-Chiu Township, Pintung County in southern Taiwan.8 In 1987, a major DENV-1 outbreak occurred in the southern part of Taiwan. After this, a dengue outbreak has occurred every year.9,10 Dengue hemorrhagic fever cases have been recorded since 1994. Despite this annual dengue outbreak, Taiwan is not considered yet as a dengue endemic area. The scale of each dengue outbreak depends on the appropriate control measures or other variables such as the weather.11,12 Molecular epidemiological analysis showed that multiple dengue serotype were constantly imported and circulated at the same time in this island. Different years have different combinations of dengue serotype, the incidence is increasing early in the year and declines toward the end of the year.10 In this study, the epidemiology of dengue outbreaks during the period 2002–2007 in Taiwan was characterized. Dengue in Taiwan is found to be predominantly an adult disease and the majority of the DHF/DSS cases are the elderly who are at risk of high morbidity and mortality. This adult dengue epidemiology is distinct from that of child dengue in hyper-endemic areas. Secondary infection was prevalent for serotype 2 in the year 2002 in contrast to primary infection for serotype 1 or 3 in 2004–2007.
Materials and Methods
Dengue reporting system in Taiwan.
Dengue is a category2 notifiable infectious disease. According to the Communicable Disease Prevention Act, the physicians are obliged to report the suspected dengue cases to the local health department within 24 hours of clinical diagnosis. Contacts of confirmed cases are also obliged to test their blood for dengue virus infection. The collection of serum samples and laboratory diagnosis are performed for public health surveillance purposes and enforced by the Communicable Disease Prevention Act.
This study is an effort to understand the characteristics of dengue epidemiology using these serum samples collected during 2002–2007. Unlike seroepidemiological studies, the data presented in this study were derived from routine diagnosis and analyzed anonymously. We believe the analysis of surveillance data are for public health interest and are not a research project that requires informed consent from individual patients. The laboratory diagnosis and the subsequent control measures were carried out by the central and local health environment departments, including the epidemiological investigation, mosquito eradication with insecticide treatment, and breeding source reduction.
The Taiwan Centers for Disease Control has two dengue surveillance systems. The first is a hospital-based reporting system for infectious reportable diseases and the hospital syndrome reporting system for viral hemorrhagic fever. The second is the dengue active surveillance systems, including individual self-suspected reports, the expanded epidemiological surveys for contacts of confirmed cases by local health bureaus, school and community screening systems for unknown fever, and international airport fever screening. All the sera of the reported or suspected cases are examined by Kun-Yang laboratory for northern Taiwan and Kaohsiung laboratory for southern Taiwan in the Research and Diagnostic Center. Routine dengue diagnosis includes molecular analysis using real-time reverse transcriptase-polymerase chain reaction (RT-PCR), serological analysis using captured IgM/IgG enzyme-linked immunosorbent assay (ELISA), and virus isolation by cell culture. A confirmed dengue case is defined as 1) positive for dengue virus isolation; or 2) positive for dengue virus genome by RT-PCR; or 3) positive for dengue virus-specific IgM and IgG in a single serum sample, where cross-reactions to Japanese encephalitis virus (JEV) have been excluded; or 4) 4-fold increase of dengue virus-specific IgG antibody in paired serum samples where cross-reactions to JEV have been excluded. A diagnosis report (positive, negative, or undetermined) will be completed within 6–24 hours after sample arrival and made available in the internet report system for the local health bureau and hospital for further action to be taken. Once the dengue-infected case is confirmed, the infection disease notification sheet is completed by the local health bureau or hospital with data including name, gender, sampling time, onset day, overseas travel detail, and disease symptom. Furthermore, emergency control measures, such as insecticide spread, source reduction, and expanded epidemiological investigation will be initiated to avoid local transmission. If the infected person had been traveling abroad in the 2 weeks before confirmation of infection, it will then be classified as an imported case, because the person will be considered to have been infected abroad and the dengue virus imported into Taiwan. When overseas travel is not indicated, it will be recorded as an indigenous case. For expanded epidemiological study, the staff of the local health bureau will visit and interview the index case patient and make enquiries about the patient's areas of contact or persons he or she had contact with during the active period. Blood samples will be drawn from family members, colleagues, and neighbors within a radius of 50 meters of the index case resident, who may have had contact or had fever, to understand whether any further spread of infection by a DENV-infected mosquito has occurred. The environment, including homes and working places within a radius of 50 meters of the index case resident, will be sprayed with insecticides to reduce the vector source. Once a further dengue case is found, a retrospective interview will be conducted to understand whether there are any symptoms such as fever (> 38°C), nausea, joint pain, dizziness, headaches, vomiting, abdominal pain, poor appetite, bone pain, tiredness, eyehole pain, muscle pain, or skin rash. A business Object system composed of these data is used for Centers for Disease Control and Prevention (CDC)-Taiwan dengue surveillance. The analysis of this study is derived from this database.
Dengue diagnosis.
A modified E/M-specific captured IgM and IgG ELISA was performed to measure the DENV- and JEV-specific IgM and IgG antibodies as previously described.13–17 The assay was performed by 1) measurement of DENV- and JEV-specific IgM and IgG antibodies of each serum sample simultaneously in the same ELISA plates, 2) selection of a monoclonal antibody having similar affinity to both JEV and the four DENV serotypes, 3) addition of virus-infected cell culture supernatants containing equal concentration of DENV or JEV in each well, and 4) captured IgM or IgG antibodies incubated to a cocktail of mixed viral antigens and the monoclonal antibody in a single step. For the differentiation of primary and secondary DENV infection, different laboratories used different IgM/IgG ratios; therefore, Innis and others13 and Kuno and others14 used the IgM/IgG ratio of 1.78 and 1.4, respectively. More recently, Falconar and others18 proposed to use the ratio of 2.6 to classify all primary or secondary DENV infections for early acute-phase sera (Days 1–3) by an altered ELISA. Because the protocols and the reagents are not the exact same in different laboratories, some variations of the IgM/IgG ratio would be expected and need to be adjusted. We used the IgM/IgG ratio of 1.2 as a cutoff to differentiate primary and secondary DENV infections for acute (Days 1–7) and convalescent (Days 8–45) serum samples.15 This is based on the results of comparison studies with both hemagglutination inhibition test and NS1 serotype-specific IgG ELISA, because the majority of sera tested by capture IgM and IgG ELISA can be correctly grouped into primary or secondary infection if the capture IgM/IgG ratio of 1.2 was used as the cutoff.15 The modified ELISA showed low cross-reactivity between DENV- and JEV-specific IgM and IgG antibodies and can be used reliably to differentiate primary and/or secondary JEV and DENV infections. A one-step SYBR Green I real-time RT-PCR (QuantiTect SYBR Green RT-PCR kit; Qiagen, Hilden, Germany) was performed in the Mx4000TM quantitative PCR system (Stratagene, La Jolla, CA) to detect and differentiate dengue virus serotypes in acute-phase serum samples, as described previously.17,19
Results
Dengue epidemiology in Taiwan.
In 1987, a major DENV-1 outbreak occurred in the southern part of Taiwan. Since then, dengue outbreak occurs every year; the case number in 1997–2007 is shown in Figure 1A. The greatest outbreak occurred in the year 2002. Because more detailed information was collected following the 2002 outbreak, this study reports the epidemiology from 2002 to 2007. The dengue cases were categorized into either imported cases or indigenous ones based on the virus origin of the infected person. If the infected person of the confirmed case had traveled abroad in the previous 2 weeks, it was classified as being imported from abroad. Analysis of the monthly frequency of dengue cases shows that the first indigenous index case usually occurs in May or June, infected with locally transmitted dengue virus imported from Southeast Asia.10 If the control measures are not successful, the outbreak then spreads out gradually and peaks around October (Figure 1B). When the temperature in December drops to below 15°C, the virus-carrying mosquitoes die and the outbreak gradually diminishes and vanishes in the winter. The dengue cases in January 2003 were leftover from 2002 (51 of a total of 86 cases were derived from the 2002 outbreak). The year 2003 experienced a severe acute respiratory syndrome (SARS) outbreak; therefore, more stringent control measures such as fever screen are being used. People are more alert of infectious disease, only 35 cases of a total of 86 dengue infections were confirmed in the summer or autumn. For the two competent vectors, Aedes aegypti and Aedes albopictus, that are present in Taiwan, Aedes aegypti is the major vector to transmit the dengue virus between humans and is distributed only in southern Taiwan, whereas the Ae. albopictus is distributed island-wide. Therefore, dengue cases primarily occur in the southern part of Taiwan, including Kaohsiung City, Kaohsiung County, Pingtung County, Tainan County, and Tainan City (Supplemental Table 1; available online at www.ajtmh.org). Because of multiple dengue virus importation, 3 to ~6 dengue virus strains were found to be locally transmitted each year. However, only one strain of serotype has dominated in each year, for example, DENV-2 in 2002 and 2003, DENV-1 in 2004, DENV-3 in 2005 and 2006, and DENV-1 in 2007 (Supplemental Table 2; available online at www.ajtmh.org). This pattern of dengue transmission in Taiwan has been repeated every year: beginning with importation from Southeast Asia in the early summer, spreading out through southern Taiwan in the autumn, and ending because of cold weather in the winter.
Figure 1.
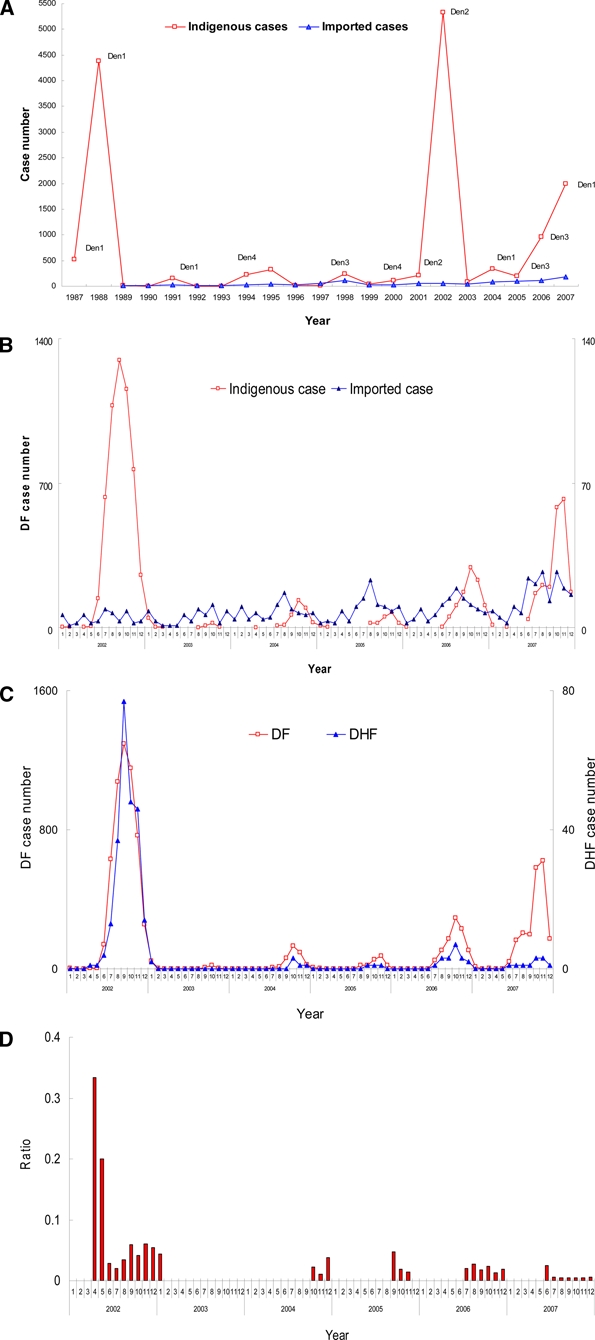
The Taiwan dengue epidemiology in 2002–2007. A, Seasonal distribution of imported and indigenous cases in 1997–2007. B, Seasonal trend of imported and indigenous cases in 2002–2007. C, Seasonal trend of DF and DHF in 2002–2007. D, Seasonal trend of DHF/DF ratio in 2002–2007. This figure appears in color at www.ajtmh.org.
Adult dengue disease in Taiwan.
In Taiwan, dengue is classified as a Type 2 infectious disease and has to be reported to CDC-Taiwan within 24 hours for further investigation. On the basis of the surveillance system records, the numbers of confirmed indigenous cases from the years 2002 to 2007 are 5,336, 86, 336, 202, 965, and 2000, respectively. Most of these cases are DF, with 241, 2, 5, 3, 19, and 11 cases of DHF/DSS. The numbers of fatal cases were 21, 1, 5, and 1 in 2002, 2003, 2006, and 2007, respectively. The percentage ratio of DF to DHF is around 0.6% to ~4.5%, with 4.5% being the higher in 2002 (Table 1). The seasonal trend of DF and DHF and the DHF/DF ratio are shown in Figure 1C and Figure 1D, respectively. Although there was a higher DHF/DF ratio in April and May, 2002, this might reflect a bias caused by the low number of cases. Similar to the data of Table 1, the DHF/DF ratio was highest in 2002 with a DENV- 2 outbreak. In 2004 (DENV-1), 2005 (DENV-3), 2006 (DENV-3), and 2007 (DENV-1), the non-serotype 2 outbreaks had a lower DHF/DF ratio of 0.6% to ~2.3%. When the age-specific prevalence of the dengue disease was analyzed, it was found that most of the DF cases were adults, with a peak in the 50–54 year age range (Figure 2A). The analysis of DHF also shows the tendency of adults or the elderly to have a greater risk of developing the severe manifestation of DHF, with this effect peaking at the 60–64 year age range (Figure 2B). For the fatal dengue, it is more apparent that those greater than 55 years of age are more susceptible to death post dengue virus infection (Figure 2C).
Table 1.
Percentage of DHF to DF and DHF case fatality in 2002–2007*
| YearNo | DF | DHF | Fatal | DHF/DF ratio | CFR fatal/DHF | |||
|---|---|---|---|---|---|---|---|---|
| Primary | Secondary | Primary | Secondary | Primary | Secondary | |||
| 2002 | 5,336 | 241 | 21 | 4.5% | 8.7% | |||
| 26% | 74% | 15% | 85% | 33% | 67% | |||
| 2003 | 86 | 2 | 1 | 2.3% | 50% | |||
| 63% | 37% | 100% | – | 100% | – | |||
| 2004 | 336 | 5 | 0 | 1.5% | – | |||
| 68% | 32% | 20% | 80% | – | – | |||
| 2005 | 202 | 3 | 0 | 1.5% | – | |||
| 63% | 37% | – | 100% | – | – | |||
| 2006 | 965 | 19 | 5 | 2% | 26.3% | |||
| 72% | 28% | 53% | 47% | 60% | 40% | |||
| 2007 | 2000 | 11 | 1 | 0.6% | 9.1% | |||
| 82% | 18% | 40%a | 60% | – | 100% | |||
DF = dengue fever; DHF = dengue hemorrhagic fever; CFR = case fatality rate.
Figure 2.
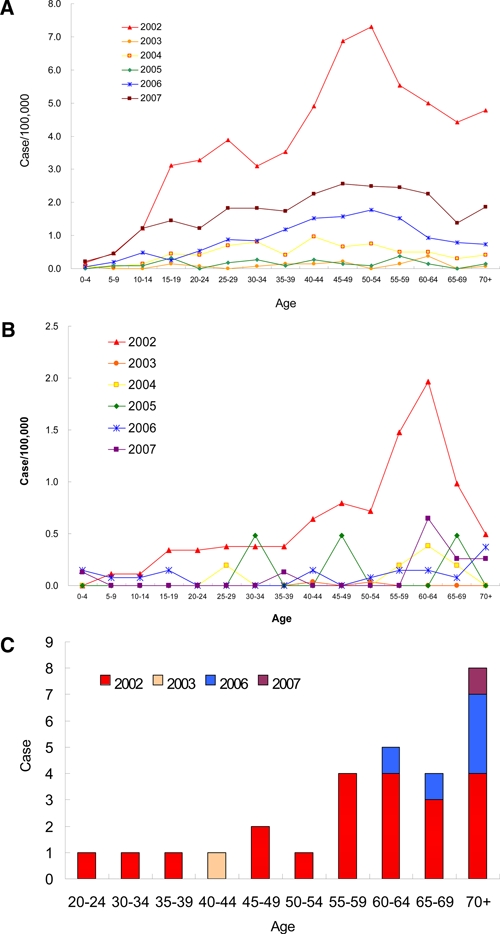
Age-specific prevalence of dengue in 2002–2007. A, Age-specific prevalence of DF in 2002–2007. The prevalence is calculated with the cases of each age range divided by the population of dengue outbreak area. The year prevalence rate is 57.5, 1.5, 7.1, 2.2, 13.2, and 25.2 per 100,000 from 2002 to 2007, respectively. B, Age-specific prevalence of DHF in 2002–2007. C, Age-specific prevalence of fatal cases in 2002–2007. This figure appears in color at www.ajtmh.org.
The dengue serological analysis can discriminate the primary infection from the secondary one based on the IgM/IgG titer ratio of anti-dengue antibodies.15–17 The infected status was determined for the dengue patients. The age distribution of primary and secondary dengue infection is shown in Figure 3. In 2002, the percentage of primary infection and secondary infection was 26% and 74%, respectively. On the other hand, in 2003, 2004, 2005, 2006, and 2007, primary infection became dominant with percentages of 63%, 68%, 63%, 72%, and 82%, respectively (Table 1). In the years 2003–2007, the majority of the dengue patients were primarily infected and in comparison with 2002 most cases were secondary infection (P < 0.001 by χ2 test). The age-specific prevalence of primary or secondary infected DF in Figure 3A reveals two different patterns for different serotypes. In the year 2002 with DENV-2, the percentage of secondary infection was 2.8-fold higher than that of the primary one. Another pattern was found in the years 2004–2007, where the dengue virus was either DENV-1 or 3 (non-serotype 2), and in which primary infection occurred more frequently than secondary infection, with around 70% being primarily infected, although the elderly (> 60 years of age) experienced more secondary infections in the years 2006 and 2007. For severe DHF, the age-specific prevalence of primary or secondary infection is shown in Figure 3B. In the year 2002, 85% of DHF patients were of secondary infection with DENV-2. In the years 2003–2007 with non-DENV 2, a total of 40 DHF cases were pooled, of which 44% were of primary infection, whereas 56% of them were of secondary infection. Among the 22 secondary-infected DHF cases, 16 cases were over 60 years of age. For the DENV-2 outbreak in 2002, the case fatality rate of DHF was 8.7% (21/242). Among the fatal cases, 67% were secondary infection, whereas 33% were primary infection. The percentage of secondary infection in fatal cases was not as high as that of DHF (67% versus 85%). On the other hand, in the non-DENV-2 outbreak in 2003–2007, the total case fatality rate (CFR) was 17.5% (7/40), higher than the 8.7% observed in the 2002 DENV-2 outbreak. Among the fatal cases, 42% (3/7) were secondary infection, whereas 58% (4/7) were primary infection (Table 1). It seems that secondary infection with DENV-2 enhances the development of DHF (P < 0.001), but not the mortality (P = 0.38 by χ2 test). However, primary infection with non-DENV-2 dengue virus in the elderly can be fatal.
Figure 3.
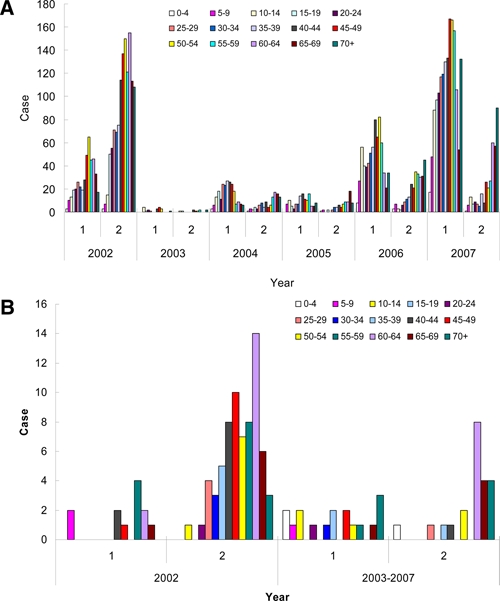
Primary/secondary dengue infection in 2002–2007. A, Age-specific prevalence of primary or secondary infection for DF in 2002–2007. B, Age-specific prevalence of primary or secondary infection for DHF in 2002–2007. The 1° and 2° denote primary and secondary infection, respectively. This figure appears in color at www.ajtmh.org.
Asymptomatic dengue during 2004–2007.
In addition to the hospital-based reporting system, an active serological surveillance was carried out in the area within a radius of 50 meters of the index case, on family members, colleagues, and neighbors who may have had contact with the index case. Table 2 shows the summary of the active surveillance report for 2004–2007. A total of 42,150 blood samples were drawn for dengue serological analysis, of which around 1.1% (464/42,150) were found to be dengue virus infected. All 464 persons did not feel sick enough to go to the hospital for medical treatment. Interviews of these confirmed dengue virus-infected persons were conducted to discover whether they had any dengue-like symptoms during the possible infection period. Among the 464 cases, 64% (297/464) recorded that they had symptoms such as fever (> 38°C), headache, myalgia, arthralgia, retro-orbital pain, maculopapular rash, petechiae, ecchymosis, vomiting, or gingival bleeding, whereas 36% (167/464) said that they did not have any symptoms at all. The age-specific distribution of the asymptomatic and symptomatic cases from this active surveillance data are shown in Figure 4A and B, respectively. The age profile of the symptomatic cases from active surveillance data are similar to the surveillance report of Figure 2A, but the proportion of young children 5–14 years of age increased. On the other hand, for the age distribution of the asymptomatic cases, the elderly (> 69 years of age) seem to have a higher frequency of asymptomatic cases (Figure 4B). Both asymptomatic and symptomatic cases can be of either primary or secondary infection, with primary infection accounting for ~70%, which is the percentage for the DENV-1 or DENV-3 infected cases in the years 2003–2007. The status of primary or secondary infection from the active surveillance system is not different from that of the surveillance reporting records. It is therefore concluded that the ratio of symptomatic to asymptomatic cases is 1.78 (64%/36%) for adult dengue in Taiwan, or that about two-thirds of the dengue-infected adults have clinical symptoms.
Table 2.
Active surveillance of dengue infection in 2004–2007
| Year | Persons screened | Dengue-infected no. and % | Symptomatic | Asymptomatic | |||
|---|---|---|---|---|---|---|---|
| Primary | Secondary | Primary | Secondary | ||||
| 2004 | 3457 | 19 | 0.5% | 15 (83%) | 3 (17%) | 0 | 1 (100%) |
| 2005 | 3659 | 17 | 0.5% | 7 (79%) | 3 (30%) | 2 (29%) | 5 (71%) |
| 2006 | 16973 | 165 | 1.0% | 101 (76%) | 32 (24%) | 20 (63%) | 12 (37%) |
| 2007 | 18061 | 263 | 1.5% | 114 (83%) | 22 (17%) | 101 (80%) | 26 (20%) |
| Total | 42150 | 464 | 1.1% | 237 (70%) | 60 (30%) | 123 (74%) | 44 (26%) |
Figure 4.
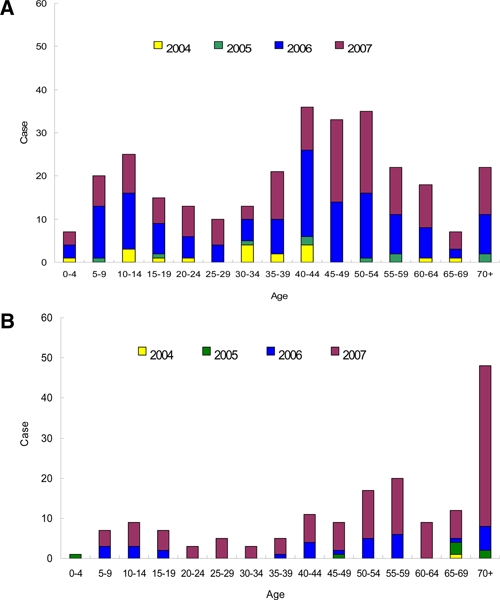
Age distribution of dengue from active surveillance in 2004–2007. A, Age distribution of symptomatic dengue-infected person in active surveillance of 2004–2007. B, Age distribution of asymptomatic dengue-infected person in active surveillance of 2004–2007. This figure appears in color at www.ajtmh.org.
Discussion
The dengue epidemiology in Taiwan is different from other locations in Southeast Asia, with a number of particular characteristics. Dengue in Taiwan is an adult infectious disease, whereas it primarily affects children in hyper-endemic Southeast Asia. The dengue-infected elderly have high morbidity and mortality rates. Secondary infection increases the disease severity, but not the mortality. Dengue-infected adults are more symptomatic. The transmission cycle in Taiwan is also unique, beginning with importation in the summer, and ending in the winter.
Among various flaviviruses in Taiwan, JEV and dengue virus are the two viruses transmitted locally in this island. Although Japanese encephalitis is an endemic disease caused by local JEV strains, dengue is not endemic and annual outbreaks are caused by different dengue virus strains imported each year, which disappear with the ending of each local outbreak. Beginning in 1968, a mass vaccination program against JEV for children was implemented in Taiwan. The annual incidence has declined dramatically to 10–35 confirmed cases after 1985. Recent seroprevalence study showed that individuals less than 10 years of age have high (80–90%) neutralizing antibody caused by vaccination, and then gradually decreases in the age group 11–40 years and to the lowest level (55%) in the age group 31–40 years.20
For the vectors to transmit the dengue virus, Ae. aegypti is mainly active indoors, whereas Ae. albopictus is mainly active outdoors. In an area where dengue is not endemic but a capable vector exists, new autochthonous cycles of infection may be established from infected travelers or immigrants who arrive from endemic areas. The dengue virus thereafter spreads out from the carrier. Aedes aegypti is more vigilant than Ae. albopictus and has repeated feeding behavior on several persons. Therefore, this feature makes Ae. aegypti a better vector to transmit dengue virus and it is responsible for most epidemics. Dengue in Taiwan is not yet an endemic disease. The environmental conditions of Taiwan including its location on a subtropical island with neighboring hyper-endemic areas, the coexistence of two vectors, and the climate that varies from a rainy, hot summer, with an average temperature of 28°C, and a dry and cold winter averaging 20°C, with occasional drops to 10–15°C, have created a scenario for a unique pattern of dengue epidemiology. All four serotypes of dengue virus were found to be transmitted and caused a local outbreak in Taiwan. For example, in the year 2005, only DENV-3 and DENV-2 caused outbreaks, each in different county areas: a major DENV-3 outbreak in Kaohsiung, and a minor DENV-2 outbreak in Tainan. However, they would disappear with the ending of each outbreak.10 The Kaohsiung area has a history of outbreaks (DENV-1 in 1988, DENV-2 in 2002). How the herd immunity affects the virus transmission is an intriguing question considering the influence of neutralizing antibody versus enhancing antibody. As dengue virus is an RNA virus that is easily mutated to adapt to the host immune pressure, there remains the question of the possible emergence of a dengue escape mutant,21 which might become more virulent and cause a more severe disease. The closed environment of Taiwan for dengue virus circulation provides an opportunity to understand how the dengue virus genome evolves to cope with the immune pressure. Two spatial-temporal patterns were reported to explain the dengue virus transmission in the 2002 Kaohsiung DENV-2 outbreak.22
The demographic pattern of dengue disease is changing. It used to be a childhood disease and the leading cause of hospitalization and death among children. However, an increasing incidence of DHF for older age groups has been reported in Bangladesh, Brazil, Indonesia, Singapore, and Cuba.23–28 Adults accounted for 82% of all cases in the Bangladesh DHF outbreak of 2000.23 In the Cuba 1997 outbreak, all DHF cases were adults, with secondary infection of DENV-2.24–26 In hyper-endemic Southeast Asia, all four dengue virus serotypes co-circulate, but in other endemic areas such as Nicaragua or Latin American countries, one serotype usually predominates in a particular year. Balmaseda and others,29 reported that the DENV-2 dominant period of 1999 to 2001 had significantly more shock, internal hemorrhage, and hospitalization duration, whereas the DENV-1 dominant 2003 was associated with increased vascular permeability. Primary infection with DENV-1 has four times greater risk of hospitalization and severe disease manifestation than primary DENV-2. Age is an important variable for the outcome of secondary dengue infection. Secondary DENV-2 caused DHF/DSS case fatality and hospitalization rates are higher both in the group of young infants 3 to 4 years of age and in the elderly of over 50 years of age in Cuba.26 In an isolated non-endemic Taiwan, Ae. aegypti has a day-biting feature by which it prefers to feed on working adults, and most people are naïve to dengue virus and have no prior anti-dengue antibody to enhance the dengue disease development. A majority of the symptomatic dengue cases are adults with a peak in the 50–54 age range, and a DHF peak in the 60–64 age range. This is in contrast to secondary-infection-associated DHF for children in hyper-endemic areas. DENV-2 in the year 2002 was associated with 74% of secondary infection and a higher DHF/DF ratio (4.5%). On the other hand, non-DENV-2 (DENV-1 or DENV-3) in the years 2004–2007 had a 70% level of primary infection, a lower DFH/DF ratio (0.6% to ~2.3%), but a higher mortality (17.5%). These data are consistent with the previous observation26,29 that primary infection of DENV-1 or DENV-3 and secondary infection of DENV-2 can lead to similar severe dengue disease or fatality. Secondary infection with DENV-2 is a significant risk factor.
The classic DF includes fever, headache, myalgia, arthralgia, retro-orbital pain, maculopapular rash, petechiae, and DHF of vascular permeability, internal hemorrhage, marked thrombocytopenia, or shock. Several reports have indicated different clinical manifestations among infants, children, and adults.30–32 Kittigul and others32 reported that hemoconcentration, thrombocytopenia, and increased liver enzyme and longer prothrombin time were reported to be significantly higher in adults than in children. For adult dengue in Taiwan, atypical lymphocytosis, activated partial thromboplastin time prolongation, alanine aminotransferase (ALT)/aspartate aminotransferase (AST) were significantly higher in DHF than in DF.33 Moreover, the DHF case fatality rate in adults was 8.7% for secondary DENV-2 in 2002 and 17.5% in primary DENV-1 or DENV-3 during 2004–2007. We have analyzed the factors associated with the severe disease and fatality of dengue-infected adults including elders with underlying diseases of hypertension, chronic renal insufficiency, or diabetes; abnormal hematological data, and elevation serum levels of AST, ALT, serum urea nitrogen (BUN) during the acute phase of infection.34 The case fatality rates of child DHF are around 0.2% to ~3.7%,35 which is lower than our adult patients. Why adult dengue patients experience high mortality is intriguing and needs further study. Taiwanese data show that secondary infection increases the risk of morbidity, but not mortality.
The early study by Burke and others36 in Thailand, reported that 87% of dengue-infected school children were either asymptomatic or minimally symptomatic. Endy and others37 reported equal incidence of inapparent and symptomatic cases for Thai children with secondary dengue infection. For adults, only 20% of dengue-infected Italian soldiers are asymptomatic in East Timor.38 The ratio of asymptomatic to symptomatic cases for travelers to Southeast Asia are between 1:0.8 and 1:3.3.39,40 The isolate outbreak for naïve adults in the Caribbean island camp participants or migrant Chinese workers in Singapore showed a high proportion of symptomatic cases with a level of 100% and 89%, respectively.41,42 Although clinically silent virus transmission is suggested or reported for children, the adults seem to be more susceptible than children to developing symptoms after dengue virus infection. The age distribution and status of primary infection of dengue-infected persons in our active surveillance system represent the general trends of dengue virus infection in Taiwan, which is not different from that of dengue-infected patients that go to the hospital for medical treatment. Most (64%) of the dengue-infected persons showed clinical symptoms after DENV-1 or DENV-3 infection. For Varicella (chickenpox), it was reported that adults are more likely to develop severe disease cases than children.43,44 It is reasonable to surmise that adults bearing a strong immune response will have a more intense immune-mediated clinical manifestation when the pathogenesis is immune-mediated.45
Urbanization, modern transportation, and global warming will all contribute to the increased incidence and geographical spread of dengue activity, even to new areas where competent vectors exist.46 The lessons learned in Taiwanese dengue research will become the model for countries or areas where dengue is not endemic, but where the vector of Ae. aegypti exists and the dengue virus can be introduced by travelers. This epidemiologic analysis of dengue disease in Taiwan not only shows a different transmission pattern, but also a different age-specific prevalence. Severe disease can be caused by secondary infection with DENV-2 or primary infection with DENV-1 or DENV-3. The adult dengue has higher morbidity or mortality than the child manifestation. Further study on this unique dengue epidemiology will shed light on our understanding of dengue disease pathogenesis.
Supplementary Material
Disclaimer: We declare that we have no conflict of interest.
Note: Supplemental tables appear at www.ajtmh.org.
Footnotes
Financial support: This work is supported by grant NHRI-98A1-PDCO-0209111 from the National Health Research Institute and grant NSC97-2745-B404-001 from National Science Council, Taiwan, Republic of China.
Authors' addresses: Chien-Chou Lin, Yh-Hsiung Huang, Pei-Yun Shu, and Ho-Sheng Wu, Centers for Disease Control, Department of Health, Taiwan, E-mails: jjlin@cdc.gov.tw, jhhuang@cdc.gov.tw, and pyshu@cdc.gov.tw. Yee-Shin Lin, Hsiao-Sheng Liu, and Huan-Yao Lei, Department of Microbiology and Immunology, College of Medicine, National Cheng Kung University, Tainan, E-mails: yslin1@mail.ncku.edu.tw, a713@mail.ncku.edu.tw, and hylei@mail.ncku.edu.tw. Trai-Ming Yeh, Department of Medical Laboratory Science and Biotechnology, College of Medicine, National Cheng Kung University, Tainan, E-mail: today@mail.ncku.edu.tw. Ching-Chuan Liu, Department of Pediatrics, College of Medicine, National Cheng Kung University, Tainan, E-mail: liucc@mail.ncku.edu.tw.
Reprint requests: Huan-Yao Lei, Department of Microbiology and Immunology, College of Medicine, National Cheng Kung University, Tainan, Taiwan, Republic of China, Tel: 886-6-2353535 Ext. 5643, Fax: 886-6-2097825, E-mail: hylei@mail.ncku.edu.tw.
References
- 1.Halstead SB. Dengue. Lancet. 2007;370:1644–1652. doi: 10.1016/S0140-6736(07)61687-0. [DOI] [PubMed] [Google Scholar]
- 2.Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med. 2004;10:S98–S109. doi: 10.1038/nm1144. [DOI] [PubMed] [Google Scholar]
- 3.Bhamarapravati N, Tuchinda P, Boonyapaknavik V. Pathology of Thailand haemorrhagic fever: a study of 100 autopsy cases. Ann Trop Med Parasitol. 1967;61:500–510. doi: 10.1080/00034983.1967.11686519. [DOI] [PubMed] [Google Scholar]
- 4.WHO . Dengue Hemorrhagic Fever: Diagnosis, Treatment, Prevention and Control. Second edition. Geneva: World Health Organization; 1997. [Google Scholar]
- 5.Shepard DS, Suaya JA, Halstead SB, Nathan MB, Gubler DJ, Mahoney RT, Wang DNC, Meltzer MI. Cost-effectiveness of a pediatric dengue vaccine. Vaccine. 2004;22:1275–1280. doi: 10.1016/j.vaccine.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 6.Halstead SB. Neutralization and antibody-dependent enhancement of dengue viruses. Adv Virus Res. 2003;60:421–467. doi: 10.1016/s0065-3527(03)60011-4. [DOI] [PubMed] [Google Scholar]
- 7.Guha-Sapir D, Schimmer B. Dengue fever: new paradigms for a changing epidemiology. Emerg Themes Epidemiol. 2005;73:1–10. doi: 10.1186/1742-7622-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu YC. Epidemic dengue 2 on Liouchyou Shian, Pingtune County in 1981. Chin J Microbiol Immunol. 1986;19:203–211. [PubMed] [Google Scholar]
- 9.Harn MR, Chiang YL, Tian MJ, Chang YH, Ko YC. The 1991 dengue epidemic in Kaohsiung City. J Formos Med Assoc. 1993;92((Suppl 1)):S39–S43. [PubMed] [Google Scholar]
- 10.Huang JH, Liao TL, Chang SF, Su CL, Chien LJ, Kuo YC, Yang CF, Lin CC, Shu PY. Laboratory-based dengue surveillance in Taiwan, 2005: a molecular epidemiologic study. Am J Trop Med Hyg. 2007;77:903–909. [PubMed] [Google Scholar]
- 11.Lei HY, Huang JH, Huang KJ, Chang CM. Current status of dengue control program in Taiwan. Dengue Bull. 2002;26:14–23. [Google Scholar]
- 12.Wu PC, Guo HR, Lung SC, Lin CY, Su HJ. Weather as an effective predictor for occurrence of dengue fever in Taiwan. Acta Trop. 2007;103:50–57. doi: 10.1016/j.actatropica.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 13.Innis BL, Nisalak A, Nimmannitya S, Kusalerdchariya S, Chongswasdi V, Suntayakorn S, Puttisri P, Hoke CH. An enzyme-linked immunosorbent assay to characterize dengue infections where dengue and Japanese encephalitis co-circulate. Am J Trop Med Hyg. 1989;40:418–427. doi: 10.4269/ajtmh.1989.40.418. [DOI] [PubMed] [Google Scholar]
- 14.Kuno G, Gómez I, Gubler DJ. An ELISA procedure for the diagnosis of dengue infections. J Virol Methods. 1991;33:101–113. doi: 10.1016/0166-0934(91)90011-n. [DOI] [PubMed] [Google Scholar]
- 15.Shu PY, Chen LK, Chang SF, Yueh YY, Chow L, Chien LJ, Chin C, Lin TH, Huang JH. Comparison of capture immunoglobulin M (IgM) and IgG enzyme-linked immunosorbent assay (ELISA) and nonstructural protein NS1 serotype-specific IgG ELISA for differentiation of primary and secondary dengue virus infections. Clin Diagn Lab Immunol. 2003;10:622–630. doi: 10.1128/CDLI.10.4.622-630.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shu PY, Chang SF, Yueh YY, Chow L, Chien LJ, Kuo YC, Su CL, Liao TL, Lin TH, Huang JH. Current status of dengue diagnosis at the Center for Disease Control, Taiwan. Dengue Bull. 2004;28:107–117. [Google Scholar]
- 17.Shu PY, Huang JH. Current advances in dengue diagnosis. Clin Diagn Lab Immunol. 2004;11:642–650. doi: 10.1128/CDLI.11.4.642-650.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falconar AK, de Plata E, Romero-Vivas CM. Altered enzyme-linked immunosorbent assay immunoglobulin M (IgM)/IgG optical density ratios can correctly classify all primary or secondary dengue virus infections 1 day after the onset of symptoms, when all of the viruses can be isolated. Clin Vaccine Immunol. 2006;13:1044–1051. doi: 10.1128/CVI.00105-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shu PY, Chang SF, Kuo YC, Yueh YY, Chien LJ, Sue CL, Lin TH, Huang JH. Development of group- and serotypespecific one-step SYBR green I real-time reverse transcription-PCR for dengue virus. J Clin Microbiol. 2003;41:2408–2416. doi: 10.1128/JCM.41.6.2408-2416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu YC, Huang YS, Chien LJ, Lin TL, Yueh YY, Tseng WL, Chang KJ, Wang GR. The epidemiology of Japanese encephalitis on Taiwan during 1966–1997. Am J Trop Med Hyg. 1999;61:78–84. doi: 10.4269/ajtmh.1999.61.78. [DOI] [PubMed] [Google Scholar]
- 21.Guzman MG, Kouri G, Halstead SB. Do escape mutants explain rapid increases in dengue case-fatality rates within epidemics? Lancet. 2000;355:1902–1903. doi: 10.1016/S0140-6736(00)02303-5. [DOI] [PubMed] [Google Scholar]
- 22.Kan CC, Lee PF, Wen TH, Chao DY, Wu MH, Lin NH, Huang SYJ, Shang CS, Fan IC, Shu PY, Huang JH, King CC, Pai L. Two clustering diffusion patterns identified from the 2001–2003 dengue epidemic, Kaohsiung, Taiwan. Am J Trop Med Hyg. 2008;79:344–352. [PubMed] [Google Scholar]
- 23.Rahman M, Rahman K, Siddque AK, Shoma S, Kamal AHM, Ali KS, Nisaluk A, Breiman RF. First outbreak of dengue hemorrhagic fever, Bangladesh. Emerg Infect Dis. 2002;8:738–740. doi: 10.3201/eid0807.010398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guzman MG, Kouri G, Valdes L, Bravo J, Alvarez M, Vazques S, Delgado I, Halstead SB. Epidemiologic studies on dengue in Santiago de Cuba. Am J Epidemiol. 1997;152:793–799. doi: 10.1093/aje/152.9.793. [DOI] [PubMed] [Google Scholar]
- 25.Vaughn DW. Invited commentary: Dengue lessons from Cuba. Am J Epidemiol. 2000152:800–803. doi: 10.1093/aje/152.9.800. [DOI] [PubMed] [Google Scholar]
- 26.Guzman MG, Kouri G, Bravo J, Valdes L, Vazquez S, Halstead SB. Effect of age on outcome of secondary dengue 2 infection. Int J Infect Dis. 2002;6:118–124. doi: 10.1016/s1201-9712(02)90072-x. [DOI] [PubMed] [Google Scholar]
- 27.Siqueira JB, Martelli CMT, Coelho GE, Simplicio ACR, Hatch DL. Dengue and dengue hemorrhagic fever, Brazil, 1981–2002. Emerg Infect Dis. 2005;11:48–53. doi: 10.3201/eid1101.031091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ooi EE, Goh KT, Gubler DJ. Dengue prevention and 35 years of vector control in Singapore. Emerg Infect Dis. 2006;12:887–893. doi: 10.3201/10.3201/eid1206.051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balmaseda A, Hammond SN, Perez L, Tellez Y, Saborio SI, Mercado JC, Cuadra R, Rocha J, Perez MA, Silva S, Rocha C, Harris E. Serotype-specific differences in clinical manifestations of dengue. Am J Trop Med Hyg. 2006;74:449–456. [PubMed] [Google Scholar]
- 30.Hammond SN, Balmaseda A, Perez L, Tellez Y, Saborio SI, Mercado JC, Videa E, Rodriguez Y, Perez MA, Cuadra R, Solano S, Rocha J, Idiaquez W, Gonzalez A, Harris E. Differences in dengue severity in infants, children, and adults in a 3-year hospital-based study in Nicaragua. Am J Trop Med Hyg. 2005;73:1063–1070. [PubMed] [Google Scholar]
- 31.Wichmann O, Hongsiriwon S, Bowonwatanuwong C, Chotivanich K, Sukthana Y, Pukrittayakamee S. Risk factors and clinical features associated with severe dengue infection in adults and children during the 2001 epidemic in Chonburi, Thailand. Trop Med Int Health. 2004;9:1022–1029. doi: 10.1111/j.1365-3156.2004.01295.x. [DOI] [PubMed] [Google Scholar]
- 32.Kittigul L, Pitakarnjanakul P, Sujirarat D, Siripanichgon K. The differences of clinical manifestations and laboratory finding in children and adults with dengue virus infection. J Clin Virol. 2007;39:76–81. doi: 10.1016/j.jcv.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 33.Liu JW, Khor BS, Lee CH, Lee IK, Chen RF, Yang KD. Dengue haemorrhagic fever in Taiwan. Dengue Bull. 2003;27:19–24. [Google Scholar]
- 34.Liu CC, Huang KJ, Huang MC, Lin JJ, Wang SM, Liu JJ, Tsai JJ, Huang JH, Lin YS, Liu HS, Yeh TM, Lei HY. High case-fatality rate of adults with dengue hemorrhagic fever during an outbreak in non-endemic Taiwan: risk factors for dengue-infected elders. Am J Infect Dis. 2008;4:10–17. [Google Scholar]
- 35.Deen JL, Harris E, Wills B, Balmaseda A, Hammond SN, Rocha C, Dung NM, Hung NT, Hien TT, Farrar J. The WHO dengue classification and case definitions: time for a reassessment. Lancet. 2006;368:170–173. doi: 10.1016/S0140-6736(06)69006-5. [DOI] [PubMed] [Google Scholar]
- 36.Burke DS, Nisalak A, Johnson DE, Scott RM. A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg. 1988;38:172–180. doi: 10.4269/ajtmh.1988.38.172. [DOI] [PubMed] [Google Scholar]
- 37.Endy TP, Chunsuttiwat S, Nisalak A, Libraty DH, Gree Sh, Rothman AL, Vaughn DW, Ennis FA. Epidemiology of inapparent and symptomatic acute dengue virus infection: a prospective study of primary school children in Kamphaeng Phet, Thailand. Am J Epidemiol. 2002156:40–51. doi: 10.1093/aje/kwf005. [DOI] [PubMed] [Google Scholar]
- 38.Peragallo MS, Nicoletti L, Lista F, D'Amelio R. Probable dengue virus infection among Italian Troops, East Timor, 1999–2000. Emerg Infect Dis. 2003;9:876–880. doi: 10.3201/eid0907.020496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cobelens FG, Groen J, Osterhaus AD, Leentvaar-Kuipers A, Wertheim-van Dillen PM, Kager PA. Incidence and risk factors of probable dengue virus infection among Dutch travelers to Asia. Trop Med Int Health. 2002;7:331–338. doi: 10.1046/j.1365-3156.2002.00864.x. [DOI] [PubMed] [Google Scholar]
- 40.Wilder-Smith A, Schwartz E. Dengue in travelers. N Engl J Med. 2008;353:924–932. doi: 10.1056/NEJMra041927. [DOI] [PubMed] [Google Scholar]
- 41.Lyerla R, Rigau-Perez JG, Vorndam AV, Reiter P, George AM, Potter I, Rm Gubler DJ. A dengue outbreak among camp participants in a Caribbean island, 1995. J Travel Med. 2000;7:59–63. doi: 10.2310/7060.2000.00022. [DOI] [PubMed] [Google Scholar]
- 42.Seet RC, Ooi EE, Wong HB, Paton NI. An outbreak of primary dengue infection among migrant Chinese workers in Singapore characterized by prominent gastrointestinal symptoms and a high proportion of symptomatic cases. J Clin Virol. 2005;33:336–340. doi: 10.1016/j.jcv.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 43.Danovaro-Holliday MC, Gordon ER, Jumaan AO, Woernle C, Judy RH, Schmid DS, Seward JF. High rate of varicella complications among Mexican-born adults in Alabama. Clin Infect Dis. 2004;39:1633–1639. doi: 10.1086/425613. [DOI] [PubMed] [Google Scholar]
- 44.Preblud SR. Age-specific risks of varicella complications. Pediatrics. 1981;68:14–17. [PubMed] [Google Scholar]
- 45.Lei HY, Yeh TM, Liu HS, Lin YS, Chen SH, Liu CC. Immunopathogenesis of dengue virus infection. J Biomed Sci. 2001;8:377–388. doi: 10.1007/BF02255946. [DOI] [PubMed] [Google Scholar]
- 46.Gubler DJ. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 2002;10:100–103. doi: 10.1016/s0966-842x(01)02288-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


