Abstract
An association between tumor necrosis factor α (TNF-α) and Entamoeba histolytica diarrhea was assessed in a cohort of 138 non-related Bangladeshi children who have been prospectively followed since 2001. Peripheral blood mononuclear cells (PBMCs) obtained at study entry were purified, cultured, and stimulated with soluble amebic antigen before cytokine measurement from supernatant. Higher levels of TNF-α were associated with increased risk of first (P = 0.01) and recurrent E. histolytica-related diarrheal episodes (P = 0.005). Children who developed E. histolytica diarrhea had significantly higher TNF-α protein levels than those who experienced asymptomatic E. histolytica infection (P value = 0.027) or no infection (P value = 0.017). Microarray studies performed using RNA isolated from acute and convalescent whole blood and colon biopsy samples revealed higher but non-significant TNF-α messenger RNA (mRNA) levels in subjects with acute E. histolytica diarrhea compared with convalescence. We conclude that there is an association between higher TNF-α production and E. histolytica diarrhea.
Introduction
Amebiasis, caused by Entamoeba histolytica, is a disease of worldwide importance and estimated to result in 40,000–100,000 deaths annually. Because the primary mode of E. histolytica transmission is through ingesting fecally contaminated food and water, its prevalence is greatest in areas with inadequate sanitation.1 The majority of infections with E. histolytica are asymptomatic, but more than 10% of infections result in diarrhea.2 Importantly, E. histolytica diarrhea in children is associated with malnourishment and stunting.3
Both increased levels of interferon-gamma (IFN-γ) from stimulated peripheral blood mononuclear cells (PBMCs)4 and a mucosal IgA antilectin antibody response5 have been previously associated with protection from E. histolytica infection. However, much information is still unknown about the aspects of the immune response that determine whether an individual with an E. histolytica infection remains asymptomatic or develops disease. Several in vitro and animal studies have evaluated the effect of tumor necrosis factor α (TNF-α) on E. histolytica with mixed results. It has been shown to both increase E. histolytica killing and worsen tissue damage.6–16 Therefore, the impact of TNF-α on disease outcome is still not clear.
The TNF-α is a pro-inflammatory cytokine, largely produced by macrophages, which can lead to tissue inflammation through the activation of macrophages, recruitment of neutrophils, and up-regulation of other pro-inflammatory mediators.17 It can also increase cell permeability, resulting in impairment of barrier function and edema formation.18 The TNF-α plays a central role in mucosal inflammation, and is elevated in the gastrointestinal tract of some forms of inflammatory colitis.19 For example, this cytokine is known to be of significant importance in the pathogenesis of inflammatory bowel disease (IBD), and anti-TNF agents have proven to be effective treatment of some individuals.20 In addition, stool TNF-α concentrations are higher in acute shigellosis compared with other forms of viral or bacterial diarrhea.21,22
This study tested the hypothesis that TNF-α promotes E. histolytica diarrhea. The PBMCs were tested for their ability to produce TNF-α in response to amebic antigen, and the association of TNF-α levels with susceptibility to invasive amebiasis measured. In addition, immunohistochemical (IHC) staining of colon biopsy samples and TNF-α messenger RNA (mRNA) levels in colon and whole blood were conducted.
Materials and Methods
Study population.
Cytokine levels were measured from the supernatant of stimulated PBMCs collected from 138 non-related children in the area of Mirpur within Dhaka, Bangladesh, between October 2001 and August 2002. After blood collection for cytokine analysis, the children were followed for an average of 1,785 days (SD ± 513 days). Monthly stool specimens were routinely obtained from all children in the cohort and tested for multiple pathogens, including E. histolytica. The children were also interviewed within their homes every other day, and stools were collected when diarrhea was reported. Height and weight of the children were obtained by research assistants at study enrollment and then every 4 months. Measurements obtained closest to the blood draw were used in analysis of this study.
The RNA for microarray analysis was isolated from whole blood obtained from seven children of the Mirpur cohort with acute E. histolytica diarrhea, and again 30 days later during convalescence. Colon biopsy samples for microarray analysis and IHC were obtained from eight subjects, ranging in age from 17 to 40, who came to the International Center for Diarrheal Diseases, Bangladesh with acute E. histolytica diarrhea. Biopsy samples were also collected 60 days later during convalescence.
Diagnosis of E. histolytica infection.
Entamoeba histolytica infection was determined using the E. histolytica II test (TechLab, Inc., Blacksburg VA), which detects amebic antigen in stool. A new E. histolytica infection was defined as a positive E. histolytica stool antigen and/or culture result following > 2 months of negative surveillance stool samples. Entamoeba histolytica diarrhea was defined as three or more unformed stools in a 24-hour period in a child diagnosed with a new episode of E. histolytica infection.
Measurement of secreted cytokines.
After isolation from whole blood, PBMCs were cultured in the presence of soluble amebic abstract (SAE) or phytohemagluttinin (PHA) for 72 hours. The resulting supernatant was frozen at −70°C until further use. Cytokine levels were subsequently measured from supernatant using a Bio-Plex human cytokine 17-plex assay (interleukin [IL]-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12, IL-13, IL-17, TNF-α, IFN-γ, granulocyte colony-stimulating factor [G-CSF], granulocyte macrophage colony-stimulating factor [GM-CSF], monocyte chemoattractant protein-1 [MCP-1], and macrophage inflammatory protein-1β [MIP-1β]) (Bio-Rad, Hercules, CA), according to manufacturer's instructions.
Microarray.
The RNA for microarray analysis was isolated from whole blood collected into Tempus tubes (Applied Biosystems, Warrington, UK) from seven children of the Mirpur cohort with acute E. histolytica diarrhea, and again 30 days later during convalescence. The Tempus spin RNA Isolation Reagent Kit (Applied Biosystems) was used to isolate blood from Tempus tubes per manufacturer's instructions.
Colon biopsy samples for microarray analysis were obtained from eight subjects, ranging in age from 17 to 40, who came to the International Center for Diarrheal Diseases, Bangladesh with acute E. histolytica diarrhea. Biopsy samples were also collected 60 days later during convalescence. Samples of colon tissue were homogenized in Trizol (Invitrogen, Carlsbad, CA), and RNA was isolated using the PicoPure RNA Isolation Kit (Molecular Devices, Sunnyvale, CA).
Microarray processing.
Samples were prepared according to Affymetrix protocols (Affymetrix, Inc., Santa Clara, CA). The RNA quality and quantity was ensured using the Bioanalyzer (Agilent, Inc., Santa Clara, CA) and NanoDrop (Thermo Scientific, Inc., Wilmington, DE), respectively. Per RNA labeling, 200 nanograms of total RNA was used in conjunction with the Affymetrix recommended protocol for the GeneChip 1.0 ST chips. The hybridization cocktail containing the fragmented and labeled complementary DNAs (cDNAs) was hybridized to the Affymetrix Human Genome ST 1.0 GeneChip. The chips were washed and stained by the Affymetrix Fluidics Station using the standard format and protocols as described by Affymetrix. The probe arrays were stained with streptavidin phycoerythrin solution (Molecular Probes, Carlsbad, CA) and enhanced by using an antibody solution containing 0.5 mg/mL of biotinylated anti-streptavidin (Vector Laboratories, Burlingame, CA). An Affymetrix Gene Chip Scanner 3000 was used to scan the probe arrays. Gene expression intensities were calculated using the GeneChip Operating software version 1.2 (GCOS 1.2, Affymetrix). The robust microarray analysis (RMA) within the Affymetrix Expression Console was used to normalize and log-transform the data and generate the .CHP files.
Immunohistochemical staining.
The IHC was performed for the detection of TNF-α in three colon biopsy samples obtained from subjects during an acute episode of E. histolytica diarrhea, and again at 60 days after recovery. Subjects with acute disease were stool antigen positive for E. histolytica, had blood and mucous in their stools, and were culture negative for other common diarrheal agents. Tissue sections were deparaffined and rehydrated before antigen retrieval using the Pascal Pressure Chamber with TRS buffer (DAKO, Denmark) for 30 seconds at 125°C and 22 psi. Slides were stained using the Dakoautostainer Universal Staining system (DAKO). Sections were incubated for 10 minutes with DAKO Dual Endogenous Enzyme Block before 30-minute incubation with the primary antibody, TNF-α (dilution 1:200). Sections were subsequently incubated for 30 minutes with DAKO Envision Dual Link (anti-mouse, rabbit antibody) before incubation with chromagen substrate diaminobenzidine tetrahydrochloride (DAB) for 10 minutes. The slides were counterstained with hematoxylin and mounted. Negative controls were performed by omitting the primary antibody. Colon tissue from subjects with ulcerative colitis was used as a positive control.
Statistical analysis.
Time to first event (E. histolytica asymptomatic infection and/or diarrhea) analysis was modeled for all cytokines using the proportional hazard model and found to be significant for TNF-α from SAE stimulated supernatant and E. histolytica diarrhea. To concisely model the association between the risk of E. histolytica diarrhea and TNF-α levels, analysis of recurrent events was performed. The inference was based on the proportional means model with the robust sandwich covariance estimate,23 which is an analogue to the Cox proportional hazards model. To examine the potential confounding effect of nutritional status, the nutrition score weight for height z-score (WHZ) closest to the blood draw was included in the model. In all of the previous survival analysis models, the continuous level of TNF-α protein was used. To illustrate the magnitude of TNF-α effect on the risk of E. histolytica diarrhea, each 1,000 pg/mL increment of TNF-α was used to calculate hazard ratio. One-way analysis of variance (ANOVA) was used to examine the difference in TNF-α protein expression from supernatant among children with a history of E. histolytica diarrhea, asymptomatic infection, or no infection. Two-sample t tests were used to compare differences between the two groups.
Microarray analysis.
The Genesifter program (Geospiza, Inc., Seattle, WA) (http://www.genesifter.net/web/) was used for analysis. A pairwise t test was used to compare TNF-α mRNA levels from acute and convalescent whole blood and colon samples.
Informed consent was obtained from adult participants and from the parents or legal guardians of minors. The studies were reviewed and approved by the Institutional Review Board at the University of Virginia and the Ethical Review Committee of the International Center for Diarrheal Disease Research, Bangladesh, Dhaka, Bangladesh.
Results
Association with survival.
The minimum value of TNF-α was 0 pg/mL, median 1,550 pg/mL, and maximum 14,915 pg/mL. Of the 138 non-related children included in this analysis, there were 107 new E. histolytica infections during the observation period, of which 25 children had diarrhea/colitis. Higher levels of TNF-α from SAE stimulated supernatant were associated with increased risk of first diarrheal episode. With each 1,000 pg/mL increase of TNF-α, the chance to have a first episode of diarrhea was elevated by 18% (hazard ratio = 1.18, 95% confidence interval [CI]: 1.04, 1.33, P = 0.01). The hazard ratio for subjects with a median TNF-α level (1,550 pg/mL) compared with those with a minimum TNF-α level (0 pg/mL) was 1.28 (95% CI: 1.06, 1.56). Figure 1 shows the steady increase of 5-year diarrhea rate (percentage of children who had diarrhea) with TNF-α level, based on the previous survival analysis model. The average 5-year diarrhea rate for children with minimal TNF-α level (0 pg/mL) is 13%, whereas it is 17% for those with median TNF-α (1,550 pg/mL). When TNF-α rises to 5,000 pg/mL, the 5-year diarrhea rate elevates to 27%.
Figure 1.
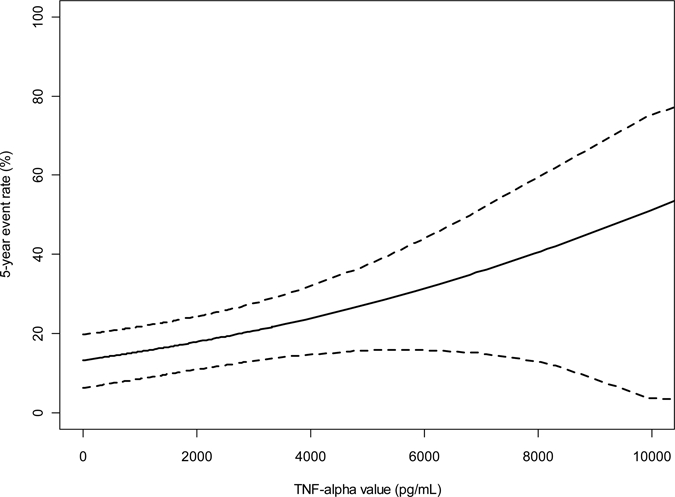
Five-year event rate for Entamoeba histolytica diarrhea based on TNF-α levels. Analysis of time to first event showed that with each 1,000 pg/mL increase of TNF-α, the chance to have E. histolytica diarrhea was elevated by 18% (hazard ratio = 1.18 and 95% confidence interval: 1.04, 1.33, P = 0.001).
After adjusting for co-infection with the soil-transmitted helminthes Ascaris lumbricoides or Trichuris trichuria, the association between TNF-α and time to first E. histolytica diarrheal episode remained significant (P = 0.01 and P = 0.02, respectively) and the hazard ratio estimates remained almost unchanged. In contrast, time to first E. histolytica asymptomatic or total infections were not associated with TNF-α levels (P = 0.30 and P = 0.15, respectively, NS). No other measured cytokine was associated with time to E.histolytica infection or diarrhea, and there was no association with TNF-α from PHA stimulated supernatant and time to E. histolytica diarrheal episode (P = 0.35). Therefore, to determine if the TNF-α responses reflected prior amebic-specific T-cell acquired memory, we additionally adjusted for baseline serum anti-E. histolytica antibodies. The association between TNF-α and time to first E. histolytica diarrheal episode also remained significant (P = 0.01).
There were 45 total episodes of diarrhea, with 12 subjects having recurrent episodes of E. histolytica diarrhea (Table 1). Higher levels of TNF-α from SAE stimulated supernatant were associated with increased risk of recurrent E. histolytica-related diarrheal episodes (P = 0.005). Analysis of recurrent events showed that with each 1,000-pg/mL increase of TNF-α, the chance to have E. histolytica diarrhea was elevated by 14% (hazard ratio = 1.14 and 95% CI: 1.04, 1.26, P = 0.005). The hazard ratio for subjects with a median TNF-α level (1,550 pg/mL) compared with those with a minimum TNF-α level (0 pg/mL) was 1.23 (95% CI: 1.07, 1.43). The TNF-α levels from PHA stimulated supernatant were not associated with recurrent episodes of E. histolytica diarrhea (P = 0.42).
Table 1.
Number and frequency of episodes of Entamoeba histolytica diarrhea among the 138 subjects in whom TNF-α levels from supernatant were obtained
| Number of episodes of E. histolytica diarrhea | Number of children | Percent of all children with an episode of E. histolytica diarrhea |
|---|---|---|
| 1 | 13 | 52 |
| 2 | 9 | 36 |
| 4 | 2 | 8 |
| 6 | 1 | 4 |
There were 617 total episodes of diarrhea from any cause among the 138 children in the cohort since 2001. To determine if the association with TNF-α was specific for E. histolytica or a general response caused by diarrhea itself, a recurrent event analysis was performed using all causes of diarrhea in the cohort since 2001. The TNF-α was not associated with total all cause diarrheal episodes (hazard ratio = 1.03, P = 0.38).
Nutritional status.
For recurrent event analysis, after adjusting for WHZ, the association between E. histolytica diarrhea and TNF-α levels remained significant (P = 0.003). Therefore, the association of TNF-α with E. histolytica diarrhea was independent of nutritional status.
Comparison of protein expression.
The TNF-α protein expression from supernatants of the SAE stimulated PBMCs obtained at study entry was compared among three groups of children: those who have never been infected with E. histolytica; children who have had asymptomatic E. histolytica infection; and children who have had E. histolytica diarrhea. Differences were seen when comparing all three groups using ANOVA (P = 0.04) (Table II). Children with E. histolytica diarrhea had significantly higher TNF-α protein levels than those with asymptomatic E. histolytica infection (P value = 0.027) and those with no E. histolytica infection (P value = 0.017) (Table II). Children with E. histolytica diarrhea also had significantly higher TNF-α protein levels than the combined groups of children with asymptomatic E. histolytica infection and no E. histolytica infection (P value = 0.011). There was no difference between children asymptomatically infected and never infected (P value = 0.51).
Table 2.
Comparison of TNF-α protein expression from supernatant of SAE stimulated PBMCs among children with a history of asymptomatic infection or no history of infection compared with symptomatic Entamoeba histolytica infection with diarrhea*
| N | Mean (SEM) | P value | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TNF-α protein (pg/mL) | No E. histolytica infection | 31 | 1697 (377) | 0.017 | Asymptomatic E. histolytica infection | 82 | 1988 (232) | 0.027 | Symptomatic E. histolytica infection | 25 | 3061 (420) | – |
The global P value = 0.04. SAE = soluble amebic abstract; PBMCs = peripheral blood mononuclear cells; SEM = standard error of the mean.
Comparison of acute and convalescent mRNA expression.
The mRNA expression was higher in acute (7.22, standard error of the mean [SEM] 0.10) compared with convalescent (7.02, SEM 0.06) whole blood samples, and acute (6.09, SEM 0.10) compared with convalescent (5.98, SEM 0.06) colon biopsy samples, but the differences were non-significant (P = 0.13 and 0.34, respectively).
Immunohistochemistry.
The TNF-α stained mononuclear cells in the lamina propria with abundant cytoplasm consistent with macrophages. No differences were observed in TNF-α staining between acute and convalescent amebic colitis (Figure 2).
Figure 2.
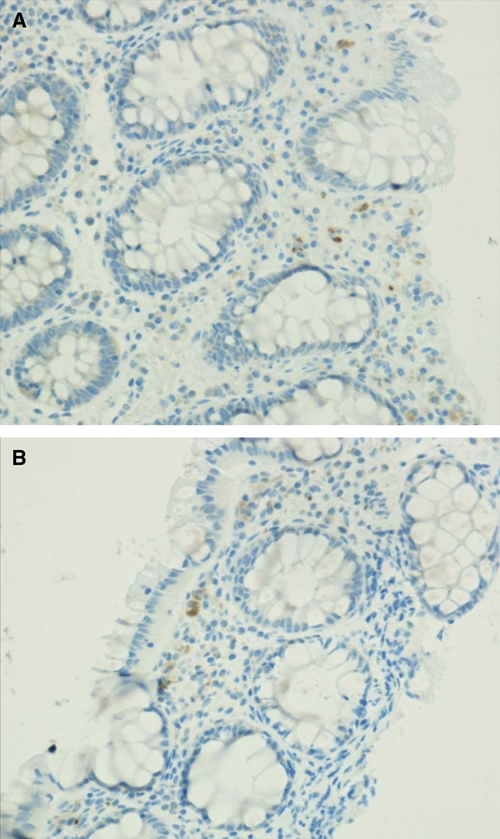
TNF-α stained mononuclear cells in the lamina propria with abundant cytoplasm consistent with macrophages in A, human colon biopsy on Day 1 of an Entamoeba histolytica diarrhea episode and B, Day 60 after recovery from E. histolytica diarrhea. This figure appears in color at www.ajtmh.org.
Conclusions
Although the majority of E. histolytica infections are asymptomatic, amebiasis remains a significant cause of morbidity and mortality in areas of the world where its prevalence is high. The host factors that influence disease development in E. histolytica infections have not been fully elucidated. This study evaluated the role of cytokines, and found an association between protein TNF-α levels from supernatant of SAE stimulated PBMCs and susceptibility to E. histolytica disease. Higher levels of TNF-α were associated with increased risk of first and recurrent E. histolytica-related diarrheal episodes, suggesting production of TNF-α may predict future susceptibility to E. histolytica diarrhea. This association was not seen with diarrhea in general from all causes. We also found no association with baseline serum anti-E. histolytica antibodies, suggesting an association with differential innate immune responses rather than prior amebic-specific T-cell acquired memory. Consistent with this, Chadee and others13 have demonstrated the E. histolytica adherence lectin (Gal-lectin) stimulates TNF-α by macrophages.
Elevated PBMC TNF-α protein expression was seen in children who had E. histolytica diarrhea compared with those who had asymptomatic infection or no infection. Higher although non-significant TNF-α mRNA expression among whole blood and colonic biopsy samples from subjects with acute versus convalescent E. histolytica diarrhea was also noted. These findings further suggest a role of TNF-α in the susceptibility to and pathogenesis of E. histolytica diarrhea. In agreement with these results, a study using human colonic xenografts reported that genes activated by TNF-α (IL-1β, IL-6, or IL-8) had increased expression during E. histolytica infection compared with control colonic samples without infection.16
Obvious differences in TNF-α staining by IHC were not apparent between acute E. histolytica diarrhea and convalescent colonic biopsy samples; however, the sample size, three subjects, was small. The biopsy samples also represent a very limited area of the colon, and may have not assessed areas with greater TNF-α variability. Furthermore, methods that quantitate differences in TNF-α levels may be more sensitive.
Previous studies have shown conflicting results regarding the role of TNF-α and amebae. Some in vitro studies have shown that TNF-α leads to increased nitric oxide (NO) production and killing of ameba.8,10–12 These findings would suggest that TNF-α may be beneficial in amebic colitis. In contrast, TNF-α has been shown to worsen tissue damage in mouse models of amebic colitis.15,16 The TNF-α also attracts E. histolytica trophozoites through chemotaxis,7 which may aid the parasite in the process of tissue invasion and colitis. On the basis of the current studies' findings, TNF-α does not appear to be protective for development of E. histolytica diarrhea and may actually increase susceptibility.
The TNF-α has been shown to play an important role in the pathogenesis of other gastrointestinal disorders such as IBD. Several studies have shown increased TNF-α levels in the serum and intestinal mucosa of individuals with IBD,24,25 and TNF-α blocking agents have been shown to result in healing of tissue inflammation in some patients. In addition to the direct affects of TNF-α itself on tissue, it may also lead to the upregulation of other pro-inflammatory cytokines and matrix metalloproteinases (MMPs). The MMPs are a family of Ca- and Zn-activated endoproteinases that are involved in physiological turnover, and mediate degradation of the extracellular matrix.26 In IBD patients, MMP-1, 3, 7, 9, and 10 are significantly increased in inflamed compared with normal colonic mucosa.27 Both MMPs and tissue inhibitor matrix metalloproteinases (TIMPs) are affected by TNF-α26; TNF-α increases the expression of MMP-3, MMP-9, and MMP-10.27,28 We have similarly seen significantly increased levels of MMPs in both colonic and PBMC mRNA expression among those with acute E. histolytica diarrhea compared with convalescence (Peterson K, Duggal P, Haque R, et al., unpublished data).
Environmental, pathogen, and host factors may each contribute to the outcome of E. histolytica infections. We have examined environmental factors including nutritional status and the contribution of co-infection with soil-transmitted helminthes. The effect of TNF-α was found to be independent. Pathogen factors, such as different parasite genotypes, could also influence the susceptibility to disease, potentially through different degrees of TNF-α stimulation. Finally, host genetic polymorphisms, such as in TNF-α or its pathways, may lead to variation in individual immune responses and contribute to the differences seen in disease susceptibility as well.
In conclusion, this study found an association between higher TNF-α production and E. histolytica diarrhea. An over-aggressive immune response from TNF-α may lead to increased inflammation and therefore disease. Further studies are warranted to clarify the role of TNF-α and its pathways in amebic colitis.
Acknowledgments
We thank the families in Mirpur, Dhaka Bangladesh for their participation in these studies. We also thank Pat Pramoonjago and Christopher A. Moskaluk for assistance with immunohistochemistry.
Footnotes
Financial support: This work is supported by NIH grant K08A1072470 to Kristine M. Peterson, grant AI043596 to William A. Petri, and in part by the intramural division of the National Human Genome Research Institute, NIH.
Authors' addresses: Kristine M. Peterson and William A. Petri Jr, Division of Infectious Diseases, University of Virginia Health Systems, Charlottesville, VA, E-mail: kmp5v@virginia.edu. Jianfen Shu, Division of Biostatistics and Epidemiology, Department of Public Health Services, University of Virginia, Charlotteville, VA. Priya Duggal, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD. Rashidul Haque and Dinesh Mondal, International Centre for Diarrheal Disease Research, Dhaka Bangladesh, Dhaka, Bangladesh.
References
- 1.Stanley SL., Jr Amoebiasis. Lancet. 2003;361:1025–1034. doi: 10.1016/S0140-6736(03)12830-9. [DOI] [PubMed] [Google Scholar]
- 2.Amidou S, Obi L, Bessong P, Stroup S, Houpt E, Guerrant R. Prevalence and species distribution of E. histolytica and E. dispar in the Venda Region, Limpopo, South Africa. Am J Trop Med Hyg. 2006;75:565–571. [PubMed] [Google Scholar]
- 3.Mondal D, Petri WA, Jr, Sack RB, Haque R. Entamoeba histolytica-associated diarrheal illness is negatively associated with the growth of preschool children: evidence from a prospective study. Trans R Soc Trop Med Hyg. 2006;100:1032–1038. doi: 10.1016/j.trstmh.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 4.Haque R, Mondal D, Shu J, Roy S, Kabir M, Davis AN, Duggal P, Petri WA., Jr Correlation of interferon-gamma production by peripheral blood mononulcear cells with childhood malnutrition and susceptibility to amebiasis. Am J Trop Med Hyg. 2007;76:340–344. [PubMed] [Google Scholar]
- 5.Haque R, Ibnekarim A, Sack B, Farr B, Ramakrishnan G, Petri WA., Jr Amebiasis and mucosal IgA antibody against the Entamoeba histolytica adherence lectin in Bangladeshi children. JID. 2001;183:1787–1793. doi: 10.1086/320740. [DOI] [PubMed] [Google Scholar]
- 6.Acevedo JA, Pacheco-Yépez J, Serrano-Luna J, Espinosa-Cantellano M, Tsutsumi V, Shibayama M. Experimental amebic liver abscess: in vivo localization of TNF-alpha. Arch Med Res. 2000;31((Suppl)):S98–S100. doi: 10.1016/s0188-4409(00)00181-8. [DOI] [PubMed] [Google Scholar]
- 7.Blazquez S, Zimmer C, Guigon G, Olivo-Marin JC, Guillén N, Labruyère E. Human tumor necrosis factor is a chemoattractant for the parasite Entamoeba histolytica. Infect Immun. 2006;74:1407–1411. doi: 10.1128/IAI.74.2.1407-1411.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denis M, Chadee K. Human neutrophils activated by interferon-gamma and tumour necrosis factor-alpha kill Entamoeba histolytica trophozoites in vitro. J Leukoc Biol. 1989;46:270–274. doi: 10.1002/jlb.46.3.270. [DOI] [PubMed] [Google Scholar]
- 9.Denis M, Chadee K. Cytokine activation of murine macrophages for in vitro killing of Entamoeba histolytica trophozoites. Infect Immun. 1989;57:1750–1756. doi: 10.1128/iai.57.6.1750-1756.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin JY, Chadee K. Macrophage cytotoxicity against Entamoeba histolytica trophozoites is mediated by nitric oxide from L-arginine. J Immunol. 1992;148:3999–4005. [PubMed] [Google Scholar]
- 11.Lin JY, Séguin R, Keller K, Chadee K. Tumor necrosis factor alpha augments nitric oxide-dependent macrophage cytotoxicity against Entamoeba histolytica by enhanced expression of the nitric oxide synthase gene. Infect Immun. 1994;62:1534–1541. doi: 10.1128/iai.62.5.1534-1541.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Séguin R, Mann BJ, Keller K, Chadee K. The tumor necrosis factor alpha-stimulating region of galactose-inhibitable lectin of Entamoeba histolytica activates gamma interferon-primed macrophages for amebicidal activity mediated by nitric oxide. Infect Immun. 1997;65:2522–2527. doi: 10.1128/iai.65.7.2522-2527.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Séguin R, Mann BJ, Keller K, Chadee K. Identification of the galactose-adherence lectin epitopes of Entamoeba histolytica that stimulate tumor necrosis factor-alpha production by macrophages. Proc Natl Acad Sci USA. 1995;92:12175–12179. doi: 10.1073/pnas.92.26.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang W, Keller K, Chadee K. Modulation of tumor necrosis factor production by macrophages in Entamoeba histolytica infection. Infect Immun. 1992;60:3169–3174. doi: 10.1128/iai.60.8.3169-3174.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Z, Mahajan S, Zhang X, Stanley SL., Jr Tumor necrosis factor alpha is a key mediator of gut inflammation seen in amebic colitis in human intestine in the SCID mouse-human intestinal xenograft model of disease. Infect Immun. 2003;71:5355–5359. doi: 10.1128/IAI.71.9.5355-5359.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Z, Stanley SL., Jr Stereotypic and specific elements of the human colonic response to Entamoeba histolytica and Shigella flexneri. Cell Microbiol. 2004;6:535–554. doi: 10.1111/j.1462-5822.2004.00381.x. [DOI] [PubMed] [Google Scholar]
- 17.Bazzoni F, Beutler B. The tumor necrosis factor ligand and receptor families. N Engl J Med. 1996;334:1717–1725. doi: 10.1056/NEJM199606273342607. [DOI] [PubMed] [Google Scholar]
- 18.Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, Said HM. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol. 2004;286:G367–G376. doi: 10.1152/ajpgi.00173.2003. [DOI] [PubMed] [Google Scholar]
- 19.Rojas-Cartagena C, Flores I, Appleyard CB. Role of tumor necrosis factor receptors in an animal model of acute colitis. Cytokine. 2005;32:85–93. doi: 10.1016/j.cyto.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Kuhbacher T, Fölsch UR. Practical guidelines for the treatment of inflammatory bowel disease. World J Gastroenterol. 2007;13:1149–1155. doi: 10.3748/wjg.v13.i8.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenberg DE, Jiang ZD, Steffen R, Verenker MP, DuPont HL. Markers of inflammation in bacterial diarrhea among travelers, with a focus on enteroaggregative Escherichia coli pathogenicity. JID. 2002;185:944–949. doi: 10.1086/339617. [DOI] [PubMed] [Google Scholar]
- 22.Nicholls S, Stephens S, Braegger CP, Walker-Smith JA, MacDonald TT. Cytokines in stools of children with inflammatory bowel disease or infective diarrhoea. J Clin Pathol. 1993;46:757–760. doi: 10.1136/jcp.46.8.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin D, Wei L, Yang I, Ying Z. Semiparametric regression for the mean and rate functions of recurrent events. J R Stat Soc, B. 2000;62:711–730. [Google Scholar]
- 24.Breese EJ, Michie CA, Nicholls SW, Murch SH, Williams CB, Domizio P, Walker-Smith JA, MacDonald TT. Tumor necrosis factor alpha-producing cells in the intestinal mucosa of children with inflammatory bowel disease. Gastroenterology. 1994;106:1455–1466. doi: 10.1016/0016-5085(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 25.Komatsu M, Kobayashi D, Saito K, Furuya D, Yagihashi A, Araake H, Tsuji N, Sakamaki S, Niitsu Y, Watanabe N. Tumor necrosis factor-alpha in serum of patients with inflammatory bowel disease as measured by a highly sensitive immuno-PCR. Clin Chem. 2001;47:1297–1301. [PubMed] [Google Scholar]
- 26.Meijer MJ, Mieremet-Ooms MA, van Hogezand RA, Lamers CB, Hommes DW, Verspaget HW. Role of matrix metalloproteinase, tissue inhibitor of metalloproteinase and tumor necrosis factor-alpha single nucleotide gene polymorphisms in inflammatory bowel disease. World J Gastroenterol. 2007;13:2960–2966. doi: 10.3748/wjg.v13.i21.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pedersen G, Saermark T, Kirkegaard T, Brynskov J. Spontaneous and cytokine induced expression and activity of matrix metalloproteinases in human colonic epithelium. Clin Exp Immunol. 2009;155:257–265. doi: 10.1111/j.1365-2249.2008.03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou L, Yan C, Gieling RG, Kida Y, Garner W, Li W, Han YP. Tumor necrosis factor-alpha induced expression of matrix metalloproteinase-9 through p21-activated kinase-1. BMC Immunol. 2009;10:15. doi: 10.1186/1471-2172-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]


