Abstract
Difficulty in controlling human Schistosoma japonicum infection is partly attributed to the presence of non-human definitive hosts. Water buffaloes are a major reservoir for transmission of S. japonicum to humans in China. However, in the Philippines, reports based on microscopic examination of buffalo stool identified a low prevalence of S. japonicum, and mathematical models using these data concluded that water buffaloes are not a major reservoir for transmission of S. japonicum to humans. We collected stool from 81 buffaloes in Macanip, Leyte, the Philippines, and assayed for S. japonicum infection by the Danish Bilharziasis Laboratory technique, the Kato-Katz technique, miracidia hatching, and a highly validated real-time polymerase chain reaction. The prevalence defined by each assay was 3.7%, 3.7%, 0%, and 51.5% respectively. Our results demonstrate that microscopic-based techniques dramatically underestimate the prevalence of S. japonicum infection in water buffaloes in the Philippines and warrant reexamination of the role of bovines in transmission of S. japonicum to humans in the Philippines.
Introduction
Schistosomiasis is one of the most prevalent parasitic diseases worldwide, with 207 million persons infected in 76 countries.1 In contrast to the other major schistosomes infecting humans, Schistosoma japonicum is a zoonosis with a wide spectrum of definitive hosts other than humans, including buffaloes, cattle, goats, sheep, rats, dogs, and cats, serving as reservoir hosts.2 Although human infection and disease caused by S. japonicum can be reduced by treatment of humans with praziquantel, control of schistosomiasis japonica remains difficult, in part, because of transmission from infected animals.3,4
The relative importance of specific host species to human transmission of S. japonicum is likely complex and may be influenced by local factors that include the number and abundance of potential host species, heterogeneity in irrigation management practices, and potential parasite genetic restrictions to infectivity of humans.5
Three recent interventional studies strongly support the contribution of water buffaloes to human transmission in the lake and marshlands region of China.6–8 In two studies, buffaloes and humans were treated with praziquantel in intervention villages, and only humans were treated in control villages. After adjusting for baseline prevalence, age, sex, and exposure, S. japonicum reinfection of humans was 48–89% lower in villages that received treatment of buffaloes and humans.6,7 In an operational research study, removing bovines and improving sanitation in intervention villages resulted in an 88% reduction in human prevalence compared with control villages.8 These findings are further supported by mathematical modeling of intra-species transmission dynamics across several ecologic settings in China, which indicate that buffaloes account for 39–99% of the S. japonicum transmission to humans.9 Based on these results, the Chinese National Schistosomiasis Control Program currently targets cattle and water buffaloes for zoonotic surveillance.8,10
In contrast to the situation in China, in the Philippines, there are no published interventional trials examining the role of non-human hosts in transmission of S. japonicum to humans. Several surveys have examined the host range of S. japonicum in the Philippines by using parasitologic and immunologic detection assays and identified dogs, cats, rats, pigs, cattle, and water buffaloes as potential hosts.4,11–18 A recent epidemiologic survey using the Danish Bilharziasis Laboratory (DBL) technique19 conducted in 50 villages in Samar, Philippines, reported a 2.1% prevalence of S. japonicum in 873 water buffaloes, which represented 49.4% of all water buffaloes in the villages.20 Prevalence in water buffaloes varied across villages, but in all villages except one, water buffaloes had the lowest prevalence of infection compared with dogs, cats, pigs, and rats. Mathematical modeling of these prevalence data suggested that water buffaloes (Bubalus bubalis) do not play a significant role in the transmission of S. japonicum to humans in Samar, the Philippines.21 These findings have been challenged because of uncertainty in the species-specific test characteristics of the diagnostic method used in the Philippines (DBL technique) compared with China (miracidia hatching).22
Traditionally, diagnosis of schistosomiasis in animals has been made by direct parasitologic techniques, including coprologic assays, such as the Kato-Katz technique and miracidia hatching. These are simple, low-cost diagnostic assays useful in areas with moderate to high infection intensity but have poor sensitivity for light infections.23
Recently, several conventional and real-time polymerase chain reaction PCR (quantitative PCR [qPCR]) methods to amplify and detect parasite DNA have been developed for all three species of schistosomes that commonly infect humans.24–34 The present study reevaluated the prevalence of S. japonicum infection in water buffaloes in a schistosomiasis-endemic area in the Philippines by using several traditional methods and a highly validated qPCR assay.32,33
Materials and Methods
Study site and stool collection.
This cross-sectional study was carried out in the village of Macanip, Jaro municipality, Leyte, the Philippines, a subsistence rice-farming village that has been the focus of schistosomiasis studies for more than 20 years. In 2001, the village level human prevalence based on duplicate Kato-Katz examination of a single stool specimen was 54%.35
In May 2008, we conducted a survey of water buffaloes in Macanip. All owners of water buffaloes were informed about the study and asked to keep their water buffaloes penned to facilitate the collection of a stool sample. Samples were collected directly from the rectums of water buffaloes into barcode-labeled cups and stored on wet ice until processed. All stools were processed within eight hours of collection. Exclusion criteria included lack of permission from the farmer and failure to pen the animals on the scheduled stool collection date.
Traditional coprologic methods for detection of S. japonicum infection.
Sixty grams of feces was extensively homogenized with a plastic spatula prior to sampling for Kato-Katz technique, DBL technique, and miracidia hatching. For the Kato-Katz assay, homogenized stool was measured onto a slide using a 50-mg template. Two slides were prepared from each sample and were stained and read according to published methods.36
The DBL technique was performed as described.37 Briefly, 5 grams of homogenized stool together with 100 mL of 1.2% saline were added to a 250-mL plastic bottle. The bottle was capped and vigorously agitated for 10 minutes. The resulting solution was poured through a series of 3 sieves of 400-μm, 250-μm, and 45-μm mesh size, respectively. The residue remaining in the 45-μm sieve was transferred to a 0.5-liter sedimentation flask with 200 mL of saline and allowed to sediment for 20 minutes in the dark. The upper 150-mL layer of saline was carefully decanted, and the remainder was poured through a 45-μm sieve. The residue was transferred from the 45-μm sieve to a 0.5-liter sedimentation flask with 200 mL of saline and allowed to sediment for 15 minutes in the dark. The upper 150-mL layer of saline was carefully decanted, and the remainder was poured through a 45-μm sieve. The residue remaining in the 45-μm sieve was washed into a 15-mL conical tube using 10 mL of saline. The 15-mL tube was centrifuged for 1 minute at 300 × g and the supernatant was discarded. The sediment was resuspended in saline to a final volume of 2.25 mL. After thoroughly mixing, 150 μL was transferred to a 1-mL microscope chamber and 850 μL of saline was added. The chamber slides were carefully mixed and the number of S. japonicum eggs was recorded. Three slides (total of 450 μL of sediment) were counted for each sample and the sum of 3 counts indicated the number of eggs per gram (EPG) of feces.
For the miracidia hatching assay, 50 grams of homogenized stool was transferred into a 250-mL plastic bottle. The fecal sample was suspended in 200 mL of dechlorinated water and allowed to sediment for 20 minutes. The upper 150-mL layer of water was carefully decanted and an additional 150 mL of dechlorinated water was added to the sample. The samples were resuspended and allowed to sediment for 20 minutes. The upper 150-mL layer of water was carefully decanted and the sediment was transferred to a 1-liter glass flask. The flask was filled with 1 liter of dechlorinated tap water and incubated under strong artificial illumination at 25°C. The presence of miracidia was assessed one, three, and five hours after incubation by ocular examination.38
Real-time PCR for detection of S. japonicum infection.
Sample preparation.
Uninfected water buffalo stools were obtained from 11 water buffaloes in a non-schistosomiasis–endemic area in China. Purified S. japonicum eggs prepared from infected mouse livers39 (provided by the Schistosomiasis Resource Center, National Institute of Allergy and Infectious Diseases, Bethesda, MD) were washed three times with phosphate-buffered saline, pH 7.4, and transferred onto glass slides for enumeration by microscopy. Enumerated eggs were washed off the slide with ASL buffer (stool lysis buffer in the QIAmp DNA stool kit; Qiagen, Valencia, CA) into microtubes for DNA extraction, followed by verification that no eggs remained on the glass slide by microscopy.
Spiked stool samples corresponding to 1 and 2 EPG were prepared by mixing 1 gram of uninfected buffalo stool with 1 or 2 eggs, homogenizing extensively with a plastic spatula, freezing at –80°C for more than 1 hour, thawing, and transferring 200 mg of stool to a new microtube. The 200-mg stool samples were further homogenized in 1.4 mL of ASL buffer and DNA was extracted. Spiked stool samples corresponding to 5, 10, 20, 40, and 80 EPG were prepared by mixing 200 mg of uninfected water buffalo stool with enumerated S. japonicum eggs and processed as above. Spiked samples in ASL buffer were prepared by adding enumerated, purified eggs to 1.4 mL of ASL buffer, vortexing, and DNA extraction.
Genomic DNA extraction.
Genomic DNA was extracted from adult worms, purified eggs, or stool samples using QIAamp DNA Stool Mini Kit (Qiagen). The protocol “Isolation of DNA from Stool for Pathogen Detection” was used with two modifications: 1) samples were incubated at 95.0°C for 20 minutes with vortexing every 5 minutes (instead of an incubation for 5 minutes at 70°C) and 2) washing of the purification column with buffer AW1 was increased from once to three times. Genomic DNA was eluted with 200 μL AE buffer at room temperature for 5 minutes.
Quantitative PCR.
We used a previously described qPCR specific for S. japonicum that amplifies an 82-base-pair sequence in the mitochondrial nicotinamide adenine dinucleotide dehydrogenase I gene using SYBR Green for detection.32,33 The primer pair SjqPCR-F (5′-TGR TTT AGA TTT GGG TGT GC-3′) and SjqPCR-R (5′-AAC CCC CAC AGT CAC TAG CAT AA-3′) was synthesized (Invitrogen, Carlsbad, CA) as described.32 The qPCR was performed in TempAssure PCR 8-tube strips (USA-Scientific Inc., Ocala, FL) in a total reaction volume of 50 μL that contained 1 × SYBR® GreenER qPCR™ SuperMix Universal (Invitrogen), 200 nM of each primer, and 2 μL of extracted DNA as a template plus Ultra Pure BSA (Ambion, Austin, TX) to a final concentration of 0.1 mg/mL. The PCR program was 95.0°C for 15 minutes; 40 cycles at 95.0°C for 15 seconds and 60.0°C for 1 minute; and a melting point analysis of 60.0–95.0°C over 15 minutes by using a Mastercycler® ep RealPlex (Eppendorf, Hamburg, Germany).
DNA extracted from spiked stool samples with 1, 2, 5, 10, 20, 40, 80 EPG were amplified in triplicate with each assay to construct a standard curve for quantification of unknown samples. The PCRs for all unknown samples were carried out in duplicate. DNA extracted from the 11 uninfected water buffaloes served as negative controls.
A qPCR result was considered positive if its cycle threshold (Ct) value was less than mean Ct value of the negative controls and its melting point was within ±0.9°C of the expected melting temperature of 70.6°C. An unknown sample result was regarded positive when at least one of the two duplicate samples was positive. The estimated EPG by qPCR was calculated based on the standard curve for each plate and recorded as the average value of the duplicate assays.
Statistical analysis.
Results of microscopy and qPCR were stored in a Microsoft (Redmond, WA) Excel databases and analyzed in JMP v8 (SAS Institute, Cary, NC). Standard curves relating log(EPG + 1) and Ct were calculated in JMP by using linear regression. Multivariate regression was used to evaluate for the presence of PCR inhibitors by fitting a linear model with log(EPG + 1) as the outcome and Ct as the predictor. The interaction term (sample type × Ct) was added to the model and evaluated for significance. Potential sampling bias by availability of samples for qPCR analysis was evaluated by contingency table analysis. Statistical significance was considered at a P value < 0.05.
Results
Study site and sample collection.
In May 2008, there were 358 households that owned 91 water buffaloes in Macanip. We collected stool from 81 buffaloes and performed the Kato-Katz technique, the DBL technique, and miracidia hatching assays in the field. We performed qPCR assays on a subset of 66 buffaloes whose stool samples were available for genomic DNA extraction. In contingency table analysis, the prevalence of S. japonicum infection by the Kato-Katz technique, the DBL technique, and miracidia hatching did not differ between the 66 buffaloes analyzed by qPCR compared with the 15 buffaloes that were not analyzed by qPCR (P = not significant).
Validation of the qPCR assay—linearity, false-positive results, and assessment of inhibitors.
To assess linearity, false-positive results, and potential impact of stool-derived PCR inhibitors, we performed parallel qPCR assays using DNA purified from spiked stool samples, uninfected stool samples, and DNA obtained from purified eggs. The qPCR assay was applied to DNA extracted from 25 independently prepared samples of enumerated, purified eggs in buffer, and 25 samples of enumerated, purified eggs homogenized in uninfected buffalo stool. Samples corresponding to 1, 2, 10, 50 (±2) and 250 (±5) eggs were evaluated. All samples were positive by the qPCR, which indicated a sensitivity of 100%, even for samples with only 1 egg. There was an excellent linear relationship between Ct and log(egg count + 1) in DNA samples obtained from purified eggs and spiked stool samples (R2 = 0.94 and R2 = 0.94, respectively, Figure 1).
Figure 1.
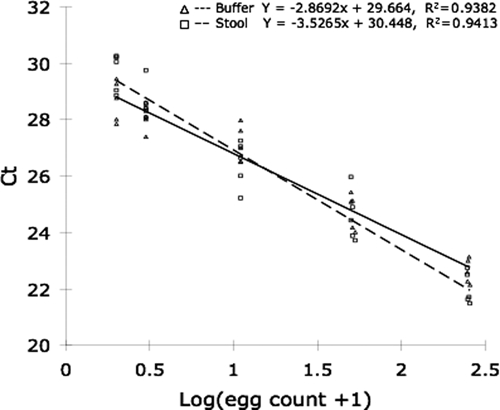
Amplification efficiency of quantitative polymerase chain reaction (qPCR) assay using stool or buffer as matrix. Twenty-five samples prepared in stool (squares) and 25 samples prepared in buffer (triangles) were evaluated in triplicate by qPCR. Samples contained 1, 2, 10, 50, or 250 enumerated, purified Schistosoma japonicum eggs. Regression lines and equations are indicated for each sample matrix.
To assess specificity, we performed qPCR assays on 11 water buffalo stools obtained from animals living in non-endemic areas. All 11 samples were negative by qPCR, which was in agreement with the published specificity of 100% for this assay.32
Multivariate regression models showed that the DNA source (coded as an interaction term with Ct) was a significant predictor of the efficiency of amplification (P = 0.02). This analysis indicates that the sample matrix influences the slope of the regression line relating Ct with egg count (Figure 1). Unexpectedly, for low egg counts, DNA extracted from stool amplified more efficiently than DNA extracted from buffer, and the opposite trend was observed at high egg counts. Although the magnitude of this matrix effect is not likely to be of inferential significance, we constructed all standard curves using DNA extracted from stool samples spiked with purified, enumerated eggs.
Validation of the qPCR assay—spike recovery.
Using genomic DNA obtained from stool samples spiked with defined EPGs as standards, we performed spike-recovery experiments on 3–5 independently spiked samples for each EPG tested (1, 2, 5, 10, 20, 40, and 80 EPG). The comparison of spiked EPG (enumerated by microcopy) with recovered EPG (calculated from standard curve) was excellent, with R2 ranging from 0.98 to 0.99 (Table 1).
Table 1.
Recovery of Schistosoma japonicum eggs spiked into uninfected buffalo stool by qPCR*
| EPG† | No. samples prepared‡ | Measured EPG by qPCR | |
|---|---|---|---|
| Mean ± SD | Range | ||
| 1 | 3 | 0.9 ± 0.4 | 0.8–1.3 |
| 2 | 3 | 2.5 ± 1.0 | 1.6–3.8 |
| 5 | 5 | 7.5 ± 1.2 | 6.2–8.7 |
| 10 | 5 | 10.0 ± 2.1 | 7.4−13.0 |
| 20 | 5 | 20.8 ± 4.6 | 14.2–26.9 |
| 40 | 5 | 34.5 ± 3.8 | 29.0–36.4 |
| 80 | 5 | 82.2 ± 10.1 | 70.8–98.2 |
qPCR = quantitative polymerase chain reaction; EPG = eggs per gram (of feces).
EPG of purified enumerated S. japonicum eggs spiked into uninfected buffalo stool.
Each sample was tested in triplicate.
Prevalence and intensity of S. japonicum infection in water buffaloes.
We assessed the prevalence of S. japonicum in water buffaloes using traditional coprologic and qPCR methods. The prevalence (no. positive/no. examined) of S. japonicum infection was 3.7% (3 of 81), 3.7% (3 of 81), and 0% (0 of 81) by the DBL technique, the Kato-Katz technique, and miracidia hatching, respectively. We performed qPCR on a subset of 66 of 81 stool samples and identified a prevalence (no. positive/no. examined) of 51.5% (34 of 66) (Figure 2). Of the 34 positive samples, 30 were positive in both qPCR replicates, and 4 were positive in only 1 of the 2 replicates analyzed.
Figure 2.
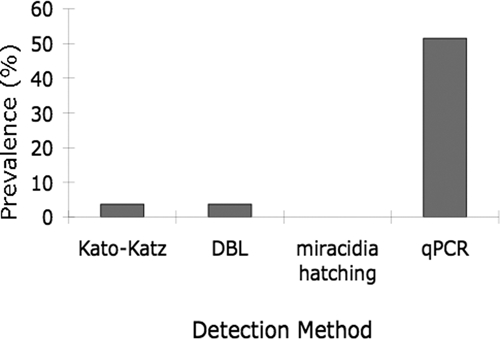
Prevalence of Schiostosoma japonicum in water buffaloes in Leyte, the Philippines, as assessed by traditional coprologic and quantitative polymerase chain reaction detection methods.
The geometric mean intensity of infection measured by qPCR was 2.05 EPG in the 34 positive stools with two samples exceeding 5 EPG (Figure 3). These two samples were also positive by the Kato-Katz and DBL techniques.
Figure 3.
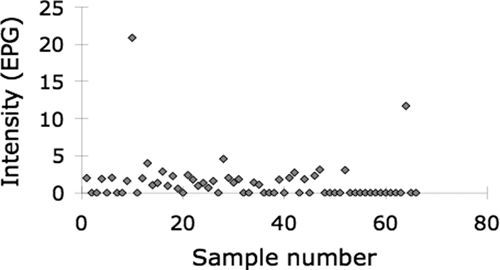
Intensity of Schistosoma japonicum infection in water buffaloes in Leyte, Philippines, quantified by quantitative polymerase chain reaction.
Discussion
Despite repeated chemotherapy of humans with praziquantel, intensity, prevalence, and morbidity caused by schistosomiasis remain unacceptably high in many disease-endemic areas.1,40 Particular difficulty in controlling S. japonicum has been attributed to the presence of non-human hosts. Recently, several interventional trials that used either treatment6,7 or removal8 of water buffaloes from disease-endemic areas have convincingly demonstrated the role of this domesticated animal in contributing to transmission of S. japonicum to humans in China. These findings have been further supported by mathematical modeling approaches, which also identify water buffaloes as an important reservoir for transmission to humans.9 Together, these results have reinvigorated research to develop a vaccine for bovine schistosomiasis based on its direct veterinary application and its human transmission–blocking activity.41,42
In the Philippines, water buffaloes, dogs, pigs, cattle, and rats have been recognized as hosts for S. japonicum for decades.18 However, no interventional trials on any potential reservoir species have been published. Recently, a survey of potential host species for S. japonicum in Samar, the Philippines, found a relatively low prevalence of S. japonicum infection in water buffaloes (2.1%), and modeling efforts using these data did not identify buffaloes as a major source of transmission to humans.21
Based on the low prevalence of buffalo schistosomiasis reported in Samar, and the choice of diagnostic test for this survey (DBL technique),20 we reexamined the prevalence of schistosomiasis in water buffaloes in the Philippines. We found a 51.5% prevalence of infection with S. japonicum in the 66 water buffaloes assayed by a highly validated qPCR-based diagnostic method. This prevalence was significantly higher than the prevalence determined by any of the three traditional coprologic detection methods in the same study samples. We note that the prevalence (51.5%) and geometric mean intensity of infection (2.05 EPG) in bovines in our study is comparable to that observed in the interventional studies in China (prevalence = 11.5–29.3%, 0.53–7.2 geometric mean EPG).43 However, this comparison must be made with caution given the different detection assays used in the two studies.
Traditional coprologic detection techniques are inexpensive, widely available, and have sufficient sensitivity and specificity for use in disease-endemic areas that are characterized by moderate-to-high intensity infections. Recently, their deficiencies for diagnosis in disease-endemic areas characterized by low prevalence with low-intensity infections have been highlighted.23
The Kato-Katz fecal smear technique is the least expensive and most commonly used method for detecting human intestinal schistosomiasis,36,44 but shows low sensitivity in human studies.45,46 In a study comparing the Kato-Katz method (duplicate 42-mg fecal smears) and a nylon bag miracidia hatching test (using 30 grams of feces) to detect S. japonicum infection of humans, Yu and others concluded that the Kato-Katz technique was more sensitive than hatching and was not susceptible to the intra-analytic variables such as temperature, water quality, and dietary factors that influence the hatching method.47
The DBL technique was developed to diagnose S. japonicum infection in pigs by the Danish Center for Experimental Parasitology. The method is based on filtration, sedimentation, and centrifugation and therefore enables evaluation of a larger fecal specimen than the Kato-Katz method (5 grams versus 50 mg, respectively).19 This assay has been compared with the Bell method for stool samples obtained from seven experimentally infected pigs and found to be more sensitive.19 To date, the linearity, sensitivity, and specificity of the assay using laboratory-based spike-recovery approaches have not been examined in any host species. This finding is particularly germane given that the method was developed for pigs, a host with low cellulose content in its stool. Because the dense fibrous capsule surrounding S. japonicum eggs renders them adherent,19,48,49 differential sensitivity of the DBL technique across host species, with particularly low sensitivity in ruminants, may be expected because of egg adherence to cellulose with variable removal during the filtration steps.
In a recent paper, a latent-class statistical approach has been used to estimate the sensitivity and specificity of the DBL technique in several host species, including water buffaloes.37 The change in prevalence observed in 91 water buffaloes was evaluated as the number of stools examined per animal increased from one to five. These input data were analyzed with a Bayesian iterative model to estimate sensitivity and specificity. Using this approach, the estimated sensitivity of the DBL technique in water buffaloes was 78% and 98.9% when one or three stools were examined, respectively. We note that these estimates were not confirmed with actual measures of sensitivity by spike-recovery studies using purified S. japonicum eggs and uninfected buffalo stool. Similarly, the statistical method used has not been validated against traditional laboratory based assessments50,51 and there are debates regarding the utility of such methods with imperfect diagnostic tests.52,53
In the present study, we used a highly validated qPCR specific for S. japonicum to detect water buffalo infection in the Philippines. This assay was developed for the sensitive and specific detection of S. japonicum in human stool samples32 and was subsequently validated in a pig model of S. japonicum.33 This assay does not detect S. mansoni, Ascaris, Tricuris, hookworms, or Taenia.32,33 In addition, the assay did not detect Clonorchis sinensis or Fasciola hepatica when 8 ng of genomic DNA isolated from adult worms was used as template (data not shown). We observed significantly improved linearity of the assay compared with published results (Figure 1).32 We attribute this improvement to exhaustive homogenization of stools prior to sampling and modifications to the DNA extraction protocol (see Materials and Methods).
In spike-recovery experiments, the qPCR assay showed excellent sensitivity, linearity, and recovery (Table 1) in buffalo stool samples and buffer as matrix (Figure 1). In these experiments, all samples, including stool samples with only 1 EPG, were positive by qPCR, which indicated a sensitivity of 100%, even at low infection intensities. We detected an effect of matrix on efficiency of amplification, although this effect was minor and addressed by using stool as matrix for all standard curves. The specificity of the qPCR has been examined by using genomic DNA from a variety of helminthes.32 In addition, we extended the assay's specificity assessment by evaluating stools from 11 buffaloes with life histories that excluded habitation in S. japonicum-endemic areas. All 11 buffaloes were negative by qPCR assay and no false-positive results were obtained with other helminth samples, which indicated a specificity of 100%.
Our qPCR results showed a remarkably high (51.5%) prevalence of S. japonicum infection in comparison with the Kato-Katz, miricidial hatching, and DBL techniques. Given the low sensitivity of the Kato-Katz technique at low intensities of infection,45,46 this finding was an expected result. Our inability to hatch miricidia from any of the qPCR-positive buffalo stool samples, despite extensive efforts, may reflect low sensitivity of miricidial hatching at low infection intensities,47 difficulties with water quality or temperature,47 or a strain-specific feature differentiating S. japonicum from China and the Philippines, as observed for cercarial shedding from snails.54 Based on the high estimated sensitivity of the DBL technique in buffaloes (78% for a single stool),37 the discordant results between the DBL technique and the highly validated qPCR in our study were striking. Because the test characteristics of the DBL technique based on traditional spike-recovery methods remain unknown, this discordance supports the assertion that the DBL technique has low sensitivity at low infection intensities in buffaloes. When compared with the qPCR assay as a referent, the DBL technique had a sensitivity of 8.8% and a specificity of 100% in the 66 buffaloes in our study sample that were analyzed by both methods.
A limitation of the current study is that the qPCR assay detects S. japonicum DNA in stool samples. It does not differentiate between viable eggs in stool samples and several other possibilities, including non-viable eggs in stool samples and/or S. japonicum DNA in stool samples that are derived from adult worms. We note that these possibilities characterize non-permissive hosts, and long-term survival of S. japonicum adult worms in naturally infected non-permissive hosts has not been described.55 Importantly, this limitation does not influence the interpretation of qPCR-based prevalence data for buffaloes, but is critical from a public health perspective because only viable eggs in stool samples could contribute to transmission.
Although mathematical modeling of epidemiologic data suggest that water buffaloes do not play a significant role in the transmission of S. japonicum in Samar, Philippines,20,21 our study demonstrates that the coprologic technique used dramatically underestimates the prevalence of S. japonicum in water buffaloes. Given that buffaloes excrete 200-fold more feces than humans (50–60 kg/day versus 250 grams/day),56 even the low infection intensities detected in the present study constitute significant environmental contamination.43 Because essentially all eggs excreted by buffalo enter the environment, only formidable genetic barriers would preclude transmission to humans. We therefore conclude that interventional studies are warranted to quantify the impact of buffalo treatment, vaccination, or removal on reducing transmission of S. japonicum to humans in the Philippines.
Acknowledgments
We thank the villagers in Macanip and the veterinary team at Leyte State University for performing the buffalo stool collection; Professor Jiao-Jiao Lin and his veterinary team at Shanghai Veterinary Research Institute for providing the uninfected buffalo stools; and Dr. Fred Lewis and Dr. Y.-S. Liang (Schistosomiasis Resource Center, National Institute of Allergy and Infectious Diseases) Professor L. Fu (Department of Pathogen Biology, Xuzhou Medical College, China) and Dr. Ana Espino (Laboratory of Molecular Parasitology and Immunology, University of Puerto Rico School of Medicine) for kindly providing experimental materials.
Footnotes
Financial support: This study was supported by National Institutes of Health (grant RO1AI48123) and the Natural Science Foundation of China (grant 30671836).
Authors' addresses: Hai-Wei Wu, Center for International Health Research, Rhode Island Hospital, Providence, RI, Department of Pediatrics, Brown University Medical School, Providence, RI, and Department of Pathogen Biology, Nanjing Medical University, Nanjing, Jiangsu, China, E-mail: haiwei_wu@brown.edu. Yung-Fang Qin, Jiangsu Provincial Center for Disease Prevention and Control, Nanjing, Jiansu, China, E-mail: fangia2004@yahoo.com.cn. Kai Chu, Rui Meng, Yun Liu, and Min-Jun Ji, Department of Pathogen Biology, Nanjing Medical University, Nanjing, Jiangsu, China, E-mails: chukai19812007@163.com, merit3413@163.com, liuyun1258@163.com, and jiminjun@njmu.edu.cn. Stephen T. McGarvey, International Health Institute, Brown University, Providence, RI, E-mail: stephen_mcgarvey@brown.edu. Remigio Olveda and Luz Acosta, Research Institute for Tropical Medicine, FILINVEST Corporate City, Alabang, Muntinlupa City, Philippines, E-mails: remi_olveda@yahoo.com.ph and lpacosta@yahoo.com. Tomas Fernandez, College of Veterinary Medicine, Visayas State University, ViSCA, Baybay, Leyte, Philippines, E-mail: tfernandez@yahoo.com. Jennifer F. Friedman, Center for International Health Research, Rhode Island Hospital, Providence, RI, and Department of Pediatrics, Brown University Medical School, Providence, RI, E-mail: jennifer_friedman@brown.edu. Jonathan D. Kurits, Center for International Health Research, Rhode Island Hospital, Providence, RI, and Department of Pathology and Laboratory Medicine, Brown University Medical School, Providence, RI, E-mail: jonathan_kuritis@brown.edu.
Reprint requests: Jonathan D. Kurtis, Department of Pathology and Laboratory Medicine, Brown University Medical School, Rhode Island Hospital, 55 Claverick Street, Providence, RI 02903, E-mail: jonathan_kurtis@brown.edu.
References
- 1.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6:411–425. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- 2.Ross AG, Sleigh AC, Li Y, Davis GM, Williams GM, Jiang Z, Feng Z, McManus DP. Schistosomiasis in the People's Republic of China: prospects and challenges for the 21st century. Clin Microbiol Rev. 2001;14:270–295. doi: 10.1128/CMR.14.2.270-295.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou XN, Guo JG, Wu XH, Jiang QW, Zheng J, Dang H, Wang XH, Xu J, Zhu HQ, Wu GL, Li YS, Xu XJ, Chen HG, Wang TP, Zhu YC, Qiu DC, Dong XQ, Zhao GM, Zhang SJ, Zhao NQ, Xia G, Wang LY, Zhang SQ, Lin DD, Chen MG, Hao Y. Epidemiology of schistosomiasis in the People's Republic of China, 2004. Emerg Infect Dis. 2007;13:1470–1476. doi: 10.3201/eid1310.061423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blas BL, Rosales MI, Lipayon IL, Yasuraoka K, Matsuda H, Hayashi M. The schistosomiasis problem in the Philippines: a review. Parasitol Int. 2004;53:127–134. doi: 10.1016/j.parint.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Wang TP, Vang Johansen M, Zhang SQ, Wang FF, Wu WD, Zhang GH, Pan XP, Ju Y, Ornbjerg N. Transmission of Schistosoma japonicum by humans and domestic animals in the Yangtze River Valley, Anhui province, China. Acta Trop. 2005;96:198–204. doi: 10.1016/j.actatropica.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 6.Guo J, Li Y, Gray D, Ning A, Hu G, Chen H, Davis GM, Sleigh AC, Feng Z, McManus DP, Williams GM. A drug-based intervention study on the importance of buffaloes for human Schistosoma japonicum infection around Poyang Lake, People's Republic of China. Am J Trop Med Hyg. 2006;74:335–341. [PubMed] [Google Scholar]
- 7.Gray DJ, Williams GM, Li Y, Chen H, Forsyth SJ, Li RS, Barnett AG, Guo J, Ross AG, Feng Z, McManus DP. A cluster-randomised intervention trial against Schistosoma japonicum in the People's Republic of China: bovine and human transmission. PLoS One. 2009;4:e5900. doi: 10.1371/journal.pone.0005900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang LD, Chen HG, Guo JG, Zeng XJ, Hong XL, Xiong JJ, Wu XH, Wang XH, Wang LY, Xia G, Hao Y, Chin DP, Zhou XN. A strategy to control transmission of Schistosoma japonicum in China. N Engl J Med. 2009;360:121–128. doi: 10.1056/NEJMoa0800135. [DOI] [PubMed] [Google Scholar]
- 9.Gray DJ, Williams GM, Li Y, McManus DP. Transmission dynamics of Schistosoma japonicum in the lakes and marshlands of China. PLoS One. 2008;3:e4058. doi: 10.1371/journal.pone.0004058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mao SP. Epidemiology and control of schistosomiasis in the People's Republic of China. Mem Inst Oswaldo Cruz. 1987;82((Suppl 4)):77–82. doi: 10.1590/s0074-02761987000800012. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto J, Kirinoki M, Kawai S, Chigusa Y, Ilagan EJ, Ducusin BE. Prevalence of schistosomiasis japonica among schoolchildren and animal reservoirs in Oiental Mndoro, Philippines. Jpn J Trop Med Hyg. 1999;27:175–180. [Google Scholar]
- 12.Fedorko JM. Schistosoma japonicum in the black rat, Rattus rattus mindanensis, from Leyte, Philippines in relation to Oncomelania snail colonies with reference to other endoparasites. Southeast Asian J Trop Med Public Health. 1999;30:343–349. [PubMed] [Google Scholar]
- 13.Fernandez TJ, Petilla T, Banez B. An epidemiological study on Schistosoma japonicum in domestic animals in Leyte, Philippines. Southeast Asian J Trop Med Public Health. 1982;13:575–579. [PubMed] [Google Scholar]
- 14.Dumag PU, Gajudo CE, Sena CY, Cardenas EC, Fementira EB. Epidemiology of animal schistosomiasis in the Philippines. Philipp J Animal Industry. 1981;36:1–23. [Google Scholar]
- 15.Kamiya H, Tada Y, Matsuda H, Tanaka H, Blas BL, Nosenas JS, Santos AT., Jr Annual fluctuation of Schistosoma japonicum infection in field rats, Rattus rattus mindanensis, in Dagami, Leyte, Philippines. Jpn J Exp Med. 1980;50:375–382. [PubMed] [Google Scholar]
- 16.Oshima S, Yasuraoka K, Irie Y, Blas BL, Nosenas JS, Santos AT., Jr Final localization of Schistosoma japonicum in the lungs of field rats, Rattus mindanensis, in Leyte, Philippines. Jpn J Exp Med. 1978;48:503–507. [PubMed] [Google Scholar]
- 17.Cabrera BD, Valeza F, Santos AT, Jr, Cruz I. Current status of schistosomiasis japonica in Sorsogon Province, Republic of the Philippines. Southeast Asian J Trop Med Public Health. 1978;9:86–92. [PubMed] [Google Scholar]
- 18.Pesigan TP, Farooq M, Hairston NG, Jauregui JJ, Garcia EG, Santos AT, Santos BC, Besa AA. Studies on Schistosoma japonicum infection in the Philippines. 1. General considerations and epidemiology. Bull World Health Organ. 1958;18:345–455. [PMC free article] [PubMed] [Google Scholar]
- 19.Willingham AL, Johansen MV, Barnes EH. A new technique for counting Schistosoma japonicum eggs in pig feces. Southeast Asian J Trop Med Public Health. 1998;29:128–130. [PubMed] [Google Scholar]
- 20.Fernandez TJ, Jr, Tarafder MR, Balolong E, Jr, Joseph L, Willingham AL, III, Belisle P, Webster JP, Olveda RM, McGarvey ST, Carabin H. Prevalence of Schistosoma japonicum infection among animals in fifty villages of Samar province, the Philippines. Vector Borne Zoonotic Dis. 2007;7:147–155. doi: 10.1089/vbz.2006.0565. [DOI] [PubMed] [Google Scholar]
- 21.Riley S, Carabin H, Belisle P, Joseph L, Tallo V, Balolong E, Willingham AL, Fernandez TJ, Gonzales RO, Olveda R, McGarvey ST. Multi-host transmission dynamics of Schistosoma japonicum in Samar Province, the Philippines. PLoS Med. 2008;5:e18. doi: 10.1371/journal.pmed.0050018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McManus DP, Williams GM, Li Y. Multi-host transmission dynamics of Schistosoma japonicum: the China perspective. PLoS Med. 2008;5:e18. doi: 10.1371/journal.pmed.0050018. Reader responses. March 31, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bergquist R, Johansen MV, Utzinger J. Diagnostic dilemmas in helminthology: what tools to use and when? Trends Parasitol. 2009;25:151–156. doi: 10.1016/j.pt.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Abath FG, Gomes AL, Melo FL, Barbosa CS, Werkhauser RP. Molecular approaches for the detection of Schistosoma mansoni: possible applications in the detection of snail infection, monitoring of transmission sites, and diagnosis of human infection. Mem Inst Oswaldo Cruz. 2006;101((Suppl 1)):145–148. doi: 10.1590/s0074-02762006000900023. [DOI] [PubMed] [Google Scholar]
- 25.Pontes LA, Dias-Neto E, Rabello A. Detection by polymerase chain reaction of Schistosoma mansoni DNA in human serum and feces. Am J Trop Med Hyg. 2002;66:157–162. doi: 10.4269/ajtmh.2002.66.157. [DOI] [PubMed] [Google Scholar]
- 26.Xia CM, Rong R, Lu ZX, Shi CJ, Xu J, Zhang HQ, Gong W, Luo W. Schistosoma japonicum: a PCR assay for the early detection and evaluation of treatment in a rabbit model. Exp Parasitol. 2009;121:175–179. doi: 10.1016/j.exppara.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 27.Gomes AL, Melo FL, Werkhauser RP, Abath FG. Development of a real time polymerase chain reaction for quantitation of Schistosoma mansoni DNA. Mem Inst Oswaldo Cruz. 2006;101((Suppl 1)):133–136. doi: 10.1590/s0074-02762006000900021. [DOI] [PubMed] [Google Scholar]
- 28.Pontes LA, Oliveira MC, Katz N, Dias-Neto E, Rabello A. Comparison of a polymerase chain reaction and the Kato-Katz technique for diagnosing infection with Schistosoma mansoni. Am J Trop Med Hyg. 2003;68:652–656. [PubMed] [Google Scholar]
- 29.Sandoval N, Siles-Lucas M, Perez-Arellano JL, Carranza C, Puente S, Lopez-Aban J, Muro A. A new PCR-based approach for the specific amplification of DNA from different Schistosoma species applicable to human urine samples. Parasitology. 2006;133:581–587. doi: 10.1017/S0031182006000898. [DOI] [PubMed] [Google Scholar]
- 30.ten Hove RJ, Verweij JJ, Vereecken K, Polman K, Dieye L, van Lieshout L. Multiplex real-time PCR for the detection and quantification of Schistosoma mansoni and S. haematobium infection in stool samples collected in northern Senegal. Trans R Soc Trop Med Hyg. 2008;102:179–185. doi: 10.1016/j.trstmh.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 31.Gobert GN, Chai M, Duke M, McManus DP. Copro-PCR based detection of Schistosoma eggs using mitochondrial DNA markers. Mol Cell Probes. 2005;19:250–254. doi: 10.1016/j.mcp.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Lier T, Simonsen GS, Haaheim H, Hjelmevoll SO, Vennervald BJ, Johansen MV. Novel real-time PCr for detection of Schistosoma japonicum in stool. Southeast Asian J Trop Med Public Health. 2006;37:257–264. [PubMed] [Google Scholar]
- 33.Lier T, Johansen MV, Hjelmevoll SO, Vennervald BJ, Simonsen GS. Real-time PCR for detection of low intensity Schistosoma japonicum infections in a pig model. Acta Trop. 2008;105:74–80. doi: 10.1016/j.actatropica.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Sandoval N, Siles-Lucas M, Lopez Aban J, Perez-Arellano JL, Garate T, Muro A. Schistosoma mansoni: a diagnostic approach to detect acute schistosomiasis infection in a murine model by PCR. Exp Parasitol. 2006;114:84–88. doi: 10.1016/j.exppara.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 35.Leenstra T, Acosta LP, Wu HW, Langdon GC, Solomon JS, Manalo DL, Su L, Jiz M, Jarilla B, Pablo AO, McGarvey ST, Olveda RM, Friedman JF, Kurtis JD. T-helper-2 cytokine responses to Sj97 predict resistance to reinfection with Schistosoma japonicum. Infect Immun. 2006;74:370–381. doi: 10.1128/IAI.74.1.370-381.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- 37.Carabin H, Balolong E, Joseph L, McGarvey ST, Johansen MV, Fernandez T, Willingham AL, Olveda R. Estimating sensitivity and specificity of a faecal examination method for Schistosoma japonicum infection in cats, dogs, water buffaloes, pigs, and rats in western Samar and Sorsogon Provinces, the Philippines. Int J Parasitol. 2005;35:1517–1524. doi: 10.1016/j.ijpara.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 38.Ministry of Health C. Manual for Schistosomiasis Control. Shanghai: Publishing House for Sciences and Technology; 2000. [Google Scholar]
- 39.Lewis F.1998Animal Models for Infectious diseases Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W, Coico R, Current Protocols in Immunology New York: John Wiley and Sons, Inc.,S49 [Google Scholar]
- 40.Bergquist R, Utzinger J, McManus DP. Trick or treat: the role of vaccines in integrated schistosomiasis control. PLoS Negl Trop Dis. 2008;2:e244. doi: 10.1371/journal.pntd.0000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Da'dara AA, Li YS, Xiong T, Zhou J, Williams GM, McManus DP, Feng Z, Yu XL, Gray DJ, Harn DA. DNA-based vaccines protect against zoonotic schistosomiasis in water buffalo. Vaccine. 2008;26:3617–3625. doi: 10.1016/j.vaccine.2008.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McManus DP. Prospects for development of a transmission blocking vaccine against Schistosoma japonicum. Parasite Immunol. 2005;27:297–308. doi: 10.1111/j.1365-3024.2005.00784.x. [DOI] [PubMed] [Google Scholar]
- 43.Gray DJ, Williams GM, Li Y, Chen H, Li RS, Forsyth SJ, Barnett AG, Guo J, Feng Z, McManus DP. A cluster-randomized bovine intervention trial against Schistosoma japonicum in the People's Republic of China: design and baseline results. Am J Trop Med Hyg. 2007;77:866–874. [PMC free article] [PubMed] [Google Scholar]
- 44.Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 45.Doenhoff MJ, Chiodini PL, Hamilton JV. Specific and sensitive diagnosis of schistosome infection: can it be done with antibodies? Trends Parasitol. 2004;20:35–39. doi: 10.1016/j.pt.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 46.Lin DD, Liu JX, Liu YM, Hu F, Zhang YY, Xu JM, Li JY, Ji MJ, Bergquist R, Wu GL, Wu HW. Routine Kato-Katz technique underestimates the prevalence of Schistosoma japonicum: a case study in an endemic area of the People's Republic of China. Parasitol Int. 2008;57:281–286. doi: 10.1016/j.parint.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 47.Yu JM, de Vlas SJ, Jiang QW, Gryseels B. Comparison of the Kato-Katz technique, hatching test and indirect hemagglutination assay (IHA) for the diagnosis of Schistosoma japonicum infection in China. Parasitol Int. 2007;56:45–49. doi: 10.1016/j.parint.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 48.Cort WW. Notes on the eggs and miracidia of the human schistosomes. Univ Calif Publ Zool. 1919;18:509–519. [Google Scholar]
- 49.Ford JW, Blankespoor HD. Scanning electron microscopy of the eggs of three human schistosomes. Int J Parasitol. 1979;9:141–145. doi: 10.1016/0020-7519(79)90105-x. [DOI] [PubMed] [Google Scholar]
- 50.Genser B, Truschnig-Wilders M, Stunzner D, Landini MP, Halwachs-Baumann G. Evaluation of five commercial enzyme immunoassays for the detection of human cytomegalovirus-specific IgM antibodies in the absence of a commercially available gold standard. Clin Chem Lab Med. 2001;39:62–70. doi: 10.1515/CCLM.2001.014. [DOI] [PubMed] [Google Scholar]
- 51.Joseph L, Gyorkos TW, Coupal L. Bayesian estimation of disease prevalence and the parameters of diagnostic tests in the absence of a gold standard. Am J Epidemiol. 1995;141:263–272. doi: 10.1093/oxfordjournals.aje.a117428. [DOI] [PubMed] [Google Scholar]
- 52.Andersen S. Re: “Bayesian estimation of disease prevalence and the parameters of diagnostic tests in the absence of a gold standard. Am J Epidemiol. 1997;145:290–291. doi: 10.1093/oxfordjournals.aje.a009102. [DOI] [PubMed] [Google Scholar]
- 53.Joseph L. Re: “Bayesian estimation of disease prevalence and the parameters of diagnostic tests in the absence of a gold standard”—The First Author Replies. Am J Epidemiol. 1997;145:291. doi: 10.1093/oxfordjournals.aje.a117428. [DOI] [PubMed] [Google Scholar]
- 54.Hope M, Duke M, McManus DP. A biological and immunological comparison of Chinese and Philippine Schistosoma japonicum. Int J Parasitol. 1996;26:325–332. doi: 10.1016/0020-7519(95)00133-6. [DOI] [PubMed] [Google Scholar]
- 55.He YX, Salafsky B, Ramaswamy K. Host–parasite relationships of Schistosoma japonicum in mammalian hosts. Trends Parasitol. 2001;17:320–324. doi: 10.1016/s1471-4922(01)01904-3. [DOI] [PubMed] [Google Scholar]
- 56.Guo JG, Ross AG, Lin DD, Williams GM, Chen HG, Li Y, Davis GM, Feng Z, McManus DP, Sleigh AC. A baseline study on the importance of bovines for human Schistosoma japonicum infection around Poyang Lake, China. Am J Trop Med Hyg. 2001;65:272–278. doi: 10.4269/ajtmh.2001.65.272. [DOI] [PubMed] [Google Scholar]


