Abstract
Secondary or reactive hemophagocytic syndrome (HPS) is frequently related to viral infections. However, the novel swine-origin influenza A (H1N1) virus associated HPS has never been reported. On October 10, 2009, a 17-year-old female child with no past medical history, complaining of severe asthenia, pneumonia, myalgia, and high fever, was admitted to our department, and H1N1 DNA was detected. Five days after her hospitalization, all signs and symptoms aggravated into HPS. After treatment for H1N1 influenza, the patient had a recovery and clearance of H1N1 infection 10 days after hospitalization. Three weeks later, the patient was discharged without any complaints, indicating the etiological role of H1N1infection in HPS.
Introduction
Hemophagocytic syndrome (HPS) is a clinical condition characterized by infiltration of the bone marrow and reticuloendothelial system by macrophages and activated histiocytes, leading to uncontrolled phagocytosis of platelets, erythrocytes, lymphocytes, and precursor cells. This syndrome is classified as primary or acquired, the latter being more frequent.1 The acquired type is associated with several etiologies, including viral, bacterial, fungal, and protozoa infections, malignancies (lymphomas), and other conditions like rheumatic diseases. Among these viral infections, the most likely to be associated with hemophagocytic are Epstein–Barr virus (EBV), cytomegalovirus (CMV), human herpesvirus type 6 (HHV-6), and human herpesvirus type 8 (HHV-8).2 However, the novel swine-origin influenza A (H1N1) virus with hemophagocytic has never been reported.
Materials and Methods
Informed consent was obtained from all human adult participants and from parents or legal guardians of minors with the name of the appropriate institutional review board that approved the project. We report a case of HPS associated with the new H1N1 virus infection. A 17-year-old female child with no past medical history complained of severe asthenia, pneumonia, myalgia, and high fever and was hospitalized for the first time on October 10, 2009. During 21 days, her H1N1 DNA was monitored.
Results
The patient's severe asthenia, pneumonia, myalgia, and high-fever symptoms developed 10 days before her hospitalization. After admission in our hospital, chest contrast-enhanced computerized tomography (CT) scan revealed interstitial pneumonia. She was given an empiric treatment of pneumonia based on tienam in 10 days. Initial and further laboratory data are described in Table 1.
Table 1.
Laboratory findings in a patient with HPS and H1N1
| Laboratory finding | Oct. 10 | Oct. 20 | Normal range |
|---|---|---|---|
| Hb (g/L) | 78 | 97 | 131–172 |
| Leukocyte (cells/L) | 0.5 × 109 | 0.9 | 3.97–9.15 |
| Platelet (×103/L) | 28 × 109 | 21 | 85–303 |
| DB (μmol/L) | 47 | 19 | 3.0–17.0 |
| AST (U/L) | 52 | 23 | 0–33 |
| ALT (U/L) | 23 | 227 | 0–27 |
| Fe (ng/mL) | 1,500 | 20–200 | |
| Reticulocyte (%) | 0.87 | 0.85 | 0.5–1.5 |
| TG (mg/dL) | 2.35 | 2.23 | 0.4–1.5 |
| CRP (mg/L) | 214.4 | 0–10 | |
| LDH (U/L) | 1,657 | 760 | 230–460 |
Hb = hemoglobin; DB = direct bilirubin; IDB = indirect bilirubin; AST = aspartate aminotransferase; ALT = alanine aminotransferase; Fe = ferritin; TG = triglycerides; CRP = C-reactive protein; LDH = lactate dehidrogenase.
Ten days later, her signs and symptoms aggravated with severe jaundice and hepatosplenomegaly. Serology testing was negative for toxoplasmosis, CMV, rubella, hepatitis C virus (HCV), EBV, human immunodeficiency virus (HIV), human t-cell leukemia virus (HTLV) I/II, and autoimmune antibodies. H1N1 influenza was diagnosed after positive-DNA H1N1 virus detection. The antibiotic treatment was stopped. Oseltamivir was administered for H1N1 infection until laboratory tests were negative for H1N1 virus. After admission for 10 days, the patient still had fever, and two units of blood sample were collected because of persistent, poorly tolerated severe anemia (Hb = 79 g/L). HPS was diagnosed after bone-marrow aspiration confirmed the hemophagocytosis. A corticosteroid treatment with prednisone (1 mg/kg/day) was implemented during 10 days. The H1N1 infection vanished because of the response to oseltamivir. Her respiratory function recovered slowly through continuous non-invasive ventilation and oxygen therapy with face mask. After corticosteroid administration, all the symptoms such as fever, jaundice, pneumonia, and asthenia improved, and the biochemical parameters progressively turned to normal (Table 1). Three weeks later, the patient was discharged without any complaints.
Discussion
The sudden emergence and rapid spread of oseltamivir-resistant H1N1 viruses with neuraminidase (NA) gene H274Y amino acid substitution has been the hallmark of global seasonal influenza since January 2008.3–5 Viruses carrying this mutation are widely presumed to exhibit attenuated pathogenicity,6 compromised transmission,7 and reduced lethality.8 The case described herein has complied with all diagnostic criteria and is the first case of H1N1 virus-associated HPS to be reported.
The HPS was first described in 1979 in immunosuppressed patients with viral infections.9 It is characterized by fevers, lymphadenopathy, hepatosplenomegaly, maculopapular rash, cytopenias, and hyperferritinemia caused by dysregulated activation and proliferation of macrophages, leading to uncontrolled phagocytosis of platelets, erythrocytes, lymphocytes, and their hematopoietic precursors throughout the reticuloendothelial system. Familial HPS seems to have a genetic etiology, whereas acquired HPS may be associated with malignancy, autoimmune disease, or infection.
The pathophysiological mechanism for HPS remains poorly understood. In patients with HPS, the expression of major histocompatibility complex MHC-I and MHC-II molecules increased, suggesting that splenic macrophages were activated.10
The prognosis of the HPS relies mainly on the severity and duration of the cytopenias. Long-term neutropenia increases the risk of severe infections from gram-positive bacteria like Staphylococcus aureus. Thrombocytopenia should be monitored because of the possibility of spontaneous bleeding, which can occur in the central nervous system (CNS) as a fatal outcome. Anemia is a risk factor for infections and can induce organ dysfunctions, such as heart failure and tissue hypoxia. In addition to cytopenias, some cases of HPS developed disseminated intravascular coagulation and fatal organic dysfunction because of severe sepsis, which contributed to the mortality of more than 40% in the largest series.11
The treatment of HPS is not well-defined because of lack of controlled studies.12 Some supportive therapies corrected the severe cytopenias and treated the causal infection.
The effective use of prednisolone or plasma exchange against HPS has been reported;13–16 however, whether or not these treatments were effective against HPS in our patient is still unknown.17
H1N1 influenza is one of many infections that can cause a secondary hemophagocytic syndrome, and it is possible that the patient carried falciparum H1N1 infection with continuing anemia after successful anti-virus therapy.
We report a case of H1N1 virus-associated HPS, which is an uncommon event described in critical patients and thus, might be underdiagnosed. Treatment of the underlying infection can improve clinical outcomes, and therefore, early diagnosis and treatment of H1N1 virus-associated HPS are essential in clinical practice (Figure 1).
Figure 1.
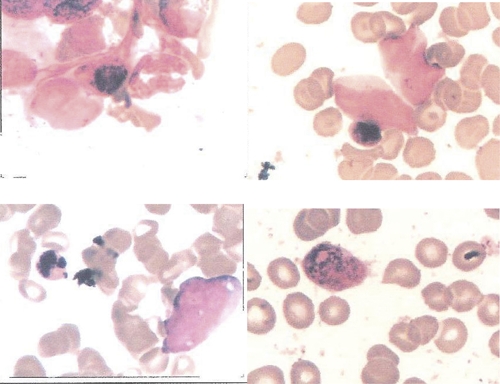
Bone-marrow smear results. Blank arrow, macrophages with hemophagocytosis (HE staining ×400). This figure appears in color at www.ajtmh.org.
Acknowledgments
The authors thank Profs. Huang-Mao, Zhang-Jinsong, Xue, and Cao-Quan for their kindly clinical assistance. The authors thank Prof. Zhang-Jianfu for his laboratorial assistance.
Footnotes
Author's address: Wei Zhao, ICU of Second Affiliated Hospital of Southeast University, Nanjing, 210003 China, E-mail: Doctor0219@163.com.
References
- 1.Fisman DN. Hemophagocytic syndromes and infection. Emerg Infect Dis. 2000;6:601–608. doi: 10.3201/eid0606.000608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neofytou E, Sourvinos G, Asmarianaki M, Spandidos DA, Makrigiannakis A. Prevalence of human herpes virus types 1–7 in the semen of men attending an infertility clinic and correlation with semen parameters. Fertil Steril. 2008;85:420–421. doi: 10.1016/j.fertnstert.2008.03.074. [DOI] [PubMed] [Google Scholar]
- 3.Lackenby A, Hungnes O, Dudman SG, Meijer A, Paget WJ, Hay AJ, Zambon MC. Emergence of resistance to oseltamivir among influenza A(H1N1) viruses in Europe. Euro Surveill. 2008;13:pii8026. doi: 10.2807/ese.13.05.08026-en. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization 2008Epidemic and Pandemic Alert Response: Influenza A (H1N1) Virus Resistance to Oseltamivir Avaliable at: http://www.who.int/csr/disease/influenza/h1n1_table/en/index.html Accessed February 10, 2009
- 5.Centers for Disease Control and Prevention 2008CDC Issues Interim Recommendations for the Use of Influenza Antiviral Medications in the Setting of Oseltamivir Resistance Among Circulating Influenza A (H1N1) Viruses, 2008–09 Influenza Season Available at: http://www2a.cdc.gov/HAN/ArchiveSys/ViewMsgV.asp?AlertNum=00279 Accessed February 10, 2009
- 6.Ives JA, Carr JA, Mendel DB, Tai CY, Lambkin R, Kelly L, Oxford JS, Hayden FG, Roberts NA. The H274Y mutation in the influenza A/H1N1 neuraminidase active site following oseltamivir phosphate treatment leave virus severely compromised both in vitro and in vivo. Antiviral Res. 2002;55:307–317. doi: 10.1016/s0166-3542(02)00053-0. [DOI] [PubMed] [Google Scholar]
- 7.Herlocher ML, Truscon R, Elias S, Yen HL, Roberts NA, Ohmit SE, Monto AS. Influenza viruses resistant to the antiviral drug oseltamivir: transmission studies in ferrets. J Infect Dis. 2004;190:1627–1630. doi: 10.1086/424572. [DOI] [PubMed] [Google Scholar]
- 8.Yen HL, Ilyushina NA, Salomon R, Hoffmann E, Webster RG, Govorkova EA. Neuraminidase inhibitorresistant recombinant A/Vietnam/1203/04 (H5N1) influenza viruses retain their replication efficiency and pathogenicity in vitro and in vivo. J Virol. 2007;81:12418–12426. doi: 10.1128/JVI.01067-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Risdall RJ, McKenna RW, Nesbit ME, Nesbit ME, Krivit W, Balfour HH, Jr, Simmons RL, Brunning RD. Virus-associated hemophagocytic syndrome: a benign histiocytic proliferation distinct from malignant histiocytosis. Cancer. 1979;44:993–1002. doi: 10.1002/1097-0142(197909)44:3<993::aid-cncr2820440329>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 10.Kereveur A, McIlroy D, Samri A, Oksenhendler E, Clauvel JP, Autran B. Up-regulation of adhesion and MHC molecules onsplenic monocyte/macrophages in adult haemophagocytic syndrome. Br J Haematol. 1999;104:871–877. doi: 10.1046/j.1365-2141.1999.01247.x. [DOI] [PubMed] [Google Scholar]
- 11.Dellinger RP. Inflammation and coagulation: implications for the septic patient. Clin Infect Dis. 2003;36:1259–1265. doi: 10.1086/374835. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe M, Shibuya A, Okuno J, Maeda T, Tamama S, Saigenji K. Hepatitis A virus infection associated with hemophagocytic syndrome: report of two cases. Intern Med. 2002;41:1188–1192. doi: 10.2169/internalmedicine.41.1188. [DOI] [PubMed] [Google Scholar]
- 13.Koizumi K, Yokoyama K, Nishio M, Shibata S, Ozutumi K, Yamaguchi M, Sato N, Yasukouchi T, Sawada K, Koike T. A case with virus-associated hemophagocytic syndrome (VAHS) complicated by rhabdomyolysis which were associated with herpes-simplex virus infection. Jpn J Clin Hematol. 1996;37:40–45. [PubMed] [Google Scholar]
- 14.Fujiki R, Shiraishi K, Noda K, Ohshita Y, Fukahori S, Johjima H, Tanaka K, Rikimaru T, Aizawa H. A case of hemophagocytic syndrome associated with military tuberculosis. Kekkaku. 2003;78:443–448. [PubMed] [Google Scholar]
- 15.Goto S, Aoike I, Shibasaki Y, Morita T, Miyazaki S, Shimizu T, Suzuki M. A successfully treated case of disseminated tuberculosis-associated hemophagocytic syndrome and multiple organ dysfunction syndrome. Am J Kidney Dis. 2001;38:E19. doi: 10.1053/ajkd.2001.27727. [DOI] [PubMed] [Google Scholar]
- 16.Sanada S, Ookawara S, Shindo T, Morino K, Ishikawa H, Suzuki M. A case report of the effect of plasma exchange on reactive hemophagocytic syndrome associated with toxic shock syndrome. Therap Aphre Dialy. 2004;8:503–506. doi: 10.1111/j.1774-9987.2004.00188.x. [DOI] [PubMed] [Google Scholar]
- 17.Aouba A, Noguera ME, Clauvel JP, Quint L. Hemophagocytic syndrome associated with Plasmodium vivax infection. Br J Haematol. 2000;103:832–833. doi: 10.1046/j.1365-2141.2000.01968.x. [DOI] [PubMed] [Google Scholar]


