Abstract
The objective of this study was to identify the factors that determine serum homocysteine concentrations in Korean population. In a community-based study, 871 participants completed detailed questionnaires and physical examination. We found that increased age, male sex, family history of stroke, deficiencies of serum folate and vitamin B12, and elevated serum creatinine significantly increased the risk of hyperhomocysteinemia. However, hormonal and behavioral factors (smoking, alcohol drinking, coffee consumption, and sedentary time) were not associated with the risk of hyperhomocysteinemia. The risk of hyperhomocysteinemia was steeply increased in subjects with two or more risk factors among four selected risk factors (deficiencies of serum folate and vitamin B12, elevated creatinine, and family history of stroke) compared to subjects who did not have any risk factors, especially subjects over the age of 65 yr (odds ratio [OR], 33.5; 95% confidence interval [CI], 3.71-302.0 in men; OR, 39.2; 95% CI, 7.95-193.2 in women). In conclusion, increased age, male sex, family history of stroke, deficiencies of serum folate and vitamin B12, and elevated serum creatinine are important determinants of serum homocysteine concentrations with interaction effects between these factors.
Keywords: Homocysteine, Hyperhomocysteinemia, Folic Acid, Vitamin B12, Creatinine, Stroke, Behavior, Hormones, Korea
INTRODUCTION
Death rates from coronary heart disease (CHD) have declined substantially in the United States and Western countries in recent decades (1). In contrast, the Asia-Pacific region accounts for about half of the global burden of cardiovascular disease (CVD) (2). Moreover, recent mortality from ischemic heart disease has increased 2.4-fold since 1990 in Korea (3). The decline of mortality from CHD in Western countries is attributable to changes in risk factors and medical therapies based on a variety of evidence (1). Conversely, the increase in the death rate from CHD in Asia may be due to a gradual increased exposure to Westernized lifestyles.
Several clinical trials have reported no association between homocysteine-lowering therapy and reduction of CVD events (4, 5). However, these trials lacked sufficient statistical power to confirm the association between serum homocysteine levels and CVD (6). Hyperhomocysteinemia is widely recognized as a major independent risk factor for CVD, comparable to the effect of smoking or hyperlipidemia (7). Moreover, hyperhomocysteinemia is an easily modifiable risk factor for CVD (8). Therefore, a study identifying factors that effectively lower the serum homocysteine levels in an Asian sample with an increased risk for CHD is warranted. In particular, the factors that determine serum homocysteine levels, including lifestyle factors, need to be ascertained.
The results of previous work have suggested that serum concentrations of homocysteine may be influenced by the following factors: demographic factors (increased age, male sex, and ethnic origin); genetic variations (the methylenetetrahydrofolate reductase and cystathionine β-synthase gene); physiological and clinical factors (reduced renal function, increasing muscle mass, and deficiencies in folate, vitamin B12, and vitamin B6); disease states (diabetes mellitus, hypothyroidism, nephropathy or end-stage renal disease, and obesity); hormonal factors (menopause, thyroid deficiency, and some oral contraceptive use); lifestyle factors (smoking, excessive alcohol consumption, low intake of folate and B-vitamins, excessive coffee consumption, and lack of physical activity); and several drugs (including folate-, vitamin B12- and vitamin B6-antagonists) (8, 9).
Several studies have attempted to determine the factors involved in regulating homocysteine in Asia; however, most have been based on medical patients or pregnant women. A few population-based studies have been conducted, but had small population samples (10, 11) or were restricted to associations with physiological factors, such as serum folate and vitamin B12 concentrations (12). To date, no comprehensive studies on the various factors that determine the serum concentrations of homocysteine have been carried out in Korea. Therefore, we assessed the acquired (i.e., physiological and clinical factors, disease state, and hormonal factors) and behavioral determinants of homocysteine concentration and interaction effects between these factors through a comprehensive survey of a community-based Korean population.
MATERIALS AND METHODS
Subjects
This study, which is part of a population-based cohort study on CVD, was conducted in a rural area (Yangpyeong County) of Korea. All adults who were greater than 20 yr old in the targeted villages were invited to the Health Examination Service in cooperation with the Yangpyeong Public Health Center. Among a total of 907 adults who participated in this comprehensive examination survey from February 2001 to February 2002, serum samples from 889 subjects were available for the analysis of serum total homocysteine (tHcy), folate, and vitamin B12. A total of 18 participants were excluded from the final analysis due to the absence of clinical data (three people) and incomplete questionnaires (15 people). Therefore, 871 subjects were included in the current study. The mean age of 364 men was 55.8±13.3 yr (range 19-88) and of 507 women was 54.9±13.5 yr (range 19-87). The study protocol was approved by the Institutional Review Board of Hanyang University Hospital (2002-2).
Data collection
All screenings and interviews were performed by trained staff in the Yangpyeong Community Health Center of Hanyang University. A detailed description of this study's methodology has been published in our previous study aimed at establishing a reference value for serum tHcy using the same sample (13).
Questionnaire
Face-to-face interviews were conducted by trained interviewers. The questionnaire was composed of demographics (age, sex, marital status, occupation, education, and income), family history, medical history, reproductive history (menopause, oral contraceptive use), lifetime tobacco usage, alcohol consumption, physical activity, and coffee consumption (consumption frequency per month, week, or day).
Physical examinations
After changing into supplied clothing, each participant's height and weight were measured according to the study protocol. Body mass index (BMI) was calculated as the ratio of weight to height squared (kg/m2).
Blood sample collection and biochemical analysis
Venous blood samples were obtained after at least an eight-hour fast. Within two hours after blood collection, one 10-mL plain tube was centrifuged at 2,500-3,000 rpm for 10 min and the serum fraction was separated into several aliquots. The serum was put in ice and transported to the laboratory. Serum creatinine was assayed using a Hitachi 747 analyzer (Hitachi High-Technologies Corporation, Tokyo, Japan) on the same day it was drawn. The remnant blood samples were then stored at -70℃, and then the serum concentrations of tHcy, folate, and vitamin B12 were determined using a microparticle immunoassay (MPIA) on an Axsym machine (Abbott Diagnostics, Abbott Park, IL, USA).
Statistical analyses
In our previous study (13) aimed at establishing a reference value for serum tHcy, we used 506 out of 889 subjects as a reference population. These subjects had adequate serum levels of folate (≥11.3 nM/L), vitamin B12 (≥185 pM/L), and creatinine (≤110 µM/L for men and ≤90 µM/L for women), after excluding individuals with a medical history of angina or myocardial infarction from the study sample. The sex-specific 2.5th and 97.5th percentile values for the tHcy concentration in the reference population were then determined as reference intervals according to the guidelines of the Clinical and Laboratory Standards Institute (14). Levels that exceeded the sex-specific 97.5th percentile values (15.31 µM/L for men, 12.69 µM/L for women) were defined as hyperhomocysteinemia. Serum folate was classified as deficient (<6.8 nM/L), borderline (6.8-11.2 nM/L), or normal (≥11.3 nM/L) (15). Serum vitamin B12 was classified as deficient (<148 pM/L), borderline (148-184.9 pM/L), or normal (≥185 pM/L) (16). Elevated levels of serum creatinine were defined as >110 µM/L for men and >90 µM/L for women (17). The subjects were divided into three categories on the basis of BMI: ≤normal weight (<23 kg/m2), overweight (23-24.9 kg/m2), or obese (≥25 kg/m2) according to the re-defined WHO criterion for obesity in the Asia Pacific region (18).
To improve the normality of the skewed serum tHcy, folate, vitamin B12, and creatinine concentration distributions, these values were logarithmically transformed and are presented as geometric means with 95% confidence intervals. Significant differences between men and women were compared using a t-test for continuous variables and a chi-square-test for categorical variables. Significant mean differences in serum tHcy, folate, and vitamin B12 concentrations among the four age groups (<45, 45-54, 55-64, ≥65 yr) were compared using ANOVA and the P values for trends were calculated by linear regression. P values less than 0.05 were considered statistically significant. Multivariate logistic regression was used to calculate the odds ratios (OR) and the corresponding 95% confidence intervals (CI) adjusted for the factors associated with hyperhomocysteinemia. Previous studies have reported that smokers (19) and alcoholics (20) have lower blood and tissue concentrations of folate and vitamin B12. These findings suggest the possibility that the effects of smoking and alcohol consumption on tHcy levels may be mediated in part through folate. For this reason, we excluded folate and vitamin B12 in the statistical model for examining the effect of smoking and drinking. Analyses were conducted using SPSS 12.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
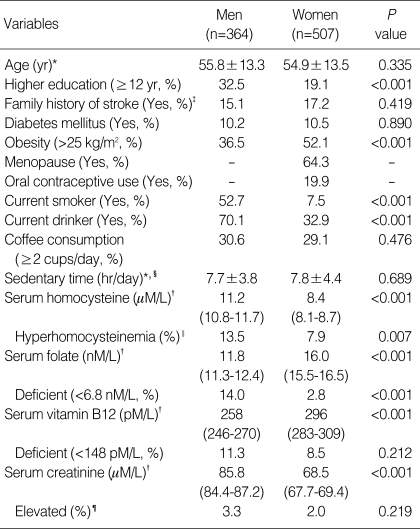
The distributions of the general characteristics and variables that were expected to contribute to serum homocysteine concentration are shown in Table 1. The mean tHcy concentration was higher in men compared to women (11.2 µM/L for men, 8.4 µM/L for women, P<0.001). Also, the prevalence of hyperhomocysteinemia among men (13.5%) was significantly higher than that of women (7.9%). The rates of higher education and current smoking and alcohol drinking as well as the mean concentration of serum creatinine were significantly higher in men compared to women, while the prevalence of obesity (>25 kg/m2) and the mean concentrations of serum folate and vitamin B12 were significantly higher in women compared to men. Only 14% of the men and 2.8% of the women were classified as folate deficient on the basis of a 6.8 nM/L cutoff point.
Table 1.
Distribution of the general characteristics and serum concentration of homocysteine, folate, vitamin B12, and creatinine in the study subjects
*arithmetic mean±SD; †geometric mean (95% confidence interval); ‡Only first-degree relatives were included, §sum of the time spent sedentary while eating at a table, watching TV, driving the car and taking the bus, sitting indoors (reading a book, knitting, or talking, etc.), and working in a seated position; ∥cut-off points of hyperhomocysteinemia >15.31 µM/L for men and >12.69 µM/L for women; ¶>110 µM/L for men and >90 µM/L for women; P values for differences between men and women were calculated by a t-test for continuous variables and by a χ2-test for categorical variables.
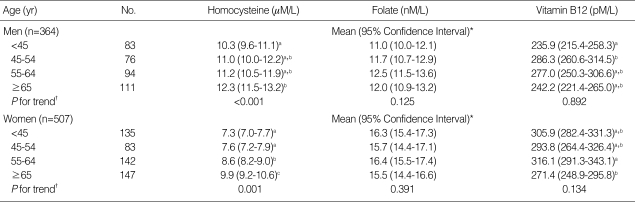
Table 2 shows the means and 95% CI for the serum concentrations of tHcy, folate, and vitamin B12 according to four age groups (<45, 45-54, 55-64, ≥65 yr). The mean tHcy concentration increased significantly with age in both men (P for trend <0.001; 10.3 µM/L for men <45 yr vs. 12.3 µM/L for men ≥65 yr) and women (P for trend= 0.001; 7.3 µmM/L for women <45 yr vs. 9.9 µM/L for women ≥65 yr). However, the serum concentrations of folate and vitamin B12 were not associated with increased age.
Table 2.
Serum concentrations of homocysteine, folate, and vitamin B12 by sex and age
Different letters indicates significant differences according to ANOVA (P<0.05); *geometric mean (95% confidence interval); †P for trends were calculated by linear regression.
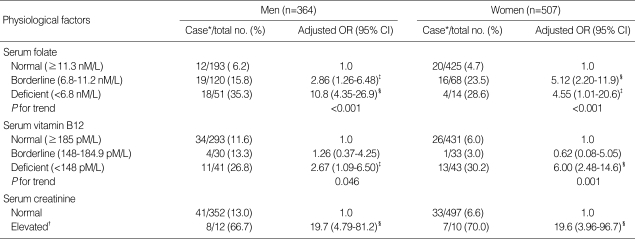
The associations between hyperhomocysteinemia and serum folate, vitamin B12, or creatinine levels are described in Table 3. Both folate deficiency (OR, 10.8; 95% CI, 4.35-26.9 in men; OR, 4.55; 95% CI, 1.01-20.6 in women) and vitamin B12 deficiency (OR, 2.67; 95% CI, 1.09-6.50 in men; OR, 6.00; 95% CI, 2.48-14.6 in women) were significantly associated with hyperhomocysteinemia. The prevalence of hyperhomocysteinemia was highly increased with the degree of folate deficiency (6.2% for men and 4.7% for women in the normal group vs. 35.3% for men and 28.6% for women in the deficient group) and vitamin B12 deficiency (11.6% for men and 6.0% for women in the normal group vs. 26.8% for men and 30.2% for women in the deficient group). Elevated serum creatinine was also highly associated with the risk of hyperhomocysteinemia (OR, 19.7; 95% CI, 4.79-81.2 in men; OR, 19.6; 95% CI, 3.96-96.7 in women).
Table 3.
Risk of hyperhomocysteinemia in relation to serum levels of folate, vitamin B12, and creatinine by sex
OR, odds ratio adjusted for age and serum concentrations of folate, vitamin B12, and creatinine; *cut-off points of hyperhomocysteinemia were >15.31 µM/L for men and >12.69 µM/L for women; †>110 µM/L for men and >90 µM/L for women; ‡P<0.05; §P<0.01.
As shown in Table 4, the risk of hyperhomocysteinemia was significantly increased when both serum folate and vitamin B12 were deficient (OR, 31.7; 95% CI, 7.20-139.8). In addition, the interaction between folate and vitamin B12 concentrations was significant (P<0.001).
Table 4.
Interaction effects of serum folate and vitamin B6 levels on hyperhomocysteinemia
OR, odds ratio adjusted for sex, age, and serum creatinine levels; *P<0.01; †interaction between folate and vitamin B12 was statistically significant (P<0.001).
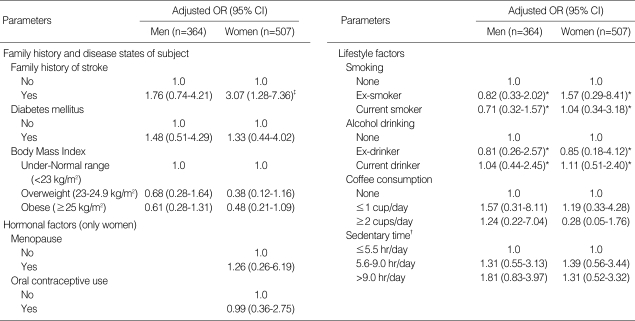
Table 5 shows the associations between hyperhomocysteinemia and family history, disease status, or hormonal and lifestyle factors. Only a family history of stroke was significantly associated with the risk of hyperhomocysteinemia in women (OR, 3.49; 95% CI, 1.34-9.06). Diabetes mellitus, BMI, hormonal factors (menopause and oral contraceptive use), and behavioral factors (smoking, alcohol drinking, coffee consumption, and sedentary time) were not associated with the risk of hyperhomocysteinemia.
Table 5.
Risk of hyperhomocysteinemia in relation to family history, disease state, and hormonal and lifestyle factors by sex
OR, odds ratio adjusted for age, serum concentrations of folate, vitamin B12, and creatinine; *adjusted for only age and serum creatinine; †sum of the time spent sedentary while eating at a table, watching TV, driving the car and taking the bus, sitting indoors (reading a book, knitting, or talking, etc.), and working in a seated position, which was classified into tertile groups; ‡P<0.05.
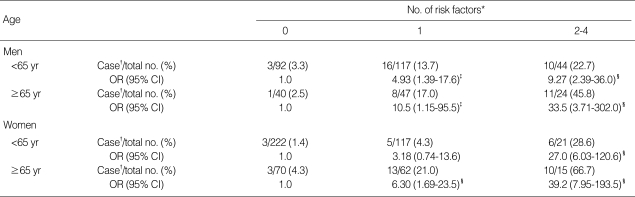
Table 6 shows the risks of hyperhomocysteinemia according to the total number of the four selected risk factors (serum folate and vitamin B12 deficiency, elevated creatinine, and family history of stroke) in each sex and age group. In contrast to subjects without any risk factors, the risk of hyperhomocysteinemia was steeply increased in subjects with two or more risk factors. This was especially true in both men and women over the age of 65 (OR, 33.5; 95% CI, 3.71-302.0 in men; OR, 39.2; 95% CI, 7.95-193.2 in women).
Table 6.
Risk of hyperhomocysteinemia in relation to the number of the four selected risk factors by sex and age
*Folate deficiency (<6.8 nM/L), vitamin B12 deficiency (<148 pM/L), elevated creatinine (>110 µM/L for men and >90 µM/L for women), and family history of stroke; †cut-off points of hyperhomocysteinemia were >15.31 µM/L for men and >12.69 µM/L for women; OR, odds ratio adjusted for age within two age groups (<65 yr and ≥65 yr); ‡P<0.05; §P<0.01.
DISCUSSION
The mean serum concentrations of tHcy in our study population (11.2 µM/L in men, 8.4 µM/L in women) were lower than those of healthy adults in Japan (21) and north China (12). However, the mean concentrations from our study population were higher than those of healthy adults in the US (22) and were similar to the Singapore Chinese (23) and a rural population of Costa Rica (24). In addition, the serum concentration of tHcy in our subjects were similar to that of 195 healthy Koreans selected in a hospital setting (10), but lower than that of 204 healthy Korean visitors to a medical center (11). These differences in tHcy concentrations can be attributed to variations in the blood concentrations of folate and vitamin B12 in the population of each country. Folate deficiency was not common in our study sample; only 14% of men and 2.8% of women were classified as folate deficient (<6.8 nM/L), compared to 37% in the north Chinese population (12) and 31% of urban women and 40% of rural women in Costa Rica (24) using the same criteria. This lower prevalence of serum folate deficiency seems to be due to higher consumption of vegetables, including kimchi, in Koreans. Kimchi is one of the main sources of folate in Korean foods (25). Additionally, the serum folate deficiency rate in our rural population was lower than that of urban Koreans (17.7% in men and 7.7% in women), who were also found to have a higher mean tHcy concentration than that of our subjects (11). Based on the 2005 Korea National Health and Nutrition Survey (KNHANES), this finding can be explained by the greater consumption of vegetables and kimchi in the rural population compared to the urban population (25). In our study, serum deficiency of folate or vitamin B12 was significantly associated with the risk of hyperhomocysteinemia. Furthermore, the risk of hyperhomocysteinemia was largely increased in subjects with deficient concentrations of folate and vitamin B12 compared with persons who have adequate concentrations of both vitamins (OR, 31.7; 95% CI, 7.20-139.8). The interaction between folate and vitamin B12 concentrations was significant. These results can be explained by homocysteine metabolism, in which the remethylation process of homocysteine is dependent on a vitamin B12-denpendent enzyme and folate in the form of 5-methyltetrahydrofolate (8).
In our study, serum tHcy concentrations increased significantly with age in both men and women, whereas serum concentrations of folate and vitamin B12 did not decrease with age. This indicates that the serum tHcy levels in the older age group may be influenced by other factors related to homocysteine metabolism rather than vitamin levels, such as the decreased activity of several related enzymes (26), physiologic decline in renal function (27), and hormonal changes (28) in the elderly. In our study, the risk of hyperhomocysteinemia was more dramatically increased in women over 65 yr of age than in men of the same age group (OR, 6.03; 95% CI, 2.02-19.7 in women over 65 yr; OR, 2.74; 95% CI, 1.04-7.22 in men over 65 yr), but the age effect in women disappeared after controlling for menopause (data not shown). Mechanisms of increased tHcy levels after menopause are largely unknown. Previous work suggests that higher postmenopausal tHcy levels may be related to the lower methionine transamination or estrogen deficiency (28).
With regard to the factor of sex, the mean concentration of tHcy in men was significantly higher than the concentrations measured in women. This can be partly explained by the significantly lower serum folate and vitamin B12 concentrations in men compared to women. However, the mean difference in tHcy concentrations between men and women still remained significant after controlling for serum folate and vitamin B12 levels (data not shown). This residual sex difference may be explained by tHcy formation in connection with the creatine/creatinine synthesis that is proportional to muscle mass (8). It remains to be established whether this difference is caused by the smoking, alcohol consumption, and dietary habits of men. However, our study could not find any associations between these habits and hyperhomocysteinemia in either sex.
In our study, none of the behavioral factors (smoking, alcohol consumption, coffee consumption, and physical inactivity) were associated with the risk of hyperhomocysteinemia. Relationships between cigarette smoking and plasma homocysteine concentrations are controversial (12, 21, 23, 29). In our study, the mean serum folate concentrations of current smokers were significantly lower than those of non-smokers and ex-smokers. Furthermore, subjects with lower folate concentrations showed considerably higher mean tHcy concentrations. Considering this, we expected that current smokers would have an increased risk of hyperhomocysteinemia. Contrary to our expectations, however, smoking status was not associated with the risk of hyperhomocysteinemia. Instead, the prevalence of hyperhomocysteinemia was lower in male smokers. This may be explained by the specific characteristics of smokers, such as age. The mean age (52.7±13.4 yr) of male smokers was significantly lower than the mean age (59.2±12.2 yr) of male non- and ex-smokers. However, the association between smoking and hyperhomocysteinemia before and after controlling for age was similar. There is a possibility that a residual confounding effect of other factors related to age could still be present. In addition, this unexpected result related to smoking may be due to reverse causation in that subjects with diabetes mellitus or obesity, factors considered to increase risk of hyperhomocysteinemia, may have changed their behavior to improve their health. Although our result was not significant, cigarette smoking may be indirectly associated with tHcy concentrations via folate; a Chinese study showed that the positive effect of smoking on tHcy concentrations disappeared after adjusting for plasma folate concentrations (23). Likewise, in other studies (10, 12, 21, 23), we have not found any association between alcohol consumption and tHcy concentrations. Besides smoking and alcohol consumption, little information has been collected on coffee consumption and physical inactivity as determinants of homocysteine concentrations in the general Asian population (10, 23). Although coffee consumption (30) and physical inactivity (29) have been shown to increase the risk of hyperhomocysteinemia in some Europeans and Americans, a Chinese study (23) did not detect any association between behavioral factors (daily coffee consumption, weekly exercise, cigarette smoking, and alcohol consumption) and plasma tHcy concentrations after adjusting for plasma folate concentrations. In addition, another Korean study also reported that behavioral factors, such as cigarette smoking, alcohol consumption, and coffee consumption, did not influence plasma tHcy levels (10).
Although serum concentrations of folate and vitamin B12 appear to be strong determinants of hyperhomocysteinemia, the risk of hyperhomocysteinemia cannot be explained by a single factor; Homocysteine increased with age, regardless of the lack of associations between age and serum folate and vitamin B12 concentrations. Also, differences in tHcy concentrations between the sexes remained after controlling for serum folate and vitamin B12. In line with this, we hypothesized that the risk of hyperhomocysteinemia may be increased more by several combined factors than by any single factor. Considering this explanation, we estimated the risk of hyperhomocysteinemia according to the number of risk factors (deficiencies in serum folate and vitamin B12, elevated serum creatinine, and family history of stroke). We found that the more risk factors a participant had, the higher the risk for hyperhomocysteinemia. This finding was especially pronounced in elderly men and women.
One of the limitations of this study is that we cannot clarify the causality of folate and vitamin B12 in hyperhomocysteinemia due to the cross-sectional design of the study. Also, the representativeness of our study population as well as sample size is lack. Regardless of these limitations, this study is the first study to explore the determining factors for serum tHcy concentrations in a community-based study with Korean sample. In the future, we will follow up this cohort to confirm the associations between serum tHcy concentrations and the onset or death from CHD.
In conclusion, increased age, male sex, family history of stroke, deficiencies of serum folate and vitamin B12, and elevated serum creatinine are important determinants of serum tHcy concentrations with interaction effects between these factors in a community-based Korean population.
Footnotes
This work was supported by grant number FG01-4-04 of the 21C Frontier Functional Human Genome Project from the Ministry of Education Science and Technology in Korea.
References
- 1.Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, Giles WH, Capewell S. Explaining the decrease in U.S. deaths from coronary disease, 1980-2000. N Engl J Med. 2007;356:2388–2398. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 2.Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet. 1997;349:1269–1276. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- 3.Korea National Statistical Office. Annual Report on the Cause of Death Statistics in 2006. Daejeon, Korea: Korea National Statistical Office; 2007. [Google Scholar]
- 4.Wang X, Qin X, Demirtas H, Li J, Mao G, Huo Y, Sun N, Liu L, Xu X. Efficacy of folic acid supplementation in stroke prevention: a meta-analysis. Lancet. 2007;369:1876–1882. doi: 10.1016/S0140-6736(07)60854-X. [DOI] [PubMed] [Google Scholar]
- 5.Bonaa KH, Njolstad I, Ueland PM, Schirmer H, Tverdal A, Steigen T, Wang H, Nordrehaug JE, Arnesen E, Rasmussen K. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354:1578–1588. doi: 10.1056/NEJMoa055227. [DOI] [PubMed] [Google Scholar]
- 6.McNulty H, Pentieva K, Hoey L, Ward M. Homocysteine, B-vitamins and CVD. Proc Nutr Soc. 2008;67:232–237. doi: 10.1017/S0029665108007076. [DOI] [PubMed] [Google Scholar]
- 7.Graham IM, Daly LE, Refsum HM, Robinson K, Brattstrom LE, Ueland PM, Palma-Reis RJ, Boers GH, Sheahan RG, Israelsson B, Uiterwaal CS, Meleady R, McMaster D, Verhoef P, Witteman J, Rubba P, Bellet H, Wautrecht JC, de Valk HW, Sales Luis AC, Parrot-Rouland FM, Tan KS, Higgins I, Garcon D, Medrano MJ, Candito M, Evans AE, Andria G. Plasma homocysteine as a risk factor for vascular disease. The European Concerted Action Project. JAMA. 1997;277:1775–1781. doi: 10.1001/jama.1997.03540460039030. [DOI] [PubMed] [Google Scholar]
- 8.Refsum H, Ueland PM, Nygard O, Vollset SE. Homocysteine and cardiovascular disease. Annu Rev Med. 1998;49:31–62. doi: 10.1146/annurev.med.49.1.31. [DOI] [PubMed] [Google Scholar]
- 9.Nygard O, Vollset SE, Refsum H, Brattstrom L, Ueland PM. Total homocysteine and cardiovascular disease. J Intern Med. 1999;246:425–454. doi: 10.1046/j.1365-2796.1999.00512.x. [DOI] [PubMed] [Google Scholar]
- 10.Lim HS, Nam KS, Heo YR. The relationships of health-related life-styles with homocysteine, folate, and vitmain B12 status in Korean adults. Korean J Community Nutr. 2001;6:507–515. [Google Scholar]
- 11.Min H. Folate status and plasma homocysteine concentration of Korean adults. Korean J Nutr. 2001;34:393–400. [Google Scholar]
- 12.Hao L, Ma J, Zhu J, Stampfer MJ, Tian Y, Willett WC, Li Z. High prevalence of hyperhomocysteinemia in Chinese adults is associated with low folate, vitamin B-12, and vitamin B-6 status. J Nutr. 2007;137:407–413. doi: 10.1093/jn/137.2.407. [DOI] [PubMed] [Google Scholar]
- 13.Kim JU, Kim HJ, Choi BY. Normal value for serum homocysteine and the prevalence of hyperhomocysteinemia in a rural population. Korean J Epidemiol. 2008;30:100–109. [Google Scholar]
- 14.Clinical and Laboratory Standards Institute. How to Define and Determine Reference Intervals in the Clinical Laboratory; Approved Guideline. Wayne, Pennsylvania: Clinical and Laboratory Standards Institute; 2000. [Google Scholar]
- 15.Herbert V. Folic acid. In: Shils ME, Olson JA, Shike M, Ross AC, editors. Modern Nutrition in Health and Disease. 9th ed. Baltimore: Williams & Wilkins; 1999. pp. 433–446. [Google Scholar]
- 16.Weir DG, Scott JM. Vitamin B12 "cobalamin". In: Shils ME, Olson JA, Shike M, Ross AC, editors. Modern Nutrition in Health and Disease. 9th ed. Baltimore: Williams & Wilkins; 1999. pp. 447–458. [Google Scholar]
- 17.Selhub J, Jacques PF, Rosenberg IH, Rogers G, Bowman BA, Gunter EW, Wright JD, Johnson CL. Serum total homocysteine concentrations in the third National Health and Nutrition Examination Survey (1991-1994): population reference ranges and contribution of vitamin status to high serum concentrations. Ann Intern Med. 1999;131:331–339. doi: 10.7326/0003-4819-131-5-199909070-00003. [DOI] [PubMed] [Google Scholar]
- 18.Steering Committee of the Western Pacific Region of the World Health Organization; The International Association for the Study of Obesity and the International Obesity Task Force. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment. Melbourne: Health Communications Australia Pty Ltd; 2000. pp. 8–56. [Google Scholar]
- 19.Piyathilake CJ, Macaluso M, Hine RJ, Richards EW, Krumdieck CL. Local and systemic effects of cigarette smoking on folate and vitamin B-12. Am J Clin Nutr. 1994;60:559–566. doi: 10.1093/ajcn/60.4.559. [DOI] [PubMed] [Google Scholar]
- 20.Hamid A, Wani NA, Rana S, Vaiphei K, Mahmood A, Kaur J. Down-regulation of reduced folate carrier may result in folate malabsorption across intestinal brush border membrane during experimental alcoholism. FEBS J. 2007;274:6317–6328. doi: 10.1111/j.1742-4658.2007.06150.x. [DOI] [PubMed] [Google Scholar]
- 21.Adachi H, Hirai Y, Fujiura Y, Matsuoka H, Satoh A, Imaizumi T. Plasma homocysteine levels and atherosclerosis in Japan: epidemiological study by use of carotid ultrasonography. Stroke. 2002;33:2177–2181. doi: 10.1161/01.str.0000026861.18199.89. [DOI] [PubMed] [Google Scholar]
- 22.Shimakawa T, Nieto FJ, Malinow MR, Chambless LE, Schreiner PJ, Szklo M. Vitamin intake: a possible determinant of plasma homocyst(e)ine among middle-aged adults. Ann Epidemiol. 1997;7:285–293. doi: 10.1016/s1047-2797(97)00004-5. [DOI] [PubMed] [Google Scholar]
- 23.Saw SM, Yuan JM, Ong CN, Arakawa K, Lee HP, Coetzee GA, Yu MC. Genetic, dietary, and other lifestyle determinants of plasma homocysteine concentrations in middle-aged and older Chinese men and women in Singapore. Am J Clin Nutr. 2001;73:232–239. doi: 10.1093/ajcn/73.2.232. [DOI] [PubMed] [Google Scholar]
- 24.Kim MK, Ordovas JM, Selhub J, Campos H. B vitamins and plasma homocysteine concentrations in an urban and rural area of Costa Rica. J Am Coll Nutr. 2003;22:224–231. doi: 10.1080/07315724.2003.10719297. [DOI] [PubMed] [Google Scholar]
- 25.Korea Ministry of Health and Welfare. The Third Korea National Health and Nutrition Examination Survey (KNHANES III) Kwacheon, Korea: Ministry of Health and Welfare; 2006. [Google Scholar]
- 26.Gartler SM, Hornung SK, Motulsky AG. Effect of chronologic age on induction of cystathionine synthase, uroporphyrinogen I synthase, and glucose-6-phosphate dehydrogenase activities in lymphocytes. Proc Natl Acad Sci USA. 1981;78:1916–1919. doi: 10.1073/pnas.78.3.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brattstrom L, Lindgren A, Israelsson B, Andersson A, Hultberg B. Homocysteine and cysteine: determinants of plasma levels in middle-aged and elderly subjects. J Intern Med. 1994;236:633–641. doi: 10.1111/j.1365-2796.1994.tb00856.x. [DOI] [PubMed] [Google Scholar]
- 28.Hak AE, Polderman KH, Westendorp IC, Jakobs C, Hofman A, Witteman JC, Stehouwer CD. Increased plasma homocysteine after menopause. Atherosclerosis. 2000;149:163–168. doi: 10.1016/s0021-9150(99)00321-4. [DOI] [PubMed] [Google Scholar]
- 29.Nygard O, Vollset SE, Refsum H, Stensvold I, Tverdal A, Nordrehaug JE, Ueland M, Kvale G. Total plasma homocysteine and cardiovascular risk profile. The Hordaland Homocysteine Study. JAMA. 1995;274:1526–1533. doi: 10.1001/jama.1995.03530190040032. [DOI] [PubMed] [Google Scholar]
- 30.Nygard O, Refsum H, Ueland PM, Stensvold I, Nordrehaug JE, Kvale G, Vollset SE. Coffee consumption and plasma total homocysteine: The Hordaland Homocysteine Study. Am J Clin Nutr. 1997;65:136–143. doi: 10.1093/ajcn/65.1.136. [DOI] [PubMed] [Google Scholar]