Abstract
Endoscopic mucosal resection (EMR) results in the formation of iatrogenic gastric ulcers and the optimal treatments for such ulcers are still unclear. We aimed to evaluate the efficacy of rebamipide in the management of EMR-induced ulcers by comparing it with an H2 receptor antagonist. After EMR, patients were randomly assigned into either rebamipide or famotidine groups. All patients received a one-week lansoprazole 30 mg q.d. therapy followed by three-week famotidine (20 mg b.i.d.) or rebamipide (100 mg t.i.d.) therapy. Four weeks after the treatments, ulcer sizes, stages, bleeding rates, and ulcer-related symptoms were compared using endoscopy and a questionnaire. A total of 63 patients were enrolled in this study. Finally, 51 patients were analyzed, 26 in rebamipide and 25 in famotidine group. Baseline characteristics were not significantly different between the two groups. Four weeks after EMR, the two groups were comparable in terms of ulcer reduction ratio (P=0.297), and ulcer stage (P=1.000). Moreover, no difference was observed with regard to ulcer-related symptoms, drug compliance, adverse drug event rates, and bleeding rates. Our data suggest that rebamipide is not inferior to famotidine in healing iatrogenic gastric ulcers, and could be a therapeutic option in the treatment of such ulcers.
Keywords: Rebamipide; Stomach Ulcer, Endoscopic Mucosal Resection; H2 Receptor Antagonist
INTRODUCTION
Endoscopic mucosal resection (EMR) has been established as a therapeutic alternative for early gastric cancer (EGC) confined to the mucosa and its premalignant lesions (1). For EGC, EMR provides a survival rate of more than 90% relative to that of surgery if the technique is applied with the appropriate indication (2). Moreover, morbidity and mortality associated with surgery can be avoided, and specimens can be obtained for accurate staging (3). The frequency of EMR has increased worldwide and the emergence of a newer technique, endoscopic submucosal dissection (ESD), extended the indication criteria of EMR (4). However, this procedure inevitably results in formation of large ulcers at the resected area, which occasionally bleed.
The characteristics and healing mechanism of EMR-induced ulceration are not fully understood. Furthermore, the duration of treatment and optimal treatment options are controversial. At present, it is evident that an abundant blood supply at the margin of EMR-induced ulcer is an important factor in promoting ulcer healing. Healing of gastric ulcers requires angiogenesis in the granulation tissue at the base of the ulcer, together with replication of epithelial cells at the ulcer margins and subsequent reestablishment of glandular architecture (5). Epithelial and endothelial cell proliferation is largely driven by various growth factors. In the case of angiogenesis, vascular endothelial growth factor appears to be among the most important (6). In addition, fibroblast growth factor-2 and platelet derived growth factor are known to play an important role in gastric ulcer healing (7, 8).
Rebamipide is a mucosal protective and ulcer healing drug that stimulates prostaglandin generation in gastric mucosa, and improves not only speed but also quality of ulcer healing (9). Supporting this, experimental studies have indicated that rebamipide treatment directly stimulated angiogenesis and activated pro-angiogenic growth factors (10). Theoretically, rebamipide can be a potential therapeutic agent in EMR-induced gastric ulcers, however there have been no studies concerning its efficacy in EMR-induced ulcers. Accordingly, we aimed to evaluate the efficacy of rebamipide on EMR-induced ulcers by comparing it to famotidine, an H2 receptor antagonist (H2RA) through a prospective randomized study.
MATERIALS AND METHODS
Patients
The patients who underwent EMR including ESD for gastric adenoma or early gastric cancer confined to the mucosa were consecutively enrolled in this study between December 2006 and April 2008 at the Severance Hospital in Korea. Patients 18 yr of age or older were eligible to participate in this study. Exclusion criteria included patients taking H2RA, proton pump inhibitors (PPI), nonsteroidal anti-inflammatory drugs (NSAIDs), anticoagulants, or glucocorticoids of which potency is equivalent to prednisolone ≥10 mg within the past seven days. Furthermore, patients who should continue use of ulcer-inducing medications during the study, such as aspirin, NSAIDs, glucocorticoids, or anticoagulants, who had past surgical history of the stomach or esophagus, and women who were lactating, pregnant, or intended to become pregnant during the study period, were also excluded. At enrollment, baseline characteristics such as sex, age, and comorbid diseases were recorded. The institutional review board of the Severance Hospital approved the study protocol and all patients provided written informed consent to participate in this study (4-2006-0005).
Endoscopic mucosal resection
EMR was done by one of the following methods: 1) ESD using an insulation-tipped electrosurgical knife, 2) precutting and resection using a snare (EMR-P), 3) EMR using a transparent cap (EMR-C), or 4) snare polypectomy. Before EMR, the maximal diameter and lesion location were recorded. After EMR, ulcer dimensions were recorded. Ulcer dimensions were calculated by multiplying the maximal diameter by the perpendicular diameter. Diameter was measured with biopsy forceps (Olympus, Tokyo, Japan) placed on the ulcer surface, using the width between fully-opened forceps (7 mm) (7). Rapid urease test (CLOtest®; Kimberley-Clark, Draper, UT, USA) was performed with specimens obtained from the lesser curvature of the antrum for evaluation of Helicobacter pylori infection status. Hemoglobin and coagulation profile (prothrombin time and activated partial thromboplastin time) were also checked one day before EMR.
Study protocol
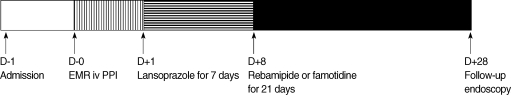
Patients were randomly assigned into either the rebamipide or famotidine group after EMR (Fig. 1). All EMR procedures were performed by one specialized endoscopist. All patients received pantoprazole 40 mg intravenous, b.i.d. on the day EMR was performed. Starting the next day, lansoprazole (30 mg) was administered once daily to patients in both groups for seven days. Two days after the EMR, patients were discharged if no bleeding or perforation occurred. After completing one week of lansoprazole treatment, patients received a three week treatment of rebamipide 100 mg t.i.d. or famotidine 20 mg b.i.d. During the study period, patients were instructed not to take any other medications that may affect ulcer healing.
Fig. 1.
Flow diagram of the study.
EMR, endoscopic mucosal resection; PPI, proton pump inhibitor; iv, intravenous.
Primary objectives included the evaluation of gastric ulcer healing and clinical symptoms. Gastric ulcer healing was assessed by endoscopic examination 4 weeks after EMR. Ulcer healing was assessed by measuring changes in both ulcer stage and size. Gastric ulcer stage was classified by using a six-stage system as proposed by Sakita and Fukutomi: active (A1, A2), healing (H1, H2), and scarring (S1, S2) (11). Ulcer dimensions were measured with biopsy forceps, by the same method as that used at the time of EMR. Endoscopists were not informed about the drugs the patients had received at the time of EMR or endoscopy.
Clinical symptoms, drug adverse events, complications, and drug compliance were assessed by a physician via an interview and questionnaire at an outpatient clinic. Epigastric pain was recorded using a four-grade system (12).
Statistical analyses
Baseline characteristics of patients were compared by either the Fisher's exact test or t-test. Ulcer reduction ratios were calculated by dividing the ulcer dimension at four weeks after EMR by the initial ulcer dimension (13). Ulcer reduction ratios were compared by the Mann-Whitney test. The stage of ulcer, symptoms during treatment, and frequency of adverse events were compared by the Fisher's exact test. P values of less than 0.05 were considered to be statistically significant. Statistical analysis was performed using SPSS for Windows (version 12.0; SPSS Inc., Chicago, IL, USA).
RESULTS
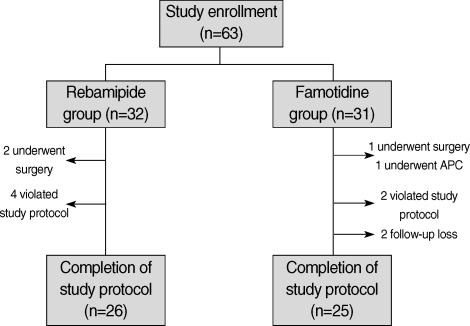
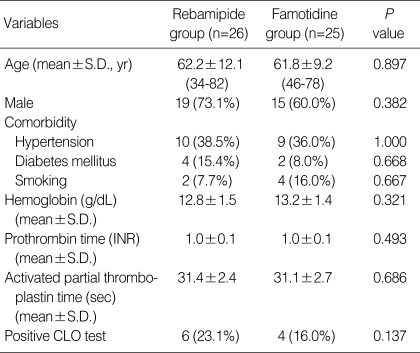
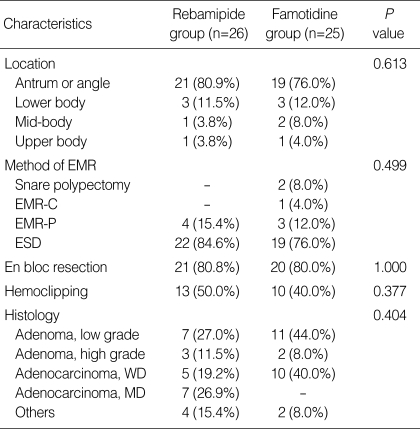
A total of 63 patients were enrolled in this study and were randomly assigned to each group at a ratio of 32:31. Twelve patients in the two groups (6 in rebamipide group and 6 in famotidine group) were excluded from the final analyses because four underwent additional gastrectomy or endoscopic treatments after confirmation of cancer involvement in the resection margin, six had violated study protocol or taken inadequate medications, and two were lost during follow-up (Fig. 2). In baseline characteristics such as sex, age, comorbidity, blood test, and rapid urease test positivity, there were no significant differences between the two groups (Table 1). Moreover, there were no significant differences between the two groups with regard to the characteristics of gastric ulcers created by EMR such as size, location, EMR method for lesion, hemoclipping after EMR, and histopathology (Table 2).
Fig. 2.
Flow diagram of patient enrollment, assignment, and completion of the study.
APC, argon plasma coagulation.
Table 1.
Demographic and clinical characteristics of patients in both groups
INR, international normalized ratio; CLO, campylobacter-like organism.
Table 2.
Characteristics of gastric ulcers created by EMR
EMR, endoscopic mucosal resection; EMR-C, EMR using a transparent cap; EMR-P, EMR by precutting and resecting using a snare; ESD, endoscopic submucosal dissection; WD, well differentiated; MD, moderately differentiated.
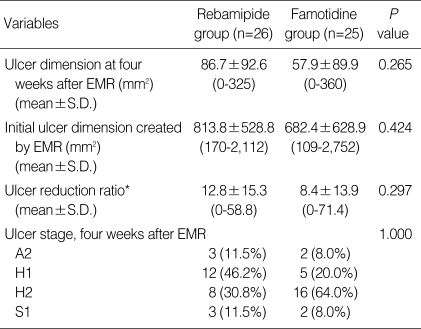
The stages of the 51 ulcers at follow-up endoscopy were compared between the two groups. There was no significant difference in the distribution of ulcer stages between the two groups (P=1.000). Furthermore, ulcer reduction ratios were found to be similar between the two groups (P=0.297) (Table 3).
Table 3.
Comparison of ulcer size, ulcer stage, and ulcer reduction ratio
*Ulcer reduction ratio is defined as ulcer dimension at four weeks after EMR/initial ulcer dimension created by EMR×100.
EMR, endoscopic mucosal resection.
To specifically evaluate the effect of rebamipide in large iatrogenic ulcers, we performed subgroup analysis for patients who underwent ESD. Twenty-two out of 26 (84.6%) in rebamipide group and 19 out of in 25 (76.0%) in famotidine group underwent ESD. Baseline characteristics were comparable between the two groups (data not shown). Ulcer reduction ratios were not significantly different between the two groups (9.98 vs. 9.93, P=0.991). Furthermore, the distribution of ulcer stages was not significantly different (P=0.746) (three in A2, 10 in H1, 6 in H2, and 3 in S1 stage; rebamipide group vs. 2 in A2, 5 in H1, 11 in H2, and 1 in S1 stage; famotidine group).
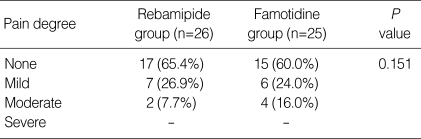
The distribution of epigastric pain during the period of anti-ulcer medication was not different between the two groups (Table 4). Bleeding after EMR and side effects of the study drugs were not found in any of the 51 patients. Drug compliance was evaluated by counting remaining tablets. Drug compliance was considered good if drug intake was more than 75%. Compliance for therapy, i.e., the percentage of tablets taken (>75%), was 86.7% (26/30 patients) and 92.6% (25/27 patients) in the rebamipide and famotidine group, respectively, which was not significantly different (P=0.673).
Table 4.
The severity of epigastric pain during the treatment period
None, without epigastric pain; Mild, minimal epigastric pain; Moderate, with epigastric pain without disturbing activities of daily living; Severe, with epigastric pain with disturbing activities of daily living.
DISCUSSION
The aim of this study was to evaluate the efficacy of rebamipide in ulcer healing after EMR by comparing it to famotidine, which has already proven to be effective in the healing of EMR-induced or peptic ulcers. PPI and H2RA are therapeutic agents for peptic ulcer diseases and PPI had been reported to be more effective than H2RA in healing such ulcers (14-18). Recently, an increase in the incidence of EMR therapy for gastric neoplasia has led to the increased interest in the treatment of artificial ulcers after EMR. Recent studies have demonstrated that PPI and H2RA are also effective in EMR-induced ulcer healing. Some of those studies revealed that PPI might be more potent than H2RA in the healing of artificial ulcers created by EMR, especially larger ulcers after ESD (4, 5, 8), whereas others have suggested that H2RAs are comparable to PPIs in preventing bleeding and accelerating ulcer healing with lower costs (19). Despite the fact that both drugs are potent acid inhibitors, they are quite expensive. In addition, PPIs are more expensive than H2RAs. Currently, Korean national health insurance covers PPIs and H2RA in cases of peptic ulcers, but not in those of EMR-induced ulcers. PPI or H2RA treatment after EMR costs over twice as much as peptic ulcer cases in Korea.
Although suppressors of acid secretion have been a mainstay for the promotion of ulcer healing for three decades, a better understanding of the mechanisms underlying mucosal defense has led to the development of novel anti-ulcer therapies. Rebamipide has been studied in various clinical situations as an adjunct to anti-secretory therapy for the prevention of gastric mucosal damage induced by NSAIDs, and the prevention and treatment of peptic ulcer diseases. Although clinical studies of rebamipide regarding peptic ulcers are still limited, the efficacy of rebamipide in peptic ulcers caused by H. pylori have been demonstrated. Several Japanese investigations have demonstrated that rebamipide significantly promoted gastric ulcer healing following one week of eradication therapy compared to placebo (1, 8). Furthermore, rebamipide is covered by health insurance in the case of EMR-induced ulcers, and is less expensive than H2RA in Korea. An economic benefit would be expected if rebamipide is used in the management of EMR induced ulcers since it has a similar efficacy to PPIs or H2RAs. Based on this background, we hypothesized that the efficacy of rebamipide could be equal to that of H2RA as a treatment option for EMR-induced gastric ulcers. To prove this, we compared the efficacy and tolerability of rebamipide with those of famotidine. Our data analysis of 51 patients indicated that the efficacy of rebamipide was comparable to famotidine in terms of ulcer healing and relief of ulcer-related symptoms. Moreover, drug side effect rates were negligible and compliance to drug treatment was excellent in both groups. In terms of costs and benefits, the total costs required from the first day of EMR to day 28 were 19,320 won for famotidine and 15,750 won for rebamipide. The cost-benefit ratio was significantly higher for rebamipide; rebamipide could heal ulcers at only 81.5% of the cost required by the famotidine treatment.
These results that ulcer healing of patients in rebamipide group was comparable to those in famotidine group, may be partly explained by studies indicating that iatrogenic ulcers, such as EMR-induced ulcers, heal faster than peptic ulcers. The mechanisms of EMR-induced ulcer healing are not fully understood, but some studies have found that they heal faster, and recur less often (13).
For one week after EMR, we randomly administered lansoprazole to all patients enrolled in the study regardless of their treatment group. The incidence of bleeding after EMR had been reported at 1.2-11.6% of patients in Japan (20). ESD is a more complex procedure with a greater rate of complications. One study performed in Korea reported that the rate of bleeding after ESD reached 41.6% (21). Prevention of bleeding after EMR is crucial. Therefore, we were concerned about the potential bleeding complications, which led to all patients receiving lansoprazole during the first week after EMR to reduce the risk of delayed bleeding. Therefore, this study did not demonstrate what proportion one week of PPI contributed to the iatrogenic ulcer healing after EMR. The effect of rebamipide on iatrogenic ulcers created by EMR would have been better proved if we compared it with H2RA alone without the use of PPI for one week. A study initiated in Korea demonstrated that for EMR-induced ulcers, treatment with omeprazole for one week is equivalent to the treatment with omeprazole for four weeks, suggesting that shorter treatment duration might be sufficient (13). However, in that study, ESD cases were not included, and the mean size of ulcer created by EMR was much smaller than those in the present and other studies. To address these concerns, we additionally performed a subgroup analysis in which only patients with ESD were included. After ESD, a larger artificial ulceration is created than those produced by snare polypectomy or EMR-P. We found no overall differences between the rebamipide and famotidine group in terms of ulcer stage or ulcer reduction ratio in the subgroup analysis. Moreover, since all ESD procedures were performed by one specialized endoscopist, ESD procedure was considered to be standardized. Therefore, in our study, although PPI might have affect the ulcer healing in part, our aim to evaluate the efficacy of rebamipide compared to H2RA could be accomplished by investigating relatively large iatrogenic ulcer created by ESD.
Factors that induce peptic ulcers include stress, chronic alcohol consumption, intake of NSAIDs or aspirin, H. pylori infection, and smoking (22). We excluded patients who should continue to take NSAIDs, antiplatelet agents or glucocorticoids. With regards to the proportion of current smokers, there were no statistically significant differences between the 2 groups (2 in rebamipide vs. 4 in famotidine group) (Table 1). After 4 weeks after EMR, 5 patients (3 in rebamipide, 2 in famotidine group) showed that their ulcers stayed active stage (A2) (Table 3). All these patients were not current smokers and on good compliance to drugs. However, initial ulcer sizes were greater with their mean size of 1,186 mm2, ranging from 552 to 2009. No clinical factors were found to be statistically significant in relation to the ulcer healing stage after 4 weeks. This observation, although inadequate to be concluded on the statistical evidences, suggests that ulcer healing rate is mostly influenced by initial ulcer size, but not by clinical or personal factors.
Although the present study is the first investigation that demonstrates the efficacy of rebamipide in terms of ulcer healing after EMR, some limitations exist. The sample size used was small and considerable numbers of patients (19.0% of the initially included patients) were excluded from analysis. Supplementary experimental investigations, such as measurement of growth factor expression in patients with EMR-induced ulcer, would provide a better understanding of the mechanisms underlying artificial ulcer healing. Considering the endemic H. pylori infection in Korea, the proportion of positive CLO test in the present study was relatively low. Atrophy of background gastric mucosa combined to gastric neoplasm would contribute to a higher rate of false negative of CLO test. However, we did not evaluate the histological examination of the surrounding gastric mucosa or serologic test for H. pylori. Finally, hemoclipping is expected to influence on ulcer healing and delayed bleeding either positively or negatively. It was performed in 40-50% of patients in our study. Although the proportion of patients who underwent hemoclipping after EMR was not significantly different in the 2 groups, it might have influenced the artificial ulcer healing.
In conclusion, our data suggest that rebamipide is not inferior to famotidine in terms of ulcer healing after EMR, and may be an alternative for the treatment of EMR-induced ulcers.
Footnotes
This work was supported by Yonsei University Research Fund of 2006 (6-2006-0108).
References
- 1.Conio M, Ponchon T, Blanchi S, Filiberti R. Endoscopic mucosal resection. Am J Gastroenterol. 2006;101:653–663. doi: 10.1111/j.1572-0241.2006.00424.x. [DOI] [PubMed] [Google Scholar]
- 2.Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225–229. doi: 10.1136/gut.48.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee JH, Kim JJ. Endoscopic mucosal resection of early gastric cancer: Experiences in Korea. World J Gastroenterol. 2007;13:3657–3661. doi: 10.3748/wjg.v13.i27.3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanaka M, Ono H, Hasuike N, Takizawa K. Endoscopic submucosal dissection of early gastric cancer. Digestion. 2008;77(Suppl 1):23–28. doi: 10.1159/000111484. [DOI] [PubMed] [Google Scholar]
- 5.Wallace JL. Recent advances in gastric ulcer therapeutics. Curr Opin Pharmacol. 2005;5:573–577. doi: 10.1016/j.coph.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Szabo S, Vincze A, Sandor Z, Jadus M, Gombos Z, Pedram A, Levin E, Hagar J, Iaquinto G. Vascular approach to gastroduodenal ulceration: new studies with endothelins and VEGF. Dig Dis Sci. 1998;43(9 Suppl):40S–45S. [PubMed] [Google Scholar]
- 7.Esaki M, Aoyagi K, Matsumoto T, Kuwano Y, Shimizu M, Fujishima M. Effects of omeprazole and famotidine on fibroblast growth factor-2 during artificial gastric ulcer healing in humans. Eur J Gastroenterol Hepatol. 2002;14:365–369. doi: 10.1097/00042737-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Piazuelo E, Lanas A, Jimenez P, García-Gonzalez A, Esteva F. In vitro wound repair by human gastric fibroblasts: implications for ulcer healing. Dig Dis Sci. 1998;43:1230–1240. doi: 10.1023/a:1018803707179. [DOI] [PubMed] [Google Scholar]
- 9.Arakawa T, Kobayashi K, Yoshikawa T, Tarnawski A. Rebamipide: overview of its mechanisms of action and efficacy in mucosal protection and ulcer healing. Dig Dis Sci. 1998;43(9 Suppl):5S–13S. [PubMed] [Google Scholar]
- 10.Tarnawski AS, Chai J, Pai R, Chiou SK. Rebamipide activates genes encoding angiogenic growth factors and Cox2 and stimulates angiogenesis: a key to its ulcer healing action? Dig Dis Sci. 2004;49:202–209. doi: 10.1023/b:ddas.0000017439.60943.5c. [DOI] [PubMed] [Google Scholar]
- 11.Sakita T, Fukutomi H. Endoscopic diagnosis. In: Yoshitoshi Y, editor. Ulcer of the stomach and duodenum. Tokyo: Nankodo; 1971. pp. 198–208. [Google Scholar]
- 12.Ye BD, Cheon JH, Choi KD, Kim SG, Kim JS, Jung HC, Song IS. Omeprazole may be superior to famotidine in the management of iatrogenic ulcer after endoscopic mucosal resection: a prospective randomized controlled trial. Aliment Pharmacol Ther. 2006;24:837–843. doi: 10.1111/j.1365-2036.2006.03050.x. [DOI] [PubMed] [Google Scholar]
- 13.Lee SY, Kim JJ, Lee JH, Kim YH, Rhee PL, Paik SW, Rhee JC. Healing rate of EMR-induced ulcer in relation to the duration of treatment with omeprazole. Gastrointest Endosc. 2004;60:213–217. doi: 10.1016/s0016-5107(04)01683-9. [DOI] [PubMed] [Google Scholar]
- 14.Bate CM, Wilkinson SP, Bradby GV, Bateson MC, Hislop WS, Crowe JP, Willoughby CP, Peers EM, Richardson PD. Randomised, double blind comparison of omeprazole and cimetidine in the treatment of symptomatic gastric ulcer. Gut. 1989;30:1323–1328. doi: 10.1136/gut.30.10.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walan A, Bader JP, Classen M, Lamers CB, Piper DW, Rutgersson K, Eriksson S. Effect of omeprazole and ranitidine on ulcer healing and relapse rates in patients with benign gastric ulcer. N Engl J Med. 1989;320:69–75. doi: 10.1056/NEJM198901123200201. [DOI] [PubMed] [Google Scholar]
- 16.Schepp W, Classen M. Pantoprazole and ranitidine in the treatment of acute duodenal ulcer. A multicentre study. Scand J Gastroenterol. 1995;30:511–514. doi: 10.3109/00365529509089781. [DOI] [PubMed] [Google Scholar]
- 17.Lauritsen K, Rune SJ, Wulff HR, Olsen JH, Laursen LS, Havelund T, Astrup L, Bendtsen F, Linde J, Bytzer P. Effect of omeprazole and cimetidine on prepyloric gastric ulcer: double blind comparative trial. Gut. 1988;29:249–253. doi: 10.1136/gut.29.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lauritsen K, Rune SJ, Bytzer P, Kelbaek H, Jensen KG, Rask-Madsen J, Bendtsen F, Linde J, Højlund M, Andersen HH. Effect of omeprazole and cimetidine on duodenal ulcer. A double-blind comparative trial. N Engl J Med. 1985;312:958–961. doi: 10.1056/NEJM198504113121505. [DOI] [PubMed] [Google Scholar]
- 19.Yamaguchi Y, Katsumi N, Tauchi M, Toki M, Nakamura K, Aoki K, Morita Y, Miura M, Morozumi K, Ishida H, Takahashi S. A prospective randomized trial of either famotidine or omeprazole for the prevention of bleeding after endoscopic mucosal resection and the healing of endoscopic mucosal resection-induced ulceration. Aliment Pharmacol Ther. 2005;21(Suppl 2):S111–S115. doi: 10.1111/j.1365-2036.2005.02484.x. [DOI] [PubMed] [Google Scholar]
- 20.Kojima T, Parra-Blanco A, Takahashi H, Fujita R. Outcome of endoscopic mucosal resection for early gastric cancer: review of the Japanese literature. Gastrointest Endosc. 1998;48:550–554. doi: 10.1016/s0016-5107(98)70108-7. [DOI] [PubMed] [Google Scholar]
- 21.Jang JS, Lee EJ, Lee SW, Lee JH, Roh MH, Han SY, Choi SR, Jeong JS. Endoscopic submucosal dissection for early gastric cancer and gastric adenoma. Korean J Gastroenterol. 2007;49:356–363. [PubMed] [Google Scholar]
- 22.Maity P, Biswas K, Roy S, Banerjee RK, Bandyopadhyay U. Smoking and the pathogenesis of gastroduodenal ulcer--recent mechanistic update. Mol Cell Biochem. 2003;253:329–338. doi: 10.1023/a:1026040723669. [DOI] [PubMed] [Google Scholar]