Abstract
A new oxygen sensor, compound 2, was synthesized through a chemical modification of a popularly used oxygen sensor of platinum(II)-5,10,15,20-tetrakis-(2,3,4,5,6-pentafluorophenyl)-porphyrin (PtTFPP). The new sensor compound 2 possesses four crosslinkable methacrylate functional moieties, enabling it to be polymerized and crosslinked with other monomers for polymer sensing film (also called membrane) preparation. Using this characteristic, compound 2 was covalently bonded to hydrophilic poly(2-hydroxyethyl methacrylate)-co-polyacrylamide (referred to as PHEMA to simplify) and hydrophobic polystyrene (PS) films. To better understand the advantages and disadvantages of chemical crosslinking approaches and the influence of polymer matrices on sensing performance, PtTFPP was physically incorporated into the same PHEMA and PS matrices to compare. Response to dissolved oxygen (DO), leaching of the sensor molecules from their matrices, photostability of the sensors, and response time to DO changes were studied. It was concluded that the chemical crosslinking of the sensor compound 2 in polymer matrices: (i) alleviated the leaching problem of sensor molecules which usually occurred in the physically doped sensing systems and (ii) significantly improved sensors’ photostability. The PHEMA matrix was demonstrated to be more suitable for oxygen sensing than PS, because for the same sensor molecule, the oxygen sensitivity in PHEMA film was higher than that in PS and response time to DO change in the PHEMA film was faster than that in PS. It was the first time oxygen sensing films were successfully prepared using biocompatible hydrophilic PHEMA as a matrix, which does not allow leaching of the sensor molecules from the polymer matrix, has a faster response to DO changes than that of PS, and does not present cytotoxicity to human lung adenocarcinoma epithelial cells (A549). It is expected that the new sensor compound 2 and its similar compounds with chemically crosslinking characteristics can be widely applied to generate many interesting oxygen sensing materials for studying biological phenomena.
1. Introduction
Measurement of dissolved oxygen (DO) has attracted great attention because DO is highly related to water quality, food freshness, medication, scientific understanding of cell respiration and metabolism, and other physiological and biological applications.1 Various types of oxygen sensors have been developed based on mechanisms such as pressure, electrochemistry, and fluorescence quenching (an optical approach).1 Because sensors based on emission quenching are non-invasive or minimally invasive, disposable, easily miniaturized (down to sub-micrometer), and simple to process (as a coating or solid layer on optical fibers or surfaces for remote measurement of chemical and biochemical parameters), the optical approach1b to DO measurement most interested us among the various mechanisms being used. We are using the optical oxygen sensors integrated with lab-on-a-chip devices for single cell metabolism studies.1j, 1k
Ruthenium(II) polypyridyl complexes2, platinum (II) and palladium (II) porphyrins3, and cyclometalated complexes of platinum (II) and iridium (III)4 are typical chemical materials acting as fluorescence-based oxygen sensors because of their long triplet state lifetime in the ranges of a few to tens of microseconds and sufficient triplet-triplet energy transfer from these compounds to oxygen molecules. This results in a decrease of the oxygen sensors’ emission intensities and a shortening of their lifetimes by oxygen. Among the many oxygen sensors, platinum(II)-5,10,15,20-tetrakis-(2,3,4,5,6-pentafluorophenyl)-porphyrin (PtTFPP) is believed to be an excellent sensor due to its good response to oxygen concentrations and high photostability as compared with others.5 In order to realize the measurement of DO in liquids, oxygen sensing molecules were usually physically incorporated and dispersed in various hydrophobic matrices with good oxygen permeability2–4 including silicon rubbers, silica gels, organosilicas, polystyrene, cellulose derivatives, and polyurethane-type hydrogels.
Biocompatible polymers, such as collagen, poly(2-hydroxyethyl methacrylate) (PHEMA), and poly[N-(2-hydroxypropyl) methacrylamide] (PHPMA) have been well applied as hydrogels and scaffolds to study cell growth, proliferation, differentiation, and also in studying cell function in 3D environments.6 However, there is almost no report about using these biocompatible hydrogels as matrices for optical sensing studies because of the concern of significant leaching of the physically dispersed sensor molecules from these hydrophilic matrices. The leaching is undesired for applications for DO measurements because it may result in problems of signal instability, inaccuracy of measurements, decreased long-term applicability, and toxicity for cells. Successful preparation of optical oxygen sensors using the biocompatible gels as matrices with good oxygen sensitivity, photostability, reasonable response time, non-cytotoxicity and non-leaching of the sensors from their matrices is highly expected for bioapplications.
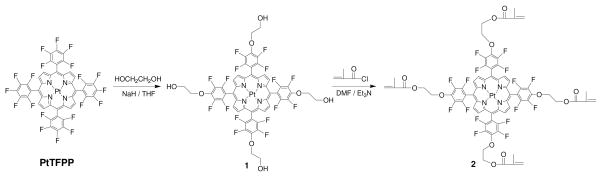
Herein, we developed a new oxygen sensor 2 shown in Scheme 1, which is a modified PtTFPP possessing four methacrylate moieties which can be easily mixed with hydrogel precursors (e.g. a mixture of 2-hydroxyethyl methacrylate (HEMA) and acrylamide) in a solvent (e.g. N,N′-dimethylformamide (DMF)) and polymerized/crosslinked to generate polymer hydrogels (e.g, PHEMA hydrogel) with chemically bonded sensors. Using about 20-wt% of acrylamide as a co-monomer with HEMA is for improving the matrix’s ion permeability for a further integration of the sensing film with other sensors such as pH, K+, Ca2+, and Na+ ion sensors for simultaneous measurement of multi-parameters. The polymers can be prepared with controlled thickness as thin films or membranes. For a demonstration of excellent characteristics of the covalently bonded sensors in the PHEMA films, we also: (1) chemically immobilized sensor monomer 2 to the popularly used hydrophobic polystyrene (PS) matrix to study the influence of matrices on sensing performance and (2) physically dispersed PtTFPP, the precursor for compound 2, in the PS and PHEMA matrices to compare the covalently bonded and physically incorporated sensors on sensors’ photostability and leaching. Interferences by ions, pH values, media, and temperatures on the PHEMA sensing films with covalently linked sensor compound 2 were studied. Toxicity of the new sensor film with covalently bonded sensor molecules to human lung cancer A549 cells was also evaluated.
Scheme 1.
2. Results and discussion
Synthesis of the new sensor 2
As outlined in Scheme 1, PtTFPP was first chemically modified using anhydrous ethylene glycol at a basic condition to generate compound 1. The fluoride at para position of the meso-pentafluorophenyl group has a much higher chemical reactivity than other fluorides at the phenyl rings. This characteristic has been used to modify 5,10,15,20-tetrakis-(2,3,4,5,6-pentafluorophenyl)-porphyrin (TFPP) and platinum (II)-5,10,15,20-tetrakis-(2,3,4,5,6-pentafluorophenyl)-porphyrin (PtTFPP).7 After purification through column chromatography, 1 was then used to react with methacryloyl chloride to generate monomer 2 possessing four methacrylate moieties.
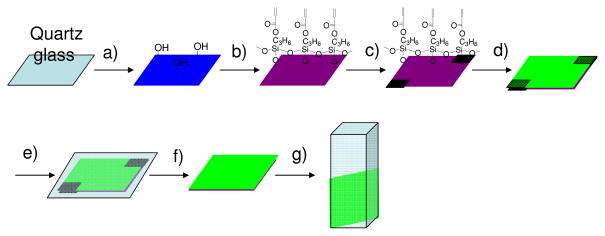
Sensing film (membrane) preparation
A schematic procedure for the membrane preparation was given in Figure 1.
Figure 1.
Schematic illustration of the preparation of sensing membranes. a) oxygen plasma treatment to generate active hydroxyl groups; b) vapor deposition of thin TMPSA layer; c) 25-μm tape used to control membrane thickness; d) sensor solution dispensed onto modified quartz surface; e) solution covered with a cover glass and polymerized at 80°C for 1.5 hours; f) cover glass and tape removed; film rinsed using methanol and double-distilled water; and g) sensing membrane on quartz substrate immersed into liquid in cuvette for fluorescence measurements.
To achieve a stable polymer membrane on a substrate for optical characterization, a quartz substrate was modified with an acrylate-containing silane, trimethylsilylpropyl acrylate (TMPSA), using a vapor deposition approach.8 The silane groups were anchored on the quartz surface with active hydroxyl groups generated by oxygen plasma treatments. The acrylate moiety can be further polymerized with other monomers to chemically graft the sensing membranes on the quart surface. Contact angle of the quartz surface after the TMPSA modification was measured to be 60°, whereas the contact angle of oxygen plasma-treated quartz before the vapor deposition was approximately 10°. This difference indicated the successful surface modification of the quartz surface using TMPSA. In order to provide the polymer films with a good mechanical stability, ethyloxylate trimethylolpropane triacrylate (SR454) was used as a crosslinker. The membranes (films F1 to F8, Table 1) were prepared through a solution polymerization of the monomers of 2, S or HEMA/acrylamide, and SR454 in DMF via a variation of sensors, matrices and sensor concentrations. Thermal polymerization initiated by azobisisobutyronitrile (AIBN) under nitrogen was used to cure the monomers for polymer immobilization on the quartz substrate. Thermal polymerization was used for polymer thin film generation not the popularly used photopolymerization approach in order to avoid the potential photo-bleaching problem for sensors9 under short wavelength (usually 365 nm or 254 nm) photo-excitation. For a faster reaction, the polymerization was performed at 80°C. It was found that under such conditions, uniform polymer membranes grafted on quartz surfaces were obtained after polymerization for 1.5 hours under nitrogen. Typical pictures of a thin film with and without excitation by 365 nm were given in supporting information (S-Figure 1). The film thickness distribution was also given in S-Figure 1. Increasing the polymerization time was not found to improve the film stability or vary the sensing performance. For our sensing studies, the thickness of the sensor membranes was controlled to approximately 25 μm.
Table 1.
Responses of the sensing films to dissolved oxygen in pH 7.0 B-R buffer.
| Samples | Sensor | Matrix | Con. b | λexc | λem |
KSV: (kPa−1) at various excitation wavelengths |
|||
|---|---|---|---|---|---|---|---|---|---|
| 394 nm | 405 nm | 508 nm | 514 nm | ||||||
| F1 | 2 | PHEMA a | 0.1 | 394 | 649 | 0.0874 (0.9978) e | |||
| F2 | 2 | PHEMA a | 1.0 | 394 | 649 | 0.0894; (0.9962) | 0.0876; (0.9977) | 0.0851; (0.9963) | 0.0826; (0.9962) |
| F3 | 2 | PHEMA a | 10.0 | 389d | 649 | 0.073; (0.928) | |||
| F4 | PtTFPP | PHEMA a | 1.0 | 394 | 650 | 0.1061; (0.9968) | 0.1016; (0.9977) | 0.1027; (0.9963) | 0.0979; (0.9962) |
| F5 | 2 | PS | 0.1 | 395 | 650 | 0.0402; (0.9983) | |||
| F6 | 2 | PS | 1.0 | 395 | 650 | 0.0392; (0.9987) | 0.0380; (0.9987) | 0.0377; (0.9984) | 0.0374; (0.9982) |
| F7 | 2 | PS | 10.0 | 389d | 650 | 0.0378; (0.990) | |||
| F8 | PtTFPP | PS | 1.0 | 394 | 650 | 0.045; (0.9979) | |||
for simplification, the film of PHEMA and acrylamide copolymer was called PHEMA.
Con.: concentration in mg/g of sensor/matrix;
λex in nm: only the Soret band was listed in the table, the Q bands at 508 and 540 nm were not listed.
broad peak.
numbers in the parentheses are the correlation coefficients (R2) of the fitting.
Optical properties of the sensing films
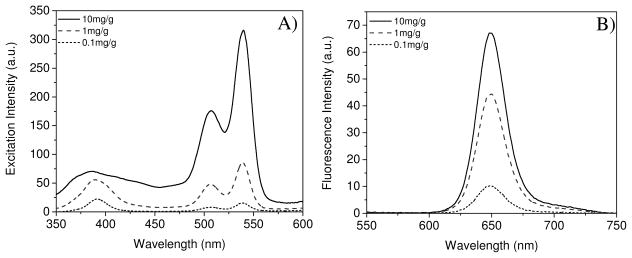
Concentration dependent excitation and emission spectra of sensor monomer 2 covalently bonded into poly(2-hydroxyethyl methacrylate)-co-polyacrylamide (referred to as PHEMA to simplify) (F1, F2 and F3 in Table 1) and PS (F5, F6 and F7 in Table 1) matrices were investigated in Britton-Robinson (B-R) pH 7.0 buffer. Typical spectra of F1, F2 and F3 were given in Figure 2. Three peaks at ~390 nm, 508 nm, and 540 nm were observed in the excitation spectra corresponding to the Soret band and Q bands of platinum porphyrins.10 Red emission was observed with a peak at 649 nm. The PtTFPP physically dispersed in the PHEMA hydrogel also exhibits similar photophysical properties (Table 1). Detailed studies about the structures influence on the photophysical properties of PtTFPP, compound 1 and compound 2 in solutions and films were given in supporting information, S-Figure 2 and S-Figure 3. The fluorescence intensities did not have a linear dependence on the sensors’ concentrations, showing a possibility of self-absorption11 of the sensor molecules in the films. This may result in the self-quenching of the sensors, especially at higher concentration, such as 10 mg/g (sensor/gel), where the Soret-band around 390 nm became broad.
Figure 2.
Concentration dependent excitation (A) and emission spectra (B) of the sensors 2 covalently crosslinked on PHEMA matrix (films F1, F2 and F3). The units mg/g in the figures are weight ratios of the sensor in milligrams to polymer matrix in gram scale. For F1: 0.1 mg/g; F2: 1.0 mg/g; F3:10.0 mg/g.
Typical oxygen sensing (film F2) for dissolved oxygen
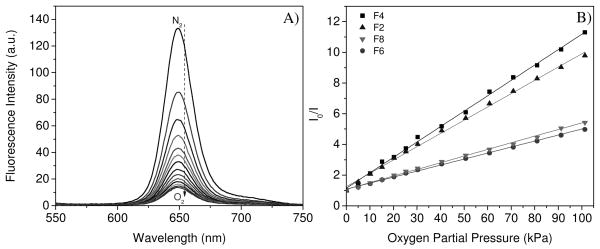
A mixture of oxygen and nitrogen gas was used to saturate aqueous solutions to tune dissolved oxygen concentrations by bubbling. Figure 3A shows typical DO response using chemically bonded 2 in PHEMA hydrogel F2. Fluorescence intensity of the oxygen sensor decreased with an increase in the dissolved oxygen concentration, showing the good oxygen diffusion to the sensing membrane in aqueous solutions. Because the PHEMA hydrogel swells significantly in water12, the dissolved oxygen can interact efficiently with the sensor molecules to quench sensor’s emissions. It has been well known the PHEMA hydrogel is an excellent material exhibiting good oxygen permeability for contact lenses.13
Figure 3.
Typical response to DO using F2 (covalently crosslinked 2 in PHEMA) in B-R pH 7.0 buffer (A) and the Stern-Volmer fittings (B) for F2, F4 (physically doped PtTFPP in PHEMA), F6 (covalently crosslinked 2 in PS) and F8 (physically doped PtTFPP in PS) excited at 405 nm. I0 is the emission intensity in the deoxygenated buffer by flushing with nitrogen. I is the emission intensity of oxygen-containing solutions equilibrated with different oxygen partial pressures.
Intensity ratios (I0/I) of the F2 (Figure 3B) followed the Stern-Volmer equation with a correlation coefficient (R2) exceeding 0.997 as given in equation 1.
| (1) |
where KSV is the Stern-Volmer quenching constant. I0 and I are the steady-state luminescence signals at 650 nm measured in deoxygenated and oxygen-containing solutions, respectively. Strictly speaking, the sensing membrane responds to DO ([O2]). However, since the actual oxygen concentration diffused into the polymer membrane was unknown, the partial pressure (pO2) of oxygen in the gas used to saturate the buffer was applied in the above equation. The utilization of the oxygen partial pressure rather than the dissolved oxygen concentration in solutions has been widely accepted and used by other scientists1b, 9, 14, since the DO is proportional to the oxygen partial pressure. Standard atmosphere pressure of 101.3 kPa corresponds to an oxygen partial pressure of 21.3 kPa, and DO in air-saturated water at 20 °C under the standard pressure is 9.23 mg/L or 9.23 ppm.15
Excitation wavelengths had a slight effect on the Stern-Volmer quenching constants (Table 1). Besides the two wavelengths of the absorbance maxima at the Soret-band (394 nm) and the Q-band (508 nm), two widely applied laser excitation wavelengths (405 and 514 nm) in confocal fluorescence microscopy were chosen for comparison. Only slightly smaller quenching constants were observed when the excitation wavelengths were longer.
Concentration dependent Stern-Volmer quenching constants were also given in Table 1. It was found that the KSV had some differences at different concentrations. Especially, at a high concentration of 10 mg/g (sensor/gel), the quenching constant was much smaller, probably due to the self-aggregations of the sensor molecules in the membranes. Based on these results, the ratio of sensors to matrices of 1 mg/g for sensing performance investigation was usually chosen in order to achieve a good signal-to-noise ratio.
Effects of chemically bonded approach, physically doped method, and the matrices on oxygen sensing
All of the sensor films from F1 to F8 (Table 1) showed linear Stern-Volmer responses to DO. However, sensitivity to DO was related to the film preparation approaches and matrices. The influence was reflected in the KSV quenching constants. A larger KSV indicated a higher sensitivity to DO. Typical plots using films F2 (chemically crosslinked 2 in PHEMA), F4 (physically incorporated PtTFPP in PHEMA), F6 (chemically bonded 2 in PS) and F8 (physically doped PtTFPP in PS) with the same sensor concentration (1 mg sensor in 1 g matrix) and the same excitation wavelength (405 nm) were given in Figure 3B, and more detailed data were given in Table 1.
Under a same condition, KSV of F4 is larger than F2. Similarly, KSV of F8 is larger than F6. These results showed that the chemical crosslinking of the sensor molecules 2 in the PHEMA and PS matrices made the films less sensitive to oxygen than the sensor molecules PtTFPP dispersed physically in the same matrices. The chemical crosslinking of the sensor molecules in the matrices probably restricted the molecule movements inside the matrix and slightly prohibited the interactions with oxygen molecules, resulting in a smaller quenching constant.
For the same sensor using same film preparation method, the KSV in PHEMA is almost twice of the KSV in PS (F2 vs. F6 or F4 vs. F8), showing a significant influence of the polymer matrices on the oxygen sensing and suggesting the PHEMA hydrogel is a much better matrix for DO sensing than the hydrophobic PS. One reason is that the oxygen diffusion coefficient of PS (1.1 × 10−8 to 2.3 × 10−7 cm2/s) 16 is much smaller than that of the swollen PHEMA (8 × 10−7 ~ 2.2 × 10−6 cm2/s) 12 in aqueous solutions.
Leaching study of the sensing membranes
The membranes with chemically bonded and physically trapped sensors were immersed in pH 7 B-R buffer and methanol, respectively, at room temperature to study the leaching of sensor molecules from polymer matrices. If the sensors molecules leach out of the sensing membranes and disperse in the polar solvents such as water and methanol, the total emission intensity of the sensing membranes and media will decrease as the emission of the oxygen sensor molecules will be quenched significantly by polar solvents. Significant emission decays, 15% for F4 in B-R buffer over 48 hours and 67% for F8 in methanol over only 16 hours, were observed. Meanwhile, relatively weak PtTFPP emission was observed in B-R buffer and methanol, indicating that PtTFPP molecules leached out from their matrices. These results clearly showed the instability of sensing films made through the physical trapping approach when used for dissolved oxygen study, whereas sensing films with covalently bonded sensors alleviated the leaching problem. For the sensor films F2 and F6, there was no observed emission intensity difference before and after 48 hours of soaking in buffer or methanol.
Response time studies using F2 and F6
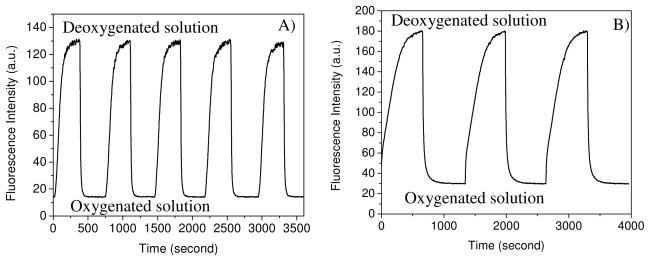
Figure 4 shows the response time and operational stability of the sensing films F2 (2 crosslinked in PHEMA) and F6 (2 crosslinked in PS) in pH 7.0 B-R buffer, which was conducted by measuring the intensity signal during alternated saturation of the solution with oxygen and nitrogen.
Figure 4.
Response time of the film F2 with covalently crosslinked 2 in PHEMA (A) and F6 with covalently crosslinked 2 in PS (B) to deoxygenated solution using nitrogen gas and oxygenated solution using oxygen gas.
For F2, the response times, t95 (i.e. the time for 95% of the total change in fluorescence intensity to occur) and t99 (i.e. the time for 99% of the total change in fluorescence intensity to occur), were 50 seconds and 70 seconds from the deoxygenated buffer to 100% oxygenated solution (Figure 4A), respectively. Recovery times from the oxygenated solution to deoxygenated solution were slower with a t95 of 240 seconds and t99 of 285 seconds. For F6, the response time t95 was 84 s and t99 was 182 s. The recovery times were much slower with a t95 of 420 s and t99 of 540 s (Figure 4B). Slow response times of sensors in PS for DO were previously reported in the range of 2 to 5 minutes2d, 17a, although the same sensors can respond much more rapidly to oxygen gas in the range of 2 to 5 seconds. Some silica gel sensing films were reported to have slow DO response time of about 2 minutes.2e, 17b Another type of sensing film using Nafilon2a, 17c as the matrix was also reported to have a response time of 1–2 minutes to DO. Therefore, the PHEMA hydrogel-based optical sensing film exhibits good response time (50 s for t95) to DO, enabling a new avenue for dissolved oxygen sensing. The response time can be even faster through a decrease in film thickness, an increase in the nitrogen/oxygen bubbling velocity of our setting up (see experimental section for details), or a use of microspheres of the PHEMA gel dispersed in aqueous solution. For example, Borisov et al 18 recently reported a fast DO response optical sensor (about 4 second) using polystyrene-block-polyvinylpyrrolidione beads with physically incorporated PtTFPP suspended in liquids.
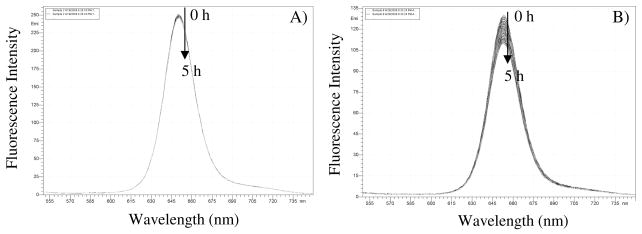
Photostability of the sensing membranes (F2 vs. F4) in aqueous solutions
Short term photostability was studied in pH 7.0 B-R buffer using F2 and F4 at ambient air condition. The films were continuously exposed by excitation light at 405 nm (0.2 mW/cm2) over a period of 5 h with 150 measurements. It was observed that F2 (Figure 5A) was quite stable under our experimental conditions with a less than 1% total intensity decrease, which corresponded to a variation of less than 0.0067% per measurement. A dramatically different result was observed for F4 under the same experimental conditions, where 16% of total emission decay (Figure 5B) was observed corresponding to a decay of 0.11% per measurement.
Figure 5.
Photostability of covalently crosslinked 2 in PHEMA (F2, A) and physically doped PtTFPP in PHEMA (F4, B) in pH 7.0 B-R buffer with 5 hours of continuous exposure at 405 nm (0.2 mW/cm2).
The photostability study strongly indicated that chemical bonding of the sensors with their matrix could improve the sensors’ photostability. It was pointed out by Lee et al.5 that molecular movement in the sensor film is a important factor affecting the sensors’ photostability. We believe the crosslinking of the sensors within the polymer matrices restricts the sensor molecules’ movement and results in the sensors being less reactive toward photo-oxidation and reduction to increase their photostability as compared to the physically incorporated PtTFPP in the same matrices.
Interference tests for F2 film
As stated above, we successfully prepared the chemically bonded sensing films using the PHEMA hydrogel as the matrix and demonstrated that these new sensing films exhibited reasonable oxygen response, excellent photostability, non-leaching character and good response time. The interference by ions, pH values, mediums, and temperature was further studied using the film F2.
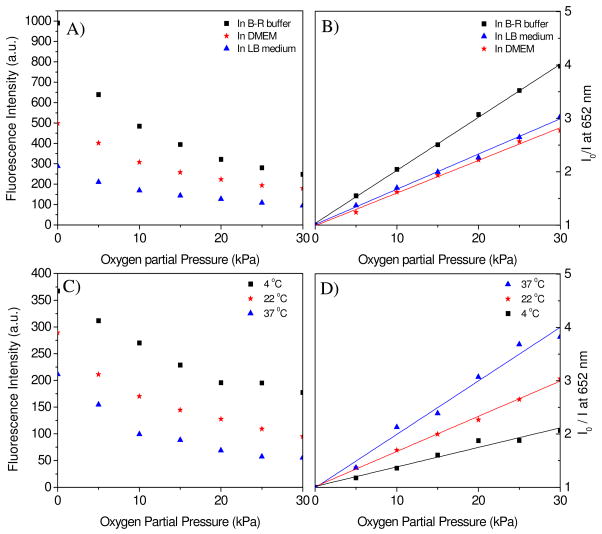
Interference by ions, pH values, and amino acids in media often occur when the sensors are applied in various liquid environments. Interference is generally significantly reduced, but not always removed completely, upon the immobilization of sensor molecules in various polymer matrices. 1 mM NaCl, KCl, and CaCl2 in the B-R pH 7 buffers, as well as varied pH values from 2 to 9, have no obvious influence on the emission intensity (with a emission variation of less than 1%) as compound 2 is neutral and has no pH, sodium, potassium, or calcium ion-sensitive groups or ligands. On the contrary, Dulbecco’s Modified Eagle’s Medium (DMEM) and Lysogeny Broth medium (LB) have significant influence on the emission intensity. 30% fluorescence intensity decay in DMEM and 60% decay in LB medium were observed as compared to that in B-R buffer (Figure 6A), showing that combinations of salts, amino acids, vitamins, sugars, proteins and extract of yeast present in the media likely interact with the sensors. Similar influences of LB medium in decreasing emission intensities of PtTFPP sensors dispersed in the polystyrene-block-polyvinylpyrrolidone beads were reported by Borisov et al.18
Figure 6.
Medium influence (A and B) on the oxygen sensing at room temperature (22°C) and temperature effect (C and D) on the oxygen sensing in LB medium. F2 film was used for this study. The fluorescence intensities at 652 nm were given in the left y-axis. Ratios of I0/I were given in the right y-axis. I0 is the emission intensity in the deoxygenated buffer by flushing with nitrogen. I is the emission intensity of oxygen-containing solutions equilibrated at different oxygen partial pressures. Fitting of the Stern-Volmer plots (B and D) is performed using equation 1.
Media not only decreased the emission intensities as compared to that in B-R buffer, but also affected the oxygen responses. Figure 6A shows the emission intensities of the F2 film and Figure 6B gives Stern-Volmer fittings of the film in the three different aqueous solutions at the same oxygen partial pressures. It was found that the sensing film had the highest Stern-Volmer quenching effect in the B-R buffer and the lowest quenching effect in DMEM medium. Table 2 gives the detailed KSV values. On the other hand, high temperature decreased the sensor’s fluorescence intensity (Figure 6C) but increased the oxygen quenching effect (Figure 6D), which is a common phenomenon for many oxygen sensors in thin films14b, 14c, most likely due to an enhanced interaction of the sensing moieties with oxygen molecules at high temperature. This indicates that when used for practical DO detection, careful calibration in the specific environment is necessary and the temperature should be controlled precisely to eliminate experimental error.
Table 2.
Stern-Volmer quenching constants (KSV in kPa−1) of the sensing film F2 in B-R buffer, DEME and LB medium at 4, 22 and 37 °C.
| DMEM | LB medium | B-R buffer (pH 7.5) | |
|---|---|---|---|
| 37 °C | 0.091 (0.971) | 0.10 (0.984) | 0.13 (0.975) |
| 22 °C | 0.061 (0.998) | 0.066 (0.997) | 0.088 (0.997) |
| 4 °C | 0.029 (0.990) | 0.029 (0.989) | 0.038 (0.964) |
values in parentheses are the correlation coefficients (R2) of the Stern-Volmer fitting.
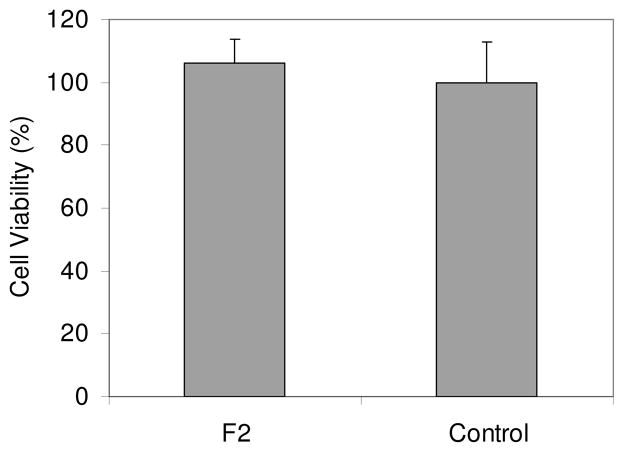
Toxicity to cells
Toxicity to A549 cancer cells was investigated using F2 films evaluated by a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) viability assay. The MTT assay is based on intracellular reduction of a tetrazolium dye to a formazan product measured spectrophotometrically, and is used for high-throughput screening.19 No inhibition for cell proliferation or cytotoxicity was observed after 24 hours of incubation of the cancer cells in the presence of the sensing membranes (Figure 7). The non-toxicity demonstrated a good biocompatibility of the new sensing membranes. Although the oxygen sensor contains the heavy metal platinum atom, it is chemically linked with the polymer matrix to prevent its leaching into the medium, which endows the sensing membranes to be nontoxic.
Figure 7.
Viability (MTT) of A549 cells incubated in the presence of the sensing membrane F2 in DMEM for 24 hours.
Storage stability
All of the sensing films can be stored in the dark at room temperature for at least 6 months. Because of the non-leaching character of the chemically conjugated sensing membranes, the films of 2 in PHEMA and PS matrices can be stored in aqueous solution for at least 3 months without alteration of their sensing performances.
Validation
The oxygen response functions were tested in pH 7.5 buffers equilibrated with oxygen and nitrogen gas mixtures with 5, 8, 20, and 27% of oxygen corresponding to pO2 of 5.1, 8.1, 20.2 and 27.4 kPa using F2 film. Experiments were performed at room temperature. The tested results are given in Table 3. The numbers in the parentheses in the table are the differences from the measured values and the adjusted values of the tested solutions. It can be seen that the sensing films gave good DO determinations not exceeding ±7.4% of the set values.
Table 3.
| Set value | Determined valuec | Difference in % |
|---|---|---|
| 5.1 | 5.2 (+0.1) | +2.0 |
| 8.1 | 7.5 (−0.6) | −7.4 |
| 20.2 | 20.6 (+0.4) | +2.0 |
| 27.4 | 28.8 (+1.4) | +5.1 |
pO2 represents the oxygen partial pressure in kPa;
experiment performed at atmosphere pressure;
values in the parentheses were the differences between the measured and set values.
3. Conclusion
A new oxygen sensor was successfully prepared through a simple chemical modification of a popularly used oxygen sensor PtTFPP. Using the reactivity of methacrylate active groups, the new sensor was able to be chemically embedded into hydrophilic PHEMA and hydrophobic PS for dissolved oxygen sensing studies. Physically incorporated PtTFPP, which is the precursor of the new sensor, in the same PHEMA and PS was also evaluated as a counterpart for a demonstration of the advantages of the chemically conjugated approach. Results showed that the chemically bonded sensors (i) alleviated the leaching problem of the sensors from their matrices and (ii) improved sensors’ photostability. It was also demonstrated that the biocompatible PHEMA gel is a good matrix for oxygen sensing, because the ability for dissolved oxygen to diffuse in the PHEMA matrix is significantly larger than that in the PS matrix, resulting in faster response time and higher oxygen sensitivity for dissolved oxygen in the PHEMA matrix than in the hydrophobic PS matrix. pH values did not interfere with the sensors. However, the media (LB and DMEM) had influences on the emission intensity and response to oxygen, suggesting that the components in the media (protein, amino acids, sugar, and/or yeast extract) likely interact with the sensor molecules. Temperature is critical in affecting the quenching velocity. Higher temperature results in a faster quenching velocity although the emission intensity decreases.
Thus, we have studied new hydrophilic PHEMA hydrogel-based oxygen sensing films in detail. We have also demonstrated the advantages of the chemically crosslinked oxygen sensor films. It is expected that using the crosslinking method to chemically conjugate oxygen sensor molecules to the biocompatible hydrophilic matrix will provide a new avenue to broaden the oxygen sensor’s applications in biological fields. Using the sensing film, which is biocompatible and suitable as a 3D scaffold for tissue engineering, for more understanding of cell growth, proliferation and metabolism is expected and in progress.
4. Experimental section
4.1 Materials and reagents
All chemicals and solvents were of analytical grade and were used without further purifications. Anhydrous ethylene glycol, sodium hydride (NaH), methacryloyl chloride, N,N′-dimethyl formamide (DMF), trimethylsilylpropyl acrylate (TMSPA), styrene, HEMA, azobisisobutyronitrile (AIBN), and acrylamide were commercially available from Aldrich and used without further purification. Ethyloxylate trimethylolpropane triacrylate (SR454®) was a commercial product of Sartomer Company (Exton, PA). PtTFPP was commercially available from Frontier Scientific Inc. (Logan, UT). Doubly distilled water was used for the preparation of the buffer solutions. The pH values were determined with a digital pH meter (Thermo Electron Corporation, Beverly, MA) calibrated at room temperature (23 ± 2 °C) with standard buffers of pH 10.01, 7.00, and 4.01. Britton-Robinson (B-R) buffers composed of acetic acid, boric acid, phosphoric acid, and sodium hydroxide were used for tuning pH values. Calibration gases (nitrogen and oxygen, each of 99.999% purity) were purchased from AIR Liquide America, LP (Houston, TX). Exact gas percentage was controlled using a home-made gas manipulator. All measurements of sensing behaviors were carried out at atmospheric pressure (760 mmHg, or 101 kPa) at room temperature (23 ± 2°C) unless specified. Quartz glass from University Wafer (South Boston, MA) was cut into squares of 1.31 cm × 1.31 cm and 0.36 cm × 0.36 cm using a dicer (Microautomation, Billerica, MA) for fluorescence measurement. DMEM was purchased from Invitrogen (Carlsbad, California). LB medium was made of 10 g tryptone, 5 g yeast extract and 10 g NaCl in 1 L distilled water.
4.2 Instruments
Varian liquid-state NMR operated at 400 MHz for 1H NMR, 100 MHz for 13C NMR, and 376 MHz for 19F NMR were used for spectra measurements. Matrix Assisted Laser Desorption/Ionization Time-of-Flight (MALDI-TOF) Mass Spectrometry was performed by the ASU Mass Spectrometry Laboratory. An oxygen plasma cleaner (Harrick Plasma, Ithaca, NY) was used for quartz glass surface activation. Shimadzu RF-5301 spectrofluorophotometer (Shimadzu Scientific Instruments, Columbia, MD) was used for fluorescence measurements. For an easy measurement of the films in liquid solutions, the quartz glass was cut with a dicer into squares of 1.31 cm × 1.31 cm, which can fit diagonally into quartz fluorescence cuvettes to enable the sensing membrane be positioned at an angle of 45° to the excitation light. Contact angle measurements were made using an FTA 1000C instrument (NanoDispense Inc, Fremont, CA). Temperature was adjusted from 20 to 37 °C using a Fisher Scientific Isotemp 202S water bath with a temperature variation accuracy of ± 0.5°C.
4.3 Quartz substrate modification using TMSPA
In order to achieve stable polymer membranes on the quartz glasses, the quartz surface was modified with TMSPA. Detergent and acetone-cleaned quartz glass was treated with oxygen plasma for ~ 30 minutes to generate active hydroxyl groups. Immediately following plasma treatment, the quartz glass was placed in a vacuum chamber to graft a thin layer of TMSPA on its surface under vacuum for 20 h using a known vapor deposition approach.8
4.4 Preparation of PHEMA polymer membranes
0.1 mg, 1 mg, or 10 mg of the sensor monomer 2 or PtTFPP, HEMA (800 mg), acylamide (150 mg), SR454 (50 mg), and AIBN (10 mg) were dissolved in 1 mL DMF as stock solutions. 10 μL of the stock solution were added onto the surface of the TMSPA-modified quartz glass and covered with a clean but untreated cover slip to make a sandwich structure. The thickness was controlled using 25-μm Kapton tape (DuPont, Wilmington, DE). The sandwich set-up was placed into a vacuum oven, which was evacuated and refilled with nitrogen three times. Polymerization was carried out under nitrogen at 80°C for 1.5 hours in the oven. The quartz glasses with polymer membranes were removed from the oven, with Kapton tape and non-surface modified glass being removed from the polymerized membrane surface. The uniform polymer membranes on the quartz glasses were washed three times using methanol to remove any remaining non-polymerized monomers and residual DMF. The films were dried and stored in the dark at room temperature.
4.5 Preparation of PS polymer membranes
The preparation of the PS polymer membranes was similar to that of PHEMA, however, styrene was used instead of HEMA and acrylamide.
4.6 Release study
The sensor membranes were immersed in the B-R pH 7.5 buffer and methanol for a certain time. Spectra were taken under the same optical set-up as before. For test whether there is any sensors’ emission in the buffer or methanol, the sensing film was taken out from the cuvette and the emission of the liquids were measured.
4.7 Photostability study
The sensing membranes in pH 7.0 B-R buffer were continuously exposed by excitation light at 405 nm (0.2 mW/cm2) over a period of 5 h. The membrane fluorescence intensity was recorded with an interval of 2 min.
4.8 Response time study
Sensing films were placed diagonally into a quartz cuvette with approximately 2 mL of B-R buffer (pH 7). Emission measurements were taken every four seconds at the sensor’s peak emission at 650 nm excited at 405 nm. To vary the concentration of dissolved oxygen in the buffers, a gas manifold was used to control gas flow through a tube and needle to bubble the buffer. Holes were installed in the cuvette cap to allow the needle into the cuvette and gas flow out. The needle was positioned so the bubbling would occur in the buffer just above the sensor film to decrease the possible interference by the gas bubbles, which may affect the fluorescence measurements. Oxygen and nitrogen flow rates were set at 66 standard cubic centimeters per minute. In all trials, the buffer was first saturated with oxygen before beginning the measurements. Measurements began with the change from 100% oxygen flow into the buffer to 100% nitrogen, and then continued as the gas flow composition was switched between oxygen and nitrogen. The intervals of gas flow depended on the type of film. For the PHEMA films, 360 seconds was the interval for nitrogen and oxygen bubbling; for the PS films, 660 seconds was used as the interval.
4.9 MTT assay for cytotoxicity study
The assay was performed by an in vitro MTT-based toxicology assay kit (Sigma). A549 cancer cells (American Type Culture Collection (ATCC), Manassas, VA) were cultured in DMEM supplemented with 10% fetal bovine serum, 5% penicillin, 2 mM L-glutamine (Sigma), and incubated at 37 °C in a 5% CO2 atmosphere. The cells were seeded onto 96-well plates at 10,000 cells per well, and incubated for 1 day. The sensing membranes (0.36 cm × 0.36 cm) on the quartz glasses were immersed into the DMEM growth medium and the cells with the sensing membranes were incubated in the 96-well plates. 24 Hours later, the membranes were removed from the 96-well plates. The medium in the wells were removed and the cells were washed with PBS buffer and then incubated in fresh DMEM medium (100 μL) and 10 μL of MTT solution (5 mg/mL) in 5% CO2 at 37°C for another 3 h. 60 μl of the culture medium was removed and 50 μl of DMSO was added to each well to dissolve the internalized purple formazan crystals by gentle pipetting up and down. The absorbance was measured at a wavelength of 490 nm using SpectraMax 190 from Molecular Devices (Downingtown, PA, USA). Each experiment was conducted twice in triplicate. The result was expressed as a percentage of the absorbance of the blank control.
4.10 Synthesis of compound 1
180 mg of 40% NaH oil (3 mmol) was added to a solution of 2.2 g of ethylene glycol (35.48 mmol) in 30 mL dry THF. The mixture was stirred at room temperature for 30 minutes, and then PtTFPP (720 mg, 0.63 mmol) was added in one portion. The mixture was stirred at room temperature for 24 hours to complete the reaction. After THF was removed, the residue was poured into sodium chloride solutions. The product was extracted into ethyl acetate, dried over Na2SO4, and purified using column chromatography with chloroform/methanol (10:1 by volume) to get 600 mg of compound 1. Yield: 71%. 1H-NMR: (400 MHz, CD3COCD3, δ ppm) 9.21 (s, 8H), 4.68 (t, 8H), 4.23 (m, 4H), 4.08 (t, 8H). 13C NMR (100 MHz, CD3COCD3, δ in ppm): 150.53, 148.10, 145.35, 144.30, 142.89, 142.75, 134.29, 115.29, 115.09, 114.90, 109.88, 79.62, 63.80. 19F-NMR (376 MHz, CD3COCD3, δ in ppm, CF3COOH = −76.55 ppm): −136.96 (8F, dd, JF-F = 14 Hz, JF-F = 28 Hz), −153.29 (8F, dd, JF-F = 14 Hz, JF-F = 28 Hz). MALDI-TOF-Mass: C52H29N4O8F16Pt: calc: 1335.85; Measured: 1335.16.
4.11 Synthesis of compound 2
Compound 1 (50 mg, 0.037 mmol) and 300 mg triethylamine (2.97 mmol) were dissolved in 5mL DMF and cooled to 0°C. To this solution, 100 μL (~108 mg, 1.0 mmol) of methacryloyl chloride in 500 μL of THF were added slowly. The mixture was stirred at room temperature overnight. Thin layer chromatography (TLC) was used to verify that the reaction was completed. The mixture was poured into water. Organic product was extracted into ethyl acetate, dried over Na2SO4. After the ethyl acetate was removed, the residue was washed with hot methanol twice and filtered to yield 40 mg sensor 2 with a yield of 64%. 1H-NMR (400 MHz, DMSO-d6, δ ppm): 9.22 (s, 8H), 6.16 (s, 4H), 5.79 (s, 4H), 4.82 (t, 8H), 4.60 (t, 8H), 1.96 (s, 12H). 13C NMR (100 MHz, DMSO-d6, δ in ppm): 166.93, 147.66, 145.28, 142.78, 141.49, 140.29, 138.77, 136.20, 132.56, 126.65, 112.73, 112.53, 107.09, 73.59, 64.10, 18.40. 19F-NMR (376 MHz, DMSO-d6, δ in ppm, CF3COOH = −76.55 ppm): −141.11 (8F, d, JF-F = 18 Hz), −156.91 (8F, d, JF-F = 18 Hz).
Supplementary Material
Acknowledgments
This work was supported by a grant from the NIH National Human Genome Research Institute, Centers of Excellence in Genomic Science, Grant Number 5 P50 HG002360. We also would like to thank Dr. Yongzhong Li at Center for Ecogenomics for the evaluation of the toxicity of the sensor films.
Footnotes
Supporting Information Available: Pictures of a typical thin film, absorbance and emission spectra of PtTFPP, compound 1, and compound 2 in THF solutions, and the photo-stability of PtTFPP in 5% THF-containing B-R 7 buffer. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Amao Y. Mirochim Acta. 2003;143:1. [Google Scholar]; (b) Nagl S, Wolfbeis OS. Analyst. 2007;132:507. doi: 10.1039/b702753b. [DOI] [PubMed] [Google Scholar]; (c) Hitoshi H, Tsuneo I, Toshiaki M, Kazahiro K. J Oceanogr. 2006;62:99. [Google Scholar]; (d) Clark LC. Trans Am Soc Art Int Org. 1956;2:41. [Google Scholar]; (e) Evans NTS, Naylor PFF. Resp Physiol. 1967;3:38. doi: 10.1016/0034-5687(67)90021-7. [DOI] [PubMed] [Google Scholar]; (f) Huch R, Lubbers DW, Huch A. Arch Dis Child. 1974;49:213. doi: 10.1136/adc.49.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Köneke R, Comte A, Jürgens H, Kohls O, Lam H, Scheper T. Chem Eng Tech. 1999;22:666. [Google Scholar]; (h) Mitsubayashi K, Wakabayashi Y, Murotomi D, Yamada T, Kawase T, Iwagaki S, Karube I. Sensors Actuators B. 2003;95:373. [Google Scholar]; (i) Wang XD, Chen X, Xie ZX, Wang XR. Angew Chem Int Ed. 2008;47:7450. doi: 10.1002/anie.200801733. [DOI] [PubMed] [Google Scholar]; (j) Lidstrom ME, Meldrum DR. Nature Rev Microbiology. 2003;1:158. doi: 10.1038/nrmicro755. [DOI] [PubMed] [Google Scholar]; (k) Molter TW, McQuaide SC, Suchorolski MT, Strovas TJ, Burgess LW, Meldrum DR, Lidstrom ME. Sensors Actuators B. 2009;135:678. doi: 10.1016/j.snb.2008.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) García-Fresnadillo D, Marazuela MD, Moreno-Bondi MC, Orellana G. Langmuir. 1999;15:6451. [Google Scholar]; (b) Amao Y, Okura I. Sensors Actuators B. 2003;88:162. [Google Scholar]; (c) Xu H, Aylott JW, Kopelman R, Miller TJ, Philbert MA. Anal Chem. 2001;73:4124. doi: 10.1021/ac0102718. [DOI] [PubMed] [Google Scholar]; (d) McEvoy AK, McDonagh C, MacCraith BD. J Sol-Gel Science Technol. 1997;8:1121. [Google Scholar]; (e) Roche P, Al-Jowder R, Narayanaswamy R, Young J, Scully P. Anal Bioanal Chem. 2006;386:1245. doi: 10.1007/s00216-006-0787-5. [DOI] [PubMed] [Google Scholar]
- 3.(a) Borisov SM, Vasylevska AS, Krause C, Wolfbeis OS. Adv Funct Mater. 2006;16:1536. [Google Scholar]; (b) Kimura F, Khalil G, Zettsu N, Xia Y, Callis J, Gouterman NM, Dalton L, Dabiri D, Rodriguez M. Meas Sci Technol. 2006:254. [Google Scholar]; (c) Köse ME, Carrol BF, Schanze KS. Langmuir. 2005;21:9121. doi: 10.1021/la050997p. [DOI] [PubMed] [Google Scholar]; (d) McDonagh C, Burke CS, MacCraith BD. Chem Rev. 2008;108:400. doi: 10.1021/cr068102g. [DOI] [PubMed] [Google Scholar]
- 4.(a) Vander Donckt E, Camerman B, Herne R, Vandeloise R. Sensors Actuators B. 1996;32:121. [Google Scholar]; (b) DeRosa M, Mosher P, Yap G, Foscaneanu K, Crutchley R, Evans C. Inorg Chem. 2003;42:4864. doi: 10.1021/ic026230r. [DOI] [PubMed] [Google Scholar]; (c) Borisov SM, Klimant I. Anal Chem. 2007;79:7501. doi: 10.1021/ac0710836. [DOI] [PubMed] [Google Scholar]; (d) Pauly S. Polymer Handbook. In: Brandrup J, Immergut EH, editors. Permeability and Diffusion Data. 3. Chapter 6. John Wiley & Sons; New York: 1989. pp. 435–449. [Google Scholar]
- 5.(a) Lee SK, Okura I. Anal Commun. 1997;34:185. [Google Scholar]; (b) Han BH, Manners I, Winnik MA. Anal Chem. 2005;77:8075. doi: 10.1021/ac0511703. [DOI] [PubMed] [Google Scholar]
- 6.(a) Hejcl A, Lesny P, Pradny M, Michalek J, Jendelova P, Stulik J, Sykova E. Physiol Res. 2008;57(Suppl 3):S121. doi: 10.33549/physiolres.931606. [DOI] [PubMed] [Google Scholar]; (b) Bryanta SJ, Cuy JL, Hauch K, Ratner BD. Biomaterials. 2007;28:2978. doi: 10.1016/j.biomaterials.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Schneider GB, English A, Abraham M, Zaharias R, Stanford C, Keller J. Biomaterials. 2004;25:3023. doi: 10.1016/j.biomaterials.2003.09.084. [DOI] [PubMed] [Google Scholar]
- 7.(a) Chen X, Hui L, Foster DA, Drain CM. Biochemistry. 2004;43:10918. doi: 10.1021/bi049272v. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Samaroo D, Soll CE, Todaro LJ, Drain CM. Org Lett. 2006;8:4985. doi: 10.1021/ol060946z. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Králová J, Bříza T, Moserová I, Dolenský B, Vašek P, Poučková P, Kejík Z, Kaplánek R, Martásek P, Dvořák M, Král V. J Med Chem. 2008;51:5964. doi: 10.1021/jm8002119. [DOI] [PubMed] [Google Scholar]; (d) Hirohara S, Obata M, Alitomo H, Sharyo K, Ando T, Yano S, Tanihara M. Bioconjugate Chem. 2009;20:944. doi: 10.1021/bc800522y. [DOI] [PubMed] [Google Scholar]
- 8.Hong HG, Jiang M, Sligar SG, Bohn PW. Langmuir. 1994;10:153. [Google Scholar]
- 9.Li L, Walt DR. Anal Chem. 1995;67:3746. doi: 10.1021/ac00116a021. [DOI] [PubMed] [Google Scholar]
- 10.Rumyantseva VD, Ivanovskaya NP, Konovalenko LI, Tsukanov SV, Mironov AF, Osin NS. Russ J Bioorg Chem. 2008;34:239. [PubMed] [Google Scholar]
- 11.Xiang HF, Yu SC, Che CM, Lai PT. Appl Phys Lett. 2003;83:1518. [Google Scholar]
- 12.(a) Montheard JP, Chatzopoulos M, Chappard D. Polymer Reviews. 1992;32:1. [Google Scholar]; (b) Parker JW, Cox ME. J Polym Sci A: Polym Chem. 1988;26:1179. [Google Scholar]
- 13.(a) Fornasiero F, Krull F, Prausnitz JM, Radke CJ. Biomaterials. 2005;26:5704. doi: 10.1016/j.biomaterials.2005.02.028. [DOI] [PubMed] [Google Scholar]; (b) Wang Y, Tan G, Zhang S, Guang Y. Appl Surface Sci. 2008;255:604. [Google Scholar]
- 14.(a) Wolfbeis OS. J Mater Chem. 2005;15:2657. [Google Scholar]; (b) Vasylevska GS, Borisov SM, Krause C, Wolfbeis OS. Chem Mater. 2006;18:4609. [Google Scholar]; (c) Schröder CR, Polerecky L, Klimant I. Anal Chem. 2007;79:60. doi: 10.1021/ac0606047. [DOI] [PubMed] [Google Scholar]; (d) Kocincova AS, Arain SN, Krause C, Borisov SM, Arnold M, Wolfbeis OS. Biotechnol Bioeng. 2008;100:430. doi: 10.1002/bit.21793. [DOI] [PubMed] [Google Scholar]
- 15.Rooney PC, Daniels DD. Petrol Tech Q. 1998;Q2:97. [Google Scholar]
- 16.(a) Kneas KA, Demas JN, Nguyen B, Lockhart A, Xu W, DeGraff BA. Anal Chem. 2002;74:1111. doi: 10.1021/ac010867v. [DOI] [PubMed] [Google Scholar]; (b) Gao Y, Ogilby PR. Macromolecules. 1992;25:4962. [Google Scholar]
- 17.(a) Trettnak W, Gruber W, Reininger F, O’Leary P, Klimant I. Adv Space Res. 1996;8:139. doi: 10.1016/0273-1177(95)00870-k. [DOI] [PubMed] [Google Scholar]; (b) McDonagh C, MacCraith BD, McEvoy AK. Anal Chem. 1998;70:45. doi: 10.1021/ac970461b. [DOI] [PubMed] [Google Scholar]; (c) Stein EW, Grant PS, Zhu H, McShane MJ. Anal Chem. 2007;79:1339. doi: 10.1021/ac061414z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borisov SM, Mayr T, Klimant I. Anal Chem. 2008;80:573. doi: 10.1021/ac071374e. [DOI] [PubMed] [Google Scholar]
- 19.(a) Mosmann T. J Immun Methods. 1983;65:55. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]; (b) Carmichael J, Degraff WG, Gazdar AF, Minna JD, Michell JB. Cancer Res. 1987;47:936. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.