Abstract
Nanotechnology has gained a great deal of public interest due to the needs and applications of nanomaterials in many areas of human endeavors including industry, agriculture, business, medicine and public health. Environmental exposure to nanomaterials is inevitable as nanomaterials become part of our daily life, and as a result, nanotoxicity research is gaining attention. This review presents a summary of recent research efforts on fate, behavior and toxicity of different classes of nanomaterials in the environment. A critical evaluation of challenges and future needs for the safe environmental nanotechnology has been discussed.
Keywords: Nanomaterials, silver, gold, carbon, metal oxides, toxicity, environmental impact
Introduction
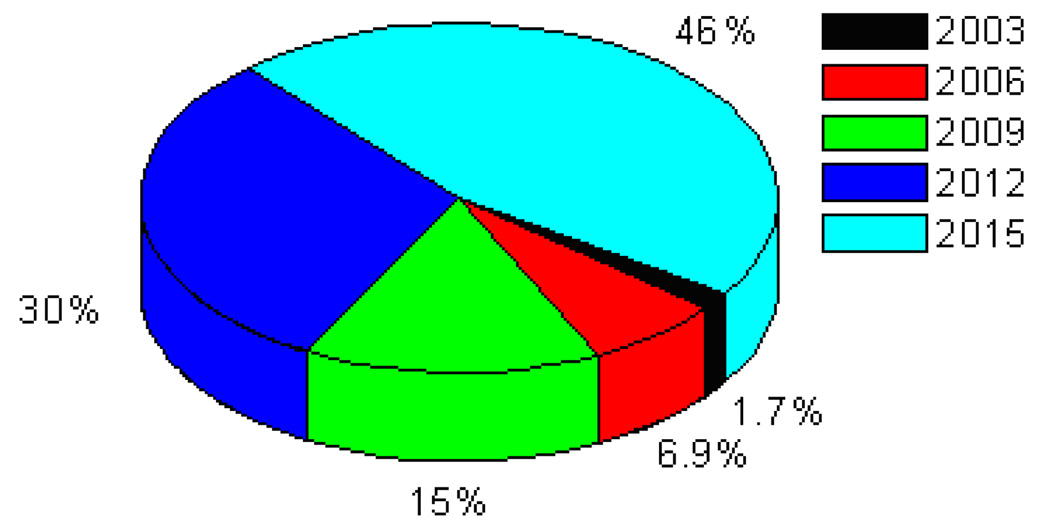
Many scientists consider nanotechnology as the next logical step in science, integrating engineering with biology, chemistry, medicine, and physics [1–30]. When the dimensions of a material become very small, its physical and chemical properties can become very different from those of the same material in bulk form. Current nanotechnology is building devices of microscopic or even molecular size, which will potentially be benefiting medicine, environmental protection, energy, and space exploration [15, 19–21, 23–25, 31–71]. With our ever increasing knowledge of nanoscience and the ability to engineer new products and services, it would not be far before the entire history can be compressed inside our pockets or the system extended by specially designed molecules that mimic the living systems. In the last couple of years, the term “Nanotechnology” has been inflated and has almost become synonymous for things that are innovative and highly promising [10, 16, 18, 25–30, 64–96]. Nanotechnology enables us to create functional materials, devices, and systems by controlling matters at the atomic and molecular scales, and to exploit novel properties and phenomena. Substantially smaller size, lower weight, more modest power requirements, greater sensitivity, and better specificity are just a few of the improvements we will see in sensor design. The fabrication of smaller and faster transistors has long been a driving force for the computer industry. As transistor sizes decrease to nanometer regime, we are approaching the point where nano-lithography will achieve the required resolution for creating these nanometer-sized devices. An obvious route when thinking about the very small is to shrink the size and cost of computers, and speed their operation phenomenally. Today's technology relies on etching patterns on silicon so that tiny electronic switches can be turned on and off, the basis for the binary code that represents everything the computer understands. Tomorrow's nanocomputers will have molecular switches, or logic rods, to place today's nanomaterial-based microbes injected into an organism to combat disease-causing bacteria and viruses, remove cancerous cells or dispense medicines. Microscopic robots may repair, or even assemble complex devices or remove harmful substances from the environment. There is no doubt that nanoscience and nanotechnology is one of the fastest growing research and technology areas. Nanotechnology has gained a great deal of public interest due to the needs and applications of nanomaterials in many areas of human endeavors including industry, agriculture, business, medicine and public health [97–101]. Between 1997 and 2005, investment in nanotechnology research and development by governments around the world soared from $432 million to about $4.1 billion, and corresponding industry investment exceeded that of the governments’ by 2005. By 2015, products incorporating nanotechnology will contribute approximately $1 trillion to the global economy (Figure 1). About two million workers will be employed in nanotechnology industries, and three times that many will have supporting jobs, as predicted by Lux Research [102].
Figure 1.
Predicted nanotechnology market based on Lux Research.
Engineered nanomaterials are rapidly becoming a part of our daily life in the form of cosmetics, food packaging, drug delivery systems, therapeutics, biosensors, and others. Since their size scale is similar to that of biological macromolecules and due to their antibacterial and odor-fighting properties, nanomaterials are extensively used for a number of commercial products such as wound dressing, detergents or antimicrobial coatings. New nanotechnology consumer products are coming on the market at the rate of 3–4 per week, a finding based on the latest update to the nanotechnology consumer product inventory maintained by the Project on Emerging Nanotechnologies [102–105]. According to the National Nanotechnology Initiative (USA) [106], thousands of tons of silica, alumina and ceria, in the form of ultrafine abrasive particle mixtures including nanoparticles, are used each year in slurries for precision polishing of silicon wafers. The manufacture of fullerenes could soon match the engineered metal oxide nanoparticles in production quantities, with the Kitakyushu plant (Mitsubishi, Japan) estimating an annual production of 1500 tons [107]. The Carbon Nanotechnology Research Institute (Japan) plans on expanding its production to 120 tons per year within the next five years. The worldwide production capacity for single-wall carbon nanotubes (SWCNT) and multi-wall carbon nanotubes (MWCNT) is estimated to be about 500 tons in 2008. During the last Summer Olympic games in Beijing, 803 products were used which contain nanomaterials, according to an analysis by the Woodrow Wilson International Center for Scholars’ Project on Emerging Nanotechnologies (PEN), a nonpartisan group [104].
Thus, the exposed population to nanomaterials continues to increase as their application expands. Despite obvious benefits of the power of small materials, there are open questions about how the nanoparticles used for day-to-day life may affect the environment. One of the crucial issues that have to be addressed in the near future, before massive fabrication of nanomaterials, is their toxicity to humans and impact on the environment. There are considerable debates regarding how the novel properties of nanomaterials could lead to adverse biological effects, with the potential to cause toxicity. One needs to understand when nanoparticles undergo biodegradation in the cellular environment, what will the cellular responses be? For example, biodegraded nanoparticles may accumulate within cells and lead to intracellular changes such as disruption of organelle integrity or gene alternations. Some of the crucial questions are: 1) Are nanomaterials more toxic than their non-nano counterparts? 2) Will nanoparticles transform in the environment into more toxic forms? Before nanomaterials are allowed to be used in daily life activities, it is important for nanotoxicology research to uncover and understand how nanomaterials influence the environment so that their undesirable properties can be avoided. To address issues concerning potential effects of emerging nanotechnologies on environment, this review discusses recent progresses on toxicity and environmental impact of nanomaterials.
Nanotechnology Promises
In 1959, Richard Feynman’s seminal talk on nanotechnology, “There’s Plenty of Room at the Bottom,” presented what was theoretically possible by manipulating matter at the atomic and molecular scales. Today, nanotechnology is an applied science, a rapidly growing industry generating a diverse array of nanoscale materials and processes. Manipulation of materials and processes on nanometer scale is opening a world of creative possibilities, and the benefits afforded by nanoscale technologies are expected to have substantial impacts on almost all industries and all areas of society. Nanotechnology is an emerging technology that not only holds promises for society, but also capable of revolutionizing our approaches to common problems. Nanotechnology is poised to have a major impact on science, food systems, agriculture, medicine, and the environment. The fabrication of smaller and faster transistors has long been a driving force for the computer industry. As transistor sizes decrease to the nanometer size regime, we are approaching the point where nano-lithography will achieve the required resolution for creating these nanometer-sized devices. An obvious outcome for making devices this small is to shrink the size and cost of computers, and at the same time, speed up their operation phenomenally. Today's technology relies on etching patterns on silicon so that tiny electronic switches can be turned on and off, the basis for the binary code that represents everything the computer understands. Tomorrow's nanocomputers will have molecular switches, or logic rods, to have today's electronic components to fit in a box of one hundredth of a cubic micron.
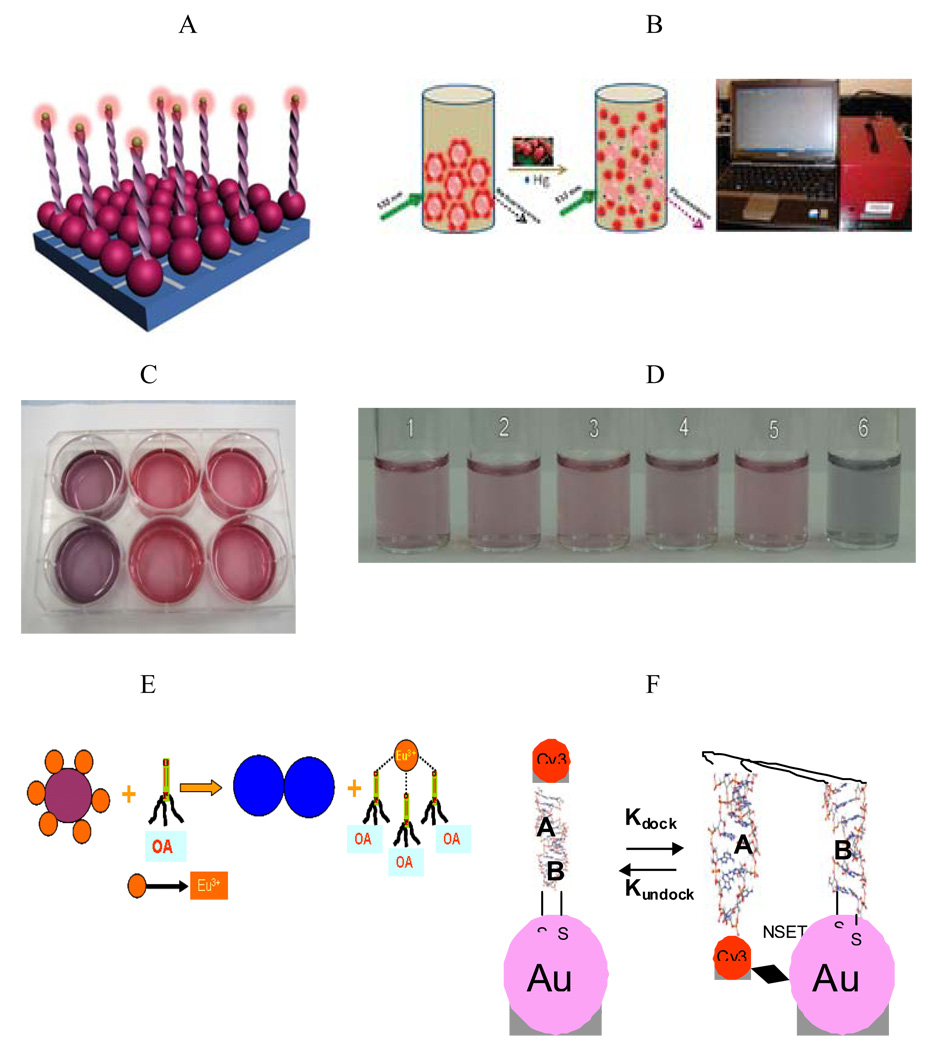
The nanoparticles are particularly useful because of their size (1 to 100 nm). Their extremely small size enables them to access a variety of biological environments; their size also endows them with valuable size dependent properties that can be exploited in applications. The benefits of nanotechnology make it ideal for sensor development, and environmental and biological monitoring (Figure 2). Finally, their large surface areas are platforms for engineering multifunctional systems for sensing.
Figure 2.
A) Schematic Representation of Gold Nanoparticle Based Surface Energy Transfer Probe for Pathogen DNA Detection, (Reprinted from reference [31] with permission). B) Schematic Representation of Gold Nanoparticle Based Mercury Detection and potable probe (Reprinted from reference [51] with permission). C) Colorimetric Assay for Pathogen RNA Detection, (Reprinted from reference [108] with permission). D) Colorimetric Assay for Mercury Detection from environmental sample, (Reprinted from reference [109] with permission) E) Schematic Representation of Gold Nanoparticle Based Organo Phosphorous Agent Detection, (Reprinted from reference [59] with permission). F) Schematic Representation of Gold Nanoparticle Based Assay for RNA Folding (Reprinted from reference [60] with permission).
There are many points of intersection between nanoscience and nanotechnology and the biological sciences [1, 14, 17, 20, 23, 24, 26, 27, 35, 74]. Indeed, the elementary functional units of biological systems comprise complex nanoscale components. Nanotechnology dreams to build repair machines of the size of a bacterium. They can enter and leave cells, destroy intruders in the blood vessels, and even check DNA for errors. Emerging biotechnology with nanoscience will allow us not only to take advantage of the improved evolutionary biological components to generate new smart sensors, but also to apply today's advanced characterization and fabrication techniques to solve environmental and biological problems. Miniaturized sensors developed through nanotechnology could also be used to detect specific bioagents accidentally or deliberately released into the environment. There will be no need for any guidance system since sensors on the front of the sub will let it try a different path if it has bumped into different objects. It will break down and release anything that its nanocomputer has been programmed for, including unwanted fat deposits. A fleet of such nanosubs could certainly cleanse the body of unwanted impurities.
Cancer nanotechnology is an interdisciplinary area of research in science, engineering, and medicine with broad applications for molecular imaging, molecular diagnosis, and targeted therapy [18, 74–76, 92, 93, 110, 111]. The basic rationale is that nanometer-sized particles, such as semiconductor quantum dots and iron oxide nanocrystals, have optical, magnetic, or structural properties that are not available from molecules or bulk solids. When linked with tumor targeting ligands such as monoclonal antibodies, peptides, or small molecules, these nanoparticles can be used to target tumor antigens as well as tumor vasculatures with high affinity and specificity. In the mesoscopic size range of 5–100 nm, nanoparticles also have large surface areas and functional groups for conjugating multiple diagnostic and therapeutic agents. Recent advances have led to bioaffinity nanoparticle probes for molecular and cellular imaging, targeted nanoparticle drugs for cancer therapy, and integrated nanodevices for early cancer detection and screening [8, 18, 92–94, 110, 112–116].
These developments raise exciting opportunities for personalized oncology in which genetic and protein biomarkers are used to diagnose and treat cancer based on the molecular profiles of individual patients. Despite the promise that nanotechnology will improve our lives, the potential risks of the technology remain largely uncertain. Do manufactured nanomaterials pose risks to the environment? One concern about small particles that are less than 10 um is that they are respirable and can reach the alveolar spaces of the lungs. While researchers try to bridge the data gap to help risk assessors, experts around the world are emphasizing the needs to regulate and oversee manufacturers to minimize potential risks from exposure to nanomaterials for workers, consumers, and wildlife.
Transport and Fate of Nanomaterials
Nanotechnology is expected to be the basis of many of the main technological innovations of the 21st century. Given the increasing rates for nanomaterial production, the potential for their release in the environment and subsequent effects on ecosystem health is becoming an increasing concern that needs to be addressed [115, 117–132]. For this purpose, it is necessary first to understand the fate and behavior of manufactured nanomaterials in the environment. We need to determine a) whether nanomaterials retain their nominal nanoscale size, original structure, and reactivity in environmental systems? b) Is their effect on environmental system different from that of larger particles of the same material? Answers to these and other questions will guide the setting of regulatory guidelines that will provide adequate protection to ecosystems while permitting the advantages that nanotechnology offers to be fully developed.
Manufactured nanomaterials will enter the environment through intentional releases as well as unintentional releases such as atmospheric emissions and solid or liquid waste streams from production facilities. In addition, nanomaterials in paints, fabrics, and personal and health care products, including sunscreens and cosmetics, enter the environment proportional to their use. Emitted nanomaterials will ultimately deposit on land and water surface. Nanomaterials reaching in the land have the potential to contaminate soil, and migrate into surface and ground waters. Particles in solid wastes, waste water effluents, direct discharges, or accidental spillages can be transported to aquatic systems by wind or rainwater runoff. The biggest release in the environment can come from spillages associated with the transportation of manufactured nanomaterials from production facilities to other manufacturing sites, intentional releases for environmental applications, and diffuse releases associated with wear and erosion from general use.
Due to the very small size of engineered nanomaterials, inhalation exposure can potentially occur to airborne particles composed of nanomaterials covering a size range from a few nanometers to several micrometers in diameter. Nanomaterials may agglomerate into larger particles or longer fiber chains, which may change their properties and may impact their behavior in the indoor and outdoor environments as well as their potential exposure and entry into the human body [115, 117–132]. They can deposit in the respiratory system and have nanostructure-influenced toxicity due to high surface area, high surface activity, unusual morphology, small diameters, or degradation into smaller particles after deposition. Particles formed from the degradation or comminuting of nanomaterials may also present a potential risk if they exhibit nanostructure-dependent biological activity. Nanoparticles have high deposition efficiencies in the lungs of healthy individuals, and even higher efficiencies in individuals with asthma or chronic obstructive pulmonary diseases [130–132]. When inhaled, nanoparticles deposit dispersedly upon the alveolar surface, likely leading to a scattered chemo-attractant signal and resulting in lower recognition and alveolar macrophage responses. Stahlhofen et al.[132] have reported that the deposition of 20 nm particles is 2.7 times greater than 100 nm particles and 4.3 times greater than 200 nm particles. Kreyling et al. [131] have shown that higher deposition efficiencies occur in patients with asthma or chronic obstructive pulmonary disease than in healthy subjects, possibly due to decreased clearance ability. They found that there was less than 25% clearance of 50- and 100-nm particles during the first 24 hr after inhalation.
Skin can be exposed to solid nanoscale particles through either intentional or nonintentional means [120–123, 133–135]. The outer skin consists of a 10 µm thick, tough layer of dead keratinized cells (stratum corneum) that is difficult to pass for particles, ionic compounds, and water-soluble compounds. Intentional dermal exposure to nanoscale materials may include the application of lotions, creams, wound dressing, detergents and socks containing silver nanomaterials. Nano TiO2 and ZnO materials can be exposed as a sunscreen component or fibrous materials coated with nanoscale substances for water or stain repellent properties. Nonintentional exposure could involve dermal contact with anthropomorphic substances generated during nanomaterial manufacture or combustion. It remains unclear whether different types of nanomaterials will penetrate the skin and have toxicological impacts includes skin or other organ cytotoxicity, through accumulation in skin or metabolism to even smaller particles or due to photoactivated nanoparticles. The conditions of use may also impact the form of the compound. During typical consumer use, the particles may be released in one particular form, but under more stressful conditions, the form may change. For example, textiles are subjected to washing, drying, and ironing. During washing, warm or hot water along with detergent may increase the release of nanomaterials. Drying textiles will subject the nanofibers to heat and agitation. Ironing applies a significant amount of heat, pressure, and abrasion. Responsible development of any new materials requires that risks to health and the general environment associated with the development, production, use and disposal of these materials are addressed. This is necessary to protect workers involved in production and use of these materials, the public, and the ecosystem. However, it also helps to inform the public about the development of these new, potentially beneficial, materials. The reported possible risks of nanomaterials are summarized in Table 1.
Table 1.
Possible Risks of Nanomaterials
| Nanomaterials | Possible Risks | References |
|---|---|---|
| Carbon nanomaterials, silica nanoparticle | Pulmonary inflammation, granulomas, and fibrosis | [118, 129, 136, 137] |
| Carbon, silver and gold nanomaterials | Distribution into other organs including the central nervous system | [118, 119, 125] |
| Quantum dots, carbon and TiO2 nanoparticles | Skin penetration | [120–123] |
| MnO2, TiO2, and carbon nanoparticles | May enter brain through nasal epithelium olfactory neurons | [118, 119, 124, 128] |
| TiO2, Al2O3, carbon black, Co, and Ni nanoparticles | May be more toxic than micron sized particles | [126, 127, 129] |
Toxicity of Nanomaterials
While nearly anything can be toxic at a high enough dose, the more relevant question is: how toxic are nanomaterials at the potential concentrations at which they might be used? Any toxic effects of nanomaterials will be specific to the type of base material, size, shape and coatings. However, to determine and understand the toxic effects of nanomaterials, strategies and interpretation of the data must be done correctly and assumptions taken into consideration. In toxicity studies of nanoparticles, different research groups used different cell lines, culturing conditions, and incubation times. With our understanding about the nature of nanoparticles during toxicity test, it is difficult to compare results from different research groups and determine whether the cytotoxicity observed is physiologically relevant. Many biological models, including cells in culture, aquatic organisms including embryonic zebrafish (Danio rerio), and whole-animal tests such as rodents, currently are used to determine potential toxicological effects of chemicals. In urban atmospheres, diesel- and gasoline-fueled vehicles and stationary combustion sources have for many years contributed particulate materials throughout a wide size range including nanomaterials. The toxic effects of such particles are still being investigated with regulatory concerns moving from the traditional particles less than 10 µm in aerodynamic diameter and below. Experimental results indicate that increased toxicity of finer-sized particles. However, to determine and understand the toxic effects of nanomaterials, strategies and interpretation of the data must be done correctly and assumptions taken into consideration. The range of nanotechnology products is very extensive and they can be broken down into a number of different compound classes, including metals, metal oxides, carbon, and semiconductor nanomaterials. The following discussions will be based on nanomaterial classes.
Metal Nanomaterials
Metallic nanoparticles are among the most widely used types of engineered nanomaterials; however, little is known about their environmental fate and effects. While bulk gold has been known to be safe, due to extraordinary properties for nanoscale particles of gold, several groups have examined the cellular uptake and cellular toxicity of gold nanoparticles [138–141]. Chithrani et al. [140] have investigated the intracellular uptake of different sized and shaped colloidal gold nanoparticles. Their results indicate that kinetics and saturation concentrations are highly dependent on the physical dimensions of the nanoparticles, with uptake half-life of 14, 50, and 74 nm gold nanoparticles being 2.10, 1.90, and 2.24 hr, respectively. They measured the absolute gold concentrations in cells by digestion and subsequent inductively coupled plasma atomic emission spectroscopy studies. They found that 50 nm spheres were taken up more quickly by cells compared to both smaller and larger spheres in the 10–100 nm range and that spheres were taken up more efficiently than nanorods that had dimensions in the 10–100 nm range. Connor et al. [139] have examined the uptake and potential toxicity of a series of gold nanoparticles in human leukemia cells. Results suggest that spherical gold nanoparticles with a variety of surface modifiers are not inherently toxic to human cells, despite being taken up into cells. Our own group investigated shape and size dependent cellular uptake and cytotoxicity of gold nanomaterials on human skin HaCaT keratinocytes [141].
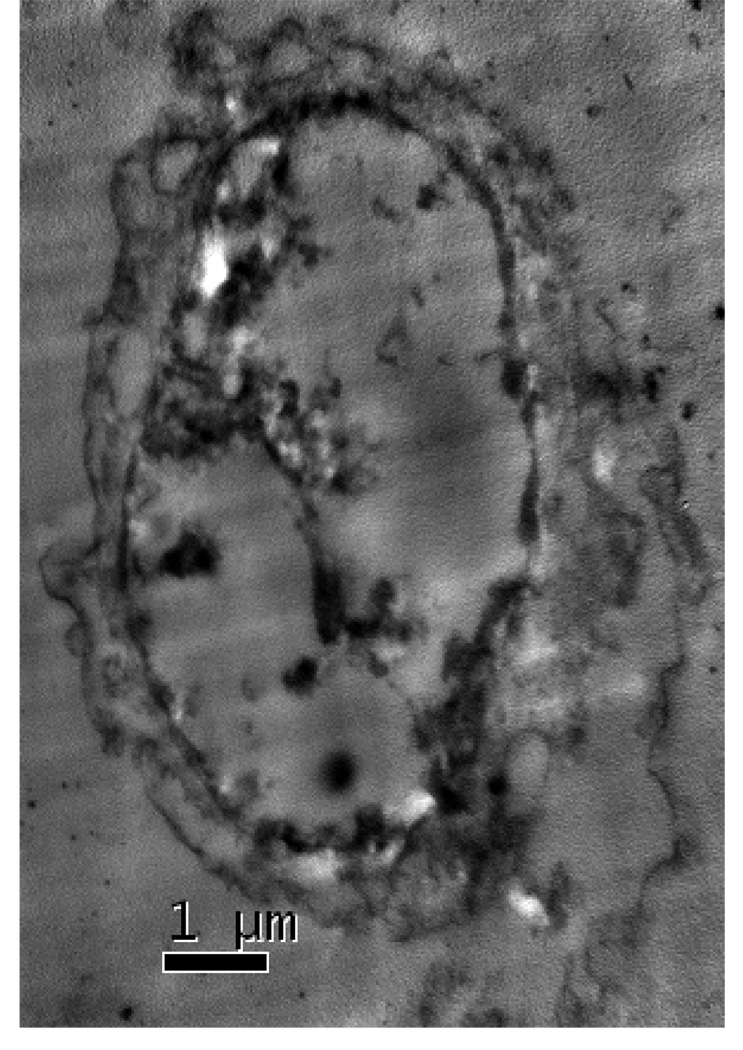
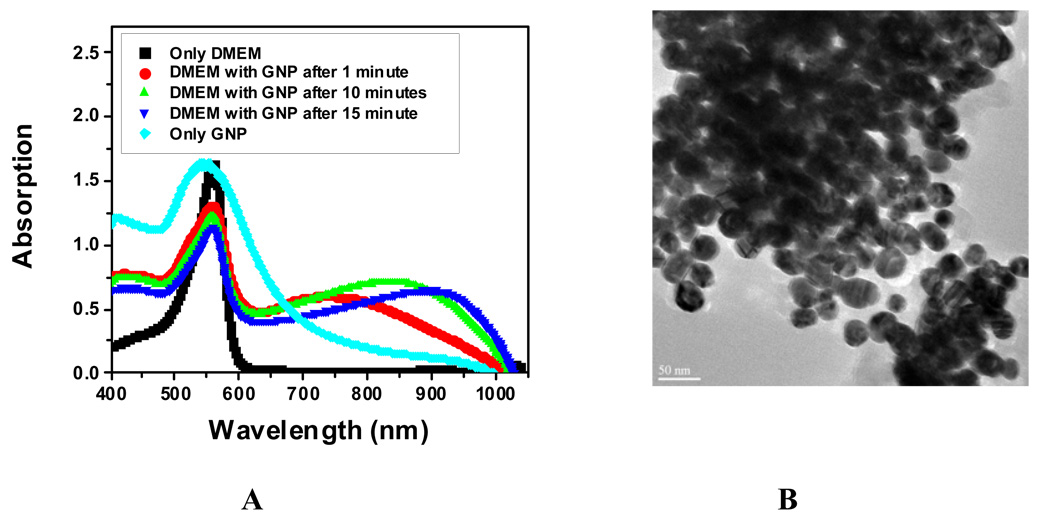
The transmission electron microscopy (TEM) data (Figure 3) shows that gold nanomaterials can penetrate through HaCaT cells easily and can accumulate in the cell nucleus. Figure 3 also indicates that gold nanomaterials are aggregated inside the cell. To understand whether gold nanomaterials are only aggregated inside the cell or may be aggregated outside the cell and then entered as aggregates, a time dependence study of gold nanopartcile’s surface plasmon resonance band (SPB) between 510–570 nm is carried out. SPB origin is attributed to the collective oscillation of the free conduction electrons induced by an interacting electromagnetic field. These resonances are also denoted as surface plasmons. The results indicate that the plasmon band shifts to longer wavelengths by about 150–400 nm (Figure 4A) in the presence of the cell culture medium DMEM, indicating strong aggregation of gold nanoparticles as it is confirmed by TEM image in Figure 4B. This aggregation is due to the presence of high concentration of sodium salt in DMEM. It also shows that the plasmon band shifts to longer wavelengths with time, indicating bigger cluster formation. Therefore, nanoparticles aggregate first outside the cell and enter the cell as aggregated form.
Figure 3.
TEM images showing gold nanomaterials uptake inside the cell. Gold nanoparticles (30 nm) are within the granular bodies. The TEM images show that gold nanoparticles are aggregated inside the cell.
Figure 4.
A: Time dependents absorption change in the presence of the cell media DMEM; B: TEM image of gold nanoparticles in the presence of DMEM (Reprinted from reference [141] with permission).
Similar results are also noted for nanorods and nanoprisms (Figure 5). We used the Olympus IX71 inverted microscope to view HaCaT cells, before and after exposure to gold nanomaterials. An illumination lamp (100 W) attached to a condenser (0.55NA) was used as the white light source. Light passes through the cell and transmitted light was collected through a 40X, 0.6NA objective and imaged in a Firewire CCD camera.
Figure 5.
Bright field confocal microscope image of A) only cell, B) only gold nanorod, C) cell incubated with Au-nanorod (4.5 aspect ratio), D) cell incubated with gold nanopartilce (20 nm), E) cell incubated with gold nanoprism (80 nm).
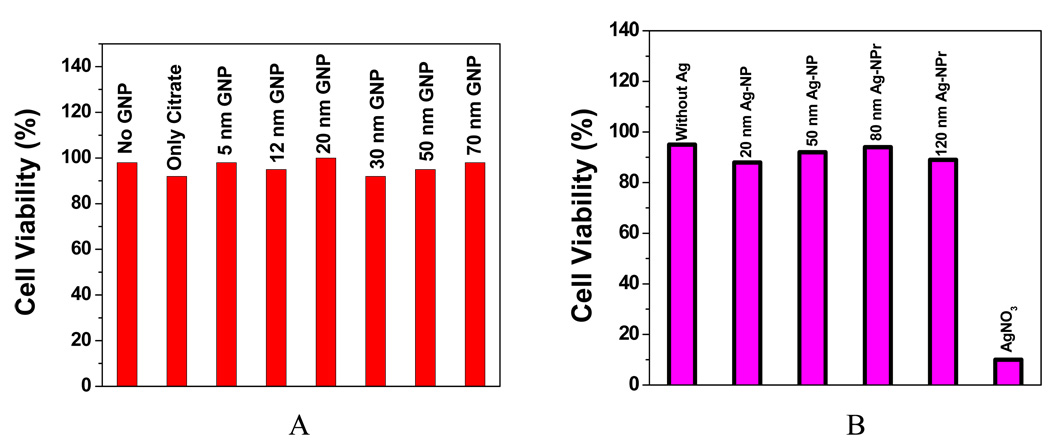
To make sure that cellular uptake reaches maximum, all our results are for 24 hr incubation. As shown in Figure 6A, no difference was found in cell viability between cell with and without gold nanoparticles of different sizes. This indicates that spherical gold nanoparticles of different sizes are not inherently toxic to human cells at our test concentration. To further confirm this, we also continuously exposed the cell for 48 hr and no toxicity was observed.
Figure 6.
A: Viability of cells incubated with gold nanoparticle of different sizes (Reprinted from reference [141] with permission); B: Viability of cells incubated with silver nanoparticles of different sizes along with silver nitrate.
Similar viability studies by Goodman et al. [142] with immune system cells also showed that gold nanoparticles were not cytotoxic and that they reduced the amount of potentially harmful reactive oxygen species in the cells. They investigated the toxicity of 2 nm gold nanoparticles functionalized with both cationic and anionic surface groups in three different cell types. The results suggested that cationic particles are generally toxic at much lower concentrations than anionic particles, which they relate to the electrostatic interaction between the cationic nanoparticles and the negatively charged cell membranes. It is important to differentiate between cytotoxicity and cellular damage. Nanoparticles that show little or no cytotoxicity via several standard assays may be still able to cause serious cellular damage. For example, 13 nm citrate-capped gold nanospheres were not toxic according to an assay in skin cells, but the particles did apparently promote the formation of abnormal actin filaments, which led to decreases in cell proliferation, adhesion, and motility as discussed by Pernodet et al [143].
Cytotoxicity also depends on the type of cells used. For example, 33 nm citrate-capped gold nanospheres were found to be not cytotoxic to baby hamster kidney and human hepatocellular liver carcinoma cells, but cytotoxic to a human carcinoma lung cell line at certain concentrations as reported by Patra et al. [144].
Exposure to high levels of silver over a long period of time can result in a condition called argyria, a blue-gray discoloration of the skin and other organs [145]. Lower-level exposure to silver is also known to cause silver to be deposited in the skin and other parts of the body. Exposure to high levels of silver in the air can result in breathing problems, lung and throat irritation, and stomach pains, while skin contact with silver can cause mild allergic reactions, such as rashes, swelling, and inflammation in some people. Carlson et al. [146] reported size-dependent cellular interactions of silver nanoparticles. Their result shows that after 24 hr of exposure, viability metrics significantly decreased with increasing dose (10–75 µg/mL) of Ag-15 nm and Ag-30 nm nanoparticles. A more than 10-fold increase of ROS levels in cells exposed to 50 µg/mL Ag-15 nm suggests that the cytotoxicity of Ag-15 nm is likely to be mediated through oxidative stress. We have reported [134] the viability of HaCaT cells exposed to silver nanoparticles and nanoprisms at 100 µg/ml with different sizes are shown in Figure 6B. To make sure that cellular uptake is at the maximum, cells were exposed to nanoparticles for 24 hr under incubation. As shown in Figure 6B, there was no difference in cell viability between cells treated or not treated with silver nanoparticles and nanoprisms of different sizes. This indicates that spherical silver nanoparticles and silver nanoprisms of different sizes are not inherently toxic to human skin keratinocyte cells. To further confirm the lack of toxicity, the exposure was continued to 48 hr and there was no observable toxicity (data not shown). However, silver nitrate in solution was highly toxic; exposure to 100 µg/ml of silver nitrate for 24 hr resulted in 90% reduction of cell viability.
Griffitt et al. [147] reported the effects of particle composition on toxicity of metallic nanomaterials in aquatic organisms. They have used zebrafish, daphnids, and an algal species as a model of various trophic levels and feeding strategies. Different organisms were exposed to silver, copper, aluminum, nickel, and cobalt as both nanoparticles and soluble salts as well as to titanium dioxide nanoparticles. Their results shows that nanosilver and nanocopper cause toxicity in all organisms tested, with 48-hr median lethal concentrations as low as 40 and 60 µg/L, respectively, in Daphnia pulex adults, whereas titanium dioxide did not cause toxicity in any of the tests. Asharani et al. [148] have reported toxicity of silver nanoparticle in zebrafish model. Using starch and bovine serum albumin (BSA) as capping agents, silver nanoparticles were synthesized to study their deleterious effects and distribution pattern in zebrafish embryos. TEM of the embryos demonstrated that nanoparticles were distributed in the brain, heart, yolk and blood of embryos as evident from the electron-dispersive x-ray analysis. Their results indicate that silver nanoparticles induce a dose-dependent toxicity in embryos, which hinders normal development. As we reported before, silver nanoparticles could be toxic because they release silver ions, which are well known for their antibacterial and other destructive behaviors.
Manufacturers of clothing articles employ nanosilver as an antimicrobial agent, but the environmental impacts of nanosilver release from commercial products are unknown. Benn et al. [149] investigated silver released from commercial clothing (socks) into water, and its fate in waste water treatment plants. Their results suggest that both colloidal and ionic silver leach from the socks. Variable leaching rates among sock types suggests that the sock manufacturing process may control the release of silver. The adsorption of the leached silver from wastewater treatment plants was used to develop a model, which predicts that a typical wastewater treatment facility could treat a high concentration of influent silver.
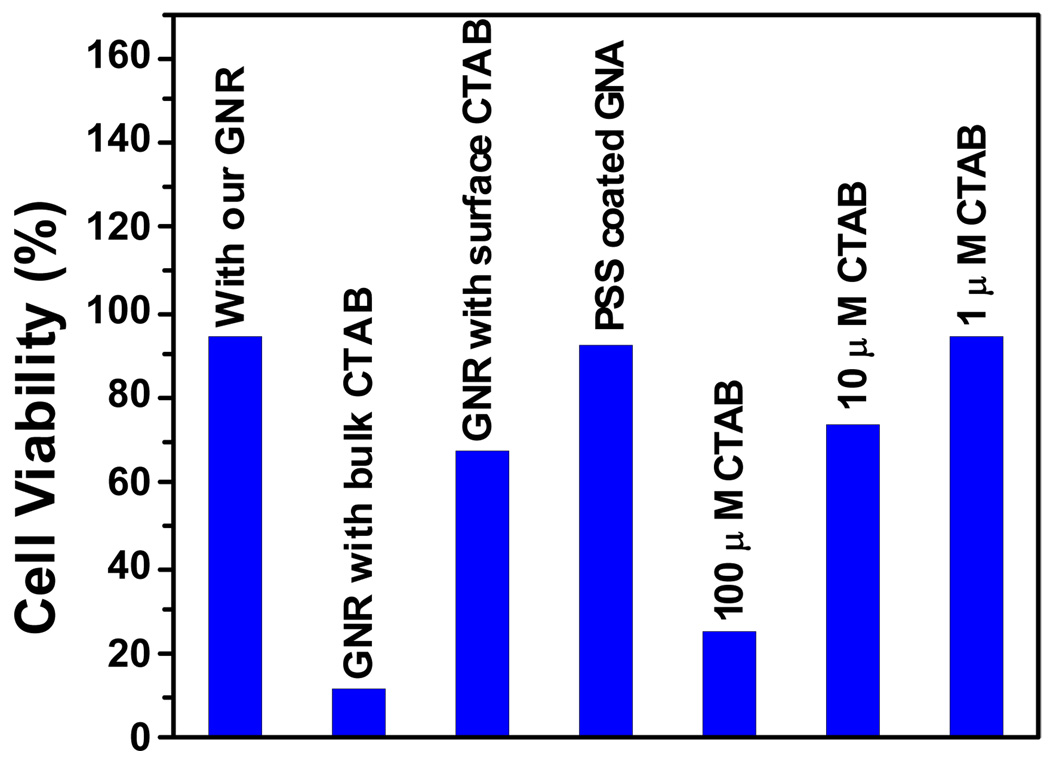
We recently reported [141] the effect of gold nanorods on human skin HaCaT keratinocyte cells (Figure 7). It indicated that gold nanorods are highly toxic. This was surprising since gold nanoparticles are not toxic, while gold nanorods, synthesized from gold nanoparticles of small size using a seed-mediated, surfactant-assisted growth method, are highly toxic. Hexadecylcetyltrimethylammonium bromide (CTAB) is a unique surfactant towards the synthesis of nanorods and is widely used. CTAB selectively forms a tightly packed bilayer on the side faces of nanorods, which leads the ends of the rods to be more exposed to facilitate anisotropic growth along the longitudinal axis. Also, CTAB-coated nanorods are positively charged. Their mutual repulsion prevents aggregation so that gold nanorods are stable in solution. Since the extra chemical present in gold nanorods that is not in gold nanoparticles is CTAB, we studied the toxicity of CTAB (Figure 7). Our results indicate that CTAB alone has the similar toxicity as the nanorods at the concentration of 100 µM. Concentration dependent study shows that CTAB is toxic even at 10 µM. In gold nanorods, there is nanorod-bound CTAB as well as excess free CTAB in solution, which was not used during nanorod formation. The excess CTAB left in the solution can be removed by centrifugation. Our data indicate that cell viability increases with centrifugation. After three centrifugations, the estimated excess CTAB in solution should be less than 1 µM. As shown in Figure 7, Figure 1 µM CTAB is not toxic. Therefore, we do not expect toxicity after three centrifugations. Our experimental data indicate there is 40% cell death even after three centrifugations (Figure 7). We believe this is due to the fact that nanorod-bound CTAB layer will eventually enter solution in the presence of cell media due to aggregation of nanorods. We tried to remove further CTAB from the nanorod-bound CTAB layer, and our experimental results shows that nanorods are not stable if CTAB is removed. This is due to the lack of repulsive interaction among individual nanorods and, as a result, nanorods break to nanoparticles and some nanorods undergo aggregation.
Figure 7.
Viability of human skin HaCaT keratinocyte cells incubated with gold nanorod (GNR) coated with CTAB, CTAB only, or PSS coated GNR (Reprinted from reference [141] with permission).
To eliminate CTAB toxicity, we have exposed the CTAB-coated gold nanorods to poly(styrenesulfonate) (PSS) polyelectrolyte solution (Figure 8A), producing extra PSS layers outside of CTAB. The extra PSS in solution was separated by centrifugation of the nanorod solution at 8000 rpm. The pellet was redispersed in N-(2-hydroxyethyl)piperazine-N’-2-ethanesulfonic acid (HEPES) solution. The nanorods prepared by seed-mediated surfactant (CTAB) methods are positively charged. The positively charged surface of the nanorods changed to a negatively charged surface when replaced with PSS.
Figure 8.
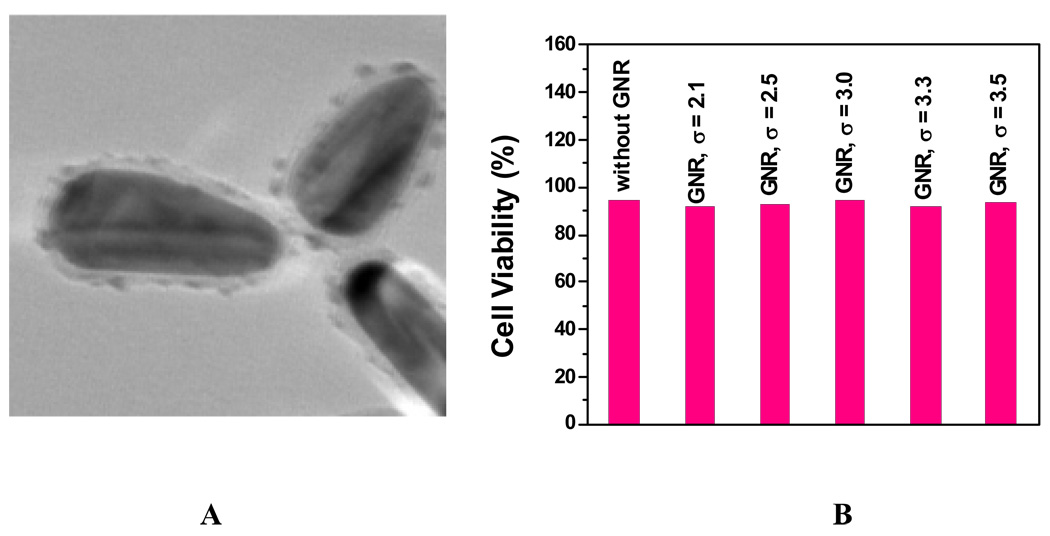
A: TEM Images of PSS-coated gold nanorods; B: Viability of cells incubated with PSS-coated gold nanorods (GNR) of different aspect ratios. (Reprinted from reference [141] with permission).
Figure 8B shows the cell viability for PSS-coated gold nanorods with different aspect ratios. Our results indicate that PSS-coated gold nanorods (TEM image in Figure 7A) are not toxic. Therefore, the toxicity of gold nanorods is due to the presence of CTAB and replacing CTAB with biocompatible and functionalization friendly stabilizing agents like PSS efficiently removed CTAB toxicity and it may be essential for using gold nanorods in living cell.
Similar results have also been reported by Niidome et al. [150]. Their results show that CTAB-capped gold nanorods are cytotoxic, as judged by the MTT assay with a different cell line, HeLa cells. Also their results show that the cytotoxicity can be reduced by over-coating gold nanorods with poly-ethylene glycol (PEG), which is well-known to reduce nonspecific binding of biological molecules to surfaces. Takahashi et al. [151] have shown that phosphatidylcholine is another biocompatible over-coating molecule that reduces the reported cytotoxicity of CTAB-coated gold nanorods. Hauck et al. [152] have shown that through the use of various layer-by-layer polyelectrolyte (PE) coating schemes, such as the common poly(diallyldimethylammonium chloride)-poly(4-styrenesulfonic acid) (PDADMAC-PSS) system, the mammalian cellular uptake of gold nanorods can be tuned from very high to very low by manipulating the surface charge and functional groups of the PEs. The toxicity of these nanorods is also examined and found to be greater than 90% viable in nearly all cases, even at very high concentrations. Their results indicate that coated gold nanorods are well suited for therapeutic applications, such as thermal cancer therapy, due to their tunable cell uptake and low toxicity.
Metal Oxide Nanomaterials
Metal oxide nanoparticles are important industrial materials widely used as additives in cosmetics, pharmaceuticals, and food colorants. Skin is usually exposed heavily to solid nanoparticles through the application of lotions or creams containing nano-TiO2 or ZnO as a sunscreen component or fibrous materials coated with nanoscale substances for water or stain repellent properties. Since the manufacture and use of nanoparticles are increasing, humans are more likely to be exposed occupationally or via consumer products and the environment. Several groups have examined the uptake and toxicity of metal oxide nanoparticles. Grassian et al. [153] reported inhalation exposure study using 2–5 nm TiO2 nanoparticles. They showed that nanoparticles aggregate to form aerosol particles in the exposure chamber with a geometric mean of the mobility diameter between 120 and 130 nm. Analysis of lung responses in mice after subacute exposures to these aggregates showed a significant but modest inflammatory response among animals necropsied at week 0, 1, or 2 after the last exposure with recovery at week 3 post-exposure. Jin et al. [154] used mouse fibroblast (L929) cells to evaluate the cytotoxicity of different concentrations of homogeneous and weakly aggregated TiO2 nanoparticles in aqueous solution. Their result shows that there is a significant increase in oxidative stress at higher TiO2 nanoparticle concentrations (>60 µg/mL). As the concentration of TiO2 nanoparticles increased in the culture medium, the levels of reactive oxygen species and lactate dehydrogenase increased. Park et al. [155] reported the cytotoxicity of TiO2 nanoparticles with the induction of ROS in cultured BEAS-2B cells. They have shown that nanoparticles penetrated into the plasma membrane and located in the peri-region of nuclear membranes, indicating that nanoparticles may have direct interactions with cellular molecules to cause adverse biological responses in cells. Their results show that with the induction of ROS, the expressions of oxidative stress-related genes including heme oxygenase-1 or inflammation-related genes including IL-8 were increased.
Limited phytotoxicity studies reported both positive and negative effects of nanoparticles on higher plants. TiO2 nanoparticles were reported to promote photosynthesis and nitrogen metabolism, and then improve growth of spinach at an optimal concentration [156, 157]. Alumina nanoparticles showed no adverse effect on the growth of California red kidney bean and ryegrass [158, 159]. However, they were reported to inhibit root elongation of corn, cucumber, soybean, cabbage, and carrot [160]. High concentrations of nano-sized ferrophase particles inhibited popcorn growth [161]. However, so far phytotoxicity mechanism remains unknown, and no information on the potential uptake of nanoparticles by plants and their subsequent fate within food chains is available. Lin et al. [162] examine cell internalization and upward translocation of ZnO nanoparticles by Lolium perenne (ryegrass). The root uptake and phytotoxicity were visualized by light, scanning electron, and transmission electron microscopies. Their result shows that in the presence of ZnO nanoparticles, ryegrass biomass significantly reduced, root tips shrank, and root epidermal and cortical cells highly vacuolated or collapsed. However, translocation factors of Zn from root to shoot remained very low under ZnO nanoparticle treatments.
Franklin et al. [163] reported comparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl2 to freshwater microalgae using the freshwater alga Pseudokirchneriella subcapitata. It revealed comparable toxicity for nanoparticulate ZnO, bulk ZnO, and ZnCl2, with a 72 hr IC50 value near 60 µg Zn/L. Cozzoli et al. [164] evaluated a human mesothelioma and a rodent fibroblast cell line for in vitro cytotoxicity tests using seven industrially important nanoparticles. Their response in terms of metabolic activity and cell proliferation of cultures exposed to 0–30 ppm nanoparticles (µg g−1) was compared to the effect of nontoxic amorphous silica and toxic crocidolite asbestos. Solubility was found to strongly influence the cytotoxic response. The result shows nanoparticle-specific cytotoxic mechanism for uncoated iron oxide and partial detoxification or recovery after treatment with zirconia, ceria, or titania. Reddy et al. [165] reported the toxicity of ZnO nanoparticles to both gram-negative and positive bacterial systems, Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus), and primary human immune cells. Collectively, their results demonstrate selectivity in the toxic nature of ZnO nanoparticles to different bacterial systems and human T lymphocytes.
Karlsson et al. [166] investigated different nanoparticles and nanotubes regarding cytotoxicity and ability to cause DNA damage and oxidative stress. Their study focused on different metal oxide nanoparticles (CuO, TiO2, ZnO, CuZnFe2O4, Fe3O4, Fe2O3), and the toxicity was compared to that of carbon nanoparticles and multi-walled carbon nanotubes (MWCNT). Their results showed that there was a high variation among different nanoparticles concerning their ability to cause toxic effects. CuO nanoparticles were the most potent regarding cytotoxicity and DNA damage. ZnO showed effects on cell viability as well as DNA damage, whereas the TiO2 particles (a mixture of rutile and anatase) only caused DNA damage. For iron oxide particles (Fe3O4, Fe2O3), no or low toxicity was observed, but CuZnFe2O4 particles were rather potent in inducing DNA lesions. Finally, the carbon nanotubes showed cytotoxic effects and caused DNA damage in the lowest dose tested. Xia et al. [167] reported oxidative stress and cytotoxicity of TiO2, ZnO, and CeO2, in RAW 264.7 and BEAS-2B cell lines. Their results indicate that ZnO induced toxicity in both cells, leading to the generation of ROS, oxidant injury, excitation of inflammation, and cell death. In contrast, CeO2 nanoparticles were taken up into caveolin-1 and LAMP-1 positive endosomal compartments, respectively, in BEAS-2B and RAW 264.7 cells, without inflammation or cytotoxicity. CeO2 also suppressed ROS production and induced cellular resistance to exogenous source of oxidative stress.
Carbon Nanomaterials
Carbon nanotubes (CNTs), with their unique one-dimensional hollow nanostructure and unusual properties, are emerging as an important new class of multifunctional building blocks for the development of nanotechnology. Recent rapid development in nanotechnology has renewed the pressing demand for large scale production of CNTs for applications in commercial products. The number of industrial scale facilities for the relatively low-cost production of multi-walled carbon nanotubes (MWCNTs) continues to grow, and therefore, professional and public exposure to MWCNTs is expected to increase significantly in the coming years. Several research groups have examined the uptake and potential hazards of CNTs, particularly MWCNTs, to humans and other biological systems. For instance, it has been demonstrated that CNTs can induce inflammatory and apoptosis responses in human T-cells [133, 168–170]. Gene expression analysis by Ding et al. [133] indicated that MWCNTs activated genes involved in cellular transport, metabolism, cell cycle regulation, and stress response in human skin fibroblasts. Magrez et al. [171] found evidence of cytotoxicity for carbon-based nanomaterials, although MWCNTs were the least toxic among the carbon nanotubes, carbon nanofibers, and carbon nanoparticles tested. In a somewhat related publication, Dumortier et al. [172] demonstrated that water-soluble CNTs functionalized with polyethylene glycol chains did not have toxic effects when tested in a wide variety of immune system cells.
Siliva et al. [173] demonstrated that ultrafine carbon particles show greater lung penetration than larger particles and are able to cross the blood-brain barrier and impact on the central nervous system. Their results indicate that toxic effects appear quickly after exposure and suggest that carbon nanoparticles travel from the lungs to the bloodstream rather than release clotting agents from the lungs. Since inhalation of asbestos fiber is known to induce asbestosis, lung cancer, and malignant mesothelioma of the pleura, there would seem to be a high probability that CNTs are also likely to have significant toxic effects on human health due to their structural resemblance to asbestos [174]. Several studies have indicated that CNTs exhibit substantial cytotoxicity in vitro, including induction of oxidative stress, inhibition of cellular proliferation, and induction of apoptosis/necrosis [173–180]. Lam et al. [137] have reported the impact of single-walled CNT (SWCNT) on lung tissue by instilling a suspension of SWCNT into the lungs of mice. Their results indicate that the SWCNT clump together into bundles and produce pulmonary inflammation together. Jia et al. [174] reported cytotoxicity results for SWCNTs, MWCNTs and fullerenes (C60) in vitro. Their results show that cytotoxicity increases by as much as ~35% when the dosage of SWCNTs increases by 11.30 µg/cm2. No significant toxicity was observed for C60 up to a dose of 226.00 µg/cm2. The cytotoxicity apparently follows a sequence on a mass basis: SWCNTs > MWCNTs > quartz > C60.
Zhu et al. [181] assessed the DNA damage response to MWCNTs in mouse embryonic stem (ES) cells. They found that MWNTs could accumulate and induce apoptosis in mouse ES cells and activate the tumor suppressor protein p53 within 2 hr of exposure. They also reported increased expression of two iso-forms of base excision repair protein 8-oxoguanine-DNA glycosylase 1 (OGG1), double strand break repair protein Rad 51, phosphorylation of H2AX histone at serine 139, and SUMO modification of XRCC4 following the treatment with MWCNTs. Carrero-Sanchez et al. [182] compared the toxicological effects between pure MWCNTs and N-doped MWCNT (CNx). Their results show that when MWCNTs were injected into the mice's trachea, the mice could die by dyspnea depending on the MWCNTs dose. However, CNx nanotubes never caused the death of any mouse. They have also found that CNx nanotubes were far more tolerated by mice when compared to MWCNTs. Extremely high concentrations of CNx nanotubes administrated directly into the mice’s trachea only induced granulomatous inflammatory responses. Muller et al. [183] reported that CNTs have the potential to induce adverse pulmonary effects, including alveolitis, fibrosis, and genotoxicity in epithelial cells. The CNTs were administered intratracheally (2 mg/rat) to Wistar rats to evaluate the short-term response (3 days) in bronchoalveolar lavage fluid. The long-term (60 days) lung response was assessed biochemically by measuring the lung hydroxyproline content and histologically. Their results show that the acute pulmonary toxicity and the genotoxicity of CNTs were reduced upon heating, but restored upon grinding, indicating that the intrinsic toxicity of CNTs is mainly mediated by the presence of defective sites in their carbon framework. Chou et al. [136] have demonstrated that intratracheal instillation of 0.5 mg of SWCNTs into male ICR mice (8 weeks old) induced alveolar macrophage activation, various chronic inflammatory responses, and severe pulmonary granuloma formation. Their experimental validation suggests that the uptake of SWCNTs into the macrophages is able to activate various transcription factors and activator protein 1 (AP-1).
Quantum Dots
Quantum Dots (QDs) are nanocrystals containing 1000 to 100,000 atoms and exhibiting unusual “quantum effects” such as prolonged fluorescence. With unique optical and electrical properties, QDs are currently applied in biomedical imaging and electronics industries. One of the more valuable properties of QDs is their fluorescence spectrum, which renders them as optimal fluorophores for biomedical imaging, e.g. fluorescent QDs can be conjugated with bioactive moieties to target specific biologic events and cellular structures such as labeling neoplastic cells, DNA, and cell membrane receptors [23, 110–114, 116, 184]. Each individual type of QD possesses its own unique physicochemical properties, which in turn determines its potential toxicity and as a result, there are discrepancies in the current literature regarding the toxicity of QDs and they can be attributed to several factors: the lack of toxicology-based studies, the variety of QD dosage/exposure concentrations reported in the literature, and the wide variation of physicochemical properties of individual QDs. Importantly, and a potential source of confusion in assessing QD toxicity, QD toxicity depends on multiple factors derived from both individual QD physicochemical properties and environmental conditions: size, charge, concentration, outer coating bioactivity, and oxidative, photolytic, and mechanical stability have all shown to be determining factors for QD toxicity [121, 135, 185–196].
Zhang et al. [135] have shown that skin penetration is one of the major routes of exposure for QDs to gain access to a biological system. QD655 and QD565 coated with carboxylic acid were studied for 8 and 24 hr in flow-through diffusion cells with flexed, tape-stripped and abraded rat skin to determine if these mechanical actions could perturb the barrier and affect penetration. These results show that the rat skin penetration of QD655 and QD565 is primarily limited to the uppermost stratum corneum layers of intact skin. Lovric et al. [185] found that CdTe QDs coated with mercaptopropionic acid (MPA) and cysteamine were cytotoxic to rat pheochromocytoma cells (PC12) in culture at concentrations of 10 µg/mL. Uncoated CdTe QDs were cytotoxic at 1 µg/mL. Cell death was characterized as chromatin condensation and membrane blebbing, symptomatic of apoptosis. The effect of QD-induced reactive oxygen species on cell death was assessed with N-acetylcysteine, bovine serum albumin (BSA), and Trolox. Hoshino et al. [186] reported that treatment with QD capping material mercaptoundecanoic acid (MUA) alone (without QD) for 12 hr caused severe cytotoxicity in murine T-cell lymphoma (EL-4) cells at 100 µg/mL. Treatment with cysteamine alone proved weakly genotoxic at 100 µg/mL (12 hr). So their cytotoxicity was attributed to QD capping material rather than the core metalloid complex itself. Shiohara et al. [187] have also observed QD-induced cytotoxicity. MUA-coated CdSe/ZnS QDs were observed to be cytotoxic to HeLa cells and primary human hepatocytes at concentrations of 100 µg/mL (MTT assay). Using primary hepatocytes as a liver model, Deufus et al. [195] found that CdSe-core QDs were indeed acutely toxic under certain conditions. They have shown that cytotoxicity of QDs can be modulated by processing parameters during synthesis, exposure to ultraviolet light, and surface coating. Their results further suggest that cytotoxicity correlates with the liberation of free Cd2+ ions due to deterioration of the CdSe lattice.
Gopee et al. [196] have demonstrated that regional lymph nodes, liver, kidney, and spleen are sentinel organs for the detection of ID administered QDs that are PEG coated and are approximately 37 nm in diameter. They have concluded that regional lymph nodes and liver can be used as sentinel organs to determine the penetration through the skin by QDs and possibly other nanoparticles. Rouse et al. [191] have investigated the effects of applied strain on QDs uptake by human epidermal keratinocytes (HEK). QDs were introduced at a concentration of 3 nM and a 10% average strain was applied to the cells. After 4 hr of cyclic strain, the cells were examined for cell viability, QDs uptake, and cytokine production. Their results show that addition of strain results in an increase in cytokine production and QDs uptake, resulting in irritation and a negative impact on cell viability. Mortensen et al. [120] have demonstrated QDs skin penetration by employing an in vivo semiconductor QDs model system. Carboxylated QDs were applied to the skin of SKH-1 mice in a glycerol vehicle with and without UVR exposure. The skin collection and penetration patterns were evaluated 8 and 24 hr after QDs application using tissue histology, confocal microscopy, and TEM with EDAX analysis. Low levels of penetration were observed in both the non-UVR exposed mice and the UVR exposed mice. Qualitatively higher levels of penetration were observable in the UVR exposed mice.
Summary, Outlook and Future Needs
In summary, we have critically discussed the fate, behavior and toxicity, of different class of nanomaterials in environment. Though several research groups have found toxic effects of nanomaterials, the causes for the toxicity are mostly unknown. There are still huge gaps in knowledge about the nature of interaction of nanoparticles with the environmental system. Much more studies are needed to evaluate the stability of these matrices in a variety of test systems to fully determine the potential for human exposure to the nanoscale components of commercially available products, as well as future products. Toxicity studies of nanoparticles using different cell lines and incubation times are increasingly being published, but due to the wide range of nanoparticle concentrations, variety of cell lines as well as culturing conditions, and lack of understanding of mechanism, it is very difficult to determine whether the toxicity observed is physiologically relevant. Importantly, analytical techniques are needed that permit real-time, in situ monitoring to optimize production processes, thus minimizing waste and energy costs as well as providing mechanistic information. Despite the progress described within this review, there are still considerable research challenges within this field that remain to be addressed. We don’t yet know which aspects of nanomaterials should be measured e.g. number, surface area or mass concentration, a combination of these, or something else entirely. Have we learned anything new in biological toxicity mechanism because of these toxicological studies? The answer, with several exceptions, is not really. Once the biomedical community embraces nanoparticles as new tools for in vivo imaging, for longer time scales, we speculate that new knowledge of how cells and organs work, both internally and externally with others, will be obtained.
The availability of routine analytical methods that address these issues is a key to gain a better understanding of the mechanisms of nanoparticle formation and reactivity. In addition, given the influence of purity on a wide range of nanoparticle properties, analytical techniques that can detect and quantify impurities will be important to pursue greener approaches. Indeed, nanoparticles are so small that they cannot be detected by optical microscopes. In addition, chemical analysis of individual nanoparticles in gases and liquids was impossible for a long time due to their low mass. The enormous diversity of engineered nanomaterials with different sizes, shapes, compositions and coatings matches and possibly exceeds that of conventional chemicals. Advances in information technology and sensor design should lead to the development of smart sensors that detect nanoparticle concentrations and determine their potential toxicity, possibly providing early indications of harm. The challenge is to reach international agreement on a battery of in vitro screening tests for human and environmental toxicity. Essential to this challenge will be the widespread and global availability of standard nanoparticle samples to allow comparison and refinement of methods across government, industry and academic laboratories. Given the similarities of the goals associated with the human health and environmental research underway in the United States, Europe, and Japan, the development of formal collaborations and government agencies in these countries should encourage these collaborations in a way that is transparent.
As shown in this review, many of the surfactants used to control and size and shape of the nanomaterials are toxic. Therefore, one needs to find alternatives to the use of surfactants, templates, or other substances to stabilize and control nanoparticle shape during synthesis. Some examples provided in this review make use of surfactants for shape-dependent nanomaterials. Surfactants are often toxic and so it must be replaced in order to incorporate new functionality on the particle surface. New biomimetic approaches wherein the molecule will be used to control shape are very important, which can show great promise in biologically derived nanoparticle production. Further research is necessary to develop these methods. There are encouraging results that suggest that the green nanoscience framework can guide design, production, and application of greener nanomaterials across the range of compositions, sizes, shapes, and functionality. Further development and application of this framework will provide research opportunities and challenges for this community for the foreseeable future.
To assess the safety of complex multi-component and multi-functional nanomaterials, scientists will need to develop validated models capable of predicting the release, transport, transformation, accumulation, and uptake of engineered nanomaterials in the environment. These models should relate physical and chemical characteristics of nanomaterials to their behaviour, allow an integrated approach to predict potential impact of engineered nanomaterials and nanoproducts, and estimate impacts within susceptible populations. Developing structure-activity relationships is needed to predict biological impacts, ecological impacts, and degradation at end-of-life. Each of these models is necessary to design nanoparticles that will have the desired human health and environmental performance to complement their physical properties.
Communicating research on nanotechnology risks and benefits outside the scientific community is challenging, but is essential for dialogues based on sound science. This means developing communication activities that enable technical information to be summarized, critiqued and ultimately synthesized for various interested parties, including decision-makers and consumers. Finally, a global understanding of nanotechnology-specific risks is essential if large and small industries are to operate on a level playing field, and developing economies are not to be denied essential information on designing safe nanotechnologies. If the global research community can take advantage of these circumstances then we can surely look forward to the advent of safe nanotechnologies.
Acknowledgements
Dr. Ray thanks NSF-PREM grant # DMR-0611539, NSF-BIO grant # 0641455, ARO grant # W911NF-06-1-0512 and NIH-SCORE grant # S06GM 008047 for their generous funding.
Footnotes
This article is not an official U.S. Food and Drug Administration (FDA) guidance or policy statement. No official support or endorsement by the U.S. FDA is intended or should be inferred.
Contributor Information
Paresh Chandra Ray, Department of Chemistry, Jackson State University, Jackson, MS 39217, USA.
Hongtao Yu, Department of Chemistry, Jackson State University, Jackson, MS 39217, USA.
Peter P. Fu, Division of Biochemical Toxicology, National Center for Toxicological Research, Jefferson, AR 72079, USA
References
- 1.Vo-Dinh T. In: Nanotechnology in Biology and Medicine: Methods, Devices, and Applications. Vo-Dinh T, editor. Boca Raton, FL: CRC Press; 2007. [Google Scholar]
- 2.Stewart ME, Anderton CR, Thompson LB, Maria J, Gray SK, Rogers JA, Nuzzo RG. Nanostructured plasmonic sensors. Chem. Rev. 2008;108:494–521. doi: 10.1021/cr068126n. [DOI] [PubMed] [Google Scholar]
- 3.Dahl JA, Maddux BLS, Hutchison JE. Toward greener nanosynthesis. Chem. Rev. 2007;107:2891–2959. doi: 10.1021/cr050943k. [DOI] [PubMed] [Google Scholar]
- 4.Chen X, Mao SS. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007;107:2891–2959. doi: 10.1021/cr0500535. [DOI] [PubMed] [Google Scholar]
- 5.Janata J. Introduction: Modern topics in chemical sensing. Chem. Rev. 2008;108:327–328. doi: 10.1021/cr0680991. [DOI] [PubMed] [Google Scholar]
- 6.Teo BK, Sun XH. Silicon-based low-dimensional nanomaterials and nanodevices. Chem. Rev. 2007;107:1454–1532. doi: 10.1021/cr030187n. [DOI] [PubMed] [Google Scholar]
- 7.Wang J. Electrochemical glucose biosensors. Chem. Rev. 2008;108:814–825. doi: 10.1021/cr068123a. [DOI] [PubMed] [Google Scholar]
- 8.Thoumine O, Ewers H, Heine M, Groc L, Frischknecht R, Giannone G, Poujol C, Legros P, Lounis B, Cognet L, Choquet D. Probing the dynamics of protein-protein interactions at neuronal contacts by optical imaging. Chem. Rev. 2008;108:1565–1587. doi: 10.1021/cr078204m. [DOI] [PubMed] [Google Scholar]
- 9.He GS, Tan L-S, Zheng Q, Prasad PN. Multiphoton absorbing materials: Molecular designs, characterizations, and applications. Chem. Rev. 2008;108:1245–1330. doi: 10.1021/cr050054x. [DOI] [PubMed] [Google Scholar]
- 10.Hatchett DW, Josowicz M. Composites of intrinsically conducting polymers as sensing nanomaterials. Chem. Rev. 2008;108:746–769. doi: 10.1021/cr068112h. [DOI] [PubMed] [Google Scholar]
- 11.Burda C, Chen X, Narayanan R, El-Sayed MA. Chemistry and properties of nanocrystals of different shapes. Chem. Rev. 2005;105:1025. doi: 10.1021/cr030063a. [DOI] [PubMed] [Google Scholar]
- 12.Christine M, Astruc D. Gold nanoparticles: Assembly, supramolecular chemistry. Chem. Rev. 2004;104:293. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- 13.Kinbara K, Aida T. Toward intelligent molecular machines: Directed motions of biological and artificial molecules and assemblies. Chem. Rev. 2005;105:1377. doi: 10.1021/cr030071r. [DOI] [PubMed] [Google Scholar]
- 14.Mirkin CA, Letsinger RL, Mucic RC, Storhoff JJ. A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature. 1996;382:607. doi: 10.1038/382607a0. [DOI] [PubMed] [Google Scholar]
- 15.Aslan K, Geddes CD. Metal-enhanced fluorescence: An emerging tool in biotechnology. Curr. Opn. Biotech. 2005;16:55–62. doi: 10.1016/j.copbio.2005.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aslan K, Huang J, Wilson GM, Geddes CD. Metal-enhanced fluorescence-based RNA sensing. J. Am. Chem. Soc. 2006;128:4206–4207. doi: 10.1021/ja0601179. [DOI] [PubMed] [Google Scholar]
- 17.Aslan K, Lakowicz JR, Geddes CD. Plasmon light scattering in biology and medicine: New sensing approaches, visions and perspectives. Curr. Opn. Chem. Bio. 2005;9:538–544. doi: 10.1016/j.cbpa.2005.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bagalkot V, Zhang L, Levy-Nissenbaum E, Jon S, Kantoff PW, Langer R, Farokhzad OC. Quantum dot-aptamer conjugates for synchronous cancer imaging, therapy, and sensing of drug delivery based on bi-fluorescence resonance energy transfer. Nano Lett. 2007;7:3065–3070. doi: 10.1021/nl071546n. [DOI] [PubMed] [Google Scholar]
- 19.Francisco GJ, Viana BP, Jose R. Gold nanoparticle based systems in genetics. Curr. Pharmacogen. 2007;5:39–47. [Google Scholar]
- 20.Zhang C-Y, Yeh H-C, Kuroki MT, Wang T-H. Single-quantum-dot-based DNA nanosensor. Nat. Mat. 2005;4:826–831. doi: 10.1038/nmat1508. [DOI] [PubMed] [Google Scholar]
- 21.Rosi NL, Mirkin CA. Nanostructures in biodiagnostics. Chem. Rev. 2005;105:1547. doi: 10.1021/cr030067f. [DOI] [PubMed] [Google Scholar]
- 22.Alivisatos AP, Johnson KP, Peng X, Wislon TE, Bruchez MP, Schultz PG. Organization of 'nanocrystal molecules' using DNA. Nature. 1996;382:609–611. doi: 10.1038/382609a0. [DOI] [PubMed] [Google Scholar]
- 23.Alivisatos P. The use of nanocrystals in biological detection. Nat. Biotechnol. 2004;22:47–52. doi: 10.1038/nbt927. [DOI] [PubMed] [Google Scholar]
- 24.Benoit D. Quantum dots: DNA detectives. Nat. Mat. 2005;4:797–798. doi: 10.1038/nmat1520. [DOI] [PubMed] [Google Scholar]
- 25.Seferos DS, Giljohann DA, Hill HD, Prigodich AE, Mirkin CA. Nano-flares: Probes for transfection and mRNA detection in living cells. J. Am. Chem. Soc. 2007;129:15477–15479. doi: 10.1021/ja0776529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sassolas A, Leca-Bouvier BD, Blum LJ. DNA biosensors and microarrays. Chem. Rev. 2008;108:109–139. doi: 10.1021/cr0684467. [DOI] [PubMed] [Google Scholar]
- 27.Borisov SM, Wolfbeis OS. Optical biosensors. Chem. Rev. 2008;108:423–461. doi: 10.1021/cr068105t. [DOI] [PubMed] [Google Scholar]
- 28.Famulok M, Hartig JS, Mayer G. Functional aptamers and aptazymes in biotechnology, diagnostics, and therapy. Chem. Rev. 2007:3715–3743. doi: 10.1021/cr0306743. [DOI] [PubMed] [Google Scholar]
- 29.Ghosh SK, Pal T. Interparticle coupling effect on the surface plasmon resonance of gold nanoparticles: From theory to applications. Chem. Rev. 2007;107:4797–4862. doi: 10.1021/cr0680282. [DOI] [PubMed] [Google Scholar]
- 30.Kumar A, Jakhmola A. RNA-mediated fluorescent Q-PbS nanoparticles. Langmuir. 2007;23:2915–2918. doi: 10.1021/la0628975. [DOI] [PubMed] [Google Scholar]
- 31.Griffin J, Singh AK, Senapati D, Lee E, Gaylor K, Jones-Boone J, Ray PC. Sequence specific HCV-RNA quantification using size dependent nonlinear optical properties of gold nanoparticles. Small. 2008 doi: 10.1002/smll.200801334. In press. [DOI] [PubMed] [Google Scholar]
- 32.Griffin J, Singh AK, Senapati D, Rhodes P, Mitchell K, Robinson B, Yu E, Ray PC. Size and distance dependent NSET ruler for selective sensing of hepatitis C virus RNA. Chem. Eur. J. 2008 doi: 10.1002/chem.200801812. Online early view. [DOI] [PubMed] [Google Scholar]
- 33.Cao YWC, Jin RC, Mirkin CA. Nanoparticles with Raman spectroscopic fingerprints for DNA and RNA detection. Science. 2002;297:1536–1540. doi: 10.1126/science.297.5586.1536. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Zhang MA, Young O, Lyandres RP, Van Duyne MA. Rapid detection of an anthrax biomarker by surface-enhanced Raman spectroscopy. J. Am. Chem. Soc. 2005;127:4484. doi: 10.1021/ja043623b. [DOI] [PubMed] [Google Scholar]
- 35.Ryan BC, Kwong GA, Radu CG, Witte ON, Heath JR. DNA -encoded antibody libraries: A unified platform for multiplexed cell sorting and detection of genes and proteins. J. Am. Chem. Soc. 2007;129:1959–1967. doi: 10.1021/ja065930i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodrigo M, Pedro B, Leandro R, Goncalo D, Leonardo S, Ricardo F, Elvira F. Amorphous/nanocrystalline silicon biosensor for the specific identification of unamplified nucleic acid sequences using gold nanoparticle probes. Appl. Phys. Lett. 2007;90 023903/1-023903/3. [Google Scholar]
- 37.Du B-A, Li Z-P, Liu C-H. One-step homogeneous detection of DNA hybridization with gold nanoparticle probes by using a linear light-scattering technique. Angew. Chem. Int. Ed. 2006;45:8022–8025. doi: 10.1002/anie.200603331. [DOI] [PubMed] [Google Scholar]
- 38.Hong OE, Lee MY, Nam D, Yoon SH, Kim HC. Inhibition assay of biomolecules based on fluorescence resonance energy transfer (FRET) between quantum dots and gold nanoparticles. J. Am. Chem. Soc. 2005;127:3270–3271. doi: 10.1021/ja0433323. [DOI] [PubMed] [Google Scholar]
- 39.Reinhard BM, Siu M, Agarwal H, Alivisatos AP, Liphardt J. Calibration of dynamic molecular rulers based on plasmon coupling between gold nanoparticles. Nano Lett. 2005;5:2246–2252. doi: 10.1021/nl051592s. [DOI] [PubMed] [Google Scholar]
- 40.Yun CS, Javier A, Jennings T, Fisher M, Hira S, Peterson B, Hopkins N, Reich O, Strouse GF. Nanometal surface energy transfer in optical rulers, breaking the FRET barrier. J. Am. Chem. Soc. 2005;127:3115–3119. doi: 10.1021/ja043940i. [DOI] [PubMed] [Google Scholar]
- 41.Dubertret B, Calame M, Libchaber AJ. Single-mismatch detection using gold-quenched fluorescent oligonucleotides. Nat. Biotechnol. 2001;19:365–370. doi: 10.1038/86762. [DOI] [PubMed] [Google Scholar]
- 42.Gaylord BS, Bazan GC, Heeger AJ. DNA hybridization detection with water-soluble conjugated polymers and chromophore-labeled single-stranded DNA. J. Am. Chem. Soc. 2003;125:896–900. doi: 10.1021/ja027152+. [DOI] [PubMed] [Google Scholar]
- 43.Nam JM, Thaxton CS, Mirkin CA. Nanoparticle-based bio-bar codes for the ultrasensitive detection of proteins. Science. 2003;301:1884–1886. doi: 10.1126/science.1088755. [DOI] [PubMed] [Google Scholar]
- 44.Maxwell DJ, Taylor JR, Nie S. Self-assembled nanoparticle probes for recognition and detection of biomolecules. J. Am. Chem. Soc. 2002;124:9606. doi: 10.1021/ja025814p. [DOI] [PubMed] [Google Scholar]
- 45.Li H, Rothberg LJ. DNA sequence detection using selective fluorescence quenching of tagged oligonucleotide probes by gold nanoparticles. Anal. Chem. 2004;76:5414. doi: 10.1021/ac049173n. [DOI] [PubMed] [Google Scholar]
- 46.Fan C, Wang S, Hong JW, Bazan GC, Plaxco KW, Heeger AJ. Beyond superquenching: Hyper-efficient energy transfer from conjugated polymers to gold nanoparticles. Proc. Natl Acad. Sci. USA. 2003;100:6297–6301. doi: 10.1073/pnas.1132025100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aldaye FA, Sleiman HF. Dynamic DNA templates for discrete gold nanoparticle assemblies: Control of geometry, modularity, write/erase and structural switching. J. Am. Chem. Soc. 2007;129:4130–4131. doi: 10.1021/ja070017i. [DOI] [PubMed] [Google Scholar]
- 48.Jennings TL, Singh MP, Strouse GF. Fluorescent lifetime quenching near d = 1.5 nm gold nanoparticles: Probing NSET validity. J. Am. Chem. Soc. 2006;128:5462. doi: 10.1021/ja0583665. [DOI] [PubMed] [Google Scholar]
- 49.Jain PK, Huang W, El-Sayed MA. On the universal scaling behavior of the distance decay of plasmon coupling in metal nanoparticle pairs: A plasmon ruler equation. Nano Lett. 2007;7:2080. [Google Scholar]
- 50.Seelig J, Leslie K, Renn A, Kuhn S, Jacobsen V, van de Corput M, Wyman C, Sandoghdar V. Nanoparticle-induced fluorescence lifetime modification as nanoscopic ruler: demonstration at the single molecule level. Nano Lett. 2007;7:685. doi: 10.1021/nl0627590. [DOI] [PubMed] [Google Scholar]
- 51.Darbha GK, Ray A, Ray PC. Gold nanoparticle-based miniaturized NSET Probe for rapid and ultra-sensitive detection of mercury in soil, water and fish. ACS Nano. 2007;3:208–214. doi: 10.1021/nn7001954. [DOI] [PubMed] [Google Scholar]
- 52.Rex M, Hernandez FE, Campiglia AD. Pushing the limits of mercury sensors with gold nanorods. Anal. Chem. 2006;78 doi: 10.1021/ac051166r. 445-4. [DOI] [PubMed] [Google Scholar]
- 53.Ray PC, Fortner A, Darbha GK. Gold nanoparticle based FRET assay for the detection of DNA cleavage. J. Phys. Chem. B. 2006;110:20745–20748. doi: 10.1021/jp065121l. [DOI] [PubMed] [Google Scholar]
- 54.Ray PC. Label-free diagnostics of single base-mismatch DNA hybridization on gold nanoparticles using hyper-Rayleigh scattering technique. Angew. Chem. Int. Ed. 2006;45:1151–1154. doi: 10.1002/anie.200503114. [DOI] [PubMed] [Google Scholar]
- 55.Tiwari VS, Tovmachenko O, Darbha GK, Hardy W, Singh JP, Ray PC. Non-resonance SERS effects of silver colloids with different shapes. Chem. Phys. Lett. 2007:77–82. [Google Scholar]
- 56.Kim CK, Kalluru RR, Singh JP, Fortner A, Griffin J, Darbha GK, Ray PC. Gold nanoparticle based miniaturized laser induced fluorescence probe for specific DNA hybridization detection: Studies on size dependent optical properties. Nanotechnology. 2006;17:3085. [Google Scholar]
- 57.Ray PC, Darbha GK, Ray A, Hardy W, Walker J. Gold nanoparticle based FRET Probe for multiplexed hybridization detection: accurate identification of bio-agents DNA. Nanotechnology. 2007;18:375504–375510. [Google Scholar]
- 58.Ray PC, Darbha GK, Ray A, Walker J, Hardy W, Perryman A. Gold nanoparticle based FRET for DNA detection. Plasmonics. 2007;2:173–183. [Google Scholar]
- 59.Dasary SSR, Rai US, Yu H, Anjaneyulu Y, Dubey M, Ray PC. Gold nanomaterial based surface-enhanced fluorescence assay for detection of organophosphorus agents. Chem. Phys. Lett. 2008;460:187–190. doi: 10.1016/j.cplett.2008.05.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Griffin J, Ray PC. Gold nanoparticle based NSET for monitoring Mg2+ dependent RNA folding. J. Phys. Chem. B. 2008;112:11198–11201. doi: 10.1021/jp8059322. [DOI] [PubMed] [Google Scholar]
- 61.Pons T, Medintz IL, Sapsford KE, Higashiya S, Grimes AF, English DS, Mattoussi H. On the quenching of semiconductor quantum dot photoluminescence by proximal gold nanoparticles. Nano Lett. 2007;7:3157–3164. doi: 10.1021/nl071729+. [DOI] [PubMed] [Google Scholar]
- 62.Wang L, Yan R, Huo Z, Wang ZJ, Bao J, Wang S, Peng Q, Li Y. Fluorescence resonance energy transfer biosensor based on upconversion-luminescent nanoparticles. Angew. Chem. Int. Ed. 2005;117:6208–6211. doi: 10.1002/anie.200501907. [DOI] [PubMed] [Google Scholar]
- 63.Dulkeith E, Morteani AC, Niedereichholz T, Klaar TA, Feldmann J, Levii SA, Reinhoudt DN. Fluorescence quenching of dye molecules near gold nanoparticles: Radiative and nonradiative effects. Phys. Rev. Lett. 2002;89:203202–203205. doi: 10.1103/PhysRevLett.89.203002. [DOI] [PubMed] [Google Scholar]
- 64.Forster T. Intermolecular energy transference and fluorescence. Ann. Phys. 1948;2:55–7. [Google Scholar]
- 65.Wark AW, Lee HJ, Qavi AJ, Corn RM. Nanoparticle-enhanced diffraction gratings for ultrasensitive surface plasmon biosensing. Anal. Chem. 2007;79:6697–6701. doi: 10.1021/ac071062b. [DOI] [PubMed] [Google Scholar]
- 66.Churaman W, Currano L, Singh AK, Rai US, Dubey M, Amirtharaj P, Ray PC. Understanding the high energetic behavior of nano-energetic porous silicon. Chem. Phys. Lett. 2008;464:198–201. [Google Scholar]
- 67.Jennings TL, Schlatterer JC, Singh MP, Greenbaum NL, Strouse GF. NSET molecular beacon analysis of hammerhead RNA substrate binding and catalysis. Nano Lett. 2006;6:1318–1324. doi: 10.1021/nl052458a. [DOI] [PubMed] [Google Scholar]
- 68.Sönnichsen C, Reinhard BM, Liphardt J, Alivisatos P. A molecular ruler based on plasmon coupling of single gold and silver nanoparticles. Nat. Biotech. 2005;23:741–745. doi: 10.1038/nbt1100. [DOI] [PubMed] [Google Scholar]
- 69.Kim JH, Chaudhary S, Ozkan M. Multicolour hybrid nanoprobes of molecular beacon conjugated quantum dots: FRET and gel electrophoresis assisted target DNA detection. Nanotechnology. 2007;18:195105. [Google Scholar]
- 70.Bates AD, Callen BP, Cooper JM, Cosstick R, Geary C, Glidle A, Jaeger L, Pearson JL, Proupin-Perez M, Xu C, Cumming DRS. Construction and characterization of a gold nanoparticle wire assembled using Mg2+-dependent RNA-RNA interactions. Nano Lett. 2006;6:445–448. doi: 10.1021/nl052316g. [DOI] [PubMed] [Google Scholar]
- 71.Zhang C-Y, Johnson LW. Quantum-dot-based nanosensor for RRE IIB RNA-rev peptide interaction assay. J. Am. Chem. Soc. 2006;128:5324–5325. doi: 10.1021/ja060537y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Skewis LR, Reinhard BM. Spermidine modulated ribonuclease activity probed by RNA plasmon rulers. Nano Lett. 2008;8:214–220. doi: 10.1021/nl0725042. [DOI] [PubMed] [Google Scholar]
- 73.Gill R, Willner I, Shweky I, Banin U. Fluorescence resonance energy transfer in CdSe/ZnS-DNA conjugates: Probing hybridization and DNA cleavage. J. Phys. Chem. B. 2005;109:23715–23719. doi: 10.1021/jp054874p. [DOI] [PubMed] [Google Scholar]
- 74.Cissell KA, Rahimi Y, Shrestha S, Hunt EA, Deo SK. Bioluminescence-based detection of microRNA, miR21 in breast cancer cells. Anal. Chem. 2008;80:2319–2325. doi: 10.1021/ac702577a. [DOI] [PubMed] [Google Scholar]
- 75.El-Sayed IH, Huang X, El-Sayed MA. Surface plasmon resonance scattering and absorption of anti-EGFR antibody conjugated gold nanoparticles in cancer diagnostics: Applications in oral cancer. Nano Lett. 2005;5:829–834. doi: 10.1021/nl050074e. [DOI] [PubMed] [Google Scholar]
- 76.Medley CD, Smith JE, Tang Z, Wu Y, Bamrungsap S, Tan W. Gold nanoparticle-based colorimetric assay for the direct detection of cancerous cells. Anal. Chem. 2008;80:1067–1072. doi: 10.1021/ac702037y. [DOI] [PubMed] [Google Scholar]
- 77.Yu C, Nakshatri H, Irudayaraj J. Identity profiling of cell surface markers by multiplex gold nanorod probes. Nano Lett. 2007;7:2300–2306. doi: 10.1021/nl070894m. [DOI] [PubMed] [Google Scholar]
- 78.Quarta A, Corato RD, Manna L, Argentiere S, Cingolani R, Barbarella G, Pellegrino T. Multifunctional nanostructures based on inorganic nanoparticles and oligothiophenes and their exploitation for cellular studies. J. Am. Chem. Soc. 2008 doi: 10.1021/ja800102v. [DOI] [PubMed] [Google Scholar]
- 79.Loo L, Guenther RH, Lommel SA, Franzen S. Encapsidation of nanoparticles by red clover necrotic mosaic virus. J. Am. Chem. Soc. 2007;129:11111–11117. doi: 10.1021/ja071896b. [DOI] [PubMed] [Google Scholar]
- 80.Koyfman AY, Braun G, Magonov S, Chworos A, Reich NO, Jaeger L. Controlled spacing of cationic gold nanoparticles by nanocrown RNA. J. Am. Chem. Soc. 2005;127:11886–11887. doi: 10.1021/ja051144m. [DOI] [PubMed] [Google Scholar]
- 81.Li H, Rothberg L. Detection of specific sequences in RNA using differential adsorption of single-stranded oligonucleotides on gold nanoparticles. Anal. Chem. 2005;77:6229–6233. doi: 10.1021/ac050921y. [DOI] [PubMed] [Google Scholar]
- 82.Dillenback LM, Goodrich GP, Keating CD. Temperature-programmed assembly of DNA:Au nanoparticle bioconjugates. Nano Lett. 2008;8:1556. doi: 10.1021/nl0508873. [DOI] [PubMed] [Google Scholar]
- 83.Medintz IL, Berti L, Pons T, Grimes AF, English DS, Alessandrini A, Facci P, Mattoussi H. A reactive peptidic linker for self-assembling hybrid quantum dot-DNA bioconjugates. Nano Lett. 2007;7:1741–1748. doi: 10.1021/nl070782v. [DOI] [PubMed] [Google Scholar]
- 84.Numnuam A, Chumbimuni-Torres KY, Xiang Y, Bash R, Thavarungkul P, Kanatharana P, Pretsch E, Wang J, Bakker E. Potentiometric detection of DNA hybridization. J. Am. Chem. Soc. 2008;130:410–411. doi: 10.1021/ja0775467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kumar A, Kumar V. Self-assemblies from RNA-templated colloidal CdS nanostructures. J. Phys. Chem. C. 2008;112:3633–3640. [Google Scholar]
- 86.Liu Y, Zhong M, Shan G, Li Y, Huang B, Yang G. Biocompatible ZnO/Au nanocomposites for ultrasensitive DNA detection using resonance Raman scattering. J. Phys. Chem. B. 2008;112:6484–6489. doi: 10.1021/jp710399d. [DOI] [PubMed] [Google Scholar]
- 87.Long H, Kudlay A, Schatz GC. Molecular dynamics studies of ion distributions for DNA duplexes and DNA clusters: Salt effects and connection to DNA melting. J. Phys. Chem. B. 2006;110:2918–2926. doi: 10.1021/jp0556815. [DOI] [PubMed] [Google Scholar]
- 88.Wang H, Tessmer I, Croteau DL, Erie DA, Van Houten B. Functional characterization and atomic force microscopy of a DNA repair protein conjugated to a quantum dot. Nano Lett. 2008;8:1631–1637. doi: 10.1021/nl080316l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lassiter JB, Aizpurua J, Hernandez LI, Brandl DW, Romero I, Lal S, Hafner JH, Nordlander P, Halas NJ. Close encounters between two nanoshells. Nano Lett. 2008;8:1212–1218. doi: 10.1021/nl080271o. [DOI] [PubMed] [Google Scholar]
- 90.Tam F, Goodrich GP, Johnson BR, Halas NJ. Plasmonic enhancement of molecular fluorescence. Nano Lett. 2007;7:496–501. doi: 10.1021/nl062901x. [DOI] [PubMed] [Google Scholar]
- 91.Wang Y, Qian W, Tan Y, Ding S. A label-free biosensor based on gold nanoshell monolayers for monitoring biomolecular interactions in diluted whole blood. Biosens. Bioelectron. 2008;28:1166–1170. doi: 10.1016/j.bios.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 92.Loo C, Lowery A, Halas N, West J, Drezek R. Immunotargeted nanoshells for integrated cancer imaging and therapy. Nano Lett. 2005;5:709–711. doi: 10.1021/nl050127s. [DOI] [PubMed] [Google Scholar]
- 93.Gobin AM, Lee MH, Halas NJ, James WD, Drezek RA, West JL. Near-infrared resonant nanoshells for combined optical imaging and photothermal cancer therapy. Nano Lett. 2007;4:1929–1934. doi: 10.1021/nl070610y. [DOI] [PubMed] [Google Scholar]
- 94.Su C-H, Sheu H-S, Lin C-Y, Huang C-C, Lo Y-W, Pu Y-C, Weng J-C, Shieh D-B, Chen J-H, Yeh C-S. Nanoshell magnetic resonance imaging contrast agents. J. Am. Chem. Soc. 2007;129:2139–2146. doi: 10.1021/ja0672066. [DOI] [PubMed] [Google Scholar]
- 95.Sassolas A, Leca-Bouvier BD, Blum LJ. A biosensors and microarrays. Chem. Rev. 2008;108 doi: 10.1021/cr0684467. [DOI] [PubMed] [Google Scholar]
- 96.Maynard AD. Safe handling of nanotechnology. Nature. 2006;444:267–269. doi: 10.1038/444267a. [DOI] [PubMed] [Google Scholar]
- 97.Monica JC, Heintz ME, Lewis PT. The perils of pre-emptive regulation. Nat. Nanotech. 2008;2:68–70. doi: 10.1038/nnano.2007.15. [DOI] [PubMed] [Google Scholar]
- 98.Zhao Y, Xing G, Chai Z. Are carbon nanotubes safe? Nat. Nanotech. 2008;3:191–192. doi: 10.1038/nnano.2008.77. [DOI] [PubMed] [Google Scholar]
- 99.Lewinski N, Colvin V, Drezek R. Cytotoxicity of nanoparticles. Small. 2008;4:26–39. doi: 10.1002/smll.200700595. [DOI] [PubMed] [Google Scholar]
- 100.Colvin VL. The potential environmental impact of engineered nanmoaterials. Nat. Biotech. 2003;21:1166–1170. doi: 10.1038/nbt875. [DOI] [PubMed] [Google Scholar]
- 101.Borm PJA, Robbins D, Haubold S. The potential risks of nanomaterials: A review carried out for ECETOC. Particle Fibre Toxicol. 2006;3:11–35. doi: 10.1186/1743-8977-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.http://www.luxresearchinc.com.
- 103.The Royal Society. London: 2004. [Google Scholar]
- 104.http://www.wilsoncenter.org.
- 105.Report of the US Chemical Industry Working Group. 2003. [Google Scholar]
- 106.http://www.nano.gov.
- 107.Donaldson K, Tran C, MacNee W. Deposition and effects of fine and ultrafine particles in the respiratory tract. The European Respiratory Monograph. 2002;7:77–92. [Google Scholar]
- 108.Darbha GK, LE GE, Anderson YR, Preston F, Mitchell K, Ray PC. Miniaturized NSET sensor for microbial pathogens DNA and chemical toxins. IEEE Sensor J. 2008;8:693–701. [Google Scholar]
- 109.Darbha GK, Rai US, Singh AK, Ray PC. Highly selective detection of Hg2+ ion using NLO properties of gold nanomaterial. J. Am. Chem. Soc. 2008;130:8038–8042. doi: 10.1021/ja801412b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gao X, Cui Y, Levenson RM, Chung LW, Nie S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotechnol. 2004;22:969–976. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- 111.Wu X, Liu H, Liu J, Haley K, Treadway J, Larson J. Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nat. Biotechnol. 2003;21:41–46. doi: 10.1038/nbt764. [DOI] [PubMed] [Google Scholar]
- 112.Chan WCW, Maxwell DJ, Gao X, Bailey RE, Han M, Nie S. Luminescent quantum dots for multiplexed biological detection and imaging. Curr. Opn. Biotechnol. 2002;13:40–46. doi: 10.1016/s0958-1669(02)00282-3. [DOI] [PubMed] [Google Scholar]
- 113.Chen F, Gerion D. Fluorescent CdSe/ZnS nanocrystal-peptide conjugates for long-term, nontoxic imaging and nuclear targeting in living cells. Nano Lett. 2004;4:1827–1832. [Google Scholar]
- 114.Dubertret B, Skourides P, Norris D, Noireaux V, Brivanlou A, Libchaber A. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science. 2002;298:1759–1762. doi: 10.1126/science.1077194. [DOI] [PubMed] [Google Scholar]
- 115.Lee KJ, Nallathamby PD, Browning LM, Osgood CJ, Xu X-HN. In vivo imaging of transport and biocompatibility of single silver nanoparticles in early development of zebrafish embryos. ACS Nano. 2007;1:133–143. doi: 10.1021/nn700048y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Michalet X, Pinaud F, Bentolila L, Tsay J, Doose S, Li J. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 2005;307(5709):538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lam CW, James JT, McCluskey R, Hunter RL. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol. Sci. 2004;77:126–134. doi: 10.1093/toxsci/kfg243. [DOI] [PubMed] [Google Scholar]
- 118.Oberdorster G, Sharp Z, Atudorei A, Elder A, Gelein G, Luntsm A, Kreyling W, Cox C. Extrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats. J. Toxicol. Environ. Health Part A. 2002;65:1531–1543. doi: 10.1080/00984100290071658. [DOI] [PubMed] [Google Scholar]
- 119.Oberdorster G, Sharp Z, Atudonrei V, Elder A, Gelein R, Kreyling W, Cox C. Translocation of inhaled ultrafine particles to the brain. Inhal. Toxicol. 2004;16:437–445. doi: 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- 120.Mortensen LJ, Oberdorster G, Pentland AP, DeLouise LA. In vivo skin penetration of quantum dot nanoparticles in the murine model: The effect of UVR. Nano Lett. 2008;8:2779–2787. doi: 10.1021/nl801323y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang LW, Monteiro-Riviere NA. Assessment of quantum dot penetration into intact, tape-stripped, abraded and flexed rat skin. Skin Pharm. Physiol. 2008;21:166–180. doi: 10.1159/000131080. [DOI] [PubMed] [Google Scholar]
- 122.Baroli B, Ennas MG, Loffredo F, Isola M, Pinna R, López-Quintela MA. Penetration of metallic nanoparticles in human full-thickness skin. J. Invest. Dermatol. 2007;127:1701–1712. doi: 10.1038/sj.jid.5700733. [DOI] [PubMed] [Google Scholar]
- 123.Rouse JG, Yang J, Ryman-Rasmussen JP, Barron AR, Monteiro-Riviere NA. Effects of mechanical flexion on the penetration of fullerene amino acid-derivatized peptide nanoparticles through skin. Nano Lett. 2007;7:155–160. doi: 10.1021/nl062464m. [DOI] [PubMed] [Google Scholar]
- 124.Hussain SM, Javorina AK, Schrand AM, Duhart HM, Ali SF, Schlager JJ. The interaction of manganese nanoparticles with PC-12 cells induces dopamine depletion. Toxicol. Sci. 2006;92:456–463. doi: 10.1093/toxsci/kfl020. [DOI] [PubMed] [Google Scholar]
- 125.Semmler M, Seitz J, Erbe F, Mayer P, Heyder J, Oberdorster G, Kreyling W. Long-term clearance kinetics of inhaled ultrafine insoluble iridium particles from the rat lung, including transient translocation into secondary organs. Inhal. Toxicol. 2004;16:453–459. doi: 10.1080/08958370490439650. [DOI] [PubMed] [Google Scholar]
- 126.Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Oberdorster; Ferin J, Lehnert BE. Correlation between particle size, in vivo particle persistence, and lung injury. Environ. Health Perspect. 2004;102:173–179. doi: 10.1289/ehp.102-1567252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sharma HS, Sharma A. Nanoparticles aggravate heat stress induced cognitive deficits, blood-brain barrier disruption, edema formation and brain pathology. Prog. Brain Res. 2007;162:245–273. doi: 10.1016/S0079-6123(06)62013-X. [DOI] [PubMed] [Google Scholar]
- 129.Warheit DB, Webb TR, Colvin VC, Reed KL, Sayes CM. Pulmonary bioassay studies with nanoscale and fine-quartz particles in rats: Toxicity is not dependent upon particle size but on surface characteristics. Toxicol. Sci. 2007;95:270–280. doi: 10.1093/toxsci/kfl128. [DOI] [PubMed] [Google Scholar]
- 130.Anderson PJ, Wilson JD, Hiller FC. Respiratory tract deposition of ultrafine particles in subjects with obstructive or restrictive lung disease. Chest. 1990;97:1115–1120. doi: 10.1378/chest.97.5.1115. [DOI] [PubMed] [Google Scholar]
- 131.Kreyling WG, Semmler-Behnke M, Möller W. Ultrafine particle-lung interactions: Does size matter? J. Aerosol Med. 2006;19:74–83. doi: 10.1089/jam.2006.19.74. [DOI] [PubMed] [Google Scholar]
- 132.Stahlhofen W, Rudolf G, James AC. Intercomparison of experimental regional aerosol deposition data. J. Aerosol Med. 1989;2:285–308. [Google Scholar]
- 133.Ding L, Stilwell J, Zhang T, Elboundwarej O, Jiang H, Selegue JP, Cooke PA, Gray JW, Chen FF. Molecular characterization of the cytotoxic mechanism of multiwall carbon nanotubes and nano-onions on human skin fibroblast. Nano Lett. 2005;5:2448–2464. doi: 10.1021/nl051748o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lu W, Senapati D, Wang S, Tovmachenko O, Yu H, Ray PC. Shape dependent cellular uptake and toxic effects of silver nanomaterials on human skin HaCaT keratinocytes. J. Am. Chem. Soc. 2009 Submitted. [Google Scholar]
- 135.Zhang LW, Yu WW, Colvin VL, Monteiro-Riviere NA. Biological interactions of quantum dot nanoparticles in skin and in human epidermal keratinocytes. Toxicol. Appl. Pharmacol. 2008;228:200–211. doi: 10.1016/j.taap.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 136.Chou C-C, Hsiao H-Y, Hong Q-S, Chen C-H, Peng Y-W, Chen H-W, Yang P-C. Single-walled carbon nanotubes can induce pulmonary injury in mouse model. Nano Lett. 2008;8:437–445. doi: 10.1021/nl0723634. [DOI] [PubMed] [Google Scholar]
- 137.Lam CW, James JT, McCluskey R, Hunter RL. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intractracheal instillation. Toxicol. Sci. 2004;77:126–134. doi: 10.1093/toxsci/kfg243. [DOI] [PubMed] [Google Scholar]
- 138.Shukla R, Bansal V, Chaudhary M, Basu A, Bhonde R, Sastry M. Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: A microscopic overview. Langmuir. 2005;21:10644–10654. doi: 10.1021/la0513712. [DOI] [PubMed] [Google Scholar]
- 139.Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005;1:325–327. doi: 10.1002/smll.200400093. [DOI] [PubMed] [Google Scholar]
- 140.Chithrani BD, Ghazani AA, Chan WCW. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6:662–668. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 141.Wang S, Lu W, Tovmachenko O, Rai US, Yu H, Ray PC. Challenge in understanding size and shape dependent toxicity of gold nanomaterials in human skin keratinocytes. Chem. Phys. Lett. 2008;463:145. doi: 10.1016/j.cplett.2008.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Goodman CM, McCusker CD, Yilmaz T, Rotello VM. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjugate Chem. 2004;15:897–900. doi: 10.1021/bc049951i. [DOI] [PubMed] [Google Scholar]
- 143.Pernodet N, Fang X, Sun Y, Bakhtina A, Ramakrishnan A, Sokolov J, Ulman A, Rafailovich M. Adverse effects of citrate/gold nanoparticles on human dermal fibroblasts. Small. 2006;6:766–773. doi: 10.1002/smll.200500492. [DOI] [PubMed] [Google Scholar]
- 144.Patra HK, Banerjee S, Chaudhuri U, Lahiri P, Dasgupta AK. Cell-selective response to gold nanoparticles. Nanomedicine. 2007;3:111–119. doi: 10.1016/j.nano.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 145.White JML, Powell AM, Brady K, Russell-Jones R. Severe generalized argyria secondary to ingestion of colloidal silver protein. Clin. Exp. Dermat. 2003;28:254–256. doi: 10.1046/j.1365-2230.2003.01214.x. [DOI] [PubMed] [Google Scholar]
- 146.Carlson C, Hussain SM, Schrand AM, Braydich-Stolle LK, Hess KL, Jones RL, Schlager JJ. Unique cellular interaction of silver nanoparticles : Size -dependent generation of reactive oxygen species. J. Phys. Chem. B. 2008;112(43):13608–13619. doi: 10.1021/jp712087m. [DOI] [PubMed] [Google Scholar]
- 147.Griffitt RJ, Luo J, Gao J, Bonzongo J-C, Barber DS. Effects of particle composition and species on toxicity of metallic nanomaterials in aquatic organisms. Environ. Toxicol. Chem. 2008;27:1972–1978. doi: 10.1897/08-002.1. [DOI] [PubMed] [Google Scholar]
- 148.Asharani PV, Wu YL, Gong Z, Valiyaveettil S. Toxicity of silver nanoparticles in zebrafish models. Nanotechnology. 2008;19 doi: 10.1088/0957-4484/19/25/255102. DOI: 10.1088/0957-4484/19/25/255102. [DOI] [PubMed] [Google Scholar]
- 149.Benn TM, Westerhoff P. Nanoparticle silver released into water from commercially available sock fabrics. Environ. Sci. Technol. 2008;42:4133–4139. doi: 10.1021/es7032718. [DOI] [PubMed] [Google Scholar]
- 150.Niidome T, Yamagata M, Okamoto Y, Akiyama Y, Takahashi H, Kawano T, Katayama Y, Niidome Y. PEG-modified gold nanorods with a stealth character for in vivo applications. J. Controlled Release. 2006;114:343–347. doi: 10.1016/j.jconrel.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 151.Takahashi H, Niidome Y, Niidome T, Kaneko K, Kawasaki H, Yamada S. Modification of gold nanorods using phosphatidylcholine to reduce cytotoxicity. Langmuir. 2006;22:2–5. doi: 10.1021/la0520029. [DOI] [PubMed] [Google Scholar]
- 152.Hauck TS, Ghazani AA, Chan WCW. Assessing the effect of surface chemistry on gold nanorod uptake, toxicity, and gene expression in mammalian cells. Small. 2008;4:153. doi: 10.1002/smll.200700217. [DOI] [PubMed] [Google Scholar]
- 153.Grassian VH, O’Shaughnessy PT, Adamcakova-Dodd A, Pettibone JM, Thorne PS. Inhalation exposure study of titanium dioxide nanoparticles with a primary particle size of 2 to 5 nm. Environ. Health Perspect. 2007;115:397–402. doi: 10.1289/ehp.9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jin C-Y, Zhu B-S, Wang X-F, Lu Q-H. Cytotoxicity of titanium dioxide nanoparticles in mouse fibroblast cells. Chem. Res. Toxicol. 2008;21:1871–1877. doi: 10.1021/tx800179f. [DOI] [PubMed] [Google Scholar]
- 155.Park E-J, Yi J, Chung K-H, Rye D-Y, Choi J, Park K. Oxidative stress and apoptosis induced by titanium dioxide nanoparticles in cultured BEAS-2B cells. Toxicol. Lett. 2008;180:222–229. doi: 10.1016/j.toxlet.2008.06.869. [DOI] [PubMed] [Google Scholar]
- 156.Hong FS, Zhou J, Liu C, Yang F, Wu C, Zheng L, Yang P. Effect of nano-TiO2 on photochemical reaction of chloroplasts of spinach. Biol. Trace Elem. Res. 2005;105:269–279. doi: 10.1385/BTER:105:1-3:269. [DOI] [PubMed] [Google Scholar]
- 157.Yang F, Hong FS, You WJ, Liu C, Gao FQ, Wu C, Yang P. Influences of nano-anatase TiO2 on the nitrogen metabolism of growing spinach. Biol. Trace Elem. Res. 2006;110:179–190. doi: 10.1385/bter:110:2:179. [DOI] [PubMed] [Google Scholar]
- 158.Doshi R, Braida W, Christodoulatos C, Wazne M, O’Connor G. Nano-aluminum: transport through sand columns and environmental effects on plants and soil communities. Environ. Res. 2008;106:296–303. doi: 10.1016/j.envres.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 159.Lin DH, Xing BS. Phytotoxicity of nanoparticles: inhibition of seed germination and root elongation. Environ. Pollut. 2007;150:243–250. doi: 10.1016/j.envpol.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 160.Yang L, Watts DJ. Particle surface characteristics may play an important role in phytotoxicity of alumina nanoparticles. Toxicol. Lett. 2005;1558:122–132. doi: 10.1016/j.toxlet.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 161.Racuciu M, Creanga DE. TMA-OH coated magnetic nanoparticles internalized in vegetal tissue. Rom. J. Phys. 2007;52:395–402. [Google Scholar]
- 162.Lin D, Xing B. Root uptake and phytotoxicity of ZnO nanoparticles. Environ. Sci. Technol. 2008;42:5580. doi: 10.1021/es800422x. [DOI] [PubMed] [Google Scholar]
- 163.Franklin NM, Rogers NJ, Apte SC, Batley GE, Gadd GE, Casey PS. Comparative toxicity of nanoparticulate ZnO, Bulk ZnO, and ZnCl2 to a freshwater microalga (pseudokirchneriella subcapitata): The importance of particle solubility. Environ. Sci. Technol. 2007;41:8484–8490. doi: 10.1021/es071445r. [DOI] [PubMed] [Google Scholar]
- 164.Cozzoli PD, Curri ML, Agostiano A, Leo G, Lomascolo M. ZnO nanocrystals by a non-hydrolytic route: synthesis and characterization. J. Phys. Chem. B. 2003 [Google Scholar]
- 165.Reddy KM, Feris K, Bell J, Wingett DG, Hanley C, Punnoose A. Selective toxicity of zinc oxide nanoparticles to prokaryotic and eukaryotic systems. Appl. Phys. Lett. 2007;90:213902-1–213902-3. doi: 10.1063/1.2742324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Karlsson HL, Cronholm P, Gustafsson J, Moller L. Copper oxide nanoparticles are highly toxic: A comparison between metal oxide nanoparticles and carbon nanotubes. Chem. Res. Toxicol. 2008;21:1726–1732. doi: 10.1021/tx800064j. [DOI] [PubMed] [Google Scholar]
- 167.Xia T, Kovochich M, Liong M, Madler L, Gilbert B, Shi H, Yeh JI, Zink JI, Nel AE. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano. 2008;2:2121–2134. doi: 10.1021/nn800511k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Peters K, Unger RE, Kirkpatrick CJ, Gatti AM, Monari E. Effects of nano-scaled particles on endothelial cell function in vitro: Studies on viability, proliferation and inflammation. J. Mat. Sci.: Mat. Med. 2004;15:321–325. doi: 10.1023/b:jmsm.0000021095.36878.1b. [DOI] [PubMed] [Google Scholar]
- 169.Monteiro-Riviere NA, Nemanich RJ, Inman AO, Wang YY, Riviere JE. Multi-walled carbon nanotube interactions with human epidermal keratinocytes. Toxicol. Lett. 2005;155:377–384. doi: 10.1016/j.toxlet.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 170.Bottini M, Bruckner S, Nika K, Bottini N, Bellucci S, Magrini A, Bergamaschi A, Mustelin T. Multi-walled carbon nanotubes induce T lymphocyte apoptosis. Toxicol. Lett. 2006;160:121–126. doi: 10.1016/j.toxlet.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 171.Magrez A, Kasas S, Salicio V, Pasquier N, Seo JW, Celio M, Catsicas S, Schwaller B, Forro L. Cellular toxicity of carbon-based nanomaterials. Nano Lett. 2006;6:1121–1125. doi: 10.1021/nl060162e. [DOI] [PubMed] [Google Scholar]
- 172.Dumortier H, Lacotte S, Pastorin G, Marega R, Wu W, Bonifazi D, Briand JP, Prato M, Muller S, Bianco A. Functionalized carbon nanotubes are non-cytotoxic and preserve the functionality of primary immune cells. Nano Lett. 2006;6:1522–15228. doi: 10.1021/nl061160x. [DOI] [PubMed] [Google Scholar]
- 173.Silva VM, Corson N, Elder A, Oberdorster G. The rat ear vein model for investigating in vivo thrombogenicity of ultrafine particles (UFP) Toxicol. Sci. 2005;85:983. doi: 10.1093/toxsci/kfi142. [DOI] [PubMed] [Google Scholar]
- 174.Jia G, Wang H, Yan L, Wang X, Pei R, Yan T, Zhao Y, Guo X. Cytotoxicity of carbon nanomaterials: Single-wall nanotube, multi-wall nanotube, and fullerene. Environ. Sci. Technol. 2005;39:1378–1383. doi: 10.1021/es048729l. [DOI] [PubMed] [Google Scholar]
- 175.Murr LE, Garza KM, Soto KF, Carrasco A, Powell TG, Ramirez DA, Guerrero PA, Lopez DA, Venzor J. Cytotoxicity assessment of some carbon nanotubes and related carbon nanoparticle aggregates and the implications for anthropogenic carbon nanotube aggregates in the environment. Int. J. Environ. Res. Public Health. 2005;2:31–42. doi: 10.3390/ijerph2005010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tian F, Cui D, Schwarz H, Estrada GG, Kobayashi H. Cytotoxicity of single-wall carbon nanotubes on human fibroblasts. Toxicol. In Vitro. 2006;20:1202. doi: 10.1016/j.tiv.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 177.Worle-Knirsch JM, Pulskamp K, Krug HF. Oops they did it again! Carbon nanotubes hoax scientists in viability assays. Nano Lett. 2006;6:1261. doi: 10.1021/nl060177c. [DOI] [PubMed] [Google Scholar]
- 178.Shvedova AA, Castranova V, Kisin ER, Schwegler-Berry D, Murray AR, Gandelsman VZ, Maynard A, Baron P. Exposure to carbon nanotube material: Assessment of nanotube cytotoxicity using human keratinocyte cells. J. Toxicol. Environ. Health A. 2003;66:1909–1926. doi: 10.1080/713853956. [DOI] [PubMed] [Google Scholar]
- 179.Manna SK, Sarkar S, Barr J, Wise K, Barrera EV, Jejelowo O, Rice-Ficht AC, Ramesh GT. Single-walled carbon nanotube induces oxidative stress and activates nuclear transcription factor-κB in human keratinocytes. Nano Lett. 2005;5:1676–1684. doi: 10.1021/nl0507966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cui D, Tian F, Ozkan CS, Wang M, Gao H. Effect of single wall carbon nanotubes on human HEK293 cells. Toxicol. Lett. 2005;155:73–85. doi: 10.1016/j.toxlet.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 181.Zhu L, Chang DW, Dai L, Hong Y. DNA damage induced by multiwalled carbon nanotubes in mouse embryonic stem cells. Nano Lett. 2007;7:3592–3597. doi: 10.1021/nl071303v. [DOI] [PubMed] [Google Scholar]
- 182.Carrero-Sanchez JC, Elias AL, Mancilla R, Arrellin G, Terrones H, Laclette JP, Terrones M. Biocompatibility and toxicological studies of carbon nanotubes doped with nitrogen. Nano Lett. 2006;6(8):1609–1616. doi: 10.1021/nl060548p. [DOI] [PubMed] [Google Scholar]
- 183.Muller J, Huaux F, Fonseca A, Nagy JB, Moreau N, Delos M, Raymundo-Pinero E, Beguin F, Kirsch-Volders M, Fenoglio I, Fubini B, Lison D. Structural defects play a major role in the acute lung toxicity of multiwall carbon nanotubes: Toxicological aspects. Chem. Res. Toxicol. 2008;21:1698–1705. doi: 10.1021/tx800101p. [DOI] [PubMed] [Google Scholar]
- 184.Lidke DS, Nagy P, Heintzmann R, Arndt-Jovin DJ, Post JN, Grecco HE. Quantum dot ligands provide new insights into erbB/HER receptor-mediated signal transduction. Nat. Biotechnol. 2004;22:198–203. doi: 10.1038/nbt929. [DOI] [PubMed] [Google Scholar]
- 185.Lovric J, Bazzi HS, Cuie Y, Fortin GRA, Winnik FM, Maysinger D. Differences in subcellular distribution and toxicity of green and red emitting CdTe quantum dots. J. Mol. Med. 2005;83:377–385. doi: 10.1007/s00109-004-0629-x. [DOI] [PubMed] [Google Scholar]
- 186.Hoshino A, Hanaki K, Suzuki K, Yamamoto K. Applications of T-lymphoma labeled with fluorescent quantum dots to cell tracing markers in mouse body. Biochem. Biophys. Res. Commun. 2004;314:46–53. doi: 10.1016/j.bbrc.2003.11.185. [DOI] [PubMed] [Google Scholar]
- 187.Shiohara A, Hoshino A, Hanaki K, Suzuki K, Yamamoto K. On the cytotoxicity of quantum dots. Microbiol. Immunol. 2004;48:669–675. doi: 10.1111/j.1348-0421.2004.tb03478.x. [DOI] [PubMed] [Google Scholar]
- 188.Hardman R. A toxicological review of quantum dots: Toxicity depends on physico-chemical and environmental factors. Environ. Health Perspect. 2005;114:165–172. doi: 10.1289/ehp.8284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Guo GN, Liu W, Liang JG, He ZK, Xu HB, Yang XL. Probing the cytotoxicity of CdSe quantum dots with surface modification. Mat. Lett. 2007;61:1641–1644. [Google Scholar]
- 190.Li H, Zhou Q, Liu W. Progress in the toxicological researches for quantum dots. Sci. China, Ser. B: Chem. 2008;51:393–400. [Google Scholar]
- 191.Rouse JG, Haslauer CM, Loboa EG. Cyclic tensile strain increases interactions between human epidermal keratinocytes and quantum dot nanoparticles. Toxicol. In Vitro. 2008;22:491–497. doi: 10.1016/j.tiv.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 192.Tang M, Xing T, Zeng J, Wang H, Li C, Yin S, Yan D, Deng H, Liu J, Wang M, Chen J, Ruan D-Y. Unmodified CdSe quantum dots induce elevation of cytoplasmic calcium levels and impairment of functional properties of sodium channels in rat primary cultured hippocampal neurons. Environ. Health Perspect. 2008;116:915–922. doi: 10.1289/ehp.11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yang RSH, Chang LZ, Wu J-P, Tsai M-H, Wang H-J, Kuo Y-C, Yeh T-K, Yang CS, Lin P. Persistent tissue kinetics and redistribution of nanoparticles, quantum dot 705, in mice: ICP-MS quantitative assessment. Environ. Health Perspect. 2007;115:1339–1343. doi: 10.1289/ehp.10290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhang Y, Chen W, Zhang J, Liu J, Chen G, Pope C. In vitro and in vivo toxicity of CdTe nanoparticles. J. Nanosci. Nanotech. 2007;7:497–503. doi: 10.1166/jnn.2007.125. [DOI] [PubMed] [Google Scholar]
- 195.Derfus AM, Chan WCW, Bhatia SN. Probing the cytotoxicity of semiconductor quantum dots. Nano Lett. 2004;4:11–18. doi: 10.1021/nl0347334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gopee NV, Roberts DW, Webb P. Migration of intradermally injected quantum dots to sentinel organs in mice. Toxicol. Sci. 2007;98:249–257. doi: 10.1093/toxsci/kfm074. [DOI] [PMC free article] [PubMed] [Google Scholar]