Abstract
A dual-lipid liposome system consisting of a phospholipid and a skin ceramide extruded though a 100 nm membrane yields novel tubular and helical liposomes. These liposomes were used as templates to generate highly aspherical silica nanocapsules with length to diameter aspect ratios exceeding 10. Many of these nanocapsules have the morphology of a bulbous end attached to a long tip, mimicking microneedles attached to a reservoir. The fidelity of helical liposomes is transcribed to the silicas and the long tips indicate helically entwined left-handed silica structures. The silica coating is expected to protect and stabilize the internal contents of the liposomes, as well as enable surface functionalization for applications in drug or targeted delivery.
Templated synthesis involves aspects of directed growth and deposition of an organic or inorganic material at the solid–liquid or liquid–liquid interface. This simple concept has enabled the synthesis of ordered nanostructured inorganic materials of tunable pore sizes,1,2 as well as the transcription of complex self-assembled architectures to solid durable imprints.2,3 When colloidal bilayer systems of vesicles are used as templates, material growth occurs at the interface of a bilayer and its surrounding medium. Vesicle templates have been formed from synthetic surfactants,4 block copolymers,5 and phospholipids.6 However, in using vesicles as templates, there are inherent complications arising from the need to maintain vesicle robustness and shape during the materials synthesis process.7 Factors such as the stability of the vesicles and the nature of the growing material determine whether the topology of the vesicles can be successfully transcribed.7 During the process of templating, interactions between the growing material and the vesicles may also lead to an alteration in structure of the templated product7 or significant aggregation of the templated materials.8
Liposomes (lipid based vesicles) are potential drug delivery vehicles that allow encapsulation of drug and actives in either their internal aqueous phase or the liposome lipid bilayer.9 However, due to their inherent instability, leakage of internal contents occurs over time which greatly hampers their application.10 Hence, there is significant interest in solidifying the liposome morphology to form more robust, hollow submicron shells to encapsulate useful materials (e.g. drugs) in the interior of the nanocapsules. Liposomes have been successfully used as templates to generate such nanoscale capsules.8,11 Amorphous silica coatings allows the liposomal contents to be “locked in” and preserved for extended periods or for triggered release.8
Since liposomes made with phospholipids are typically spherical, successful instances of liposome templating in the literature are of individual spherical shells,6,11 or connected spheres.8 Spherical shells have also been reported when templating using spontaneously forming vesicles of catanionic surfactants.12 It was only recently that interesting foam-like dendritic nanostructures have been reported as a consequence of vesicle templating.13
In this paper, we report the formation and templating of elongated liposomes, some of which contain entwined helical structures. The template consists of L-α-phosphatidylcholine (PC) and a skin lipid (ceramide VI). L-α-phosphatidylcholine forms spherical liposomes. However, when L-α-phosphatidylcholine is doped with increasing amounts of ceramide VI, the liposomes transforms from a spherical shape to assume an elongated shape until the liposomes attain aspect ratios greater than 10. The morphological transformation can be explained by the packing parameters of PC and ceramide where ceramide promotes a flatter bilayer curvature compared to PC. The elongated morphology can be preserved indefinitely and our experiments have shown preservation of morphology for greater than 2 months. This provides ample time to template the liposomes using transcription methodologies to preserve template morphology. Beyond the optimum ceramide lipid level of 50 wt%, the liposome population becomes scarce and the liposomes shrink in size. This is reported in detail in a separate paper.14 To the best of our knowledge, the current study represents the first attempt of templating tubular and helical liposomes. If such liposomes can be transcribed to ceramic structures, it may be possible to develop new classes of nanocapsules with potentially increased cell penetration capability for drug delivery and diagnostics.
The synthesis of elongated tubular liposomes can be briefly summarized as follows: L-α-phosphatidylcholine and ceramide VI (chemical structures shown in Fig. 1) were combined at a 1:1 weight ratio, dissolved in a 2:1 part chloroform to methanol mixture and dried under low pressure in a standard rotary evaporator to obtain a lipid film. The dried lipid film was hydrated with distilled water at 50 °C. The liposome suspension was then extruded through a series of polycarbonate membranes (400 nm and 100 nm pore size, 11 passes each) at 55–65 °C to downsize the liposomes. The liposomes obtained after extrusion are stable for at least 2 months. To synthesize the templated materials, the extruded liposome suspension was diluted 2 fold with distilled water, after which tetraethylorthosilicate (TEOS) was added to the diluted liposome suspension (pH 5) and gently stirred for 3 to 18 days (molar ratio TEOS:lipid = 119/1). In general, a reaction period of at least 7 days is required before an even coat of silica forms to fully cover the liposomes. The samples were washed 3 times with water and once with ethanol and recovered through centrifugation (14000 rpm, 20 min) prior to imaging.
Fig. 1.
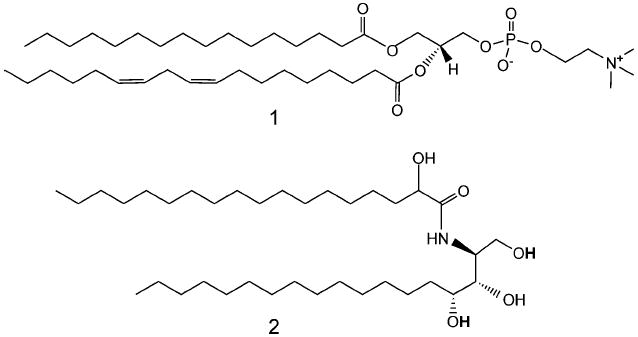
Chemical structures of L-α-phosphatidylcholine 1 and ceramide VI 2.
The morphology of the liposomes before addition of the silica precursor was characterized through cryo-transmission electron microscopy (cryo-TEM) to preserve the natural hydrated state of the materials. Briefly, cryo-TEM is done by forming a thin sample film upon rapid vitrification in liquid ethane and by preserving the sample in the frozen state throughout imaging. Fig. 2 illustrates the shape and size of the liposomes before addition of the silica precursor. The majority of liposomes have folded helical structures, are less than 500 nm in length and approximately 30 nm in inner diameter. As mentioned earlier, these liposomes are stable for more than 2 months which allows ample time to template their unique morphology.
Fig. 2.
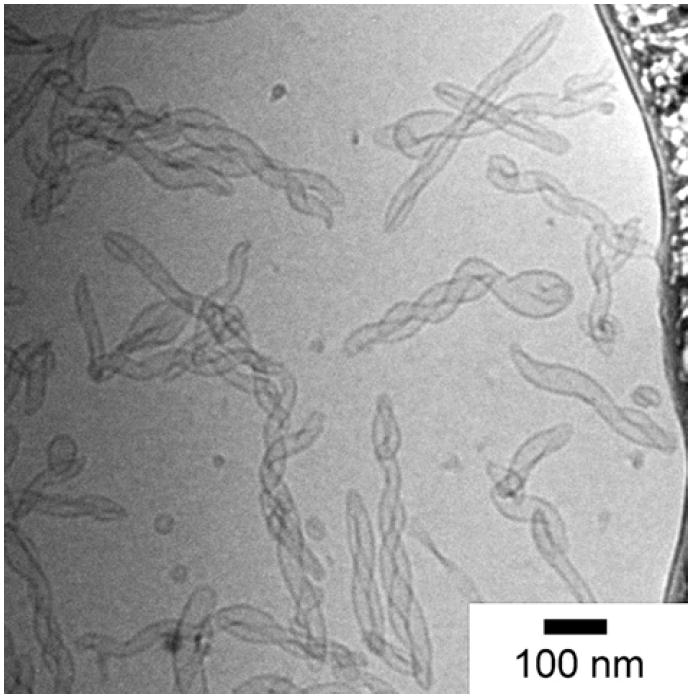
A cryo-TEM image showing elongated helical liposomes before templating with silica.
Silica templated liposomes imaged in room temperature TEM mode are presented alongside their non-templated counterparts in Figs. 3a to 3c for ease of comparison. The strong resemblance in morphology between the non-templated and templated liposomes provides evidence that the folded helical (Fig. 3a), spherical (Fig. 3b), and slightly elongated folded liposome (Fig. 3c) structures can be successfully transcribed to an inorganic material. A slight tightening of the folded helical structures is commonly observed upon templating. This tightening in structure can be rationalized in terms of interaction and further condensation of hydroxyl groups of the condensed silica network on the entwined liposome tails. Fig. 3d provides a close-up image of the tail end of the templated material. The thickness of the dense silica shell is approximately 10 nm. The superficial surface of the same silica shell appears to be coated with 5 to 8 nm spherical deposits of silica giving the silica shell a fluffy appearance. We believe that the globular silica may have arisen from colloidal silica in solution that has migrated close to the shells and condensed to form part of the silica network. This may have occurred later in the process which explains the larger size of the globular deposits as well as their positioning closer to the outer surface of the templated materials.
Fig. 3.
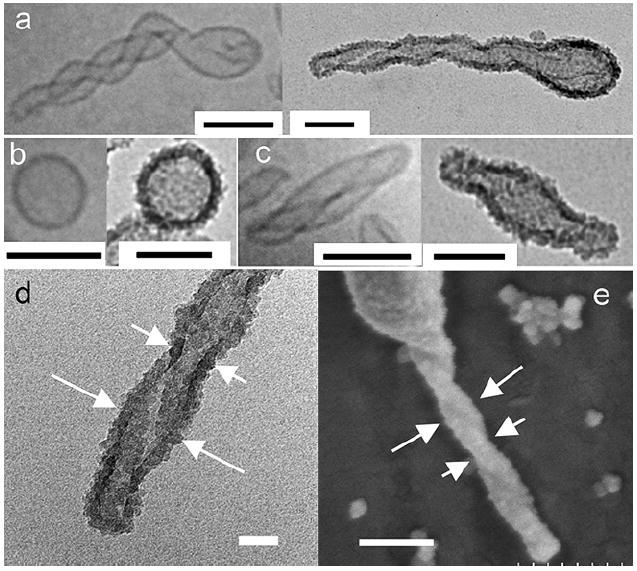
(a) Folded helical, (b) spherical and (c) slight elongated liposomes (left) and their silica templated structures (right). Scale bars in (a) to (c) are 100 nm. (d) Close-up TEM image of the tail of a silica templated helical liposome. Scale bar = 20 nm. (e) FESEM image showing the topology of a left-handed hollow silica shell. Scale bar = 150 nm. Short arrows in (d) and (e) show the narrow section of the entwined liposome tail while the long arrows point towards the wider section of the entwined tails.
The same templated materials were imaged on a field-emission scanning electron microscope to provide a complementary three-dimensional perspective of their morphology. Fig. 3e offers such a close-up view of the silica tail. Although the direction of the helix on the silica tail is not easily distinguished from the TEM images, SEM imaging indicates a left-handed helix. The periodic bulging and narrowing of the helical tail can be attributed to the entwining of the liposome tails. The narrower portion of the helical tail (shown by short arrows) is observed when a liposome tubule is located directly over another tubule when viewed from the top. The bulging section is observed when the two entwining liposome tubules are lying side by side. It is also apparent from Figs. 3a–d that the silicas appear to condense only on the outermost surface of the liposomes. Hidden regions of the lipid bilayers that are relatively less accessible do not appear to be coated by silica, which is especially true for the helical liposomes. This is illustrated in Fig. 4. Darker shades of silica are observed at the narrower portion of the tail (Fig. 5) which is in accordance with the observation that a thicker silica section arises when the liposome tubules are lying on top of each other. The pitch of the helical tail is approximated to be 100 nm from the TEM image.
Fig. 4.
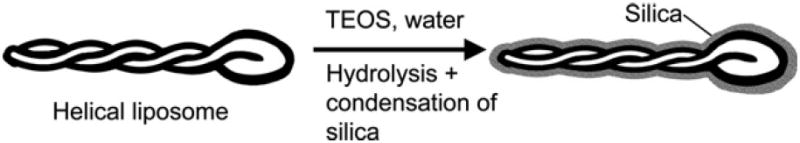
Illustration of silica templation of a double folded helical liposome.
Fig. 5.
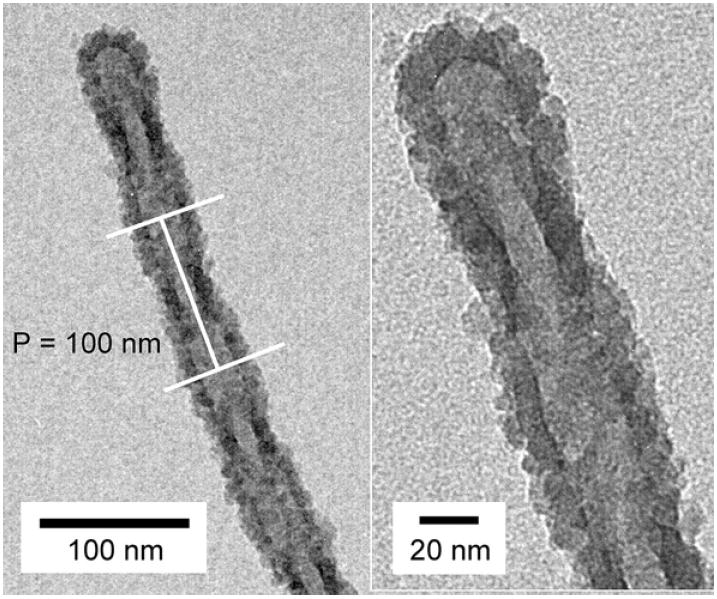
Helical silica with a pitch (P) of approx. 100 nm. The right image shows a higher magnification view of the tip of the tail.
A general overview of the templated materials is presented in Fig. 6. Hollow elongated helical silicas (>500 nm long, double black arrows), short elongated silicas (200 nm long, double black arrow heads), spherical hollow shells (100 nm wide, white arrow) and colloidal silica (10 nm, single black arrow) can all be distinguished in the same image. A number count of Fig. 6 indicates that almost 2/3 of silica nanoshells are nonspherical. In contrast, usually over 80% of the liposomes with the optimal lipid level of 50 wt% ceramide are nonspherical. The reason for this difference is unclear at present and could be due to the following possibilities: (1) some of the less elongated liposomes which may contain less ceramide tend to convert to spherical silica shells. It is also possible that upon addition of TEOS which would sequester to the lipid bilayer, some of the slightly aspherical liposomes may become more spherical. (2) Some of the tubular liposomes may break up upon the additon of TEOS to form a little debris which is clearly seen in the micrographs. We note that the molar ratio of TEOS to lipid is 119:1 and it is quite possible that TEOS can disrupt lipid structure to some degree.
Fig. 6.
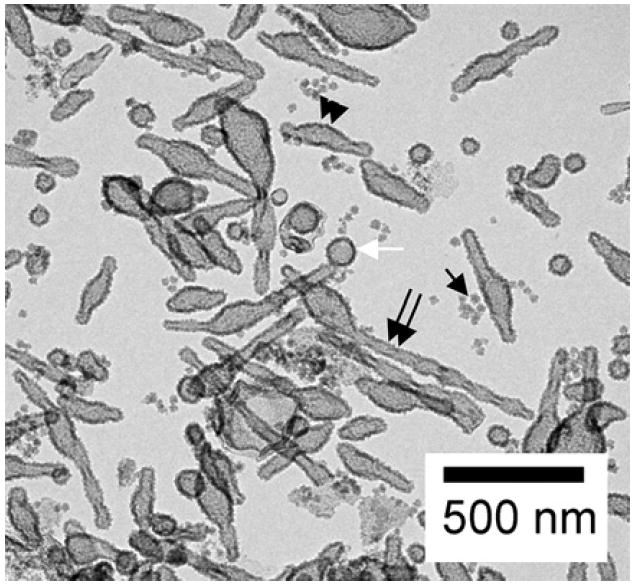
A TEM image showing colloidal silica and hollow silica shells obtained by templating aspherical and spherical liposomes.
It is also observed that many of the elongated shaped silicas possess a bulbous feature. In certain situations, the bulbous part corresponds to the region where the liposome is folded in half, e.g. in Fig. 3a. The fidelity of the bending junction becomes lost in translation from the lipid structure to the solid silica shell. In other cases, the non-uniform hardening of the liposome over the interface may lead to redistribution of lipids during the templating process and the formation of the bulbous feature.
Conclusions
In summary, we have shown that elongated liposomes as well as folded helical liposomes can be templated to form 10 nm thick hollow elongated silica shells. There are some alterations in morphology, however the helical nature of the liposomes can be successfully transcribed to silicas. Since shape plays an important role for micro- and nanoscale drug delivery carriers by influencing degradation profiles to release therapeutics and by affecting the targeting ability of the carrier, templating nonspherical liposomes is not only interesting from a materials templating standpoint but the templated nonspherical liposomes may also demonstrate delivery characteristics distinct from their spherical counterparts.15
Acknowledgments
Support from the National Institutes of Health (grant 1RO1EB006493-01) is gratefully acknowledged.
References
- 1.Kresge CT, Leonowicz ME, Roth WJ, Vartuli JC, Beck JS. Nature. 1992;359:710. [Google Scholar]
- 2.Mann S, Burkett SL, Davis SA, Fowler CE, Mendelson NH, Sims SD, Walsh D, Whilton NT. Chem Mater. 1997;9:2300. [Google Scholar]
- 3.Baral S, Schoen P. Chem Mater. 1993;5:145. [Google Scholar]; Yang YG, Suzuki M, Owa S, Shirai H, Hanabusa K. J Mater Chem. 2006;16:1644. [Google Scholar]
- 4.Jung M, Hubert DHW, Bomans PHH, Frederik P, van Herk AM, German AL. Adv Mater. 2000;12:210. [Google Scholar]; Hubert DHW, Jung M, Frederik PM, Bomans PHH, Meuldijk J, German AL. Adv Mater. 2000;12:1286. [Google Scholar]
- 5.Zhao DY, Feng JL, Huo QS, Melosh N, Fredrickson GH, Chmelka BF, Stucky GD. Science. 1998;279:548. doi: 10.1126/science.279.5350.548. [DOI] [PubMed] [Google Scholar]; Zhao DY, Huo QS, Feng JL, Chmelka BF, Stucky GD. J Am Chem Soc. 1998;120:6024. [Google Scholar]
- 6.Schmidt HT, Ostafin AE. Adv Mater. 2002;14:532. [Google Scholar]
- 7.Hubert DHW, Jung M, German AL. Adv Mater. 2000;12:1291. [Google Scholar]
- 8.Steinberg Y, Schroeder A, Talmon Y, Schmidt J, Khalfin RL, Cohen Y, Devoisselle JM, Begu S, Avnir D. Langmuir. 2007;23:12024. doi: 10.1021/la702311f. [DOI] [PubMed] [Google Scholar]
- 9.Khan DR, Rezler EM, Lauer-Fields J, Fields GB. Chemical Biology & Drug Design. 2008;71:3. doi: 10.1111/j.1747-0285.2007.00610.x. [DOI] [PubMed] [Google Scholar]
- 10.Zuidam NJ, Gouw HK, Barenholz Y, Crommelin DJ. Biochim Biophys Acta. 1995;1240:101. doi: 10.1016/0005-2736(95)00180-5. [DOI] [PubMed] [Google Scholar]; Grit M, Crommelin DJ. Chem Phys Lipids. 1993;64:3. doi: 10.1016/0009-3084(93)90053-6. [DOI] [PubMed] [Google Scholar]; Cevc G, Richardsen H. Advanced Drug Delivery Reviews. 1999;38:207. doi: 10.1016/s0169-409x(99)00030-7. [DOI] [PubMed] [Google Scholar]
- 11.Begu S, Durand R, Lerner DA, Charnay C, Tourne-Peteilh C, Devoisselle JM. Chem Commun. 2003:640. doi: 10.1039/b210927a. [DOI] [PubMed] [Google Scholar]; Begu S, Girod S, Lerner DA, Jardiller N, Tourne-Peteilh C, Devoisselle JM. J Mater Chem. 2004;14:1316. [Google Scholar]; Begu S, Pouessel AA, Lerner DA, Tourne-Peteilh C, Devoisselle JM. J Control Release. 2007;118:1. doi: 10.1016/j.jconrel.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 12.Hentze HP, Raghavan SR, McKelvey CA, Kaler EW. Langmuir. 2003;19:1069. [Google Scholar]; McKelvey CA, Kaler EW, Zasadzinski JA, Coldren B, Jung HT. Langmuir. 2000;16:8285. [Google Scholar]; Kepczynski M, Ganachaud F, Hemery P. Adv Mater. 2004;16:1861. [Google Scholar]; Kepczynski M, Lewandowska J, Romek M, Zapotoczny S, Ganachaud F, Nowakowska M. Langmuir. 2007;23:7314. doi: 10.1021/la063442i. [DOI] [PubMed] [Google Scholar]; Lootens D, Vautrin C, Van Damme H, Zemb T. J Mater Chem. 2003;13:2072. [Google Scholar]
- 13.Song YJ, Steen WA, Pena D, Jiang YB, Medforth CJ, Huo QS, Pincus JL, Qiu Y, Sasaki DY, Miller JE, Shelnutt JA. Chem Mater. 2006;18:2335. [Google Scholar]
- 14.Xu P, Tan G, Zhou J, He J, Lawson L, McPherson G, John V. Langmuir. in revision. [Google Scholar]
- 15.Champion JA, Katare YK, Mitragotri S. J Control Release. 2007;121:3. doi: 10.1016/j.jconrel.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]; Denkbas EB, Ottenbrite RM. J Bioact Compat Pol. 2006;21:351. [Google Scholar]


