Abstract
Background: The Women's Health Initiative Dietary Modification Trial tested the effects on chronic disease of a dietary pattern lower in fat and higher in vegetables, fruit, and grains.
Objective: The objective was to evaluate the effects of dietary carbohydrate changes on lipids and lipoprotein composition.
Design: Postmenopausal women were randomly assigned to an intervention or a comparison group for a mean of 8.1 y. Lipoprotein analyses and subclasses were based on subsamples of 2730 and 209 participants, respectively.
Results: At year 6, the total reported fat intake was 7.8% lower and carbohydrate intake was 7.6% higher in the intervention group than in the comparison group. Triglyceride change between groups differed by 2.3, 3.8, and −0.8 mg/dL at 1, 3, and 6 y, respectively, and HDL-cholesterol change differed by −1.6, −0.7, and −1.0 mg/dL at 1, 3, and 6 y, respectively. Changes did not differ by age, ethnicity, or obesity. In diabetic intervention women who were white, the triglyceride difference between the intervention and comparison groups was 33.8 mg/dL, whereas in black women with diabetes (n = 50 in the intervention group; n = 83 in the comparison group), the triglyceride difference was 6.4 mg/dL (P for 3-factor interaction = 0.049). No significant changes were observed in apolipoprotein or lipoprotein particles. Reductions in LDL cholesterol varied by quartile of reported lowering of saturated or trans fat.
Conclusions: The replacement of 7–8% of fat intake with complex carbohydrates over 6 y was not associated with clinically adverse effects on triglycerides, HDL cholesterol, or lipoprotein subclasses. Diabetic white women with higher triglyceride concentrations may have greater increases in triglycerides.
See corresponding editorial on page 829.
INTRODUCTION
The link between diet and plasma lipids and lipoproteins has been recognized since the 1950s (1). Since then, observational studies have established a link between dietary saturated and trans fats and LDL cholesterol (2). Some studies of increased carbohydrate intakes, especially of sugars, have resulted in elevated triglycerides and sometimes lower HDL-cholesterol concentrations and changes in LDL-cholesterol composition (3–7). More recent studies have focused on glycemic index (GI) and glycemic load (GL). In observational studies, GI and/or GL generally have shown positive associations with triglycerides (8–11) and inverse associations with HDL cholesterol (9, 12–15). The effects of reduced-GI and/or reduced-GL diets on HDL cholesterol and triglycerides in randomized controlled trials have been less consistent. All of the trials that examined either carbohydrate intake or GI/GL have been short (<1 y), and most have been conducted in overweight or obese participants (9, 10, 15).
The Women's Health Initiative (WHI) Dietary Modification (DM) Trial tested the effects of a dietary pattern low in total fat, along with increased vegetables, fruit, and grains, on chronic diseases in postmenopausal women during a mean 8.1 y of follow-up (16, 17). A recent publication showed no reduction of cardiovascular disease (CVD); trends toward greater reductions in LDL cholesterol and coronary heart disease risk were observed only in those who reported lower intakes of saturated fat or trans fat or higher intakes of vegetables and fruit (18). Despite the increased carbohydrate intake in the intervention group, no end-of-trial changes in triglycerides or HDL cholesterol were observed (18). In this article, the effects of the WHI diet, particularly the effects of the carbohydrates on lipids and lipoprotein composition, were evaluated.
SUBJECTS AND METHODS
Recruitment and dietary intervention
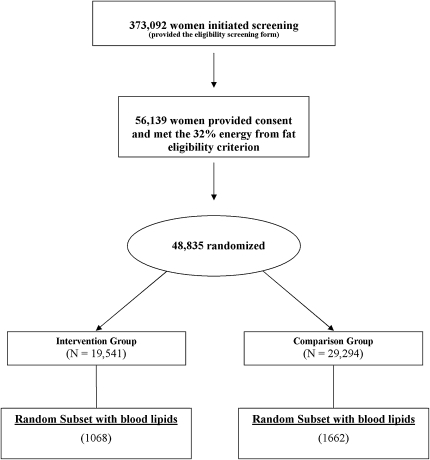
Details of the WHI DM study design and methods were published previously (16, 17). All women provided written informed consent, and the study was approved by the local institutional review boards as well as by the Coordinating Center Institutional Review Board and the National Institutes of Health (NIH). Briefly, 48,835 women aged 50–79 y were enrolled between 1993 and 1998 at 40 clinical centers throughout the United States and were randomly assigned to an intervention group (DM-I) (40%, n = 19,541) or a usual diet comparison group (DM-C) (60%, n = 29,294) (Figure 1) by using a randomized permuted block algorithm with a block size of 8, stratified by clinical center site and age group. Ethnicity was classified by self-report with the use of options listed on the personal data form completed by all participants at baseline.
FIGURE 1.
Participant flow in the Dietary Modification Trial of the Women's Health Initiative.
Eligibility criteria included being postmenopausal and consuming at baseline a diet with a fat intake ≥32% of total energy, as assessed by a food-frequency questionnaire (FFQ). Major exclusions for WHI included prior breast or colorectal cancer, other cancers except nonmelanoma skin cancer in the past 10 y, medical conditions with predicted survival <3 y, and adherence concerns such as alcoholism. Additional DM trial–specific exclusions included type 1 diabetes and frequent consumption of meals prepared away from home. The analyses in this manuscript are based on the subsample of 2730 women for whom plasma lipoproteins were measured. This subsample was chosen at random, with overselection for minority women.
The intervention was designed to promote dietary change, with the goals of reducing total fat intake to 20% of energy and increasing vegetable and fruit intakes to ≥5 servings and grain intake to ≥6 servings/d. The intervention did not include total energy reduction or weight-loss goals. Although not a separate focus of the intervention, it was presumed that by reducing total fat intake to 20% of energy, saturated fat would also be reduced to 7% of energy.
The intensive behavioral modification program included 18 group sessions in the first year and quarterly maintenance sessions thereafter, led by specially trained and certified nutritionists. Each participant was assigned her own fat gram goal, calculated on the basis of height. Participants self-monitored their daily fat gram intake and servings of vegetables, fruit, and grains. No formal intervention regarding saturated fat, cholesterol, trans fatty acids, or other known atherogenic factors was provided. Details of the intervention strategies and limitations were published previously (17, 19, 20).
Group activities were supplemented during the intervention by individual interviews that used validated reflective listening techniques, targeted message campaigns, and personalized feedback on fat intake. Individual contacts were completed by telephone or mail. The DM-C participants received a copy of the Dietary Guidelines for Americans and other health-related materials, but had no contact with the nutritionists. DM trial participants were invited to participate concurrently in a WHI hormone therapy (HT) trial (estrogen alone or estrogen plus progestin) (20). Participation in a trial of calcium and vitamin D supplementation was offered after 1 y (21). Of all DM participants, 42.2% joined the dietary trial only, 16.5% were in the dietary trial and the hormone therapy trial, 51.6% were in the dietary and the calcium trials, and 10.3% were in all 3 trials. Details of the hormone therapy and calcium trials were published previously (20, 21). The results of this report were not influenced by assignment to hormone or calcium/vitamin D randomization group.
On the basis of the intent-to-treat criteria, the participants were followed from the date of entry until death, loss to follow-up, request for no further contact, or to the trial's planned completion date, regardless of their adherence to the dietary intervention. All dietary trial participants were contacted by clinic staff at 6-mo intervals to provide information on health outcomes. Height, weight, waist circumference, and blood pressure were measured at annual visits by using standardized procedures. Physical activity was assessed at baseline and years 1, 3, 6, and 9; questions assessed walking and sports, and hours of activity per week were calculated for each participant. Physical activity was expressed as metabolic equivalents per week for the analysis. Diabetes was defined as self-report of physician diagnosis, use of hypoglycemic medication, or fasting glucose >125 mg/dL.
All DM participants completed an FFQ, designed specifically for the study (22) at baseline and 1 y. Thereafter, one-third of the participants completed the FFQ each year in a rotating sample; completion rates were 100% at baseline and 81% thereafter. Follow-up dietary intake data were computed from FFQs administered from years 5 to 7 (designated as year 6 follow-up), thus including all participants. The methods for assigning the GI and GL values used in the WHI FFQ were reported previously (23). Four-day food records (4DFRs) were provided by all women before randomization and by 4.6% at year 1. The analyses are based on FFQ data, but the results did not differ in those for whom 4DFR data were available. Fasting blood samples were collected at baseline and year 1 from all DM participants and from 5.8% (n = 2816) of a subsample of women at years 3 and 6. The subsample was randomly chosen with an oversampling of minority women, where the odds for selection were 6-fold higher than for white women. The analyses are based on the subsample of DM participants who had blood samples analyzed at baseline and years 1, 3, and 6.
Laboratory methods
Blood samples were drawn after the subjects fasted overnight (≥12 h) at all time points and were stored at −70°C until assayed. All lipids and lipoprotein subfractions were analyzed from EDTA-treated plasma. Total cholesterol and triglycerides were measured enzymatically, HDL cholesterol was measured by manganese sulfate precipitation, and LDL cholesterol was calculated according to the method of Friedewald (24). HDL2 and HDL3 were measured by ultracentrifugation and lipoprotein(a) [Lp(a)] by gel electrophoresis (25, 26).
Lipoprotein subclassification (type, size, and concentration) was performed on samples from 209 participants at baseline and year 1 who were in the hormone trials and also in the DM trial, as part of a nested case-control study of CVD, biomarkers, and hormone therapy. Lipoprotein particles were quantified via nuclear magnetic resonance (NMR) spectroscopy (27) by using an automated commercially available assay (LipoScience Inc, Raleigh, NC). Details of the NMR methodology were published previously (27).
Data analysis
All primary analyses were based on the intent-to-treat principle. Baseline values and nutrition data and lipid and lipoprotein values taken at the various time intervals were compared between randomization groups in the 5.8% subsample. Participants with missing values for total cholesterol or triglycerides at baseline were excluded (n = 86). A total of 2730 participants were included in the analyses of baseline lipid values and 2423, 2157, and 1009 participants for years 1, 3, and 6, respectively. Because hormone use influences apolipoprotein and lipoprotein distribution, the analyses of these variables were performed first by using only the women in the control groups (n = 99) of the hormone trials and then using all 209, with adjustment for hormone use.
Secondary analyses compared changes in the intervention and comparison groups stratified by baseline characteristics (eg, ethnicity, age, and body mass index) and other characteristics known to influence lipoproteins. For the analysis of baseline percentage of energy intake from fat, carbohydrate, and other dietary components, data from baseline 4DFRs were also used, as described previously (16, 17). The possibility of subgroup effects was explored by testing for interactions in expanded models. Thirty-two subgroups were tested; thus, ≥2 would be expected to be significant by chance alone at the 0.05 level of significance. Secondary analyses also were conducted to examine the relation in the intervention group between lipid and lipoprotein changes at years 3 and 6 and quartiles of specific nutrient intakes at year 1 (ie, percentage of energy from fat, vegetables/fruit, and grain). Other dietary components not specific to the intervention but believed to influence lipoproteins were similarly assessed, including saturated fats, polyunsaturated fats, trans fatty acids, fiber, total carbohydrate, simple sugars, GI, GL, and Katan Index (ΔLDL = 1.28Δsaturated fat – 0.24Δmonounsaturated fat − 0.55Δpolyunsaturated fat) (29). The analyses were adjusted for energy intake, baseline lipoprotein values, and known correlates of trial adherence; the lipoprotein changes in the comparison group were used as the reference. Statistical analyses were performed by using SAS (version 9.1; SAS Institute Inc, Cary, NC).
RESULTS
The prespecified randomly selected subset for whom blood data were available was ethnically diverse (51% minority) and included a range of education and income levels; 3.8% had a history of CVD (Table 1). Baseline characteristics were similar between the intervention and comparison groups, except for the prevalence of hypertension (P = 0.02). The women in this subset and the whole DM trial reflect the characteristics of the general population of women of this age throughout the United States, except that they had somewhat higher obesity, income, and education levels and lower smoking rates (17). Lipid-lowering medication use was reported by 12% of women in both the intervention and comparison groups.
TABLE 1.
Baseline characteristics of participants in the Dietary Modification Trial who provided blood samples (n = 2730), by randomization assignment1
| Intervention | Comparison | P value2 | |
| No. of subjects randomly assigned | 1068 | 1662 | — |
| Age (y) | 61.6 ± 6.93 | 61.8 ± 6.9 | 0.44 |
| Race-ethnicity [n (%)]4 | 0.86 | ||
| White | 522 (48.9) | 807 (48.6) | |
| Black | 294 (27.5) | 485 (29.2) | |
| Hispanic | 127 (11.9) | 178 (10.7) | |
| American Indian/Alaska Native | 27 (2.5) | 47 (2.8) | |
| Asian/Pacific Islander | 79 (7.4) | 114 (6.9) | |
| BMI [n (%)] | 0.65 | ||
| <25 kg/m2 | 230 (21.7) | 356 (21.5) | |
| 25 to <30 kg/m2 | 377 (35.5) | 561 (33.9) | |
| ≥30 kg/m2 | 455 (42.8) | 736 (44.5) | |
| Current smoker [n (%)] | 85 (8.1) | 119 (7.3) | 0.44 |
| Alcohol intake [n (%)] | 0.15 | ||
| 0 g/d | 541 (50.9) | 834 (50.4) | |
| >0 to <1.2 g/d | 154 (14.5) | 284 (17.2) | |
| ≥1.2 g/d | 368 (34.6) | 538 (32.5) | |
| Hypertension [n (%)] | 412 (43.0) | 722 (47.7) | 0.02 |
| Lipid-lowering medication use [n (%)] | 108 (11.7) | 171 (11.8) | 0.95 |
| Diabetes, history or fasting glucose >125 mg/dL [n (%)] | 114 (10.9) | 180 (11.0) | 0.92 |
| Enrolled in hormone therapy trial [n (%)] | 0.02 | ||
| Not enrolled | 721 (67.5) | 1065 (64.1) | |
| Assigned to active hormone therapy | 161 (15.1) | 322 (19.4) | |
| Assigned to placebo | 186 (17.4) | 275 (16.6) | |
| Enrolled in calcium and vitamin D trial [n (%)] | 0.30 | ||
| Not enrolled | 549 (51.4) | 807 (48.6) | |
| Assigned to active calcium and vitamin D | 264 (24.7) | 422 (25.4) | |
| Assigned to placebo | 255 (23.9) | 433 (26.1) | |
| Postmenopausal hormone use ever [n (%)] | 540 (50.6) | 838 (50.4) | 0.94 |
| Hysterectomy [n (%)] | 472 (44.2) | 792 (47.7) | 0.08 |
| Activity (METs/wk) | 9.5 ± 11.7 | 9.4 ± 11.9 | 0.85 |
| History of cardiovascular disease [n (%)] | |||
| MI | 15 (1.4) | 24 (1.4) | 0.93 |
| Stroke | 12 (1.1) | 26 (1.6) | 0.34 |
| CABG or PCI | 11 (1.0) | 18 (1.1) | 0.87 |
| Metabolic syndrome [n (%)]5 | 391 (36.6) | 608 (36.6) | 0.99 |
Participants are those with known values for triglycerides or total, LDL, or HDL cholesterol. METs, metabolic equivalent tasks; MI, myocardial infarction; CABG, coronary artery bypass surgery; PCI, percutaneous coronary intervention.
values are from chi-square tests.
Mean ± SD (all such values).
Ethnicity was unknown for 1.8% of participants.
Defined retrospectively by the Adult Treatment Plan III criteria.
Nutrient data based on the FFQs for both groups at baseline and at years 1, 3, and 6 are shown in Table 2. Taken as a whole, no meaningful changes were seen in any of the self-reported dietary components in the DM-C group, whereas the DM-I group experienced changes in all dietary components at 1, 3, and 6 y. Mean total fat intake fell from 38% to 25% of energy in year 1 and was 10.4% lower in the DM-I group than in the DM-C group at year 1. By year 6, mean total fat intake had risen to 29% of daily calories in the DM-I group, with a difference of 7.8% between the 2 groups (all P < 0.001). Saturated, monounsaturated, polyunsaturated, and trans fats all decreased proportionately, with greater differences in year 1 between the DM-I and DM-C groups, but significant decreases were maintained through year 6 (all P < 0.001). Carbohydrate intake increased from 46% to 58% of daily calories in year 1 and was 10% higher than in the DM-C group; the difference between groups was 7.6% by year 6 (P < 0.001 for all years). GI did not change, but sugar intake increased to 28.1% of energy and was 4.7% higher. GL was 14.1 units higher in year 1 in the DM-I group than in the DM-C group. Differences in sugar intake and GL were 4.0% and 6.7 units at year 6, respectively. Vegetable/fruit intake was higher in the intervention group by 1.3 servings/d throughout the trial. Fiber intake in the DM-I group was 3.6 g/d higher at year 1 and 2.6 g/d higher at year 6, compared with the DM-C group. Reported declines in nutrient differences between the groups from year 1 to year 6 were gradual, with intermediate values for all nutrients in year 3.
TABLE 2.
Baseline and follow-up nutrient intakes among participants who provided blood samples1
| Year 1 |
Year 3 |
Year 6 |
|||||||||
| Baseline |
Absolute difference between groups2 | Absolute difference between groups2 | Absolute difference between groups2 | ||||||||
| DM-I | DM-C | DM-I | DM-C | DM-I | DM-C | DM-I | DM-C | ||||
| Total energy (kcal) | 1801 | 1763 | 1506 | 1582 | −75.5 (−130.3, −20.6) | 1485 | 1576 | −91.1 (−105.1, −77.1) | 1424 | 1526 | −101.8 (−154.4, −49.2) |
| Total fat (% of energy) | 37.7 | 38.2 | 25.3 | 35.7 | −10.4 (−11.0, −9.8) | 26.5 | 36.0 | −9.5 (−9.7, −9.3) | 29.3 | 37.0 | −7.8 (−8.4, −7.1) |
| Saturated fat (% of energy) | 12.4 | 12.6 | 8.3 | 11.8 | −3.5 (−3.8, −3.3) | 8.7 | 12.1 | −3.3 (−3.4, −3.3) | 9.4 | 12.2 | −2.8 (−3.1, −2.5) |
| Monounsaturated fat (% of energy) | 14.6 | 14.7 | 9.4 | 13.7 | −4.3 (−4.5, −4.0) | 9.8 | 13.8 | −3.9 (−4.0, −3.8) | 11.1 | 14.3 | −3.2 (−3.5, −2.9) |
| Polyunsaturated fat (% of energy) | 7.9 | 7.8 | 5.4 | 7.4 | −1.9 (−2.1, −1.8) | 5.6 | 7.3 | −1.7 (−1.7, −1.7) | 6.3 | 7.6 | −1.3 (−1.5, −1.1) |
| P:S (% of energy) | 0.7 | 0.6 | 0.7 | 0.7 | 0.0 (0.0, 0.1) | 0.7 | 0.6 | 0.0 (0.0, 0.0) | 0.7 | 0.7 | 0.0 (0.0, 0.1) |
| Total trans fatty acid (% of energy) | 2.8 | 2.8 | 1.7 | 2.6 | −0.9 (−1.0, −0.8) | 1.7 | 2.4 | −0.7 (−0.7, −0.7) | 1.8 | 2.4 | −0.6 (−0.7, −0.5) |
| Protein (% of energy) | 16.4 | 16.3 | 17.5 | 16.5 | 0.9 (0.7, 1.2) | 17.6 | 17.0 | 0.7 (0.6, 0.7) | 17.7 | 17.0 | 0.7 (0.4, 1.0) |
| Carbohydrate (% of energy) | 46.0 | 45.7 | 58.0 | 47.9 | 10.0 (9.3, 10.7) | 56.4 | 47.0 | 9.3 (9.1, 9.5) | 53.9 | 46.3 | 7.6 (6.8, 8.4) |
| Sugar (% of energy) | 21.6 | 21.6 | 28.1 | 23.4 | 4.7 (4.1, 5.3) | 27.8 | 23.1 | 4.8 (4.6, 4.9) | 26.8 | 22.8 | 4.0 (3.3, 4.6) |
| Glycemic index | 53.0 | 53.2 | 52.7 | 52.8 | −0.0 (−0.3, 0.3) | 52.1 | 52.0 | 0.1 (−0.0, 0.2) | 51.3 | 51.6 | −0.4 (−0.7, 0.0) |
| Glycemic load | 101 | 99 | 106 | 92 | 14.1 (10.5, 17.7) | 100 | 88 | 11.3 (10.4, 12.2) | 90 | 83 | 6.7 (3.4, 9.9) |
| Dietary fiber (g) | 15.4 | 14.8 | 17.8 | 14.3 | 3.6 (3.0, 4.1) | 17.9 | 14.8 | 3.1 (2.9, 3.3) | 16.7 | 14.1 | 2.6 (2.0, 3.2) |
| Soluble fiber (g) | 4.2 | 4.0 | 4.8 | 3.9 | 0.9 (0.8, 1.1) | 4.8 | 4.0 | 0.8 (0.8, 0.8) | 4.4 | 3.8 | 0.7 (0.5, 0.8) |
| Cholesterol (mg) | 264 | 264 | 180 | 237 | −56.8 (−67.8, −45.7) | 185 | 240 | −54.4 (−57.4, −51.5) | 196 | 243 | −47.0 (−58.3, −35.6) |
| Vegetables and fruit (servings/d) | 3.6 | 3.4 | 4.9 | 3.6 | 1.3 (1.2, 1.5) | 5.1 | 3.9 | 1.3 (1.2, 1.3) | 4.9 | 3.6 | 1.3 (1.1, 1.4) |
| Grains (servings/d) | 4.9 | 4.7 | 5.2 | 4.2 | 0.9 (0.7, 1.2) | 4.6 | 4.0 | 0.7 (0.6, 0.7) | 4.2 | 3.8 | 0.5 (0.3, 0.7) |
| Whole grains (servings/d) | 1.2 | 1.2 | 1.4 | 1.1 | 0.3 (0.2, 0.4) | 1.3 | 1.1 | 0.3 (0.3, 0.3) | 1.3 | 1.0 | 0.2 (0.2, 0.3) |
| Soy (servings/wk)3 | 0.2 | 0.2 | 0.3 | 0.2 | 0.1 (0.0, 0.2) | 0.3 | 0.2 | 0.1 (0.1, 0.1) | 0.4 | 0.3 | 0.1 (−0.0, 0.2) |
| Nuts (servings/wk) | 1.5 | 1.4 | 0.5 | 1.2 | −0.6 (−0.8, −0.5) | 0.8 | 1.5 | −0.7 (−0.8, −0.7) | 1.1 | 1.6 | −0.6 (−0.8, −0.4) |
| Fish (servings/wk) | 2.1 | 2.0 | 2.0 | 1.8 | 0.2 (0.0, 0.4) | 2.0 | 1.9 | 0.0 (0.0, 0.1) | 2.2 | 2.1 | 0.1 (−0.1, 0.3) |
All values are means or means (95% CIs). Nutrient intakes were available for 1063 and 1656 participants at baseline, 982 and 1490 at year 1, 746 and 1094 at year 3, and 881 and 1373 at year 6 in the intervention and comparison groups, respectively. Data on servings of types of food were available for 1063 and 1656 participants at baseline, 981 and 1490 at year 1, 760 and 1101 at year 3, and 878 and 1368 at year 6 in the intervention and comparison groups, respectively. DM-I and DM-C, Women's Health Initiative Dietary Modification Trial participants in the intervention and control groups, respectively; P:S, ratio of polyunsaturated to saturated fatty acids.
All differences are significant at < 0.001 from a 2-sample t test, except for total energy (0.005), glycemic index (0.93), soy (0.05), and fish (0.04) at year 1; total energy (0.10), glycemic index (0.02), soy (0.009), and fish (0.12) at year 3; and glycemic index (0.06), soy (0.10), and fish (0.44) at year 6.
No soy intake was reported by 83.6%, 81.9%, 78.2%, and 76.8% of participants at baseline and years 1, 3, and 6, respectively.
During the course of the trial, women in the DM-I showed trends toward lower body weight and waist circumference (Table 3) compared with women in the DM-C; the differences were significant in the whole cohort, and weight differences were greater in women who had greater decreases in reported fat intake (28). A small difference in physical activity was observed between the groups only at year 6, with DM-C women slightly less active.
TABLE 3.
Changes in lipoproteins and other relevant variables at years 1, 3, and 6, by randomization group1
| Absolute change at year 1 |
Absolute change at year 3 |
Absolute change at year 6 |
|||||||||||||||
| Baseline |
Year 1 |
Difference between groups | Year 3 |
Difference between groups | Year 6 |
Difference between groups | |||||||||||
| DM-I | DM-C | DM-I | DM-C | DM-I | DM-C | DM-I | DM-C | DM-I | DM-C | DM-I | DM-C | DM-I | DM-C | ||||
| Weight (kg) | 77.4 | 78.5 | 75.7 | 78.0 | −1.5 | −0.4 | −1.11 (−1.73, −0.50)2 | 76.6 | 78.3 | −0.3 | 0.2 | −0.50 (−1.35, 0.34) | 77.0 | 78.4 | 0.1 | 0.3 | −0.16 (−1.03, 0.71) |
| BMI (kg/m2) | 29.7 | 30.1 | 29.0 | 29.9 | −0.6 | −0.1 | −0.45 (−0.64, −0.26)2 | 29.3 | 30.0 | −0.1 | 0.1 | −0.18 (−0.41, 0.06) | 29.7 | 30.1 | 0.2 | 0.2 | −0.03 (−0.28, 0.23) |
| Waist (cm) | 89.9 | 90.3 | 87.9 | 89.9 | −1.7 | −0.3 | −1.42 (−1.92, −0.92)2 | 89.3 | 90.9 | 0.02 | 0.7 | −0.69 (−1.35, −0.02)3 | 90.6 | 91.5 | 1.7 | 1.5 | 0.18 (−0.66, 1.01) |
| Physical activity (METs/wk) | 9.5 | 9.4 | 10.6 | 10.0 | 0.6 | 0.6 | −0.01 (−1.17, 1.14) | 11.0 | 10.5 | 1.2 | 0.9 | 0.25 (−0.75, 1.25) | 11.5 | 10.1 | 2.0 | 0.4 | 1.55 (0.47, 2.63) |
| Systolic blood pressure (mm Hg) | 127 | 129 | 126 | 127 | −1 | −3 | 1.53 (0.29, 2.77)3 | 127 | 127 | −1 | −2.5 | 1.99 (0.62, 3.36)3 | 126 | 126 | −1 | −3.1 | 1.98 (0.43, 3.54) |
| Total cholesterol (mg/dL) | 222 | 223 | 215 | 218 | −7 | −5 | −2.12 (−4.25, 0.02) | 212 | 216 | −10 | −6.9 | −3.11 (−5.81, −0.40)3 | 214 | 211 | −11 | −8.5 | −2.52 (−6.97, 1.94) |
| LDL cholesterol (mg/dL) | 133 | 134 | 126 | 129 | −7 | −6 | −1.17 (−3.13, 0.80) | 123 | 127 | −10 | −6.7 | −2.79 (−5.34, −0.24)3 | 123 | 126 | −9 | −7.6 | −0.88 (−5.11, 3.35) |
| HDL cholesterol (mg/dL) | 59 | 58 | 59 | 59 | −1 | 1 | −1.60 (−2.29, −0.90)2 | 59 | 58 | −1 | −0.0 | −0.67 (−1.50, 0.16) | 59 | 58 | −2 | −1.2 | −0.98 (−2.31, 0.34) |
| Triglycerides (mg/dL)4 | 134 | 135 | 136 | 136 | 3 | 1 | 2.33 (−1.89, 6.55) | 137 | 139 | 6 | −0.1 | 3.82 (−3.30, 10.94) | 135 | 138 | 4 | −0.1 | −0.82 (−9.21, 7.57) |
| Non-HDL cholesterol (mg/dL) | 163 | 165 | 156 | 159 | −7 | −6 | −0.70 (−2.77, 1.37) | 153 | 157 | −9 | −6.8 | −2.59 (−5.35, 0.17) | 153 | 156 | −8 | −7.5 | −1.37 (−5.91, 3.18) |
| Total:HDL cholesterol | 4.0 | 4.1 | 3.9 | 3.9 | −0.1 | −0.2 | 0.07 (0.01, 0.13)3 | 3.8 | 3.9 | −0.1 | 1.7 | −0.01 (−0.09, 0.07) | 3.8 | 3.9 | −0.1 | 3.5 | 0.00 (−0.14, 0.15) |
| Lp(a) (mg/dL)4 | 17.3 | 17.0 | 16.8 | 15.8 | −0.1 | −0.9 | 0.82 (−0.05, 1.69) | 14.7 | 15.1 | −2.1 | −2.8 | 0.68 (−0.62, 1.98) | 25.9 | 24.8 | 6.8 | 6.8 | 3.08 (0.93, 5.23) |
| HDL2 (mg/dL) | 19 | 18 | 19 | 19 | 0 | 1 | −0.70 (−1.11, −0.29)3 | 17 | 16 | −2 | −1.7 | −0.37 (−0.86, 0.12) | 17 | 16 | −2 | −1.5 | −0.44 (−1.21, 0.32) |
| HDL3 (mg/dL) | 41 | 40 | 40 | 40 | −1 | 0 | −0.83 (−1.28, −0.39)2 | 42 | 42 | 1 | 1.7 | −0.27 (−0.86, 0.33) | 42 | 42 | 0 | 0.3 | −0.27 (−1.23, 0.69) |
| VLDL-P (mmol)5 | 103 | 96 | 89 | 94 | −10 | −1 | −9.07 (−20.45, 2.30) | — | — | — | — | — | — | — | — | — | — |
| VLDL size | 47 | 47 | 51 | 49 | 4 | 1 | 3.36 (1.18, 5.54)3 | — | — | — | — | — | — | — | — | — | — |
| LDL-P (mmol)5 | 1669.8 | 1659.8 | 1705.3 | 1640.8 | 74.2 | −8.3 | 82.52 (−35.58, 200.62) | — | — | — | — | — | — | — | — | — | — |
| LDL size5 | 21.0 | 20.9 | 21.0 | 21.0 | −0.2 | 0.0 | −0.16 (−0.35, 0.03) | — | — | — | — | — | — | — | — | — | — |
| HDL-P (mmol)5 | 31.6 | 29.9 | 33.2 | 32.4 | 1.6 | 3.1 | −1.51 (−4.16, 1.14) | — | — | — | — | — | — | — | — | — | — |
| HDL size5 | 8.8 | 8.7 | 8.9 | 8.8 | 0.1 | 0.1 | −0.03 (−0.14, 0.09) | — | — | — | — | — | — | — | — | — | — |
All values are means or means (95% CIs). METs, metabolic equivalent tasks; LDL-P, LDL particle number; HDL-P, HDL particle number; VLDL-P, VLDL particle number; DM-I and DM-C, women in the intervention and comparison groups in the Women's Health Initiative Dietary Modification Trial, respectively; Lp(a), lipoprotein(a); HDL2 and HDL3, HDL subfractions isolated by ultracentrifugation.
Significant difference, P < 0.001 (2-sample t test).
Significant difference, P < 0.05 (2-sample t test).
Geometric means reported at baseline and year 1.
Lipoprotein subfraction measures are from a case-control study in the Women's Health Initiative (WHI) Hormone Trial. Available at baseline for 209 women and at year 1 for 137 women from the WHI Dietary Modification Trial. Comparison of VLDL-P change at year 1 was adjusted for baseline VLDL-P. Changes in body weight were included as a covariate when computing the changes in triglycerides and HDL cholesterol, and the results were nearly identical.
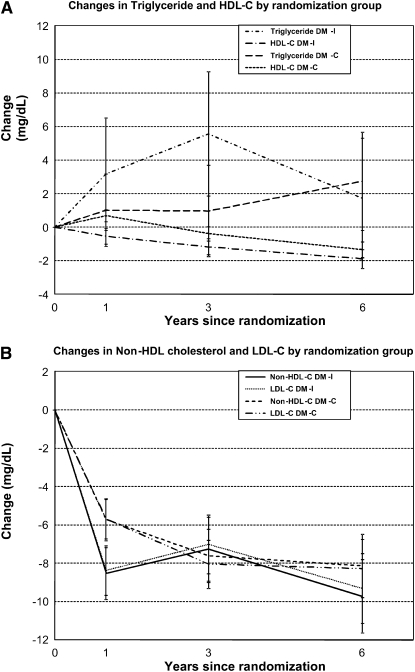
The differences in the changes in LDL cholesterol between the DM-I and DM-C groups of 1.2, 2.8, and 0.9 mg/dL after 1, 3, and 6 y (P = 0.25, 0.03, and 0.68, respectively) (Table 3 and Figure 2) were consistent with what would be predicted given the small decline in reported saturated fat intake and an accompanying decline in reported polyunsaturated fats (30). Parallel differences in non-HDL cholesterol occurred. No differences in triglyceride concentrations were observed between the DM-I and the DM-C groups, even at year 1. HDL cholesterol tended to be <1 mg/dL lower in the DM-I group than in the DM-C group. Changes in triglycerides and HDL cholesterol were nearly identical, and statistical significance remained unchanged after adjustment for weight loss. Baseline lipoprotein subfraction data were available from 209 women in the hormone trials. At year 1 only, a significant increase was observed in VLDL size as was a trend toward a lower number of VLDL particles in the DM-I than in the DM-C group; no significant changes were observed in LDL or HDL size or particle number (Table 3). Adjustment for hormone treatment did not influence the lipoprotein particle data.
FIGURE 2.
Six-year mean (±SE) changes in triglyceride and HDL-cholesterol (HDL-C) concentrations (A) and in non-HDL-cholesterol (Non-HDL-C) and LDL-cholesterol (LDL-C) concentrations (B) by randomization group. Significant differences in changes in HDL cholesterol at year 1 (P < 0.01) and in LDL cholesterol at year 3 (P < 0.05) were observed between women in the intervention and comparison groups of the Women's Health Initiative Dietary Modification Trial (DM-I and DM-C, respectively).
To determine whether changes in lipoproteins depended on the baseline characteristics of the participants, several stratified analyses were conducted comparing the DM-I with the DM-C group (Table 4). Although no ethnic differences in clinically meaningful triglyceride increases were noted, a trend toward greater difference in triglyceride changes was observed in white women than in black or Hispanic women in the intervention group (6.0 mg/dL compared with −4.1 mg/dL and −7.8 mg/dL, respectively; P for interaction = 0.13). There were no differences in triglyceride changes in obese women or in those with the metabolic syndrome. No significant differences in triglyceride changes between groups were observed when stratified by baseline triglycerides.
TABLE 4.
Changes (in mg/dL) in triglycerides (TGs) and HDL cholesterol (HDL-C) at year 1, by baseline demographic and dietary characteristics, in the intervention and comparison groups1
| Absolute change |
P value for interaction2 |
|||||||
| Intervention |
Comparison |
Absolute difference |
||||||
| Variable | TGs | HDL-C | TGs | HDL-C | TGs | HDL-C | TGs | HDL-C |
| Age at screening | 0.21 | 0.48 | ||||||
| 50–59 y | 5.7 ± 53.13 | −1.0 ± 9.1 | −0.2 ± 48.8 | 0.8 ± 8.4 | 5.89 (−0.64, 12.41)4 | −1.79 (−2.91, −0.67) | ||
| 60–69 y | 0.7 ± 58.1 | −0.6 ± 9.0 | 1.0 ± 52.6 | 1.2 ± 8.1 | −0.29 (−6.88, 6.31) | −1.76 (−2.78, −0.74) | ||
| 70–79 y | 3.8 ± 44.2 | 0.4 ± 8.4 | 3.1 ± 45.7 | 0.9 ± 8.5 | 0.67 (−8.96, 10..31) | −0.52 (−2.33, 1.29) | ||
| Race-ethnicity | 0.13 | 0.26 | ||||||
| White | 6.5 ± 60.3 | −1.7 ± 9.4 | 0.6 ± 54.1 | 0.6 ± 8.5 | 5.95 (−0.56, 12.47) | −2.28 (−3.30, −1.26) | ||
| Black | −2.0 ± 36.1 | 0.3 ± 8.2 | 2.2 ± 36.6 | 1.4 ± 8.1 | −4.13 (−9.77, 1.52) | −1.04 (−2.31, 0.22) | ||
| Hispanic | −2.0 ± 54.9 | 1.2 ± 8.6 | 5.8 ± 53.9 | 1.6 ± 7.4 | −7.82 (−21.18, 5.54) | −0.40 (−2.35, 1.55) | ||
| American Indian/Alaska Native | 4.6 ± 55.3 | 0.5 ± 7.7 | −4.6 ± 50.4 | −0.8 ± 7.6 | 9.28 (−18.81, 37.37) | 1.34 (−2.75, 5.43) | ||
| Asian/Pacific Islander | 6.8 ± 58.7 | 0.6 ± 7.3 | −5.0 ± 61.0 | 1.8 ± 9.1 | 11.73 (−6.34, 29.80) | −1.24 (−3.77, 1.28) | ||
| Unknown | 2.4 ± 74.6 | −1.6 ± 12.8 | −12.8 ± 51.6 | 2.7 ± 7.3 | 15.25 (−21.98, 52.48) | −4.34 (−10.29, 1.61) | ||
| Obesity [BMI (in kg/m2) ≥ 30] | 0.52 | 0.007 | ||||||
| Yes | 2.1 ± 57.3 | 0.2 ± 9.3 | 1.4 ± 52.1 | 0.7 ± 7.7 | 0.75 (−6.02, 7.51) | −0.47 (−1.52, 0.57) | ||
| No | 4.2 ± 52.2 | −1.2 ± 8.6 | 0.6 ± 48.8 | 1.2 ± 8.7 | 3.56 (−1.85, 8.97) | −2.42 (−3.36, −1.48) | ||
| Metabolic syndrome | 0.74 | 0.07 | ||||||
| Yes | −3.5 ± 69.7 | 1.2 ± 9.0 | −6.8 ± 62.0 | 2.0 ± 7.7 | 3.27 (−5.56, 12.10) | −0.74 (−1.84, 0.36) | ||
| No | 6.9 ± 42.8 | −1.6 ± 8.7 | 5.1 ± 41.5 | 0.4 ± 8.7 | 1.82 (−2.46, 6.09) | −2.08 (−2.97, −1.20) | ||
| Use of lipid-lowering medication | 0.53 | 0.45 | ||||||
| Yes | 10.4 ± 61.5 | 0.8 ± 10.9 | 2.9 ± 67.9 | 1.5 ± 9.4 | 7.51 (−9.81, 24.83) | −0.63 (−3.27, 2.02) | ||
| No | 2.4 ± 48.7 | −0.5 ± 8.6 | −0.7 ± 47.0 | 1.1 ± 8.1 | 3.05 (−1.37, 7.48) | −1.55 (−2.32, −0.77) | ||
| Diabetes (history or FG > 125 mg/dL) | 0.11 | 0.08 | ||||||
| Yes | 6.1 ± 56.1 | 1.6 ± 7.2 | −6.1 ± 58.8 | 1.5 ± 6.6 | 12.21 (−1.84, 26.26) | 0.14 (−1.52, 1.79) | ||
| No | 2.9 ± 53.9 | −0.9 ± 9.1 | 1.6 ± 47.6 | 0.9 ± 8.5 | 1.31 (−3.03, 5.64) | −1.83 (−2.58, −1.07) | ||
| Baseline TG | 0.48 | 0.06 | ||||||
| <108 mg/dL | 16.9 ± 27.1 | −1.7 ± 9.3 | 16.2 ± 27.8 | 0.5 ± 8.3 | 0.67 (−3 13, 4.48) | −2.20 (−3.41, −0.99) | ||
| 108 to <162 mg/dL | 8.1 ± 41.0 | −1.7 ± 8.0 | 5.3 ± 37.2 | 0.8 ± 8.6 | 2.81 (−2.71, 8.32) | −2.46 (−3.65, −1.26) | ||
| ≥162 mg/dL | −16.9 ± 77.6 | 1.7 ± 8.9 | −18.7 ± 68.8 | 1.7 ± 7.9 | 1.83 (−8.44, 12.11) | −0.02 (−1.20, 1.16) | ||
| Baseline HDL-C | 0.86 | 0.02 | ||||||
| <50 mg/dL | −4.1 ± 66.2 | 2.3 ± 8.1 | −6.0 ± 63.2 | 3.5 ± 7.1 | 1.94 (−7.16, 11.04) | −1.22 (−2.29, −0.16) | ||
| 50 to <64 mg/dL | 6.0 ± 45.4 | 0.3 ± 7.5 | 4.9 ± 44.1 | 0.9 ± 7.5 | 1.12 (−5.26, 7.49) | −0.59 (−1.66, 0.48) | ||
| ≥64 mg/dL | 8.2 ± 42.4 | −4.1 ± 9.6 | 3.9 ± 38.7 | −1.6 ± 9.3 | 4.27 (−1.34, 9.89) | −2.54 (−3.86, −1.22) | ||
| Baseline carbohydrates | 0.44 | 0.44 | ||||||
| <43.6% of energy | 0.6 ± 55.3 | −1.2 ± 8.6 | 1.6 ± 53.4 | 0.7 ± 8.4 | −0.99 (−8.67, 6.69) | −1.91 (−3.12, −0.70) | ||
| 43.6% to <49.0% of energy | 2.9 ± 56.3 | −0.3 ± 10.4 | 1.3 ± 45.3 | 1.1 ± 8.4 | 1.68 (−5.40, 8.75) | −1.47 (−2.78, −0.16) | ||
| ≥49.0% of energy | 5.6 ± 51.7 | −0.3 ± 7.8 | −0.3 ± 51.6 | 1.1 ± 8.0 | 5.86 (−1.35, 13.06) | −1.44 (−2.54, −0.33) | ||
| Baseline glycemic index | 0.72 | 0.93 | ||||||
| <51.8 | 4.6 ± 54.0 | −1.0 ± 8.1 | 2.0 ± 47.7 | 0.5 ± 8.5 | 2.60 (−4.45, 9.65) | −1.46 (−2.62, −0.30) | ||
| 51.8 to <54.6 | 4.1 ± 54.2 | −0.7 ± 9.9 | 1.7 ± 51.0 | 0.9 ± 8.4 | 2.42 (−4.41, 9.80) | −1.57 (−2.85, −0.29) | ||
| ≥54.6 | 0.3 ± 55.0 | −0.1 ± 8.7 | −1.0 ± 51.4 | 1.5 ± 7.9 | 1.32 (−6.22, 8.87) | −1.68 (−2.86, −0.50) | ||
| Baseline glycemic load | 0.84 | 0.16 | ||||||
| <76.3 | 5.9 ± 52.6 | −1.3 ± 8.5 | −0.6 ± 48.0 | 1.1 ± 8.1 | 6.51 (−0.67, 13.69) | −2.44 (−3.63, −1.25) | ||
| 76.3 to <111.0 | −1.2 ± 59.1 | −0.4 ± 10.0 | 2.4 ± 46.7 | 0.6 ± 8.7 | −3.60 (−10.87, 3.67) | −1.00 (−2.29, 0.29) | ||
| ≥111.0 | 5.0 ± 50.6 | −0.2 ± 8.1 | 0.9 ± 55.4 | 1.3 ± 8.0 | 4.10 (−3.39, 11.60) | −1.45 (−2.58, −0.31) | ||
| Baseline sugar | 0.71 | 0.34 | ||||||
| <18.8% of energy | 2.4 ± 61.0 | −1.1 ± 8.5 | 1.6 ± 52.6 | 1.0 ± 8.5 | 0.74 (−7.17, 8.66) | −2.02 (−3.22, −0.82) | ||
| 18.8% to <23.8% of energy | 3.8 ± 48.9 | −0.6 ± 8.8 | 1.7 ± 47.7 | 1.0 ± 8.3 | 2.07 (−4.78, 8.93) | −1.53 (−2.74, −0.32) | ||
| ≥23.8% of energy | 3.2 ± 52.3 | −0.2 ± 9.5 | −0.7 ± 49.9 | 1.0 ± 8.0 | 3.88 (−3.26, 11.02) | −1.25 (−2.46, −0.03) | ||
| Baseline fiber | 0.92 | 0.31 | ||||||
| <11.5 g | 4.2 ± 49.8 | −0.8 ± 10.1 | −1.3 ± 49.4 | 1.3 ± 8.1 | 5.56 (−1.62, 12.74) | −2.12 (−3.41, −0.84) | ||
| 11.5 to <16.8 g | 1.6 ± 59.3 | −0.2 ± 8.4 | 1.7 ± 45.9 | 0.8 ± 8.5 | −0.88 (−7.37, 7.21) | −0.94 (−2.14, 0.26) | ||
| ≥16.8 g | 3.5 ± 53.4 | −0.8 ± 8.3 | 2.4 ± 54.9 | 0.9 ± 8.3 | 1.15 (−6.35, 8.65) | −1.71 (−2.86, −0.56) | ||
| Baseline fat | 0.42 | 0.06 | ||||||
| <35.1% of energy | 4.2 ± 60.0 | −1.3 ± 8.3 | 1.0 ± 50.3 | 1.5 ± 8.6 | 3.12 (−4.52, 10.76) | −2.78 (−3.97, −1.59) | ||
| 35.1% to <39.7% of energy | 3.8 ± 52.2 | −0.7 ± 9.2 | 0.2 ± 50.5 | 0.7 ± 8.3 | 3.54 (−3.58, 10.67) | −1.33 (−2.55, -0.12) | ||
| ≥39.7% of energy | 1.1 ± 49.9 | 0.2 ± 9.2 | 1.3 ± 49.6 | 0.8 ± 7.9 | −0.20 (−7.39, 7.00) | −0.60 (−1.82, 0.63) | ||
| Baseline monounsaturated fat | 0.37 | 0.003 | ||||||
| <13.4% of energy | 5.2 ± 54.4 | −1.5 ± 8.6 | 2.7 ± 48.9 | 1.1 ± 8.2 | 2.50 (−4.64, 9.64) | −2.61 (−3.78, −1.44) | ||
| 13.4% to <15.3% of energy | 1.4 ± 57.8 | −1.1 ± 8.9 | −0.6 ± 52.4 | 1.4 ± 8.8 | 2.06 (−5.61 9.73) | −2.47 (−3.72, −1.23) | ||
| ≥15.3% of energy | 2.5 ± 50.3 | 0.9 ± 9.1 | 0.6 ± 48.8 | 0.5 ± 7.8 | 1.85 (−5.28, 8.99) | 0.46 (−0.74, 1.67) | ||
| Baseline saturated fat | 0.94 | 0.56 | ||||||
| <11.3% of energy | 1.7 ± 61.9 | −0.2 ± 9.2 | −0.4 ± 53.7 | 1.4 ± 8.4 | 2.08 (−5.96, 10.12) | −1.56 (−2.79, −0.32) | ||
| 11.3% to <13.3% of energy | 5.9 ± 47.1 | −0.6 ± 9.0 | 1.1 ± 45.5 | 0.8 ± 8.6 | 4.77 (−1.67, 11.22) | −1.43 (−2.65, −0.20) | ||
| ≥13.3% of energy | 1.5 ± 53.1 | −1.1 ± 8.4 | 1.8 ± 50.8 | 0.8 ± 7.9 | −0.30 (−7.74, 7.14) | −1.90 (−3.06, −0.73) | ||
| Baseline alcohol | 0.66 | 0.05 | ||||||
| 0 g/d | 1.5 ± 56.1 | −0.2 ± 8.8 | −1.9 ± 51.6 | 1.2 ± 8.4 | 3.44 (−2.75, 9.63) | −1.33 (−2.33, −0.34) | ||
| >0 to <1.2 g/d | 9.2 ± 52.1 | −0.6 ± 7.7 | 3.4 ± 51.2 | 1.7 ± 7.6 | 5.85 (−4.72, 16.41) | −2.30 (−3.87, −0.73) | ||
| ≥1.2 g/d | 2.8 ± 52.8 | −1.2 ± 9.6 | 3.7 ± 47.0 | 0.3 ± 8.4 | −0.97 (−7.83, 5.89) | −1.59 (−2.83, −0.35) | ||
FG, fasting glucose.
From linear regression models in which the change in lipoprotein is the dependent variable and independent variables include the demographic or dietary characteristic of interest, randomization assignment, and their interaction. Tests for interactions used the continuous form of the covariate except for race-ethnicity, obesity, metabolic syndrome, use of lipid-lowering medication, and diabetes.
Mean ± SD (all such values).
Mean; 95% CI in parentheses (all such values).
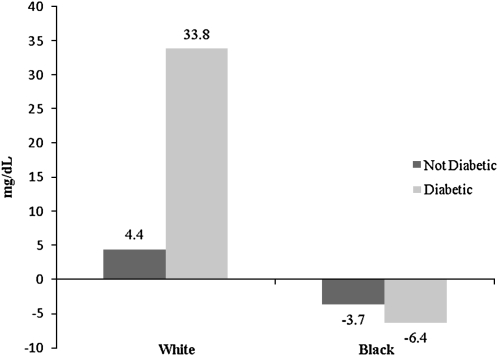
There were interactions of interest with diabetes. The triglyceride response in diabetes differed by ethnic group. In white women with diabetes (DM-I: n = 40; DM-C: n = 63), the triglyceride difference between the DM-I and DM-C groups was 33.8 mg/dL, whereas in black women with diabetes (DM-I: n = 50; DM-C: n = 83) the triglyceride difference was 6.4 mg/dL (P for 3-factor interaction = 0.049, adjusted for baseline triglycerides). Baseline triglyceride concentrations in white and black diabetic women were 215.0 and 138.4 mg/dL, respectively (Figure 3). These interactions of race with diabetes did not persist in years 3 and 6 (P for 3-factor interaction = 0.263 and 0.558). Also in women with diabetes, the triglyceride change tended to be greater in those with higher baseline triglycerides (+12 mg/dL in those with triglycerides >162 mg/dL and +1 mg/dL in those with triglycerides <108 mg/dL; P for 3-factor interaction = 0.11). In those who reported consuming more carbohydrates at baseline (> 49%), differences between groups in triglyceride changes were 5.9 mg/dL compared with −1.0 mg/dL in those with reported baseline carbohydrate consumption <44% (P for trend = NS); there was no suggestion of greater triglyceride differences in those with higher GI or GL values at baseline.
FIGURE 3.
Mean differences in triglyceride concentrations at year 1 (adjusted for baseline triglyceride concentrations) in the intervention and comparison groups of the Women's Health Initiative Dietary Modification Trial, stratified by race-ethnicity and baseline diabetes.
In the stratified analyses of baseline characteristics associated with changes in HDL cholesterol, race-ethnicity had no influence on changes in HDL cholesterol. Greater decreases (>2 mg/dL) in HDL cholesterol occurred in those who were not obese, had an HDL-cholesterol concentration ≥64 mg/dL, reported the lowest monounsaturated fat intake (P for interactions = 0.007, 0.02, and 0.003, respectively), or had the highest alcohol intake (P = 0.05). Lipid-lowering medication use did not influence changes in triglycerides and HDL cholesterol (P for interaction = 0.53 and 0.45). When baseline 4DFRs were used instead of the FFQ, the analysis of the effects of baseline intake on lipid changes did not differ (data not shown).
To determine whether changes in specific components of the diet would have a greater effect on lipoproteins, we examined changes in triglycerides, LDL cholesterol, HDL cholesterol, and non-HDL cholesterol in individuals, stratified by quartiles of reported levels of key nutrients at year 1 by using the change in the DM-C group as the reference. Models were adjusted for age, ethnicity, body mass index, hypertension, high cholesterol (defined as taking lipid-lowering medications), smoking, diabetes, physical activity, education, alcohol, hormone trial treatment arm, baseline levels of lipoprotein, and energy intake (Table 5). Changes in LDL cholesterol and non-HDL cholesterol were greater in those who reported lower levels of saturated (P < 0.001) and trans (P < 0.05) fats, and, in the case of LDL cholesterol, had the greatest change in Katan score (P < 0.001). Increases in triglycerides were greater in those who reported the highest GL (P = 0.09) and in those who decreased activity levels (11 compared with −4.0 mg/dL; P = 0.006). Similarly, decreases in HDL cholesterol were greater in those reporting the lowest total fat (P < 0.001), highest carbohydrate (P = 0.01), highest fiber (P = 0.02), and highest sugar (P < 0.001) intakes, but when trends in change in HDL cholesterol were observed, decreases were never >3 mg/dL. The same patterns were seen at years 3 and 6 with no amplification of any of the trends. Use of year 1 4DFR data showed similar trends (data not shown).
TABLE 5.
Changes in triglycerides (TGs), HDL cholesterol (HDL-C), LDL cholesterol (LDL-C), and non-HDL cholesterol (non-HDL-C) at year 1 (in mg/dL), by reported amounts of dietary components in the Women's Health Initiative Dietary Modification Trial at year 11
| Change in TGs | Change in HDL-C | Change in LDL-C | Change in non-HDL-C | |
| Total fat | ||||
| <19.5% of energy | 3.28 (−4.05, 10.61) | −1.97 (−3.18, −0.75) | −4.93 (−8.35, −1.51) | −4.17 (−7.83, −0.52) |
| 19.5% to <24.3% of energy | 6.27 (−0.71, 13.24) | −2.08 (−3.23, −0.92) | −3.94 (−7.21, −0.66) | −3.68 (−7.15, −0.21) |
| 24.3% to <29.9% of energy | 1.05 (−6.23, 8.32) | −0.24 (−1.45, 0.97) | 0.67 (−2.75, 4.09) | 1.33 (−2.29, 4.95) |
| ≥29.9% of energy | 2.57 (−4.77, 9.92) | 0.31 (−0.92, 1.53) | 1.77 (−1.68, 5.22) | 2.44 (−1.23, 6.11) |
| P for trend2 | 0.62 | <0.001 | <0.001 | 0.001 |
| Saturated fat | ||||
| <6.2% of energy | 1.79 (−5.50, 9.08) | −1.74 (−2.95, −0.53) | −5.45 (−8.87, −2.03) | −5.29 (−8.92, −1.65) |
| 6.2% to <7.9% of energy | 7.73 (0.70, 14.75) | −1.96 (−3.13, −0.80) | −3.83 (−7.12, −0.55) | −2.79 (−6.28, 0.70) |
| 7.9% to <9.9% of energy | 1.07 (−6.18, 8.33) | −0.68 (−1.89, 0.52) | 0.40 (−3.02, 3.81) | 0.74 (−2.87, 4.35) |
| ≥9.9% of energy | 2.53 (−4.76, 9.82) | 0.38 (−0.84, 1.59) | 2.36 (−1.05, 5.77) | 3.11 (−0.52, 6.75) |
| P for trend2 | 0.76 | 0.003 | <0.001 | <0.001 |
| Fruit/vegetables | ||||
| <3.0 servings/d | 1.67 (−5.54, 8.87) | −0.43 (−1.63, 0.77) | 1.38 (−1.99, 4.75) | 2.26 (−1.34, 5.86) |
| 3.0 to <4.6 servings/d | 4.29 (−2.93, 11.50) | −0.70 (−1.90, 0.50) | −2.25 (−5.65, 1.14) | −1.48 (−5.08, 2.12) |
| 4.6 to <6.4 servings/d | 0.10 (−7.05, 7.25) | −1.17 (−2.36, 0.03) | −3.17 (−6.51, 0.18) | −3.40 (−6.97, 0.16) |
| ≥6.4 servings/d | 8.14 (0.74, 15.55) | −1.83 (−3.07, −0.60) | −2.96 (−6.46, 0.54) | −1.87 (−5.56, 1.83) |
| P for trend2 | 0.34 | 0.07 | 0.05 | 0.06 |
| Fiber | ||||
| <11.6 g | 0.81 (−6.37, 8.00) | −0.08 (−1.28, 1.11) | 0.30 (−3.05, 3.66) | 0.49 (−3.09, 4.07) |
| 11.6 to <16.8 g | 6.50 (−0.74, 13.75) | −1.21 (−2.42, −0.00) | 0.14 (−3.28, 3.57) | 1.77 (−1.85, 5.38) |
| 16.8 to <22.2 g | 1.39 (−5.81, 8.58) | −0.68 (−1.87, 0.52) | −4.65 (−8.03, −1.28) | −4.46 (−8.05, −0.88) |
| ≥22.2 g | 5.02 (−2.38, 12.42) | −2.23 (−3.46, −1.00) | −2.63 (−6.12, 0.87) | −2.23 (−5.92, 1.46) |
| P for trend2 | 0.63 | 0.02 | 0.07 | 0.07 |
| Carbohydrate | ||||
| <52.4% of energy | 1.56 (−5.66, 8.77) | −0.40 (−1.60, 0.80) | 0.80 (−2.59, 4.19) | 1.51 (−2.10, 5.11) |
| 52.4% to <58.6% of energy | 0.44 (−6.91, 7.79) | −0.29 (−1.52, 0.93) | −0.27 (−3.71, 3.17) | 0.48 (−3.18, 4.14) |
| 58.6% to <63.9% of energy | 7.05 (−0.03, 14.13) | −1.15 (−2.33, 0.03) | −2.28 (−5.62, 1.06) | −1.69 (−5.22, 1.83) |
| ≥63.9% of energy | 4.15 (−3.08, 11.38) | −2.20 (−3.41, −1.00) | −4.86 (−8.25, −1.47) | −4.52 (−8.13, −0.92) |
| P for trend2 | 0.35 | 0.01 | 0.009 | 0.008 |
| Simple sugars | ||||
| <22.9% of energy | 1.18 (−6.06, 8.43) | 0.78 (−0.43, 1.98) | −1.32 (−4.75, 2.10) | −1.22 (−4.84, 2.40) |
| 22.9% to <27.7% of energy | 4.55 (−2.66, 11.77) | −0.66 (−1.86, 0.53) | 0.46 (−2.93, 3.84) | 2.00 (−1.59, 5.60) |
| 27.7% to <32.5% of energy | 1.36 (−5.93, 8.65) | −2.85 (−4.05, −1.64) | −3.44 (−6.87, −0.01) | −3.30 (−6.93, 0.34) |
| ≥32.5% of energy | 6.23 (−0.94, 13.40) | −1.36 (−2.55, −0.17) | −2.40 (−5.76, 0.95) | −1.85 (−5.43, 1.72) |
| P for trend2 | 0.43 | <0.001 | 0.32 | 0.35 |
| Polyunsaturated fat | ||||
| <4.2% of energy | 4.01 (−3.24, 11.25) | −2.20 (−3.40, −1.00) | −4.97 (−8.36, −1.58) | −4.32 (−7.94, −0.71) |
| 4.2% to <5.2% of energy | 6.95 (−0.16, 14.07) | −2.16 (−3.34, −0.98) | −2.51 (−5.85, 0.84) | −1.77 (−5.32, 1.78) |
| 5.2% to <6.5% of energy | −0.29 (−7.52, 6.94) | −0.19 (−1.39, 1.01) | −0.02 (−3.44, 3.40) | −0.03 (−3.63, 3.58) |
| ≥6.5% of energy | 2.66 (−4.66, 9.98) | 0.52 (−0.70, 1.73) | 0.88 (−2.55, 4.30) | 1.83 (−1.83, 5.49) |
| P for trend2 | 0.46 | <0.001 | 0.006 | 0.009 |
| trans Fatty acids | ||||
| <1.1% of energy | 2.64 (−4.67, 9.96) | −1.96 (−3.17, −0.74) | −4.27 (−7.67, −0.87) | −3.58 (−7.22, 0.06) |
| 1.1% to <1.5% of energy | 5.56 (−1.47, 12.58) | −2.02 (−3.19, −0.86) | −2.33 (−5.63, 0.97) | −1.81 (−5.31, 1.69) |
| 1.5% to <2.0% of energy | 4.74 (−2.61, 12.09) | −0.85 (−2.07, 0.36) | −2.70 (−6.16, 0.76) | −1.59 (−5.25, 2.07) |
| ≥2.0% of energy | 0.52 (−6.70, 7.74) | 0.76 (−0.44, 1.96) | 2.51 (−0.89, 5.91) | 2.53 (−1.07, 6.14) |
| P for trend2 | 0.63 | <0.001 | 0.006 | 0.02 |
| Glycemic index | ||||
| <50.4 | −3.66 (−10.87, 3.56) | −1.12 (−2.32, 0.09) | −2.49 (−5.88, 0.90) | −1.99 (−5.60, 1.61) |
| 50.4 to <52.8 | 9.40 (2.23, 16.57) | −1.34 (−2.54, −0.15) | −1.45 (−4.81, 1.91) | 0.26 (−3.32, 3.84) |
| 52.8 to <55.0 | 4.90 (−2.24, 12.04) | −0.28 (−1.47, 0.91) | 0.11 (−3.26, 3.47) | 0.45 (−3.11, 4.01) |
| ≥55.0 | 2.73 (−4.58, 10.05) | −1.38 (−2.61, −0.16) | −2.97 (−6.44, 0.50) | −3.19 (−6.85, 0.47) |
| P for trend2 | 0.33 | 0.90 | 0.96 | 0.68 |
| Glycemic load | ||||
| <75.4 | −1.61 (−8.83, 5.61) | −0.38 (−1.58, 0.82) | −1.70 (−5.08, 1.68) | −1.27 (−4.87, 2.34) |
| 75.4 to <99.9 | 3.24 (−4.00, 10.48) | 0.04 (−1.17, 1.25) | −2.46 (−5.87, 0.96) | −2.11 (−5.74, 1.51) |
| 99.9 to <128.8 | 5.71 (−1.50, 12.93) | −1.64 (−2.84, −0.44) | −1.29 (−4.68, 2.10) | −0.28 (−3.88, 3.32) |
| ≥128.8 | 6.49 (−0.91, 13.89) | −2.17 (−3.40, −0.94) | −1.24 (−4.73, 2.26) | −0.67 (−4.37, 3.02) |
| P for trend2 | 0.09 | 0.008 | 0.74 | 0.65 |
| Whole grains | ||||
| <0.7 serving/d | 1.14 (−6.28, 8.55) | −0.23 (−1.47, 1.00) | −0.17 (−3.64, 3.30) | 0.22 (−3.48, 3.92) |
| 0.7 to <1.2 servings/d | 4.61 (−2.42, 11.65) | −0.79 (−1.97, 0.38) | −3.73 (−7.08, −0.39) | −3.23 (−6.75, 0.29) |
| 1.2 to <1.8 servings/d | 5.71 (−1.45, 12.88) | −1.95 (−3.14, −0.76) | −0.61 (−3.97, 2.74) | 0.22 (−3.36, 3.79) |
| ≥1.8 servings/d | 2.10 (−5.21, 9.42) | −1.06 (−2.28, 0.16) | −2.24 (−5.67, 1.18) | −1.49 (−5.14, 2.16) |
| P for trend2 | 0.81 | 0.17 | 0.70 | 0.86 |
| Monounsaturated fat | ||||
| <7.0% of energy | 4.93 (−2.34, 12.20) | −2.66 (−3.86, −1.45) | −4.88 (−8.27, −1.49) | −3.75 (−7.38, −0.13) |
| 7.0% to <8.8% of energy | 4.47 (−2.66, 11.61) | −1.56 (−2.74, −0.38) | −3.63 (−6.97, −0.28) | −3.40 (−6.95, 0.16) |
| 8.8% to <11.3% of energy | 2.37 (−4.84, 9.57) | −0.51 (−1.70, 0.69) | 0.94 (−2.45, 4.32) | 1.27 (−2.32, 4.85) |
| ≥11.3% of energy | 1.67 (−5.67, 9.01) | 0.68 (−0.54, 1.90) | 1.01 (−2.45, 4.47) | 1.64 (−2.03, 5.30) |
| P for trend2 | 0.44 | <0.001 | 0.002 | 0.007 |
| Omega-3 fatty acids | ||||
| <0.7 g | 3.19 (−3.97, 10.34) | −0.91 (−2.10, 0.28) | −3.46 (−6.82, −0.11) | −2.35 (−5.92, 1.22) |
| 0.7 to <1.0 g | 0.45 (−6.77, 7.66) | −1.68 (−2.88, −0.49) | −2.30 (−5.69, 1.08) | −2.47 (−6.07, 1.12) |
| 1.0 to <1.4 g | 5.42 (−1.92, 12.75) | −1.64 (−2.86, −0.42) | −1.90 (−5.39, 1.58) | −1.97 (−5.64, 1.69) |
| ≥1.4 g | 4.55 (−2.75, 11.84) | 0.13 (−1.08, 1.35) | 1.08 (−2.33, 4.49) | 2.54 (−1.10, 6.17) |
| P for trend2 | 0.57 | 0.22 | 0.05 | 0.05 |
| Katan score for change3 | ||||
| Quartile 1 | 1.67 (−5.55, 8.89) | −2.36 (−3.57, −1.15) | −5.00 (−8.42, −1.58) | |
| Quartile 2 | 2.46 (−4.75, 9.68) | −1.54 (−2.74, −0.35) | −3.56 (−6.90, −0.21) | |
| Quartile 3 | 8.60 (1.47, 15.74) | −0.30 (−1.49, 0.89) | −1.04 (−4.46, 2.38) | |
| Quartile 4 | 0.54 (−6.75, 7.83) | 0.08 (−1.12, 1.28) | 2.68 (−0.65, 6.01) | |
| P for trend2 | 0.85 | <0.001 | <0.001 | |
| Alcohol intake | ||||
| 0 g | 4.11 (−1.26, 9.48) | −0.92 (−1.81, −0.02) | −1.68 (−4.20, 0.85) | −0.86 (−3.54, 1.83) |
| >0 to <2.6 g | 4.13 (−3.21, 11.47) | −0.91 (−2.13, 0.31) | −0.01 (−3.48, 3.46) | 0.42 (−3.25, 4.09) |
| ≥2.6 g | 0.70 (−6.74, 8.15) | −1.36 (−2.60, −0.11) | −3.10 (−6.60, 0.39) | −2.95 (−6.66, 0.77) |
| P for trend2 | 0.47 | 0.58 | 0.62 | 0.44 |
| Total recreational physical activity | ||||
| <1.9 MET-h/wk | 11.05 (1.83, 20.26) | −1.63 (−3.19, −0.06) | −3.99 (−8.44, 0.46) | −1.33 (−6.10, 3.44) |
| 1.9 to <7.5 MET-h/wk | 7.66 (−1.44, 16.77) | −1.70 (−3.25, −0.16) | 0.76 (−3.65, 5.17) | 0.70 (−4.01, 5.41) |
| 7.5 to <16.7 MET-h/wk | 0.45 (−8.28, 9.18) | −2.22 (−3.70, −0.73) | −1.89 (−6.10, 2.31) | −1.87 (−6.40, 2.66) |
| ≥16.7 MET-h/wk | −3.96 (−13.07, 5.16) | 0.55 (−1.00, 2.09) | −2.09 (−6.45, 2.27) | −1.95 (−6.66, 2.77) |
| P for trend2 | 0.006 | 0.08 | 0.77 | 0.63 |
MET-h, metabolic equivalent task hours. Changes in the intervention groups were compared with changes in the comparison group; adjusted for age, hormone therapy randomization arm, BMI, race-ethnicity, education, treated diabetes, hypertension, current smoking, high cholesterol requiring medication, total physical activity, baseline total energy, alcohol intake, and baseline lipoprotein concentration.
Determined from a linear regression model in which the dependent variable was the change in lipoprotein and covariates included a linear term for quartiles of the dietary component using the comparison group as reference and adjusted for the variables listed above.
Katan score estimates the effect of changes in carbohydrate and fatty acid intakes on lipid and lipoprotein concentrations. An estimated equation for non-HDL-C was not available.
DISCUSSION
This large long-term randomized trial of a dietary intervention ended with a 7.8% lower intake of energy from total fat and a 7.6% higher intake of carbohydrate. There was a nonsignificant trend toward lower rates of breast cancer [relative risk (RR): 0.91; 95% CI: 0.83, 1.01] (30), and lower rates of ovarian cancer (RR: 0.83; 95% CI: 0.60, 1.11) (31), but no overall effects on coronary heart disease or stroke (18). Because some studies (3, 5–7) and a major meta-analysis (4) have indicated that replacing dietary fat with carbohydrate elevates triglycerides and lowers HDL cholesterol, the present analysis was initiated to examine lipoprotein changes over a 6-y period. In this sample of postmenopausal women without severe hypertriglyceridemia at baseline, no clinically meaningful increases in triglycerides or decreases in HDL cholesterol occurred, regardless of age, race, or obesity. However, 2 interactions were potentially significant. In diabetic women with higher triglycerides at baseline, there was a 16% difference in triglyceride changes between the DM-I and DM-C groups, and white, but not black, women with diabetes experienced increases in triglyceride of 11.9% with the low-fat, high-carbohydrate diet.
Subgroup analyses explored the effects of changes in individual dietary components. The relation between carbohydrate intake and triglycerides has been the focus of much attention. Metabolic studies have shown that the increase in triglycerides can be the result of a decreased clearance of VLDL particles (32). In earlier studies, lower-fat, higher-carbohydrate diets usually contained higher amounts of simple carbohydrate and no additional fiber. More recent work has suggested that simple sugars (33, 34), and GI and/or GL might be responsible for the increases in triglycerides. Our analyses showed trends toward a greater increase in triglycerides in women reporting the highest GL. In our intervention, participants were counseled to replace dietary fat with vegetables, fruit, and grains; thus, there was not a large increase in simple sugar and no increase in GI, although the GL did increase. Thus, our results, although based solely on FFQ assessments, suggest that a dietary strategy that replaces ≈8% of fat with appropriate carbohydrates will not have clinically meaningful adverse effects on triglycerides in most individuals. Even in individuals starting at higher levels of reported carbohydrate consumption, no trend toward greater increases in triglycerides was observed. Because our highest tertile of reported baseline carbohydrate was >49% of calories, we cannot rule out effects in those with extremely high carbohydrate intakes. However, in the POUNDS LOST (Preventing Overweight Using Novel Dietary Strategies) Study, weight loss at 6 mo with a higher-carbohydrate but not with a lower-carbohydrate diet did not show clinically meaningful differences in triglycerides (8.1 mg/dL); the reported difference in dietary carbohydrate (57% compared with 43%) was almost twice that in the present study (35). Furthermore, even in those with greater reported fat reductions, there were decreases in LDL cholesterol and non-HDL cholesterol; regardless of how these decreases are achieved, they should decrease the CVD risk.
We observed minimal adverse effects of the diet on HDL cholesterol during the 6 y for which blood samples were available. Other studies of the effects of higher-carbohydrate diets on HDL cholesterol have been contradictory (4, 34). Our diet had the most adverse effect on HDL cholesterol in those who were at the lowest baseline CVD risk—those who were not obese, did not have the metabolic syndrome or diabetes, or had the highest HDL cholesterol (>64 mg/dL) or lowest triglyceride (<108 mg/dL) concentrations. Epidemiologic data have consistently shown an inverse relation between HDL cholesterol and a positive relation between triglycerides and CVD risk. Whether improving triglyceride and HDL-cholesterol concentrations with a diet intervention will reduce the CVD risk is unknown. Although no metabolic studies have been conducted, an increase in VLDL particles could conceivably result in lower HDL-cholesterol concentrations through increased cholesterol ester transfer protein (CETP) activity. As with triglycerides, our subgroup analyses, again based on an FFQ, suggest greater decreases in HDL cholesterol in the women who reported the highest GL and also in those who reported the greatest increases in simple sugar intake. However, the differences were modest (<3 mg/dL); it is likely that alterations that result in greater increases in VLDL particles will be accompanied by lower HDL cholesterol. Our study did not evaluate whether extremely high carbohydrate, GL, and sugar intakes can result in clinically meaningful decreases in HDL cholesterol. As expected, those who reported the lowest intake of total fat, including saturated and polyunsaturated fats, also had the greatest decrease in HDL cholesterol.
We explored the possibility that the low-fat diet might be detrimental to subsets of individuals. Triglyceride and HDL-cholesterol results did not vary by baseline lipid concentrations. They also did not vary by age, obesity, or alcohol intake—all variables known to affect triglycerides and HDL cholesterol. On the other hand, differences were observed related to diabetes and higher baseline triglycerides. In the DM-I group, women with diabetes had greater increases in triglycerides than did women without diabetes, although the interaction was not statistically significant, and the differences were small. The effect of increasing carbohydrate intake in diabetes differed by ethnicity. The increase in triglycerides was more marked in diabetic white women, with the increase averaging 34 mg/dL, whereas triglycerides decreased in black women with diabetes (P = 0.049 for this 3-factor interaction). This interaction may be related to the observation that, in diabetic women, the increase in triglycerides was higher in those with higher baseline triglycerides. This interaction remained significant after adjustment for baseline triglycerides. A metabolic basis may exist for the ethnic interaction. Triglyceride concentrations were lower in black than in white women; the baseline triglyceride concentration was 215 mg/dL in diabetic white women and was 138 mg/dL in diabetic black women. Because the clearance of VLDLs is higher (36), VLDL clearance may not be inhibited by increasing carbohydrate intakes in black women. Further studies are warranted to explore the effect of carbohydrate on VLDL metabolism in diabetic black and white women.
As reported previously, changes in LDL cholesterol and in non-HDL cholesterol with the WHI diet were minimal, and the ratio also did not change substantially during the trial. The intervention was designed to focus on lowering rates of breast and colorectal cancer by reducing total dietary fat. DM-I women reported saturated fat and cholesterol intakes that were <10% of total energy and <300 mg/d, respectively. However, the declines in saturated and trans fat intakes were small, and intakes of polyunsaturated fat also declined. These are the primary dietary determinants of LDL cholesterol. When the equation of Mensink and Katan (29) was applied, the observed changes in LDL cholesterol were as predicted. The subgroup analyses (Table 5) verified greater decreases in LDL cholesterol and non-HDL cholesterol with greater declines in saturated or trans fat.
Lipoprotein subfraction data were available for only 137 women at baseline and year 1. The data suggest that the diet resulted in a small increase in VLDL size, but the decrease in VLDL particles was not significant. Because increasing carbohydrate intake impairs VLDL clearance (32), larger particles may be affected more; this hypothesis must be confirmed in a larger sample. No significant changes were observed in LDL or HDL particle size or number, which was not surprising given the relatively minor differences in VLDL triglycerides.
This study had many strengths, including its randomized design, long-term follow-up, large sample size, and ethnic and socioeconomic diversity. Limitations include the relatively small numbers of women for whom lipoprotein data are available, especially for the subfractions, and the lack of targeting key nutrients relevant to lipoproteins. Data before 1 y of intervention were not available; therefore, we were not able to examine possible early changes.
This study was limited to women aged 50–79 y; there may have been greater changes in men or if the diet had been initiated at younger ages. In addition, it was not designed to assess strategies for increasing carbohydrate intake. Mean lipid concentrations at baseline were close to optimal; extrapolation of the results to a more dyslipidemic population at higher risk of CVD should be avoided. It must be stressed that we used the FFQ to assess food intake; thus, these analyses will be biased by errors in self-report (37, 38). Our validation study in a subset of women suggested that percentage of energy from fat at baseline may have been overestimated by 2–3% (39). Finally, there was major confounding of analyses of individual nutrients because of their interrelations in many foods.
In conclusion, this long-term dietary intervention in postmenopausal women, intended to reduce fat and increase vegetables, fruit, and grains, ended with a reported 7.8% decrease in energy from total fat and corresponding increases in carbohydrate and GL. The intervention did not result in clinically meaningful increases in triglycerides or decreases in HDL cholesterol over the 6 y of observation in most of the women. This study provides long-term data on the stability of lipoprotein risk factors during a dietary strategy, which promoted replacing fat with complex carbohydrates. Thus, this diet, combined with increased activity, may be used by persons choosing to restrict calories by reducing fat intake to achieve weight loss. Because therapies that lower LDL cholesterol and non-HDL cholesterol reduce CVD risk, dietary interventions should focus on strategies that affect these 2 lipid variables. The observation that this diet might induce elevations in triglycerides in white women with diabetes or in diabetic women with high triglyceride concentrations requires further investigation.
Acknowledgments
We gratefully acknowledge Rachel Schaperow, MedStar Research Institute, for editorial services.
The authors’ responsibilities were as follows—BVH, JDC, CBE, CK, JO, JBK, AR, JGR, JR, GS, JMS, and LVH: design of the experiment; BVH, JDC, CBE, CK, JO, JBK, AR, JGR, JR, GS, JMS, and LVH: data collection; BVH, JDC, CBE, CK, JO, JBK, AR, JGR, JR, GS, JMS, LVH, and MP: analysis of data; BVH, JDC, CBE, CK, JO, JBK, AR, JGR, JR, GS, JMS, and LVH: writing of the manuscript; and BVH, JDC, CBE, CK, JO, JBK, AR, JGR, JR, GS, JMS, LVH, and MP: provided significant advice or consultation. BVH has served on the advisory boards of Merck, Schering Plough, and the Egg Nutrition Council and has received research support from Merck and Pfizer. JMS has received research support from Kraft Foods Inc. JGR has received research support from Abbott, Aegerion, Astra-Zeneca, Bristol-Myers Squibb, Daiichi-Sankyo, Hoffman La Roche, Merck, and Merck Schering-Plough; has received honoraria from Merck Schering Plough; and is a consultant/advisory board member for Astra-Zeneca and Merck. The other authors had no conflicts to declare.
APPENDIX A
Long list of Women's Health Initiative investigators
Program Office (National Heart, Lung, and Blood Institute, Bethesda, MD)
Elizabeth Nabel, Jacques Rossouw, Shari Ludlam, Joan McGowan, Nancy Geller, and Leslie Ford
Clinical Coordinating Center (Fred Hutchinson Cancer Research Center, Seattle, WA)
Ross Prentice, Garnet Anderson, Andrea LaCroix, Ruth Patterson, Anne McTiernan, Barbara Cochrane, Julie Hunt, Lesley Tinker, Charles Kooperberg, Martin McIntosh, CY Wang, Chu Chen, Deborah Bowen, Alan Kristal, Janet Stanford, Nicole Urban, Noel Weiss, and Emily White; Medical Research Laboratories, Highland Heights, KY: Evan Stein and Peter Laskarzewski; San Francisco Coordinating Center, San Francisco, CA: Steven R Cummings, Michael Nevitt, and Lisa Palermo; (University of Minnesota, Minneapolis, MN: Lisa Harnack; Fisher BioServices, Rockville, MD: Frank Cammarata and Steve Lindenfelser; University of Washington, Seattle, WA: Bruce Psaty and Susan Heckbert.
Clinical centers
Albert Einstein College of Medicine, Bronx, NY: Sylvia Wassertheil-Smoller, William Frishman, Judith Wylie-Rosett, David Barad, and Ruth Freeman; Baylor College of Medicine, Houston, TX: Aleksandar Rajkovic, Jennifer Hays, Ronald Young, and Haleh Sangi-Haghpeykar; Brigham and Women's Hospital, Harvard Medical School, Boston, MA: JoAnn E Manson, Kathryn M Rexrode, Brian Walsh, J Michael Gaziano, and Maria Bueche; Brown University, Providence, RI: Charles B Eaton, Michele Cyr, and Gretchen Sloane; Emory University, Atlanta, GA: Lawrence Phillips, Vicki Butler, and Vivian Porter; Fred Hutchinson Cancer Research Center, Seattle, WA: Shirley AA Beresford, Vicky M Taylor, Nancy F Woods, Maureen Henderson, and Robyn Andersen; George Washington University, Washington, DC: Lisa Martin, Judith Hsia, Nancy Gaba, and Richard Katz; Harbor-UCLA Research and Education Institute, Torrance, CA: Rowan Chlebowski, Robert Detrano, Anita Nelson, and Michele Geller; Kaiser Permanente Center for Health Research, Portland, OR: Yvonne Michael, Evelyn Whitlock, Victor Stevens, and Njeri Karanja; Kaiser Permanente Division of Research, Oakland, CA: Bette Caan, Stephen Sidney, Geri Bailey and Jane Hirata; Medical College of Wisconsin, Milwaukee, WI: Jane Morley Kotchen, Vanessa Barnabei, Theodore A Kotchen, Mary Ann C Gilligan, and Joan Neuner; MedStar Research Institute/Howard University, Washington, DC: Barbara V Howard, Lucile Adams-Campbell, Lawrence Lessin, Cheryl Iglesia, and Linda K Mickel; Northwestern University, Chicago/Evanston, IL: Linda Van Horn, Philip Greenland, Janardan Khandekar, Kiang Liu, and Carol Rosenberg; Rush University Medical Center, Chicago, IL: Henry Black, Lynda Powell, Ellen Mason, and Martha Gulati; Stanford Prevention Research Center, Stanford, CA: Marcia L Stefanick, Mark A Hlatky, Bertha Chen, Randall S Stafford, and Sally Mackey; State University of New York at Stony Brook, Stony Brook, NY: Dorothy Lane, Iris Granek, William Lawson, Catherine Messina, and Gabriel San Roman; The Ohio State University, Columbus, OH: Rebecca Jackson, Randall Harris, Electra Paskett, W Jerry Mysiw, and Michael Blumenfeld; University of Alabama at Birmingham, Birmingham, AL: Cora E Lewis, Albert Oberman, James M Shikany, and Monika Safford; University of Arizona, Tucson/Phoenix, AZ: Cynthia A Thomson, Tamsen Bassford, Cheryl Ritenbaugh, Zhao Chen, and Marcia Ko; University at Buffalo, Buffalo, NY: Jean Wactawski-Wende, Maurizio Trevisan, Ellen Smit, Susan Graham, and June Chang; University of California at Davis, Sacramento, CA: John Robbins and S Yasmeen; University of California at Irvine, CA: F Allan Hubbell, Gail Frank, Nathan Wong, Nancy Greep, and Bradley Monk; University of California at Los Angeles, Los Angeles, CA: Lauren Nathan, David Heber, Robert Elashoff, and Simin Liu; University of California at San Diego, La Jolla/Chula Vista, CA: Robert D Langer, Michael H Criqui, Gregory T Talavera, Cedric F Garland, and Matthew A Allison; University of Cincinnati, Cincinnati, OH: Margery Gass and Nelson Watts; University of Florida, Gainesville/Jacksonville, FL: Marian Limacher, Michael Perri, Andrew Kaunitz, R Stan Williams, and Yvonne Brinson; University of Hawaii, Honolulu, HI: J David Curb, Helen Petrovitch, Beatriz Rodriguez, Kamal Masaki, and Patricia Blanchette; University of Iowa, Iowa City/Davenport, IA: Robert Wallace, James Torner, Susan Johnson, Linda Snetselaar, and Jennifer Robinson; University of Massachusetts/Fallon Clinic, Worcester, MA: Judith Ockene, Milagros Rosal, Ira Ockene, Robert Yood, and Patricia Aronson; University of Medicine and Dentistry of New Jersey, Newark, NJ: Norman Lasser, Baljinder Singh, Vera Lasser, John Kostis, and Peter McGovern; University of Miami, Miami, FL: Mary Jo O'Sullivan, Linda Parker, JoNell Potter, Diann Fernandez, and Pat Caralis; University of Minnesota, Minneapolis, MN: Karen L Margolis, Richard H Grimm, Mary F Perron, Cynthia Bjerk, and Sarah Kempainen; University of Nevada, Reno, NV: Robert Brunner, William Graettinger, Vicki Oujevolk, and Michael Bloch; University of North Carolina, Chapel Hill, NC: Gerardo Heiss, Pamela Haines, David Ontjes, Carla Sueta, and Ellen Wells; University of Pittsburgh, Pittsburgh, PA: Lewis Kuller, Jane Cauley, and N Carole Milas; University of Tennessee Health Science Center, Memphis, TN: Karen C Johnson, Suzanne Satterfield, Rongling Li, Stephanie Connelly, and Fran Tylavsky; University of Texas Health Science Center, San Antonio, TX: Robert Brzyski and Robert Schenken; University of Wisconsin, Madison, WI: Gloria E Sarto, Douglas Laube, Patrick McBride, Julie Mares, and Barbara Loevinger; Wake Forest University School of Medicine, Winston-Salem, NC: Mara Vitolins, Greg Burke, Robin Crouse, and Scott Washburn; and Wayne State University School of Medicine/Hutzel Hospital, Detroit, MI: Michael Simon.
Women's Health Initiative Memory Study
Wake Forest University School of Medicine, Winston-Salem, NC: Sally Shumaker, Stephen Rapp, Claudine Legault, Mark Espeland, and Laura Coker.
Former Principal Investigators and Project Officers
Baylor College of Medicine, Houston, TX: Jennifer Hays and John Foreyt; Brown University, Providence, RI: Annlouise R Assaf; Emory University, Atlanta, GA: Dallas Hall; George Washington University, Washington, DC: Valery Miller; Kaiser Permanente Center for Health Research, Portland, OR: Barbara Valanis; Kaiser Permanente Division of Research, Oakland, CA: Robert Hiatt; National Cancer Institute, Bethesda, MD: Carolyn Clifford†; National Heart, Lung, and Blood Institute, Bethesda, MD: Linda Pottern; University of California at Irvine, Irvine, CA: Frank Meyskens Jr; University of California at Los Angeles, Los Angeles, CA: Howard Judd†; University of Cincinnati, Cincinnati, OH: James Liu and Nelson Watts; University of Miami, Miami, FL: Marianna Baum; University of Minnesota, Minneapolis, MN: Richard Grimm; University of Nevada, Reno, NV: Sandra Daugherty†; University of North Carolina, Chapel Hill, NC: David Sheps and Barbara Hulka; University of Tennessee Health Science Center, Memphis, TN: William Applegate; University of Wisconsin, Madison, WI: Catherine Allen†; Wake Forest University School of Medicine, Winston-Salem, NC: Denise Bonds.
† Deceased. Last updated 14 May 2008.
REFERENCES
- 1.Keys A. From Naples to seven countries—a sentimental journey. Prog Biochem Pharmacol 1983;19:1–30 [PubMed] [Google Scholar]
- 2.American Heart Association Nutrition Committee, Lichtenstein AH, Appel LJ, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation 2006;114:82–96 [DOI] [PubMed] [Google Scholar]
- 3.Hellerstein MK. Carbohydrate-induced hypertriglyceridemia: modifying factors and implications for cardiovascular risk. Curr Opin Lipidol 2002;13:33–40 [DOI] [PubMed] [Google Scholar]
- 4.Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr 2003;77:1146–55 [DOI] [PubMed] [Google Scholar]
- 5.Ma Y, Li Y, Chiriboga DE, et al. Association between carbohydrate intake and serum lipids. J Am Coll Nutr 2006;25:155–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.West CE, Sullivan DR, Katan MB, Halferkamps IL, van der Torre HW. Boys from populations with high-carbohydrate intake have higher fasting triglyceride levels than boys from populations with high-fat intake. Am J Epidemiol 1990;131:271–82 [DOI] [PubMed] [Google Scholar]
- 7.Willett W, Stampfer M, Chu NF, Spiegelman D, Holmes M, Rimm E. Assessment of questionnaire validity for measuring total fat intake using plasma lipid levels as criteria. Am J Epidemiol 2001;154:1107–12 [DOI] [PubMed] [Google Scholar]
- 8.Mosdol A, Witte DR, Frost G, Marmot MG, Brunner EJ. Dietary glycemic index and glycemic load are associated with high-density-lipoprotein cholesterol at baseline but not with increased risk of diabetes in the Whitehall II study. Am J Clin Nutr 2007;86:988–94 [DOI] [PubMed] [Google Scholar]
- 9.Ebbeling CB, Leidig MM, Sinclair KB, Seger-Shippee LG, Feldman HA, Ludwig DS. Effects of an ad libitum low-glycemic load diet on cardiovascular disease risk factors in obese young adults. Am J Clin Nutr 2005;81:976–82 [DOI] [PubMed] [Google Scholar]
- 10.Sloth B, Krog-Mikkelsen I, Flint A, et al. No difference in body weight decrease between a low-glycemic-index and a high-glycemic-index diet but reduced LDL cholesterol after 10-wk ad libitum intake of the low-glycemic-index diet. Am J Clin Nutr 2004;80:337–47 [DOI] [PubMed] [Google Scholar]
- 11.Sichieri R, Moura AS, Genelhu V, Hu F, Willett WC. An 18-mo randomized trial of a low glycemic-index diet and weight change in Brazilian women. Am J Clin Nutr 2007;86:707–13 [DOI] [PubMed] [Google Scholar]
- 12.Frost G, Leeds AA, Dore CJ, Madeiros S, Brading S, Dornhorst A. Glyceamic index as a determinant of serum HDL-cholesterol concentration. Lancet 1999;353:1045–8 [DOI] [PubMed] [Google Scholar]
- 13.Slyper A, Jurva J, Pleuss J, Hoffmann R, Gutterman D. Influence of glycemic load on HDL cholesterol in youth. Am J Clin Nutr 2005;81:376–9 [DOI] [PubMed] [Google Scholar]
- 14.Liese AD, Gilliard T, Schulz M, D'Agostino RB, Wolever TMS. Carbohydrate nutrition, glycaemic load, and plasma lipids: the Insulin Resistance Atherosclerosis Study. Eur Heart J 2007;28:80–7 [DOI] [PubMed] [Google Scholar]
- 15.Maki KC, Rains TM, Kaden VN, Raneri KR, Davidson MH. Effects of a reduced-glycemic load diet on body weight, body composition, and cardiovascular disease risk markers in overweight and obese adults. Am J Clin Nutr 2007;85:724–34 [DOI] [PubMed] [Google Scholar]
- 16.The Women's Health Initiative Study Group Design of the Women's Health Initiative Clinical Trial and Observational Study. Control Clin Trials 1998;19:61–109 [DOI] [PubMed] [Google Scholar]
- 17.Ritenbaugh C, Patterson R, Chlebowski RT, et al. The Women's Health Initiative Dietary Modification Trial: overview and baseline characteristics of participants. Ann Epidemiol 2003;13:S87–97 [DOI] [PubMed] [Google Scholar]
- 18.Howard B, Van Horn L, Hsia J, et al. Low-fat dietary pattern and risk of cardiovascular disease: the Women's Health Initiative randomized controlled dietary modification trial. JAMA 2006;295:655–66 [DOI] [PubMed] [Google Scholar]
- 19.Hays J, Hunt JR, Hubbell FA, et al. The Women's Health Initiative recruitment methods and results. Ann Epidemiol 2003;13:S18–77 [DOI] [PubMed] [Google Scholar]
- 20.Stefanick ML, Cochrane BB, Hsia J, Barad DH, Liu JH, Johnson SR. The Women's Health Initiative postmenopausal hormone trials: overview and baseline characteristics of participants. Ann Epidemiol 2003;13(suppl):S78–86 [DOI] [PubMed] [Google Scholar]
- 21.Jackson RD, LaCroix AZ, Cauley JA, McGowan J. The Women's Health Initiative calcium-vitamin D trial: overview and baseline characteristics of participants. Ann Epidemiol 2003;13(suppl):S98–106 [DOI] [PubMed] [Google Scholar]
- 22.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Argus-Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol 1999;9:178–87 [DOI] [PubMed] [Google Scholar]
- 23.Neuhouser ML, Tinker LF, Thomson C, et al. Development of a glycemic index database for food frequency questionnaires used in epidemiologic studies. J Nutr 2006;136:1604–9 [DOI] [PubMed] [Google Scholar]
- 24.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without the use of preparative ultracentrifugation. Clin Chem 1972;18:499–502 [PubMed] [Google Scholar]
- 25.Anderson GL, Manson J, Wallace R, et al. Implementation of the Women's Health Initiative study design. Ann Epidemiol 2003;13:S5–17 [DOI] [PubMed] [Google Scholar]
- 26.Women's Health Initiative Women's Health Initiative scientific resources website: biospecimen collection. Bethesda, MD: National Heart, Lung, and Blood Institute, 2006 [Google Scholar]
- 27.Otvos JD. Measurement of lipoprotein subclass profiles by nuclear magnetic resonance spectroscopy. Clin Lab 2002;48:171–80 [PubMed] [Google Scholar]
- 28.Howard BV, Manson JE, Stefanick ML, et al. Low-fat dietary pattern and weight change over 7 years: the Women's Health Initiative Dietary Modification Trial. JAMA 2006;295:39–49 [DOI] [PubMed] [Google Scholar]
- 29.Mensink RP, Katan MB. Effect of dietary fatty acids on serum lipids and lipoproteins. A meta-analysis of 27 trials. Arterioscler Thromb 1992;12:911–9 [DOI] [PubMed] [Google Scholar]
- 30.Prentice RL, Prentice RL, Caan B, et al. Low-fat dietary pattern and risk of invasive breast cancer: the Women's Health Initiative randomized controlled Dietary Modification trial. JAMA 2006;295:629–42 [DOI] [PubMed] [Google Scholar]
- 31.Prentice RL, Thomson CA, Caan B, et al. Low-fat dietary pattern and cancer incidence in the Women's Health Initiative Dietary Modification Randomized Controlled Trial. J Natl Cancer Inst 2007;99:1534–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ginsberg HN, Le NA, Melish D, Steinberg D, Brown WV. Effect of a high carbohydrate diet on apolipoprotein B catabolism in man. Metabolism 1981;30:347–53 [DOI] [PubMed] [Google Scholar]
- 33.Fried SK, Rao SP. Sugars, hypertriglyceridemia, and cardiovascular disease. Am J Clin Nutr 2003;78:873S–80S [DOI] [PubMed] [Google Scholar]
- 34.Marckmann P, Raben A, Astrup A. Ad libitum intake of low-fat diets rich in either starchy foods or sucrose: effects on blood lipids, factor VII coagulant activity, and fibrinogen. Metabolism 2000;49:731–5 [DOI] [PubMed] [Google Scholar]
- 35.Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med 2009;360:859–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams-RA, Gavin JR, III, Phillips RA, et al. High-risk African Americans with multiple risk factors for cardiovascular disease: challenges in prevention, diagnosis, and treatment. Ethn Dis 2006;16:633–9 [PubMed] [Google Scholar]
- 37.Subar AF, Kipnis V, Troiano RP, et al. Using intake biomarkers to evaluate the extent of dietary misreporting in a large sample of adults: the OPEN study. Am J Epidemiol 2003;158:1–13 [DOI] [PubMed] [Google Scholar]
- 38.Willett W, Stampfer M, Chu NF, Spiegelman D, Holmes M, Rimm E. Assessment of questionnaire validity for measuring total fat intake using plasma lipid levels as criteria. Am J Epidemiol 2001;154:1107–112 [DOI] [PubMed] [Google Scholar]
- 39.Neuhouser ML, Tinker L, Shaw PA, et al. Use of recovery biomarkers to calibrate nutrient consumption self-reports in the Women's Health Initiative. Am J Epidemiol 2008;167:1247–59 [DOI] [PubMed] [Google Scholar]