Abstract
Background: Dietary deficiency in n−3 (omega-3) polyunsaturated fatty acids (PUFAs) prevails in Western populations and potentially results in adverse health outcomes. To circumvent the slow n−3 PUFA incorporation in phospholipids of key cells after oral supplementation, a new preparation for intravenous bolus injection was developed with 20 g triacylglycerols/100 mL of a mixture of 80% medium-chain triacylglycerols (MCTs) and 20% fish oil (FO) (wt:wt), and 0.4 g α-tocopherol/100 mL of the same mixture.
Objective: Our objective was to document the enrichment of n−3 PUFAs in leukocyte and platelet phospholipids after a bolus intravenous injection of MCT:FO in men.
Design: Twelve healthy male subjects received injections over a 5-min period of 50 mL of either MCT:FO or a control MCT:long-chain triacylglycerol (MCT:LCT) emulsion containing 20 g triacylglycerols/100 mL with equal amounts (wt:wt) of MCT and soybean triacylglycerols (LCT) and containing 0.02 g α-tocopherol/100 mL; after an 8-wk interval, the subjects received injections of the other preparation.
Results: Clinical and biological variables that assessed tolerance and safety remained unchanged. Plasma elimination was faster for MCT:FO than for MCT:LCT (half-life: 24.5 ± 3.5 min compared with 32.9 ± 3.0 min; P < 0.025). This was associated with a greater increase in the plasma nonesterified fatty acid concentration. The content of n−3 PUFAs, specifically eicosapentaenoic acid (20:5n−3), increased in leukocyte and platelet phospholipids within 60 min and ≥24 h after MCT:FO injection.
Conclusion: Bolus intravenous injection of a novel MCT:FO emulsion allows rapid enrichment of cells with n−3 PUFAs.
INTRODUCTION
Changes in dietary lipid intake in Western populations are characterized by increased intakes of n−6 (omega-6) polyunsaturated fatty acids (PUFAs), whereas consumption of n−3 PUFAs, and namely eicosapentaenoic (20:5n−3), docosapentaenoic (22:5n−3), and docosahexaenoic (22:6n−3) acids, has been reduced (1). These changes led to very high n−6:n−3 fatty acid ratios in plasma and cell lipids (2). The American Heart Association currently recommends increasing the consumption of n−3 PUFAs of marine origin (3). There is convincing evidence for protective effects of fish oil (FO) and derived n−3 PUFAs against inflammatory and cardiovascular diseases; such properties were first described in epidemiologic studies in the Greenland Eskimo population (4) and later confirmed by several experimental and clinical studies (5–7), generally by using oral supplementation of FO for several weeks. A consistent result of increasing n−3 PUFA intake is the reduction of sudden cardiac deaths caused by severe cardiac arrhythmias (8). Still, the efficacy of supplementation requires a number of days and weeks because n−3 PUFA incorporation in cell membranes is relatively slow after oral ingestion of FO (9, 10).
Bypassing the gastrointestinal tract with a direct administration to the systemic circulation may represent an appealing alternative for providing rapid protection for subjects at high risk of cardiac arrhythmias (eg, in selected patients undergoing anesthesia for cardiac surgery or coronary revascularization). For instance, Billman et al (11) documented protection against fatal ventricular fibrillation in a canine model of myocardial infarction by intravenous infusion of different n−3 PUFAs during the 1 h preceding a stress that combined coronary ischemia and strenuous physical exercise. However, in this model fatty acids were provided in unesterified form and bound to albumin. The infusion of a pure FO emulsion was suggested to markedly reduce the induction of ventricular arrhythmias in a small group of patients with implanted cardioverter defibrillators (12).
A new lipid emulsion was developed for counteracting the slow plasma lipolysis of pure FO preparations and to allow an intravenous injection of n−3 PUFA–rich triacylglycerols over a few minutes. This is achieved by mixing in the same particle medium-chain triacylglycerols (MCTs) and FO in a 80:20 (wt:wt) ratio. The primary aim is to promptly incorporate n−3 PUFAs into cells that are important in cardiovascular diseases and in cells regulating inflammatory and thrombotic processes. Pilot in vitro experiments documented the potential of this new emulsion to quickly and efficiently raise the n−3 PUFA content in phospholipids of cultured endothelial cells (13).
The major aim of the present investigation was to measure the incorporation of n−3 PUFAs into the phospholipids of leukocytes and platelets after a single 5-min injection of an MCT:FO emulsion in healthy volunteers.
SUBJECTS AND METHODS
Study population
The clinical studies were conducted between 11 November 2002 and 26 February 2003. The recruitment of volunteers started in September 2002. Twelve healthy male subjects volunteered to be included in the study. The criteria for inclusion were as follows: normal fasting concentration of plasma lipids (triacylglycerols, cholesterol, and phospholipids), no history of cardiovascular disease, no metabolic disorder, normal blood cell count, and routine biological variables. Criteria of exclusion were as follows: n−3 fatty acid and/or vitamin E (or multivitamin) supplementation, strict vegetarian diet, and history of alcohol or drug abuse. The subjects’ mean (±SEM) age was 29.3 ± 1.5 y. Their body weight, height, and body mass index (in kg/m2) averaged 75.8 ± 2.9 kg, 177 ± 2 cm, and 24.07 ± 0.76, respectively. At the preliminary screening (67 ± 3 d before the first lipid injection), their plasma triacylglycerol, total cholesterol, and phospholipid concentrations averaged 79.2 ± 5.5, 159.0 ± 7.0, and 175.3 ± 7.5 mg/dL, respectively. The 20:5n−3 and 22:6n −3 content in plasma phospholipids, expressed as the percentage of total fatty acid (wt:wt), averaged 1.04 ± 0.11% and 4.62 ± 0.29%, respectively.
The study protocol was approved by the Ethical Committee of Erasmus Hospital (Brussels Free University School of Medicine, Brussels, Belgium). All subjects received complete information on the potential risks and purpose of the trial and signed an informed consent form before their inclusion in the study.
Lipid emulsions
The test emulsion (MCT:FO) was a 20 g/100 mL triacylglycerol emulsion containing 80% MCT and 20% FO (wt:wt), prepared by B Braun AG (Melsungen, Germany). The control preparation [MCT:long-chain triacylglycerol (MCT:LCT)] was also a 20 g/100 mL triacylglycerol emulsion containing equal (wt:wt) amounts of MCTs and soybean triacylglycerols, which is used for parenteral nutrition (Medialipid; B Braun AG). Both preparations contained glycerol (2.5 g/100 mL), and 1.2 g/100 mL of the same egg-derived phospholipid emulsifier. The all-rac-α-tocopherol concentration was 0.4 g/100 mL in MCT:FO and 0.02 g/100 mL in MCT:LCT. The triacylglycerol fatty acid composition of the MCT:LCT and MCT:FO preparation and the diacylglycerol fatty acid composition in MCT:FO are shown in Table 1.
TABLE 1.
Fatty acid composition (weight percentage) of triacylglycerols in medium-chain triacylglycerol:long-chain triacylglycerol (MCT:LCT) and MCT:fish oil (MCT:FO) emulsions and of FO diacylglycerols
| Fatty acid | MCT:LCT | MCT:FO | FO |
| 6:0 | 0.0 | 0.1 | — |
| 8:0 | 28.0 | 53.4 | — |
| 10:0 | 20.2 | 32.0 | — |
| 12:0 | 1.3 | 0.4 | — |
| 14:0 | — | — | — |
| 16:0 | 6.8 | 0.3 | 2.4 |
| 16:1n−7 | 1.3 | 0.5 | 1.1 |
| 18:0 | 2.6 | 0.4 | 3.4 |
| 18:1n−9 | 9.6 | 0.6 | 4.2 |
| 18:2n−6 | 26.9 | 0.2 | — |
| 18:3n−6 | — | — | 1.2 |
| 20:0 | — | — | — |
| 18:3n−3 | 3.1 | — | 4.0 |
| 20:1n−9 | — | 0.3 | — |
| 18:4n−3 | — | — | 2.1 |
| 20:2n−6 | — | — | — |
| 20:3n−6 | 0.2 | — | — |
| 22:0 | — | — | — |
| 20:4n−6 | — | 0.4 | 2.3 |
| 22:1n−9 | — | 0.1 | — |
| 20:5n−3 | — | 4.9 | 36.5 |
| 24:0 | — | — | — |
| 22:3n−3 | — | — | 2.3 |
| 22:4n−6 | — | 0.6 | — |
| 22:5n−3 | — | 0.7 | 5.2 |
| 22:6n−3 | — | 5.0 | 35.4 |
| Total fatty acid concentration (mg/mL) | 177.0 | 170.4 | 12.4 |
The manufacturer provided 50-mL bottles of both preparations labeled A or B, with contents unknown to the investigators and participants. Randomization envelopes were provided by the manufacturer and were opened on the day of the first injection by the study supervisor to determine the preparation (A or B) to be injected first. Technicians and investigators remained unaware of the injected preparation until all data were collected and recorded.
Experimental protocol
The study was conducted as a double-blind, crossover, randomized control trial. All subjects received an injection of each preparation, following a random order. There was an 8-wk washout period between injections of the 2 preparations.
The subjects were requested to eat a standard low fat meal and to drink no alcoholic beverage in the evening before the test. After an overnight fast, the subjects were admitted at 0800 (day 1) in the hospital clinical research unit. They were asked to drink 200 mL of a carbohydrate-rich (6.3 g/100 mL) solution (Nutricia preoperative; Nutricia, Zoetermeer, Netherlands) 90 min before the lipid injection. Afterward, they were allowed nothing by mouth but water. They were maintained in a supine position throughout the test. One intravenous catheter was inserted in each arm. The first catheter was used for the bolus injection of lipid emulsions (50 mL injected in 5 min) and was removed. The other catheter was used for blood sampling and was kept patent during the test by a slow infusion of saline. No heparin was used.
At hourly intervals during each test and at 0800 on days 2, 3, and 8 thereafter, body temperature, heart rate and blood pressure were monitored. Likewise, blood cell counts (including hemoglobin and hematocrit determinations), coagulation tests, and routine safety laboratory variables were measured before and again at 1 and 8 h and on days 2, 3, and 8 after the injection of a lipid emulsion. After the sampling performed 8 h after the injection of the emulsion, the individuals left the hospital. They were asked to eat low-fat meals and to drink nonalcoholic beverages in the evenings of days 1 and 2.
Laboratory determinations
Plasma d-glucose (hexokinase method), insulin (radioimmunoassay), and C-peptide (radioimmunoassay) concentrations were measured by standard procedures. Triacylglycerols were enzymatically assayed with the Roche Triglycerides Glycerol blanked n°450032/Roche Precimat Glycerol n°166588 kit (Roche Diagnostics GmbH, Mannheim, Germany). The concentration of total cholesterol was measured with the Roche Cholesterol CHOD-PAP n°1489704/Roche Precimat Cholesterol n°125512 kit (Roche Diagnostics GmbH). The phospholipid concentration was measured by the BioMérieux Enzymatic Phospholipids PAP150 (BioMérieux, Lyon, France).
The content of individual fatty acids in plasma, white blood cell (WBC), and platelet phospholipids was measured by gas chromatography after separation of lipid components by thin-layer chromatography (14). The concentration of α-tocopherol in LDL, HDL, WBCs, and platelets was measured by HPLC (15). LDL sensitivity to lipid peroxidation induced by copper sulfate was assessed by using Esterbauer's method (16).
The upper bounds for the reference range of plasma insulin and C-peptide concentrations were 20 μU/mL and 4.2 ng/mL, respectively.
All data are presented as means ± SEMs, and the number of individual observations (n) is given in parentheses, as applicable. Missing variables correspond to samples that did contain enough biological material to allow analysis. The statistical significance of differences between means was assessed by using Student's t test. The statistical analysis consisted of an analysis of variance (ANOVA) for 2 × 2 periods with repeated measurements within each period. If the carryover effect was not significant, the subjects of both groups were pooled together, and an ANOVA for repeated measurements on treatment and time with interaction was performed. The pairwise comparisons were performed by using paired t tests. P < 0.05 was considered significant.
RESULTS
Clinical data
No adverse clinical effect was observed. In addition, no change of biological variables that assessed tolerance and safety were observed.
Plasma d-glucose, insulin, and C-peptide concentrations
The plasma d-glucose concentration that was measured 90 min after the ingestion of the carbohydrate solution was 0.94 ± 0.38 mmol/L higher (P < 0.025, paired comparison) than the plasma d-glucose concentration measured at screening before the study (5.02 ± 0.04 mmol/L; n = 12). This coincided with an elevated plasma insulin concentration (38 ± 11 μU/mL) and C-peptide concentration (6.51 ± 0.68 ng/mL) and a low nonesterified fatty acid concentration (108 ± 4 μM). The means for plasma d-glucose, insulin, and C-peptide concentrations were not significantly different from one another in the 2 series of experiments. The plasma d-glucose concentration decreased (P < 0.001) from 5.96 ± 0.38 mmol/L before the injection of the lipid emulsion to 4.13 ± 0.14 mmol/L 1 h thereafter. This coincided with a parallel decrease of the plasma insulin concentration with a decrease of 28 ± 11 μU/mL (P < 0.025, paired comparison) and of the C-peptide concentration with a decrease of 3.62 ± 0.62 ng/mL (P < 0.001, paired comparison). Between 1 and 8 h after the injection of the lipid emulsion, the plasma concentration of insulin remained fairly stable (decrease: 1 ± 1 μU/mL; P > 0.2), whereas the plasma concentration of C-peptide further decreased by 1.05 ± 0.27 ng/mL (P < 0.001). However, over the same period, the glycemia concentration increased by 1.12 ± 0.17 mmol/L (P < 0.001; paired comparison) to eventually reach a value of 5.24 ± 0.09 mmol/L. Such an increase was comparable in the 2 series of experiments (P > 0.6, paired comparison).
Plasma triacylglycerol concentrations
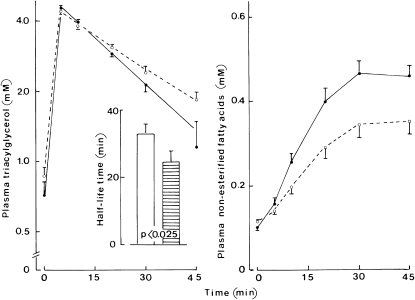
In semilogarithmic coordinates, the time course for changes in the plasma concentration of triacylglycerols is shown in Figure 1 (left panel). As estimated from the molar amount of injected triacylglycerols (15.7 and 18.7 mmol with MCT:LCT and MCT:FO, respectively) and the increment in plasma triacylglycerol concentration extrapolated to time zero, the apparent mean distribution space of the triacylglycerols was virtually identical after injection of the MCT:FO or MCT:LCT emulsion with an overall value of 3.92 ± 0.11 L. However, the clearance of plasma triacylglycerols was faster (P < 0.025, paired comparison; n = 12) with the MCT:FO emulsion [half-life (t1/2) = 24.5 ± 3.5 min; n = 12] than with the MCT:LCT emulsion (t1/2 = 32.9 ± 3.0 min; n = 12).
FIGURE 1.
Mean (±SEM) plasma triacylglycerols and nonesterified fatty acids. Left: Time course of the plasma concentration of triacylglycerols (logarithmic scale) after injection of the medium-chain triacylglycerol:long-chain triacylglycerol (MCT:LCT) emulsion (open circles and dashed line) or MCT:fish oil (MCT:FO) (closed circles and solid line) emulsion; the inset refers to the half-life for the exponential decrease in the triacylglycerol concentration between 5 and 45 min in the MCT:LCT series (open columns) and MCT:FO series (hatched column) with the corresponding P value (paired comparison). Right: Time course for the plasma concentration of nonesterified fatty acids (same presentation as in the left panel). Values refer to 12 individual determinations in all cases.
Plasma nonesterified fatty acid concentration
The basal plasma concentration of nonesterified fatty acids was comparable before the MCT:LCT (0.116 ± 0.005 mmol/L) and MCT:FO (0.101 ± 0.006 mmol/L) injections, with a paired difference of 0.014 ± 0.010 mmol/L (n = 12; P > 0.15). After lipid injection, plasma concentrations of nonesterified fatty acids increased to reach a peak value of 0.463 ± 0.032 mmol/L (n = 12) 30 min after injection of MCT:FO compared with a peak value of 0.344 ± 0.029 mmol/L (n = 12) after injection of the MCT:LCT emulsion (Figure 1). Over 45 min, the incremental area in plasma nonesterified fatty acids above the paired basal value averaged 7.08 ± 0.97 mmol ⋅ min/L (n = 12) with MCT:LCT compared with 11.53 ± 1.08 mmol ⋅ min/L (P < 0.001) with MCT:FO. This could be due to differences in the molar concentration between the 2 preparations or to a different fatty acid release by intravascular lipolysis. When divided by the molar amount of fatty acids present in the glycerides of the 2 injected emulsions, the difference between such an incremental area became less marked (with values of 0.150 ± 0.021 and 0.205 ± 0.019 ⋅ min−1⋅ L−1 in the MCT:LCT and MCT:FO series, respectively) but remained significant (P < 0.01).
n−3 PUFA composition of platelet and WBC phospholipids
Mean (±SEM) basal values for the fatty acid composition of WBC and platelet phospholipids are listed in Table 2. No significant differences were found for these values before the injection of each emulsion.
TABLE 2.
Basal fatty acid composition (weight percentage) of white blood cell and platelet phospholipids1
| Fatty acid | White blood cells | Platelets |
| 8:0 | 0.04 ± 0.01 | 0.04 ± 0.00 |
| 10:0 | 0.03 ± 0.00 | 0.04 ± 0.00 |
| 16:0 | 23.89 ± 0.38 | 17.05 ± 0.25 |
| 16:1n-7 | 0.21 ± 0.04 | 0.08 ± 0.02 |
| 18:0 | 13.77 ± 0.17 | 16.58 ± 0.17 |
| 18:1n−9 | 14.22 ± 0.32 | 13.40 ± 0.17 |
| 18:2n−6 | 15.76 ± 0.56 | 6.90 ± 0.24 |
| 18:3n−6 | 0.03 ± 0.01 | 0.05 ± 0.02 |
| 18:3n−3 | 0.07 ± 0.02 | 0.04 ± 0.00 |
| 20:4n−6 | 16.74 ± 0.50 | 29.84 ± 0.39 |
| 20:5n−3 | 0.61 ± 0.07 | 0.42 ± 0.05 |
| 22:5n−3 | 2.11 ± 0.07 | 2.40 ± 0.08 |
| 22:6n−3 | 3.55 ± 0.21 | 2.76 ± 0.16 |
| Σn−3 | 6.34 ± 0.29 | 5.61 ± 0.22 |
| Σn−6 | 32.53 ± 0.36 | 36.79 ± 0.31 |
| Σn−3/Σn−6 | 0.196 ± 0.010 | 0.154 ± 0.007 |
All values are means ± SEMs and refer to 12 individual determinations. Each of the latter determinations represents the mean of 2 measurements made in the same subject before the injection of the medium-chain triacylglycerol:long-chain triacylglycerol emulsion or medium-chain triacylglycerol:fish oil emulsion. Σ, sum of the fatty acid.
ANOVA of the 20:5n−3 relative content and total n−3 PUFA (ie, 18:3n−3, 20:5n−3, 22:5n−3, and 22:6n−3) content and the ratio between n−3 and n−6 (ie, 18:2n−6, 18:3n−6, and 20:4n−6) PUFA in platelet phospholipids indicated the absence of any significant carryover effect. The treatment response (MCT:LCT compared with MCT:FO) was highly significant for all 3 variables (P < 0.003), and its relation to time was also always significant (P < 0.03).
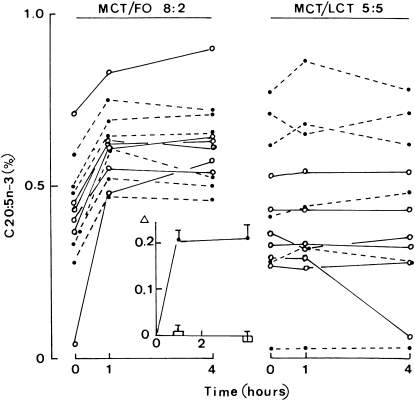
The changes of 20:5n−3 content in platelet phospholipids 1 and 4 h after the injection of the MCT:FO or MCT:LCT emulsion are shown in Figure 2. A highly significant (P < 0.001) increase was recorded in the MCT:FO series, whereas no significant change was detected in the MCT:LCT series. For the MCT:FO injection, there was a positive correlation (r = +0.890; n = 12; P < 0.001) between the individual basal values (time zero) for the 20:5n−3 content and those values recorded 1 h after injection of the MCT:FO emulsion.
FIGURE 2.
The 20:5n−3 content of platelet phospholipids. Individual values for the 20:5n−3 content of platelet phospholipids expressed as the percentage of the total weight of all phospholipid fatty acids before (time zero) and 1 or 4 h after injection of the medium-chain triacylglycerol:fish oil (MCT/FO) emulsion (left) or MCT:long-chain triacylglycerol (MCT/LCT) emulsion (right). Open circles and solid lines refer to subjects who first underwent the MCT/LCT test and 8 wk later underwent the MCT/FO test; closed circles and dashed lines refer to the opposite sequence. The inset refers to the mean (±SEM) changes (Δ) from the basal value caused by either the MCT/FO emulsion (closed circles and solid line) or MCT/LCT emulsion (open columns). Values refer to 12 individual determinations.
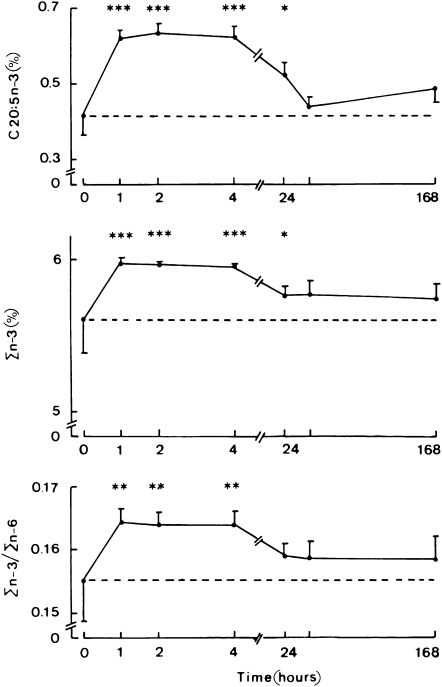
The time course for changes in the fatty acid composition of platelet phospholipids after injection of the MCT:FO emulsion is shown in Figure 3. The 20:5n−3 content, sum of all n−3 PUFAs, and ratio between all n−3 and n−6 PUFAs increased significantly within 60 min after the administration of the MCT:FO emulsion. Concentrations remained high ≥4 h postinjection and declined thereafter, remaining significantly higher overall than the basal value at day 2. In the MCT:LCT series, no significant change from the paired basal value was observed at any time for the platelet phospholipid 20:5n−3 content, total n−3 PUFA content, or ratio between all n−3 and n−6 PUFAs (data not shown).
FIGURE 3.
Mean (±SEM) weight percentage of fatty acids in platelet phospholipids. Time course for the changes in 20:5n−3 and sum of content of all n−3 fatty acids (Σn−3) of platelet phospholipids (expressed as the percentage of the total weight of all phospholipid fatty acids) and in the Σn−3:Σn−6 ratio after injection of the medium-chain triacylglycerol:fish oil emulsion. The time zero values (and horizontal dashed line) refer to the absolute measurements of each variable made before the injection of the lipid emulsion. The later values refer to the increment of each variable above the paired basal measurement and are shown together with the significance of such paired changes (*P < 0.02, **P < 0.005, ***P < 0.001). Values refer to 12 individual determinations. Note the difference in scale of the abscissa in the left and right sides of the figure.
Likewise, a significant treatment effect (P < 0.025) was observed by ANOVA when the changes in the 20:5n−3 content of WBC phospholipids recorded between 1 and 168 h after injection of either the MCT:LCT or MCT:FO emulsion were compared.
After injection of the MCT:FO emulsion, the increment in 20:5n−3 content above the paired basal value (time zero) averaged 0.10 ± 0.03% (P < 0.005), this value was reached within 60 min after the injection of MCT:FO followed by a plateau until 240 min (data not shown). In the control experiments using MCT:LCT, the corresponding increments failed to achieve significance.
n−6 PUFA composition of platelet and WBC phospholipids
Mean basal values for 18:2n−6 and 20:4n−6 in platelet and WBC phospholipids did not differ significantly in the MCT:LCT and MCT:FO series (data not shown). At ≥24 h after injection of a lipid emulsion, the mean differences from the basal value for the n−6 PUFA content of cell phospholipids were always lower in the MCT:FO series than in the MCT:LCT series. This was the case at all time points (1, 2, 4, and 24 h after injection of the lipid emulsion) for 18:n−6 and 20:4n−6 and in platelet and WBC lipids. The difference between the MCT:FO and MCT:LCT series achieved significance (P < 0.02) 2 h after injection of the lipid emulsion for the 18:2n−6 content of platelet phospholipids (−1.18 ± 0.44‰) and 48 h after injection for the 20:4n−6 content of WBC phospholipids (−13.71 ± 5.14 ‰). Even over the entire period of 168 h, the difference in the 20:5n−3 content of cell phospholipids above or below the basal value and the corresponding change in the n−6 PUFA content recorded in the same phospholipids at the same time and in the same subject after injection of the same emulsion always yielded negative products significantly different from zero whether for 18:2n−6 in platelets (−0.65 ± 0.31 × 10−6, P < 0.05) and WBCs (−9.76 ± 3.47 × 10−6; P < 0.006) or for 20:4n−6 in platelets (−2.29 ± 0.81 × 10−6; P < 0.006) and WBCs (−1.92 ± 0.93 × 10−6; P < 0.05).
α-Tocopherol content in lipoproteins and blood cells
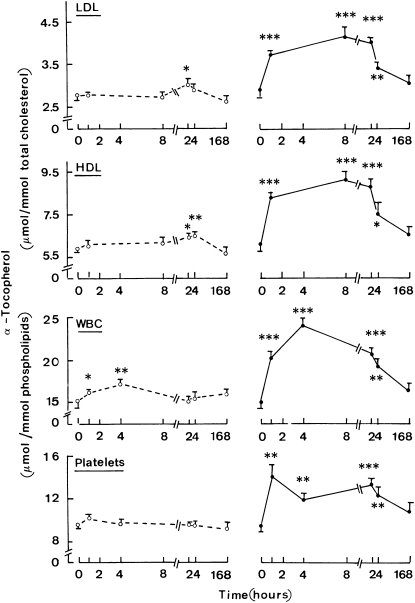
Before the injection of lipid emulsions, the α-tocopherol content of LDL or HDL was not significantly different (P > 0.5) between the MCT:LCT and MCT:FO series with values of 2.85 ± 0.13 and 5.98 ± 0.22 μmol/mmol total cholesterol (n = 12 in both cases) for LDL and HDL, respectively. A modest but significant increase in the α-tocopherol content was observed in lipoproteins 2–3 d after injection of the MCT:LCT emulsion (Figure 4). At its peak value, it represented a ≤10% relative increment in α-tocopherol content. In contrast a marked increase was observed within 60 min after the injection of MCT:FO, which contained 0.4 g α-tocopherol/100 mL as distinct from only 0.02 g α-tocopherol/100 mL in MCT:LCT. Eight hours after injection, the relative increment of α-tocopherol content in lipoproteins was close to 45–50% of the initial value. This enrichment remained significant, although it progressively decreased on days 2 and 3 (Figure 4).
FIGURE 4.
Mean (±SEM) α-tocopherol content of lipoproteins and circulating cells. Time course for the changes in the α-tocopherol content of LDL and HDL (μmol/mmol total cholesterol) and the white blood cell (WBC) and platelet phospholipids (μmol/mmol phospholipids) after injection of either the medium-chain triacylglycerol:long-chain triacylglycerol emulsion (left) or medium-chain triacylglycerol:fish oil emulsion (right). The time zero values refer to the absolute measurements of each variable made before the injection of the lipid emulsion. The later values refer to the increment of each variable above the paired basal measurement and are shown together with the significance of such paired changes (*P < 0.05, **P < 0.005, ***P < 0.001). Values refer to 12 individual determinations. Note the difference in scale of the abscissa in the left and right parts of the figure.
Before the injection of the lipid emulsion, the α-tocopherol content in blood cells was not significantly different (P > 0.6) between the MCT:LCT and MCT:FO series, with overall values of 15.07 ± 0.61 and 9.47 ± 0.28 μmol α-tocopherol/mmol phospholipids (n = 12 in both cases) for WBCs and platelets, respectively. After injection of MCT:LCT, a minor but significant increase was observed in the 2 first samples of WBCs (Figure 4). Much greater increments of the α-tocopherol content in blood cells occurred between 1 h and 3 d after injection of MCT:FO (Figure 4). At their peak values, such increments represented a 50–60% enrichment in α-tocopherol relative to basal values (time zero). These changes in the α-tocopherol content of lipoproteins and blood cells documented a significant treatment effect as judged by ANOVA.
LDL sensitivity to peroxidation
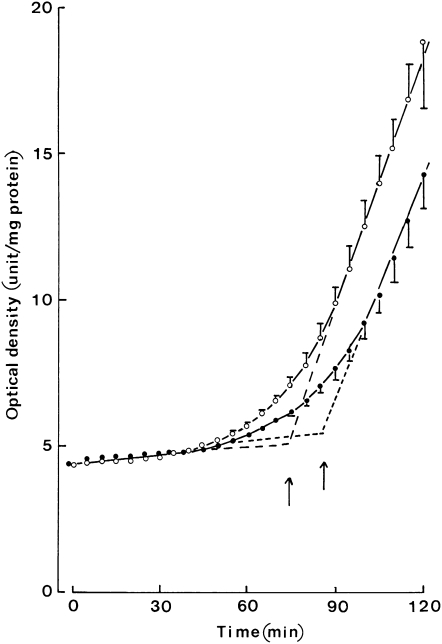
The increased α-tocopherol content of LDL after the injection of MCT:FO was associated with a decreased sensitivity to lipid peroxidation as assessed by measuring the lag time that preceded the appearance of conjugated dienes and of the propagation slope of dienes after LDL exposure to copper sulfate. For instance, 2 days after injection, the lag time for the generation of dienes by LDL was increased by ≈12 min, and the propagation slope was decreased by ≈0.0297 U ⋅ min−1 (Figure 5).
FIGURE 5.
Mean (±SEM) diene generation by LDL. Time course for the generation of dienes (expressed in arbitrary units of absorbance per milligrams of protein) by LDL separated before (open circles) or 2 d after (closed circles) injection of the medium-chain triacylglycerol:fish oil emulsion. Values refer to 12 individual determinations. The arrows correspond to the lag time for the generation of dienes from LDL.
Before the injection of lipid preparations, the lag time was not significantly different (P > 0.5) between the MCT:LCT series (72.0 ± 3.1 min; n = 12) and the MCT:FO series (74.2 ± 1.9 min; n = 12). After the injection of MCT/LCT, modest but significant (P < 0.02) increases in lag time were recorded at 8 h and at 2, 3 and 8 d (Table 3). After the injection of MCT:FO, significant increases (P < 0.025) in lag time were observed at all time points and peaking at 8 h at 16.8 ± 2.1 min (n = 12; P < 0.001). The mean increase in lag time was always higher after the injection of MCT:FO compared with after the injections of MCT:LCT; such a difference was highly significant (P < 0.001) 1 and 8 h postinjection and remained significant (P < 0.05 or less) on days 2 and 3 but not on day 8.
TABLE 3.
Lag time (min) for the peroxidation of LDL isolated before (time zero of day 1) injection of the lipid emulsion and the paired increase in such a lag time at different times after injection of the medium-chain triacylglycerol:long-chain triacylglycerol (MCT:LCT) and MCT:fish oil (MCT:FO) emulsion1
| Emulsion |
|||
| Time | MCT:LCT | MCT:FO | P |
| Day 1, hour 0 | 72.02 ± 3.10 | 74.25 ± 1.91 | NS |
| Day 1, hour 1 | +0.83 ± 1.10 | +9.86 ± 1.66 | <0.001 |
| Day 1, hour 8 | +5.94 ± 1.55 | +16.75 ± 2.14 | <0.001 |
| Day 2, hour 0 | +6.58 ± 1.34 | +13.43 ± 1.86 | <0.01 |
| Day 3, hour 0 | +4.63 ± 1.60 | +10.34 ± 1.99 | <0.04 |
| Day 8, hour 0 | +5.19 ± 1.52 | +5.57 ± 2.12 | NS |
All values are means ± SEMs and refer to 12 individual determinations. P values for differences between results obtained in the MCT:LCT and MCT:FO series were determined by Student's t test.
The basal values for the propagation slopes were also not different (P > 0.4) between the MCT:LCT (0.2692 ± 0.0124 U ⋅ min−1; n = 12) and the MCT:FO series (0.2850 ± 0.0167 U ⋅ min−1; n = 12). As previously indicated for the lag time, values of propagation slope for dienes indicated a reduced sensitivity to lipid peroxidation after the injection of MCT:FO. The difference in slope above or below paired basal measurements yielded mean negative values in the MCT:FO series at all 5 times after injection of the lipid emulsion as distinct from the mean positive values in the MCT:LCT series. For instance, 48 h after injection of a lipid emulsion, such a difference averaged −0.0350 ± 0.0167 U ⋅ min−1 (n = 12) in the MCT:FO series as distinct (P < 0.02) from +0.0217 ± 0.0131 U ⋅ min−1 (n = 12) in the MCT:LCT series. Covariance analysis indicated that, over the first 48 h after injection of a lipid emulsion, the slope of the regression line relating the difference above or below paired basal values to the time after injection of a lipid emulsion did not differ significantly in the MCT:FO and MCT:LCT series, whereas the difference in elevation of these 2 regression lines achieved significance (P < 0.02).
DISCUSSION
The current study showed the potential for a single injection of a new MCT:FO preparation in healthy volunteers to markedly raise the content of long-chain n−3 fatty acids in the phospholipids of platelets and WBCs within 60 min. The enrichment was most pronounced for 20:5n−3 and continued during the plateau phase (60–240 min) after injection of the MCT:FO emulsion to 173 ± 18% and 123 ± 5% of basal values in platelets and WBCs, respectively. The 20:5n−3 increment remained significant until day 2 (Figure 3).
The study was performed as a double-blind crossover trial, with each subject receiving an injection of the test MCT:FO preparation and of a control MCT:LCT emulsion according to a random order. This control emulsion was selected because of its substantial content [50% ( wt:wt)] of MCT and some enrichment in α-tocopherol; it has been used for >15 y in parenteral nutrition. Both preparations contained the same phospholipid emulsifier and were manufactured according to the same procedures.
The subjects were not studied in overnight fasting conditions but were asked to drink a moderate load (25 g) of carbohydrates 90 min before the injection of the lipid preparation. A study (17) suggested that this procedure may become standard practice in future immediate preoperative care of surgical patients because it markedly attenuates the catabolic postsurgical response compared with overnight fasting. Another effect of the glucose load is to increase the plasma concentrations of circulating insulin and C-peptide and to inhibit adipose tissue lipolysis by the hormone-sensitive lipase. As a result, plasma concentrations of nonesterified fatty acids were substantially reduced before a later rise corresponding to the intravascular hydrolysis of injected triacylglycerols. The latter rise coincided with an increase in the plasma d-glucose concentration, probably attributable to both increased hepatic gluconeogenesis (stimulated by the supply of glycerol as a gluconeogenic precursor and the activation of hepatic pyruvate carboxylase by fatty acid–derived acetyl coenzyme A) and reduced extrahepatic glucose use (caused by the increase in plasma unesterified fatty acid concentrations).
The half-life for the plasma clearance of triacylglycerols was shorter after the MCT:FO injections than after the MCT:LCT injection (Figure 1). This indicates that the large proportion of MCT in the test preparation totally compensated for the slow elimination of the FO component and allowed for an injection over a few minutes. Compared with the MCT:LCT injection, the MCT:FO injection was associated with a higher increment of plasma unesterified fatty acid concentration. Nevertheless, the mean plasma concentration of unesterified fatty acid remained somewhat <0.5 mmol/L. These values indicated an efficient elimination of MCT:FO from the plasma and a limited rise of the plasma unesterified fatty acid concentration. As a result, the injection was not associated with any detectable clinical or biological adverse effect. The safety of the procedure on hemostatic variables was recently documented (18).
The presence of MCTs in the test preparation may optimize the incorporation of n−3 fatty acids in cell phospholipids. The fast hydrolysis of MCT releases large amounts of medium-chain fatty acids (19), which are rapidly oxidized by many tissues and spare n−3 fatty acids from entering oxidative pathways. In addition, our groups provided evidence that direct particle uptake by cells (followed by intracellular lipolysis and fatty acid processing) represents an important pathway for the delivery of n−3 fatty acids to tissues; the rapid lipolysis of MCT leads to the formation of small-sized remnant particles (with a concentrated FO content) that can then be readily endocytosed by cells. In endothelial cells cultured in the presence of lipoprotein lipase, n−3 fatty acid incorporation in cell phospholipids was as efficient from the MCT:FO (with 20% FO) as from a pure (100%) FO emulsion (13).
Intravenous infusions of long-chain polyunsaturated n−3 fatty acids in the form of a pure FO emulsion were proposed for potential modifications of inflammatory and immune variables (20) and, more recently, for protection against severe ventricular cardiac arrhythmias (12). However, the slow plasma clearance of that emulsion requires a prolonged infusion at a slow rate (10). In contrast, the preparation used in the current study can be administered over a few minutes with a rapid n−3 fatty acid incorporation in blood cell membranes. If subsequent studies in patients could confirm the protective effects against endothelial dysfunction (or cardiac arrhythmias) as observed in animal studies (21, 22), potential indications would be patients with recent ischemic accidents or patients undergoing revascularization procedures as well as the preoperative protection of patients at high risk of developing severe arrhythmias in relation to cardiac or vascular surgery.
Although the results of large studies evaluating the potential benefit of chronic α-tocopherol supplementations at high doses have generally been disappointing (23–25), a single administration to patients undergoing an acute-phase reaction may replenish a reduced content in plasma as well as the LDL or HDL pool. The much higher content in α-tocopherol of the MCT:FO emulsion compared with the MCT:LCT emulsion limits the comparison between these 2 preparations. Indeed, injection of the MCT:FO emulsion resulted in a much more pronounced increase of α-tocopherol content in plasma LDL and HDL and in WBCs and platelets. As previously noted for n−3 fatty acids, the enrichment in α-tocopherol was already close to its highest value within 60 min after injection of the MCT:FO emulsion and only displayed an obvious, albeit partial, decline between days 2 and 3 (Figure 4). The accumulation of α-tocopherol in LDL was associated with a reduced sensitivity to lipid peroxidation as documented by the changes in the lag time preceding the rise of conjugated dienes in LDL exposed to copper sulfate (Table 3). However, the propagation slope for conjugated dienes became shallower after the MCT:FO injection and steeper after the MCT:LCT injection. The latter observation may reflect the higher relative content of PUFAs such as 18:2n−6 and 18:3 n−3 in the soybean triacylglycerols of the MCT:LCT emulsion compared with the MCT:FO emulsion.
In conclusion, the experimental procedure examined in the current article may well pave the way to a novel approach for the rapid and efficient supply of polyunsaturated n−3 fatty acids to suitable target cells in selected patients.
Acknowledgments
We are grateful to C Mélot for help and advice with statistical analyses, A Chwalik, P D'Hont, A Dufour, and E Van de Wyer for technical assistance, A Jancys and her nurses for clinical collaboration, and C Demesmaeker for secretarial help.
The authors’ responsibilities were as follows—YAC: designed the study and helped write the manuscript; RJD: designed the study; IED: supervised the experiments; LP: helped collect data; WJM: wrote the manuscript; and MH: helped write the manuscript. YAC and IED are coinventors of a patent filed by Brussels Free University that relates to the clinical use of the MCT:FO emulsion (US 10/484947; EP 02748862.6). RJD, LP, WJM, and MH had no personal or financial conflicts of interest.
REFERENCES
- 1.Kris-Etherton PM, Harris WS, Appel LJ. American Heart Association. Nutrition Committee. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002;106:2747–57 [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Ascherio A, Hu FB, et al. Interplay between different polyunsaturated fatty acids and risk of coronary heart disease in men. Circulation 2005;111:157–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krauss RM, Eckel RH, Howard B, et al. AHA Dietary Guidelines: revision 2000: A statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Circulation 2000;102:2284–99 [DOI] [PubMed] [Google Scholar]
- 4.Bang HO, Dyerberg J. Lipid metabolism and ischemic heart disease in Green lad Eskimos: Draper H. Advances in nutrition research New York, NY: Plenum Press, 1980:1–22 [Google Scholar]
- 5.Cordain L, Eaton SB, Sebastian A, et al. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr 2005;81:341–54 [DOI] [PubMed] [Google Scholar]
- 6.Browning LM. n−3 Polyunsaturated fatty acids, inflammation and obesity-related disease. Proc Nutr Soc 2003;62:447–53 [DOI] [PubMed] [Google Scholar]
- 7.Wang C, Harris WS, Chung M, et al. n−3 Fatty acids from fish or fish-oil supplements, but not alpha-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: a systematic review. Am J Clin Nutr 2006;84:5–17 [DOI] [PubMed] [Google Scholar]
- 8.Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico Dietary supplementation with n−3 polyunsaturated PUFAs and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet 1999;354:447–55 [PubMed] [Google Scholar]
- 9.Christensen MS, Høy C-E, Becker CC, Redgrave TG. Intestinal absorption and lymphatic transport of eicosapentaenoic (EPA), docosahexaenoic (DHA), and decanoic acids: dependence on intramolecular triacylglycerol structure. Am J Clin Nutr 1995;61:56–61 [DOI] [PubMed] [Google Scholar]
- 10.Oliveira FL, Rumsey SC, Schlotzer E, Hansen I, Carpentier YA, Deckelbaum RJ. Triglyceride hydrolysis of soy oil vs fish oil emulsions. JPEN J Parenter Enteral Nutr 1997;21:224–9 [DOI] [PubMed] [Google Scholar]
- 11.Billman GE, Kang JX, Leaf A. Prevention of sudden cardiac death by dietary pure ω-3 polyunsaturated fatty acids in dogs. Circulation 1999;99:2452–7 [DOI] [PubMed] [Google Scholar]
- 12.Schrepf R, Limmert T, Weber PC, Theisen K, Sellmayer A. Immediate effects of n−3 fatty acid infusion on the induction of sustained ventricular tachycardia. Lancet 2004;363:1441–2 [DOI] [PubMed] [Google Scholar]
- 13.Carpentier YA, Dupont I, Portois L, Malaisse WJ. Preclinical investigations of a medium-chain triglyceride:fish oil emulsion. III. Experiments in cultured endothelial cells. Int J Mol Med 2006;18:1177–85 [PubMed] [Google Scholar]
- 14.Dahlan W, Richelle M, Kulapongse S, Rössle C, Deckelbaum RJ, Carpentier YA. Effects of essential fatty acid contents of lipid emulsions on erythrocyte polyunsaturated fatty acid composition in patients on long-term parenteral nutrition. Clin Nutr 1992;11:262–8 [DOI] [PubMed] [Google Scholar]
- 15.Hatam LJ, Kayden HJ. A high-performance liquid chromatographic method for the determination of tocopherol in plasma and cellular elements of the blood. J Lipid Res 1979;20:639–45 [PubMed] [Google Scholar]
- 16.Puhl H, Waeg G, Esterbauer H. Methods to determine oxidation of low-density lipoproteins. Methods Enzymol 1994;233:425–41 [DOI] [PubMed] [Google Scholar]
- 17.Soop M, Nygren J, Myrenfors P, Thorell A, Ljungqvist O. Preoperative oral carbohydrate treatment attenuates immediate postoperative insulin resistance. Am J Physiol Endocrinol Metab 2001;280:E576–83 [DOI] [PubMed] [Google Scholar]
- 18.Pradier O, Portois L, Malaisse WJ, Carpentier YA. Hemostatic safety of the bolus intravenous injection of a novel medium-chain triglyceride:fish oil emulsion. Int J Mol Med 2008;22:301–7 [PubMed] [Google Scholar]
- 19.Richelle M, Carpentier YA, Deckelbaum RJ. Long- and medium-chain triacylglycerols in neutral lipid-exchange processes with human plasma low-density lipoproteins. Biochemistry 1994;33:4872–8 [DOI] [PubMed] [Google Scholar]
- 20.Mayer K, Meyer S, Reinholz-Muhly M, et al. Short-time infusion of fish-oil based lipid emulsions, approved for parenteral nutrition, reduces monocyte proinflammatory cytokine generation and adhesive interaction with endothelium in humans. J Immunol 2003;171:4837–43 [DOI] [PubMed] [Google Scholar]
- 21.Fontaine D, Otto A, Portois L, et al. Protection of aortic endothelial function in both normal and diabetic rats by intravenous bolus injection of a medium-chain triglyceride: fish oil emulsion. Int J Mol Med 2006;18:697–704 [PubMed] [Google Scholar]
- 22.Peltier S, Malaisse WJ, Portois L, et al. Acute in vivo administration of a fish oil-containing emulsion improves post-ischemic cardiac function in n-3-depleted rats. Int J Mol Med 2006;18:741–9 [DOI] [PubMed] [Google Scholar]
- 23.Stephens NG, Parsons A, Schofield PM, et al. Randomised controlled trial of vitamin E in patients with coronary disease : Cambridge Heart Antioxidant Study (CHAOS). Lancet 1996;347:781–6 [DOI] [PubMed] [Google Scholar]
- 24.Boaz M, Smetana S, Weinstein T, et al. Secondary prevention with antioxidants of cardiovascular disease in endstage renal disease (SPACE):randomised placebo-controlled trial. Lancet 2000;356:1213–8 [DOI] [PubMed] [Google Scholar]
- 25.Yusuf S, Dagenais G, Pogue J, et al. Vitamin E supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 2000;342:154–60 [DOI] [PubMed] [Google Scholar]