Abstract
Background: The overall consumption of trans fatty acids (TFAs) increases the risk of coronary artery disease. However, multiple TFA isomers exist, each with potentially different health effects. Different food sources of these specific TFA isomers are not well established.
Objective: Our objective was to determine the major independent food sources of specific TFA isomers.
Design: We investigated relations of major potential food sources of TFAs, as assessed by serial food-frequency questionnaires, with 10 plasma phospholipid TFA isomers [5 trans (t-) 18:1, 3 t-18:2, and 2 t-16:1] in 3330 older adults in the Cardiovascular Health Study, a community-based multicenter cohort. Stepwise regression was used to identify independent major food sources of individual plasma phospholipid TFA isomers, which were adjusted for demographic, lifestyle, and dietary factors.
Results: All 5 t-18:1 isomers were similarly associated with foods commonly made with partially hydrogenated vegetable oils (PHVOs), including biscuits (0.51 higher SD of total 18:1 fatty acid concentrations per serving/d, P < 0.01), chips and/or popcorn (0.33 higher SD per serving/d, P = 0.02), margarine (0.32 higher SD per serving/d, P < 0.001), fried foods (0.32 higher SD per serving/d, P = 0.04), and bakery foods (0.23 higher SD per serving/d, P = 0.02). Each of the t-18:2 isomers were associated only with bakery foods (0.50 higher SD of total 18:2 fatty acid concentrations per serving/d, P < 0.001). Ruminant foods were major correlates of t-16:1n−7, including red meats (0.72 higher SD per serving/d, P < 0.001), butter (0.43 higher SD per serving/d, P < 0.001), and higher-fat dairy (0.37 higher SD per serving/d, P < 0.001). In contrast, t-16:1n−9 were derived mainly from margarine (0.31 higher SD per serving/d, P < 0.001).
Conclusions: t-18:1 Isomers are similarly derived from multiple PHVO-containing foods. In contrast, t-18:2 and t-16:1n−9 isomers are derived from more-specific types of PHVO-containing foods. Ruminant foods are major sources of t-16:1n−7. Different TFA isomers and dietary sources should be considered when investigating health effects and interventions to lower TFAs.
INTRODUCTION
For many years, broad biochemical categorizations were used to group together cis and trans unsaturated fats until their very different health effects were identified and established over the past 2 decades. Whereas it is clear that consumption of trans fatty acids (TFAs) has adverse cardiovascular consequences (1–11), emerging evidence suggests that even this subcategorization is too broad. Similar to other fat subcategories, several different TFA isomers exist that have different carbon-chain lengths and double-bond locations—each with potentially varying biological and physiologic effects and dietary sources (5, 6, 12–15). Each of these TFA isomers may be naturally present in the diet (ie, consumed from foods containing ruminant TFAs), consumed from foods containing industrially produced partially hydrogenated vegetable oils (PHVOs), or consumed in smaller amounts from other sources, such as deodorized oils (16, 17). Whereas the total industrial TFAs consumed is consistently linked to coronary artery disease (CAD) risk (18), less is known about the health effects of specific TFA isomers. In studies evaluating TFA isomers grouped into major subclasses, total trans (t-) 18:1 and/or t-18:2 isomers were generally more strongly associated with CAD risk (13), systemic inflammation and endothelial dysfunction (5, 6, 14), sudden cardiac death (12, 15), and preeclampsia (19, 20) than were total t-16:1 isomers. However, even these major subclasses contain several individual TFA isomers (eg, at least 2 t-16:1, 3 t-18:2, and 5 t-18:1 isomers) that can be identified (21, 22).
To understand the potentially different relations of specific TFA isomers with health outcomes, the major dietary sources of each isomer in the population need to be understood. Unfortunately, the major food sources of different specific TFA isomers, or the extent to which these major food sources vary for different TFA isomers, are not well established. Such investigation has been limited by challenges in estimating the consumption of specific TFA isomers from dietary questionnaires due to limited availability of detailed and accurate food composition databases for isomer-specific TFA subclasses. In addition, measuring TFA contents of foods is limited by considerable brand-to-brand variability and, more importantly, only identifies potential TFA sources rather than what is actually consumed in the population. For example, a food that contains high TFA concentrations but is rarely consumed will contribute minimally to TFA exposure in the population. Advanced gas and thin-layer chromatographic techniques allow identification of multiple specific TFA isomers in blood (21, 22), providing objective biological markers to assess actual exposure to these individual fatty acids (23–25). The determination of which dietary sources of TFAs, both ruminant and industrial, independently contribute to individual circulating TFA isomers would assist in understanding the limited known isomer-specific associations with health outcomes, guide future investigations of health effects, and inform policy measures or other interventions to decrease consumption of specific isomers. In addition, the evaluation of the relative contribution of different dietary influences to the overall variation in blood concentrations of each TFA isomer would elucidate the extent to which nondietary factors, such as absorption, isomerization, disposition, and/or catabolism, could affect circulating concentrations. To elucidate these relations, we investigated associations between major potential food sources of TFAs and circulating biomarker concentrations of 10 specific TFA isomers in plasma phospholipids, including 5 trans isomers of oleic acid (t-18:1), 3 trans isomers of linoleic acid (t-18:2), and 2 trans isomers of palmitoleic acid (t-16:1), among 3330 US men and women in the Cardiovascular Health Study (CHS).
SUBJECTS AND METHODS
Design and population
The CHS is a community-based prospective cohort of determinants of risk factors for the onset and course of cardiovascular disease among older adults (26, 27). Briefly, 5201 men and women were randomly selected and enrolled from Medicare eligibility lists in 1989–1990, and an additional 687 African Americans were similarly enrolled in 1992–1993, from 4 US communities (Forsyth County, NC; Sacramento County, CA; Washington County, MD; and Allegheny County, PA). Eligible individuals were noninstitutionalized people aged ≥65 y, excluding those who were wheelchair bound, receiving hospice or cancer treatment, or expected to move from the study areas within 3 y. Each center's institutional review committee approved the study, and all participants gave informed consent.
Plasma phospholipid fatty acids were measured in 3419 individuals (1975 women, 1444 men) from stored blood samples taken in 1992–1993. We excluded 89 individuals with missing dietary intake information. The final population consisted of 3330 participants, including 2869 individuals randomly selected from participants with available blood samples (n = 3578) and 461 individuals from prior case-control studies of myocardial infarction. Analyses accounted for this sampling within the cohort by using inverse-probability-of-sampling weights.
Assessment of fatty acids
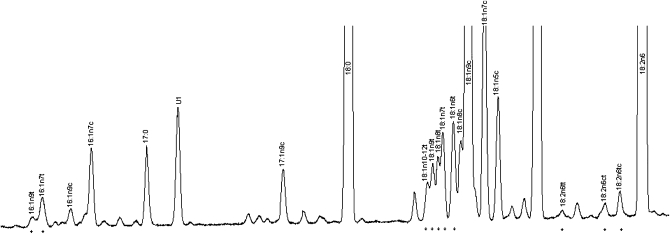
Blood was drawn after a 12-h fast and stored at −70°C before shipping, on dry ice, to the CHS Central Blood Analysis Laboratory (University of Vermont, Burlington, VT) for long-term storage at −80°C (28). Under these conditions, phospholipid fatty acids in red cells and plasma are stable during long-term storage (28–31), and in 2 prior CHS studies (12, 32) no degradation in plasma phospholipid fatty acids was observed because of lipolysis or oxidation after 10 y of storage. Plasma phospholipid fatty acids were measured at the Fred Hutchinson Cancer Research Center, and are reported as the percentage of total fatty acids. Total lipids were extracted from plasma by using the method of Folch et al (33), and phospholipids were separated from neutral lipids by one-dimensional thin-layer chromatography (TLC). Fatty acid methyl ester (FAME) samples were prepared by direct transesterification by using the method of Lepage and Roy (34) and separated by using gas chromatography with an Agilent 5890 Gas Chromatograph FID detector (Agilent, Santa Clara, CA) and a Supelco fused silica capillary column SP-2560 (100 m × 0.25 mm, 0.2 μm; Sigma-Aldrich, St Louis, MO) (initial 160°C for 16 min, ramp 3.0°C/min to 240°C, and hold for 15 min). The bis-cyanopropyl SP-2560 column was specifically designed for the separation of geometric-positional (cis/trans) isomers of FAMEs. The protocol quantified 45 distinct fatty acids peaks, including 10 TFA isomers (Figure 1).
FIGURE 1.
Representative selection of fatty acid peaks from gas chromatographic analysis in one participant, including the 10 trans fatty acid isomers (*). U1 represents an unknown fatty acid peak.
Identification, precision, and accuracy were evaluated by using model mixtures of known FAMEs and an established in-house control pool with identification confirmed by gas chromatography–mass spectrometry at the US Department of Agriculture lipid laboratory (Peoria, IL) and by silver ion TLC for TFAs (35). Pooled quality-control samples were run concurrently with study samples to confirm low batch-to-batch variation, with CVs < 3% for major fatty acids, < 9% for t-16:1, < 6% for t-18:1, and < 11% for t-18:2. Minimum laboratory drift and excellent stability during storage were confirmed in a subset of 40 individuals by comparing duplicate measurements in the same samples measured once in 1994–1996 and again in 2007. The reproducibility for the same stored sample was high, including correlations of 0.95 for TFAs (CV = 9.1%).
Assessment of foods consumed
Usual dietary intake was assessed by using reproducible and validated semiquantitative food-frequency questionnaires (FFQs) in 1989–1990 (36, 37) and again in 1996 (38, 39). For each questionnaire, participants were asked to indicate how often, on average, they had consumed usual serving sizes of various specified foods during the previous year. The 1989–1990 FFQ was a 99-item, picture-sort, interviewer-administered FFQ that included 5 responses ranging from never or <1 time/mo to almost every day. The 1996 FFQ was a 131-item, self-administered FFQ that included 9 responses ranging from never or <1 time/mo to >6 times/d. Open-ended questions elicited information on supplements, exact breakfast cereals, and fats and oils used for frying or baking. Plasma phospholipids were measured by using stored blood in 1992, at the intermediate time point between the 2 FFQs, and preliminary analyses showed that both FFQs were related to plasma phospholipid TFAs. Therefore, we averaged responses on the 2 FFQs to minimize misclassification; for participants enrolled in 1992–1993 (n = 121), the only data available were from the latter FFQ. Estimated mean (±SD) dietary TFA intake from the 2 FFQs was 3.2 ± 1.8 g/d on this basis of temporally concurrent food-composition values from the regularly updated Harvard University Nutrition database. On this basis of the FFQ estimates, major contributors to total TFA intake were bakery products (22.5% of total), fried foods eaten away from home (19.7% of total), unprocessed red meats (15.6% of total), margarine (8.4% of total), higher-fat dairy (6.3% of total), processed meats (3.8% of total), biscuits (3.2% of total), lower-fat dairy (3.0% of total), chips and/or popcorn (1.1% of total), butter (0.9% of total), fried food eaten at home (0.9% of total), and pizzas (0.8% of total). For each participant, consumption of major potential dietary sources of industrially produced and ruminant TFAs were averaged across the 2 FFQs, including spreads (ie, margarine, butter, peanut butter, and salad dressings), red and processed meats (ie, beef, pork, lamb, hamburgers, hot dogs, bacon, and other processed meats), fried foods (fried foods eaten away from home and fried foods eaten at home), dairy (ie, higher-fat milk, higher-fat cheese, ice-cream, skimmed milk, cottage, ricotta, and cream cheese), pizza (ie, cheese), bakery foods (ie, cookies, pies, pancakes, corn breads, and tortillas), chips and/or popcorn, and biscuits. Fried foods eaten at home were separately assessed as they may have included prepackaged or prepared commercial fried food or foods fried eaten at home with PHVO-containing fats. For each food and/or food group, similar food items in each FFQ were identified [eg, margarine on bread or rolls in 1989–1990 and margarine, added to food or bread, in 1996; ice cream in 1989–1990 and ice cream in 1996; and salty snacks (such as chips, popcorn) in 1989–1990 and potato chips or corn chips and popcorn in 1996] and averaged after standardization to the same serving size. Cooking fats and oils were also assessed by means of questions on consumption (yes/no) of butter, margarine, vegetable oil, vegetable shortening, and lard for frying or baking; additional information was unavailable regarding specific consumption amounts or subtypes of vegetable oils used. We evaluated the use of vegetable oils for frying or baking to assess potential contributions of oil deodorization to TFA consumption (40).
Statistical analyses
The concentration of each TFA isomer was standardized by dividing it by its SD to facilitate direct comparison of the strength of relation of different food sources with each TFA isomer. Thus, results are reported per SD difference in concentrations of each TFA isomer. Consumption of each food or food group was assessed in servings per day. We used Spearman correlations to evaluate the unadjusted interrelations between the different specific TFA isomers and between the different major dietary sources and specific TFA isomers. Backward stepwise multivariable-adjusted regression (P < 0.05 for addition, and P < 0.1 for removal) was used to assess the dietary sources independently associated with each individual plasma phospholipid TFA isomer, with potential covariates including demographics (age, sex, race, clinic site, education, income, and self-reported health), cardiovascular risk factors (prevalent CAD, stroke, diabetes, and treated hypertension), other lifestyle habits (smoking, alcohol consumption, and physical activity) and, for each food, total energy and the other potential food sources of TFAs. Missing covariate values were imputed by using age, sex, race, smoking, alcohol consumption, education, physical activity, body mass index (BMI; in kg/m2), prevalent diabetes, CAD, and stroke as predictors; the proportion of missing values that were imputed were 15% for total energy, 6% for income, 2% for smoking, 0.2% for physical activity, and 0.7% for BMI. Results were similar excluding subjects with missing values. All other variables, including foods and/or food groups, were not missing for any of the participants. Partial r2 (ΔR2) values, which were adjusted for other demographic and lifestyle factors, were evaluated to estimate the contribution of combined foods to variation in TFA concentrations. Correlations, regression coefficients, and ΔR2 values were corrected for measurement error (41, 42) by using the average of published deattenuated correlation coefficients derived from comparing estimated dietary intake from multiple weighed diet records over 1 y with estimated dietary intake from similar FFQs in other validation cohorts of US adult men and women (39, 43). Although dietary consumption patterns were generally similar in these cohorts compared with CHS, we recognized the potential limitations of using externally derived correction coefficients, particularly the potential for undercorrection for measurement error in our cohort, but this was considered the best available data to correct for dietary measurement error. All P values were 2-tailed (α = 0.05). Analyses were performed with STATA 10.0 (STATA, College Station, TX).
RESULTS
Characteristics of the 3330 participants, including 1924 men and 1406 women, are presented in Table 1. Participants were elderly, on average overweight, and of diverse socioeconomic backgrounds. The evaluated potential dietary sources of TFAs and their average (mean ± SD) weekly servings consumed were margarine (6.0 ± 4.8), butter (2.1 ± 3.6), red (2.3 ± 1.9) and processed meats (3.5 ± 3.8), fried foods (2.3 ± 2.0), higher-fat dairy (6.2 ± 4.6), lower-fat dairy (4.5 ± 3.9), bakery foods (2.8 ± 2.9), biscuits (1.1 ± 1.4), chips and/or popcorn (1.1 ± 1.6), and pizza (0.3 ± 0.5). Ranges of intakes of these different foods were generally broad (Table 1). The intakes of these different foods and/or food groups were not highly intercorrelated. For example, among all of these foods, the largest correlations were between consumption of fried foods and red meats (r = 0.4) and between consumption of cookies and pies (r = 0.4).
TABLE 1.
Characteristics of 3330 men and women with plasma phospholipid fatty acid measures in the Cardiovascular Health Study, a community-based, multicenter US cohort study1
| Values | 20th–80th Percentile | |
| Age (y) | 72.3 ± 5.22 | 68–77 |
| Women (%) | 57.8 | — |
| Nonwhite race (%) | 8.0 | — |
| Education (%) | ||
| <High school | 26.3 | — |
| High school | 27.8 | — |
| >High school | 45.9 | — |
| Income (%) | ||
| <$16,000/y | 35.0 | — |
| $16,000-$34,999/y | 38.3 | — |
| ≥$35,000/y | 26.7 | — |
| Smoking (%) | ||
| Never | 44.7 | — |
| Past | 46.8 | — |
| Current | 8.6 | — |
| BMI (kg/m2) | 26.6 ± 4.5 | 22.9–29.8 |
| Waist circumference (cm) | 97.1 ± 12.8 | 86.5–107.0 |
| Physical activity (kcal/wk) | 1068 ± 1446 | 23–1721 |
| Alcohol (%) | ||
| None | 57.3 | — |
| <1–2 drinks/wk | 29.2 | — |
| ≥3 drinks/wk | 17.1 | — |
| Prevalent coronary artery disease (%) | 19.3 | — |
| Prevalent stroke (%) | 7.4 | — |
| Diabetes (diabetes medication or fasting glucose >125 mg/dL) (%) | 16.4 | — |
| Use of antihypertensive medication (%) | 47.8 | — |
| Total energy (kcal/d) | 2017 ± 603 | 1539–2441 |
| Foods (servings/wk) | ||
| Spreads | ||
| Margarine (one pat, 5 g) | 6.0 ± 4.8 | 0.5–9.5 |
| Butter (one pat, 5 g) | 2.1 ± 3.6 | 0.1–4.0 |
| Red meats | ||
| Hamburgers (one patty, 85 g) | 1.0 ± 1.0 | 0.3–1.8 |
| Other unprocessed red meats (beef, pork, lamb) (4–6 oz/140 g) | 1.3 ± 1.3 | 0.4–2.0 |
| Total unprocessed red meats | 2.3 ± 1.9 | 0.7–3.6 |
| Hot dogs (one, 45 g) | 0.8 ± 1.3 | 0.1–0.9 |
| Bacon (2 slices, 13 g) | 0.9 ± 1.3 | 0.1–1.5 |
| Other processed meats (eg, sausage, ham, lunch meats) (one piece or slice, 27 g) | 1.8 ± 2.5 | 0.2–3.2 |
| Total processed meats | 3.5 ± 3.8 | 0.8–5.7 |
| Fried foods | ||
| Eaten away from home3 | 0.7 ± 0.7 | 0.3–1.0 |
| Eaten at home3 | 1.6 ± 1.6 | 0.4–2.6 |
| Total3 | 2.3 ± 2.0 | 0.7–3.5 |
| Dairy products | ||
| Milk (≥2% fat) (8 fl oz/244 g) | 2.2 ± 2.4 | 0.1–4.3 |
| Cheese, except cottage or ricotta (one slice or 1 oz/28 g) | 2.5 ± 2.7 | 0.5–4.0 |
| Ice cream (1/2 cup, 66 g) | 1.5 ± 1.9 | 0.1–2.5 |
| Total higher-fat dairy | 6.2 ± 4.6 | 2.2–9.6 |
| Skimmed milk (8 fl oz/244 g) | 3.3 ± 3.5 | 0.1–6.5 |
| Cottage or ricotta cheese (1/2 cup, 105 g) | 1.2 ± 1.4 | 0.1–2.5 |
| Total lower-fat dairy | 4.5 ± 3.9 | 0.6–7.1 |
| Bakery foods | ||
| Cookies, doughnuts, cake, pastry (one piece, 50 g) | 2.0 ± 2.4 | 0.4–2.8 |
| Pies (one slice, 125 g) | 0.8 ± 1.2 | 0.2–0.9 |
| Total | 2.8 ± 2.9 | 0.8–4.1 |
| Other foods | ||
| Chips4/popcorn (1 oz/28.35 g or 1 cup, 11 g) | 1.1 ± 1.6 | 0.1–1.7 |
| Biscuits4 (one piece, 55 g) | 1.1 ± 1.4 | 0.1–1.8 |
| Pizza (2 slices, 240 g) | 0.3 ± 0.5 | 0.1–0.5 |
Foods represent the average of dietary consumption at baseline (1989–1990) and in 1996.
Mean ± SD (all such values).
Information was unavailable on specific serving sizes. Food-frequency questionnaires evaluated total fried foods and fried foods eaten away from home; fried foods eaten at home were estimated from the difference.
In the United States, biscuits are a type of cracker (not cookies), and chips are crisps (not French fries).
Total TFAs represented on average 2.52% of plasma phospholipids (interquartile range = 2.0–3.0%), including an average of 0.25% total t-16:1 isomers, 2.00% total t-18:1 isomers, and 0.27% total t-18:2 isomers (Table 2). Among t-18:1 isomers, t-18:1n−6 and t-18:1n−7 were most common, but other t-18:1 isomers also appreciably contributed to the total. The total TFAs was highly correlated with total t-18:1 isomers (r = 0.99) and was modestly correlated with total t-18:2 (r = 0.62) and total t-16:1 (r = 0.45). The 5 individual t-18:1 isomers were highly intercorrelated (r > 0.85 for each). Conversely, the 2 individual t-16:1 isomers were very weakly intercorrelated (r = 0.11). Intercorrelations of the 3 individual t-18:2 isomers varied; t/c-18:2n−6n−9 was modestly correlated with c/t-18:2n−6n−9 (r = 0.71), whereas t/t-18:2n−6n−9 was not correlated with t/c-18:2n−6n−9 (r = 0.01) or c/t-18:2n−6n−9 (r = −0.09). Interestingly, t-16:1n−9 was relatively strongly correlated with each of the t-18:1 isomers (r = 0.57–0.63) and with c/t- 18:2n−6n−9 (r = 0.47). Conversely, but t-16:1n−7 was weakly correlated with t-16:1n−9 (r = 0.11), with each of the 5 t-18:1 isomers (r < 0.25 for each), and with each of the 3 t-18:2 isomers (r < 0.16 for each).
TABLE 2.
Spearman correlation coefficients between specific plasma phospholipid trans fatty acid isomers in 3330 men and women in the Cardiovascular Health Study1
|
trans 16:1 |
trans 18:1 |
trans 18:2 |
||||||||||||
| Total trans | t-16:1n−7 | t-16:1n−9 | Total t-16:1 | t-18:1n−6 | t-18:1n−7 | t-18:1n−8 | t-18:1n−9 | t-18:1n−10–12 | Total t-18:1 | t/c-18:2n−6n−9 | c/t-18:2n−6n−9 | t/t-18:2n−6n−9 | Total t-18:2 | |
| trans Fatty acid isomers | 2.52 ± 0.792 | 0.19 ± 0.05 | 0.07 ± 0.03 | 0.25 ± 0.06 | 0.61 ± 0.21 | 0.50 ± 0.18 | 0.33 ± 0.14 | 0.36 ± 0.14 | 0.21 ± 0.08 | 2.01 ± 0.71 | 0.13 ± 0.05 | 0.08 ± 0.02 | 0.05 ± 0.04 | 0.27 ± 0.09 |
| t-16:1n−7 | 0.203 | |||||||||||||
| t-16:1n−9 | 0.643 | 0.113 | ||||||||||||
| Total t-16:1 | 0.453 | 0.883 | 0.533 | |||||||||||
| t-18:1n−6 | 0.983 | 0.123 | 0.613 | 0.373 | ||||||||||
| t-18:1n−7 | 0.953 | 0.243 | 0.593 | 0.463 | 0.933 | |||||||||
| t-18:1n−8 | 0.933 | 0.203 | 0.613 | 0.433 | 0.883 | 0.863 | ||||||||
| t-18:1n−9 | 0.943 | 0.633 | 0.283 | 0.943 | 0.863 | 0.853 | ||||||||
| t-18:1n−10–12 | 0.923 | 0.063 | 0.573 | 0.293 | 0.913 | 0.823 | 0.853 | 0.913 | ||||||
| Total t-18:1 | 0.993 | 0.143 | 0.633 | 0.393 | 0.983 | 0.953 | 0.933 | 0.953 | 0.933 | |||||
| t/c-18:2n−6n−9 | 0.563 | 0.253 | 0.113 | 0.493 | 0.473 | 0.463 | 0.503 | 0.493 | 0.513 | |||||
| c/t-18:2n−6n−9 | 0.633 | 0.473 | 0.243 | 0.583 | 0.533 | 0.523 | 0.623 | 0.573 | 0.593 | 0.713 | ||||
| t/t-18:2n6n−9 | 0.143 | 0.153 | −0.054 | 0.083 | 0.103 | 0.123 | 0.153 | 0.054 | 0.113 | 0.113 | −0.093 | |||
| Total t-18:2 | 0.623 | 0.063 | 0.243 | 0.163 | 0.553 | 0.523 | 0.513 | 0.553 | 0.563 | 0.563 | 0.933 | 0.783 | 0.243 | |
Only correlations with P < 0.05 are presented.
Mean ± SD as percentage of total fatty acids (all such values).
P < 0.001.
P < 0.01.
Bivariate (unadjusted) associations between consumption of specific foods and/or food groups and each of the TFA isomers are shown in Table 3. The different dietary sources had generally similar correlations with each of the t-18:1 isomers. Total t-18:1 correlated most strongly with margarine (r = 0.27), bakery foods (r = 0.24), biscuits (r = 0.17), and fried foods eaten away from home (r = 0.16). In contrast, the dietary sources of t-16:1n−7 and t-16:1n−9 appeared very different. The most strongly correlated foods with t-16:1n−7 were butter (r = 0.25), unprocessed red meats (r = 0.26), and higher-fat dairy foods (r = 0.33). Conversely, t-16:1n−9 was correlated most strongly with consumption of margarine (r = 0.25) and bakery foods (r = 0.16). t-18:2 isomers had generally few correlations with these foods and/or foods groups; the largest correlations were with bakery foods that appeared relatively similar across the 3 different t-18:2 isomers.
TABLE 3.
Unadjusted Spearman correlation coefficients between consumption of specific foods and plasma phospholipid trans fatty acid concentrations in 3330 men and women in the Cardiovascular Health Study1
|
trans 16:1 |
trans 18:1 |
trans 18:2 |
||||||||||||
| Foods and/or food groups | Total trans | t-16:1n−7 | t-16:1n−9 | Total t-16:1 | t-18:1n−6 | t-18:1n−7 | t-18:1n−8 | t-18:1n−9 | t-18:1n−10–12 | Total t-18:1 | t/c-18:2 n−6n−9 | c/t-18:2 n−6n−9 | t/t-18:2 n−6n−9 | Total t-18:2 |
| Spreads | ||||||||||||||
| Margarine (1, 1)2 | 0.263 | 0.253 | 0.103 | 0.273 | 0.253 | 0.233 | 0.273 | 0.243 | 0.273 | 0.153 | ||||
| Butter (1, 1) | 0.253 | 0.163 | ||||||||||||
| Red meats | ||||||||||||||
| Beef, pork, lamb (2, 1) | 0.163 | 0.093 | ||||||||||||
| Hamburgers (1, 1) | 0.104 | 0.313 | 0.273 | 0.08 | 0.123 | 0.114 | 0.08 | 0.07 | ||||||
| Total unprocessed red meats (3, 2) | 0.263 | 0.193 | ||||||||||||
| Hot dogs (1, 1) | 0.094 | 0.153 | 0.104 | 0.094 | 0.123 | 0.094 | 0.094 | 0.104 | 0.07 | 0.104 | ||||
| Bacon (1, 1) | 0.084 | |||||||||||||
| Other processed meats (2, 1) | 0.153 | 0.113 | 0.06 | 0.05 | ||||||||||
| Total processed meats (4, 3) | 0.153 | 0.103 | 0.074 | 0.07 | 0.07 | |||||||||
| Fried foods | ||||||||||||||
| Eaten away from home (2, 1) | 0.163 | 0.094 | 0.183 | 0.193 | 0.153 | 0.113 | 0.113 | 0.163 | 0.113 | 0.103 | 0.123 | |||
| Eaten at home5 | 0.103 | 0.123 | 0.084 | 0.114 | 0.123 | 0.093 | 0.06 | 0.104 | ||||||
| Total (3, 3) | 0.143 | 0.104 | 0.084 | 0.163 | 0.173 | 0.143 | 0.084 | 0.094 | 0.143 | 0.07 | 0.06 | 0.094 | ||
| Dairy products | ||||||||||||||
| ≥2%-fat milk (2, 1) | 0.333 | 0.273 | 0.074 | |||||||||||
| Cheese (except cottage or ricotta cream) (1, 1) | 0.283 | 0.183 | ||||||||||||
| Ice cream (1, 1) | 0.243 | 0.173 | 0.05 | 0.074 | 0.05 | 0.084 | ||||||||
| Total higher-fat dairy (4, 3) | 0.393 | 0.293 | ||||||||||||
| Skimmed milk (1, 1) | ||||||||||||||
| Cottage or ricotta cheese (1, 1) | 0.05 | |||||||||||||
| Total lower-fat dairy (2, 2) | ||||||||||||||
| Bakery foods | ||||||||||||||
| Cookies, doughnuts, cake, pastry (1, 6)6 | 0.263 | 0.173 | 0.173 | 0.223 | 0.243 | 0.283 | 0.223 | 0.203 | 0.213 | 0.243 | 0.233 | 0.193 | 0.07 | 0.233 |
| Pies (2, 2) | 0.193 | 0.074 | 0.113 | 0.103 | 0.193 | 0.203 | 0.173 | 0.143 | 0.153 | 0.193 | 0.173 | 0.153 | 0.084 | 0.193 |
| Total (3, 8)6 | 0.253 | 0.143 | 0.163 | 0.193 | 0.243 | 0.273 | 0.223 | 0.193 | 0.203 | 0.243 | 0.223 | 0.193 | 0.074 | 0.233 |
| Other | ||||||||||||||
| Chips and/or popcorn (1, 2) | 0.05 | |||||||||||||
| Biscuits (1, 1) | 0.173 | 0.094 | 0.173 | 0.173 | 0.163 | 0.153 | 0.153 | 0.173 | 0.163 | 0.183 | 0.163 | |||
| Pizza (1, 1) | 0.153 | 0.09 | ||||||||||||
Foods and/or food groups were evaluated as the average of usual dietary consumption assessed by food-frequency questionnaires (FFQs) in 1989–1990 and 1996 and multiplied by the average number of questions on the 2 FFQs. Plasma phospholipid trans fatty acids were assessed in blood collected in 1992–1993. Only positive correlations with P < 0.05 are presented.
Numbers of questions on each FFQ in 1989–90 and 1996, respectively, are shown in parentheses (all such values).
P < 0.001.
P < 0.01.
FFQs evaluated total fried foods and fried foods eaten away from home; fried foods eaten at home were estimated from the difference.
Because of the many more questions in this category on the 1996 FFQ than on the 1989–1990 FFQ, the number of questions on the 1996 FFQ were given half weight and multiplied by the average of usual dietary consumption assessed by FFQs in 1989–1990 and 1996.
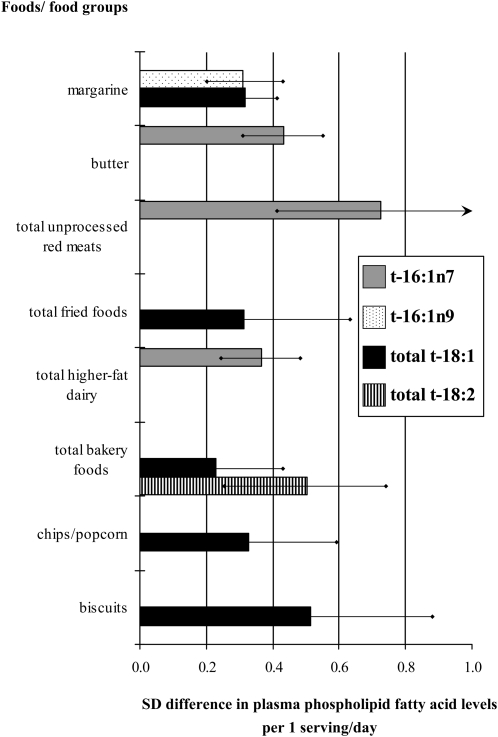
We performed separate multivariable-adjusted stepwise regression analyses to identify the independent dietary sources of each TFA isomer. The specific foods and/or food groups independently associated with each of the 5 t-18:1 isomers were similar, as were the specific foods and/or food groups independently associated with each of the 3 t-18:2 isomers (data not shown). Thus, these isomers were evaluated together in multivariable analyses as total t-18:1 and total t-18:2, respectively (Table 4). Independent dietary correlates of t-16:1n−7 and t-16:1n−9 were very different, and these isomers were considered separately (Table 4). In multivariable-adjusted analyses, each serving per day of margarine was associated with one-third SD (0.33) higher concentrations of t-18:1. Other predictors of higher t-18:1 concentrations were consumption of fried foods eaten away from home (0.68 higher SD/serving/d), biscuits (0.54 higher SD/serving/d), bakery foods (0.28 higher SD/serving/d), and chips and/or popcorn (0.28 higher SD/serving/d). The multivariable-adjusted correlates of higher t-18:2 concentrations were consumption of bakery foods (0.68 higher SD/serving/d). After multivariable-adjustment, the only food source independently associated with higher t-16:1n−9 concentrations was margarine (0.31 higher SD/serving/d). Independent correlates of higher t-16:1n−7 concentrations were very different, including consumption of butter (0.45 higher SD/serving/d), beef, pork, and lamb (0.40 higher SD/serving/d), hamburgers (1.34 higher SD/serving/d), and higher-fat dairy foods (0.31–0.52 higher SD/serving/d). When we combined similar separate foods into major food groups and performed food group-based multivariable-adjusted stepwise regression analyses, concentrations of different TFA isomers were related to specific food groups (Figure 2).
TABLE 4.
Multivariable-adjusted relations between consumption of specific foods and food groups and plasma phospholipid concentrations of trans fatty acid isomers in 3330 men and women in the Cardiovascular Health Study1
|
t-16:1n−7 |
t-16:1n−9 |
Total t-18:12 |
Total t-18:23 |
|||||
| SD difference | P | SD difference | P | SD difference | P | SD difference | P | |
| Spreads | ||||||||
| Margarine | 0.31 | <0.001 | 0.33 | <0.001 | ||||
| Butter | 0.45 | <0.001 | ||||||
| Red meats | ||||||||
| Beef, pork, lamb | 0.40 | 0.04 | ||||||
| Hamburgers | 1.34 | <0.001 | ||||||
| Hot dogs | ||||||||
| Bacon | ||||||||
| Other processed meats | ||||||||
| Fried foods | ||||||||
| Eaten away from home | 0.68 | 0.04 | ||||||
| Eaten at home | ||||||||
| Dairy products | ||||||||
| ≥2%-fat milk | 0.52 | <0.001 | ||||||
| Cheese (except cottage or cream) | 0.31 | <0.01 | ||||||
| Ice cream | 0.32 | <0.01 | ||||||
| Skimmed milk | ||||||||
| Cottage or ricotta cheese | ||||||||
| Bakery foods | ||||||||
| Cookies, brownies, cake, pastry | 0.28 | 0.02 | 0.48 | <0.01 | ||||
| Pies | 0.68 | <0.01 | ||||||
| Other | ||||||||
| Chips and/or popcorn | 0.28 | 0.04 | ||||||
| Biscuits | 0.54 | <0.01 | ||||||
| Pizza | ||||||||
| Partial ΔR2 – foods4 | 0.12 | 0.05 | 0.13 | 0.02 | ||||
| Total ΔR24 | 0.22 | 0.16 | 0.28 | 0.04 | ||||
Multivariable-adjusted SD differences in plasma phospholipid fatty acid concentrations for each serving per day of each food source based on separate stepwise multivariable regression analyses are provided. Only positive relations with P < 0.05 are shown. Potential covariates included all food sources listed in the table and age (y), sex, race (white, nonwhite), education (<high school, high school, >high school), income (<$16,000/y, $16,000–$34,999/y, ≥$35,000/y), enrollment site (4 sites), self-reported health (3 categories), smoking (never, past, current), alcohol consumption (drinks/wk), leisure-time physical activity (kcal/wk), total energy intake (kcal/d), BMI (in kg/m2), prevalent coronary artery disease, stroke, diabetes, and treated hypertension (yes/no for each). Age, sex, race, education, and total energy consumption were forced in the model.
Multivariable-adjusted results for each of the 5 individual t-18:1 isomers were similar.
Multivariable-adjusted results for the 3 individual t-18:2 isomers were similar.
Partial ΔR2 is for the sum of variance explained by the foods only. Total ΔR2 is for the sum of variance for all variables in the final model.
FIGURE 2.
Multivariable-adjusted relations between consumption of specific food groups and plasma phospholipid concentrations of trans fatty acid (TFA) isomers in 3330 men and women in the Cardiovascular Health Study. Values represent SD differences in plasma phospholipid fatty acid concentrations for each serving per day of each food with corresponding CIs based on separate stepwise multivariable regression analyses. Positive relations with P < 0.05 are shown, including between total t-18:1 and biscuits (0.51 higher SD per serving/d, P < 0.01), chips and/or popcorn (0.33 higher SD per serving/d, P = 0.02), margarine (0.32 higher SD per serving/d, P < 0.001), fried foods (0.32 higher SD per serving/d, P = 0.04), and bakery foods (0.23 higher SD per serving/d, P = 0.02); total t-18:2 and bakery foods (0.50 higher SD per serving/d, P < 0.001); t-16:1n−7 and red meats (0.72 higher SD per serving/d, P < 0.001), butter (0.43 higher SD per serving/d, P < 0.001), and higher-fat dairy (0.37 higher SD per serving/d, P < 0.001); and t-16:1n−9 and margarine (0.31 higher SD per serving/d, P < 0.001). In multivariable-adjusted analyses, total processed meats, total lower-fat dairy, and pizza were not associated with any of the plasma phospholipid TFAs. Potential covariates included all food groups shown in the figure and age (y), sex, race (white, nonwhite), education (<high school, high school, >high-school), income (<$16,000/y, $16,000–$34,999/y, ≥$35,000/y), enrollment site (4 sites), self-reported health (3 categories), smoking (never, past, current), alcohol consumption (drinks/wk), leisure-time physical activity (kcal/wk), total energy intake (kcal/d), BMI (in kg/m2), prevalent coronary artery disease, stroke, diabetes, and treated hypertension (yes/no for each). Age, sex, race, education, and total energy consumption were forced in the model.
In additional analyses, we evaluated consumption of pancakes, corn breads, tortillas, peanut butter, salad dressings (ie, mayonnaise or other creamy salad dressing), and vegetable oils as potential sources of total and individual TFA isomers. None of these foods were significantly associated with plasma phospholipid TFA concentrations in either unadjusted correlation or multivariable-adjusted analyses (data not shown). Analyses were also similar in men compared with women and after excluding individuals enrolled in 1992–1993 (n = 121) or patients with treated diabetes (n = 547) (data not shown).
DISCUSSION
To our knowledge, our findings represent the largest investigation to date of how multiple specific TFA isomers that were assessed by using objective biomarkers of exposure are interrelated with each other and to different food sources. Our results indicate that biochemical definitions, and thus scientific evaluations, that broadly categorize fats (eg, by degree of unsaturation or type of double bond only) can obscure substantial differences in dietary sources.
In this evaluation of dietary sources of 10 individual TFA isomers, several key findings were identified. First, all 5 t-18:1 isomers were highly intercorrelated and similarly associated in multivariable-adjusted analyses with foods that represent major dietary sources of PHVOs, including bakery foods, fried foods eaten away from home, biscuits, salty snacks, and margarine. This was even the case for t-18:1n−7 (vaccenic acid), which is typically considered a marker of ruminant fat. The high intercorrelations and common food sources suggest that measuring any one of the major t-18:1 isomers may be sufficient in many cohorts for investigating relations with health outcomes in epidemiologic studies. Results for t-18:1n−7 might vary in populations with very low PHVO and high ruminant fat consumption, in whom t-18:1n−7 might still be a useful marker of ruminant TFA intake. Our results suggest, however, that t-16:1n−7 may be even more useful for this purpose. Experimental studies should still consider and evaluate potentially different biological effects of each t-18:1 isomer, as these are unknown. Evidence from epidemiologic and experimental studies together can guide policy measures.
Second, the 2 t-16:1 isomers were very weakly intercorrelated, and each was associated with very different dietary sources. Ruminant foods appeared to be the major sources of t-16:1n−7, whereas margarine appeared to be the major source of t-16:1n−9. The latter fatty acid was also moderately correlated with t-18:1 isomers, consistent with a major shared dietary source. Interestingly, margarine appeared to be a common source of t-16:1n−9 and t-18:1 isomers, other dietary sources of PHVOs (ie, fried foods eaten away from home, chips and/or popcorn, biscuits, and bakery foods) were only associated with t-18:1 but not with t-16:1n−9 concentrations. The reasons for these differences, including whether t-16:1n−9 production varies in PHVOs used for margarines compared with other foods, requires further investigation. The weak intercorrelation and discordant food sources of t-16:1n−7 compared with t-16:1n−9 indicate that each isomer should be evaluated separately when considering health outcomes, biological effects, and policy implications.
Third, t/c- and c/t-18:2n−6n−9 isomers were fairly strongly intercorrelated and modestly correlated with t-18:1 isomers. In multivariable-adjusted analyses, bakery foods were their main dietary source, although these foods accounted for only 2% of the variation in total t-18:2. t/t-18:2n−6n−9 was not strongly correlated with any other TFA isomer, including other t-18:2 isomers, suggesting a relatively unique dietary source or sources compared with other TFAs. These findings indicate that the investigation of t-18:2 isomers in epidemiologic studies should consider the t/c- and c/t-isomers separately from the t/t-18:2n−6n−9 isomer. Our findings also support the importance of investigating dietary sources and biological effects of the t/t-18:2n−6n−9 isomer, particularly given biomarker findings that indicate that total t-18:2 isomers may more strongly relate to sudden cardiac death (12, 15).
For TFAs, circulating biomarker concentrations provide an objective measure of dietary consumption. In one small controlled feeding trial (n = 14) (25), raising total t-18:1 consumption by 4% of energy and c/t- and t/t-18:2n−6n−9 consumption by 1% of energy elevated their respective phospholipid concentrations by 217% and 100%. Other small controlled feeding trials (n = 11, 10) (44–45) or cross-sectional analyses (n = 129) (46) support our findings, showing elevations in circulating t-18:1 isomers after or associated with margarine intake. Recently, food sources of plasma phospholipid t-18:1n−9 (elaidic acid) and t-16:1n−7 were assessed in 2 European cohorts (47, 48). In a French cohort (n = 1114) after adjustment for age and BMI, t-18:1n−9 and t-16:1n−7 were correlated with margarine and manufactured foods but not dairy, meat, or vegetable oils; however, the results were not multivariable-adjusted for other demographic, lifestyle, or dietary factors or multiple potential food sources of TFAs simultaneously (47). In a European cohort (n = 3003), t-18:1n−9 was correlated with both margarine and dairy intake in crude (unadjusted) analyses; no multivariable-adjusted analyses were reported (48). Our results considerably extend this prior work by assessing 10 specific TFA isomer biomarkers and numerous potential food sources in a large community-based population and including adjustment for multiple demographics, lifestyles, and TFA food sources simultaneously.
Food composition studies (49–52) showed that t-18:1 isomers, ranging from n−4 to n−16, constitute the predominant TFA subclass in PHVOs and ruminant fats. In margarines, the main t-18:1 isomers are n−8 and n−9 (elaidic acid) (49). In ruminant fats, the main t-18:1 isomer is n−7 (vaccenic acid) (50), but this isomer is also present in PHVOs (49). In unadjusted analyses, consumption of some red meats was associated with t-18:1n−7 concentrations, but this association was no longer evident after multivariable adjustment for other foods. These results suggest a greater proportional consumption of t-18:1n−7 from PHVO-containing foods among these US adults. On this basis of our results, a better marker of ruminant TFA intake appears to be t-16:1n−7 that is present in lower concentrations but may be more specific to dairy and red meat than is t-18:1n–7 (52).
The major foods and food groups we assessed accounted for only a proportion of the variation in TFA concentrations. The absolute variation would be underestimated for each TFA isomer due to misclassification in dietary assessment and/or lack of assessment of food sources that were not available on the FFQ; we also had limited data on food-preparation methods. The explained variation was smallest for t-18:2 isomers, which suggests that other food sources, differences in the incorporation into different lipid fractions and tissues, and/or metabolic processes could be playing a role in the small variation explained by the dietary sources evaluated. Very little is known about TFA catabolism, potential interconversion among various isomers, or isomeric differences in incorporation into separate lipid fractions, and our findings support the need for such basic experimental research.
This investigation had several strengths. Rather than measuring the TFA content in foods and estimating exposure, we assessed the actual circulating biomarkers of TFA concentrations in 3330 free-living men and women to provide a better estimate of actual exposure to TFAs in the population. Ten different specific TFA isomers were measured, which allowed for the systematic assessment of multiple individual TFA isomers and their multiple dietary sources. We corrected for measurement error in the FFQs to provide more accurate central estimates and CIs for the observed associations. The cohort size and standardized assessment of a wide variety of participant characteristics allowed multivariable adjustment for several demographic, lifestyle, and dietary factors, including multiple potential dietary sources of TFAs, thereby minimizing residual confounding. The use of a well-described community-based multicenter cohort increased the generalizability.
There are potential limitations of the study. Although we corrected for general measurement error in the FFQs, there still could be residual dietary measurement error, and the fatty acid measurements and FFQs were not simultaneously administrated, which introduced dietary misclassification because of changes in diet over time. Thus, although our averaging of 2 FFQs reduced this temporal misclassification, the absolute correlations and variations explained are likely somewhat underestimated. In addition, the TFA content of different specific foods (eg, brands) within each food category was variable, which would further attenuate the observed associations. Therefore, to some extent our findings underestimate the magnitude of these associations, although the qualitative comparisons are still informative. Dietary questionnaires include some but not exhaustive details about specific brand and/or product names and types of vegetable oils consumed, and misclassification of specific subtypes of oils could explain the absence of significant associations with TFA concentrations. Questions on the consumption of red meat and processed meat included both ruminant (eg, beef) and nonruminant (eg, pork) sources that could attenuate associations; however, other questions (eg, on hamburgers and bacon) allowed more specific assessment of contributions from ruminant (compared with nonruminant) meats. We did not measure conjugated linoleic acid, but evidence on the importance of this fatty acid as a causal determinant of health is still relatively limited. The extent of metabolism of different TFA isomers is unknown; if substantial differences in metabolism between TFA isomers are present, this could influence our findings in unpredictable directions. These results preceded the 2006 mandated US food labeling of TFAs (53), and the extent to which potential food reformulations might alter current TFA food sources is unknown. These findings are from an older US population, and major dietary sources of TFAs may differ in younger populations or in other nations, particularly in developing countries in which a main source of TFAs may be inexpensive cooking fat (shortening) used to prepare meals in the home (17).
Overall, our findings show that individual plasma phospholipid TFA isomers have differing relations with each other and with specific food sources, thus highlighting the importance of a separate consideration of health effects, related underlying mechanisms, and policy implications of different TFA isomers. The predominant TFA subclass, t-18:1, was derived from multiple categories of PHVO-containing foods, which suggests that PHVOs are the main source of t-18:1 isomers in this cohort. In contrast, the trace fatty acids t-16:1n−9 and t-18:2 were specific to margarine and bakery foods, respectively, suggesting that different types of PHVOs used in different foods could have different contents of these TFAs. Our results indicate that t-18:1 isomers are derived from similar PHVO-containing food sources, although common dietary sources do not necessarily imply similar biological effects; that the t-16:1 isomers have distinct food sources and should be evaluated separately; and that t-16:1n−7 may be a more specific marker of ruminant TFA intake than t-18:1n−7. Our findings also identify specific gaps in our understanding of how consumption of different foods relates to different TFA isomers and subclasses. Further studies are needed to identify additional dietary sources of t-18:2, the contribution of different vegetable oils and different food brands to total and isomer-specific TFAs, and the potential influence of nondietary factors on circulating TFA concentrations. These findings are highly relevant to considering and investigating health effects of different TFA isomers and to subsequent targeted policy measures to reduce intake of specific clinically relevant TFA isomers.
Acknowledgments
We express our gratitude to the CHS participants. A full list of participating CHS investigators and institutions is available at http://www.chs-nhlbi.org.
The authors’ responsibilities were as follows—RM: study concept and design, statistical analysis, interpretation of data, drafting and critical revision of the manuscript for important intellectual content, and approval of the final manuscript for submission; IBK: interpretation of data, laboratory analysis, obtainment of funding, critical revision of the manuscript for important intellectual content, and approval of the final manuscript for submission; RNL, EBR, and FS: interpretation of data, obtainment of funding, critical revision of the manuscript for important intellectual content, and approval of the final manuscript for submission; DSS: acquisition of data, interpretation of data, obtainment of funding, critical revision of the manuscript for important intellectual content, and approval of the final manuscript for submission; XS: interpretation of data, laboratory analysis, critical revision of the manuscript for important intellectual content, approval of the final manuscript for submission; and DM: study concept and design, statistical advice, interpretation of data, obtainment of funding, drafting and critical revision of the manuscript for important intellectual content, and approval of the final manuscript for submission. DM received honoraria and travel expenses for speaking at scientific conferences and reviewing topics related to trans fat and cardiovascular disease from the World Health Organization, Nutrition Impact, and several universities and scientific organizations but has no ownership, patents, stocks, advisory board membership, or speaking board membership. RM, IBK, RNL, EBR, FS, DSS, and XS reported no conflicts of interest.
REFERENCES
- 1.Micha R, Mozaffarian D. Trans fatty acids: effects on cardiometabolic health and implications for policy. Prostaglandins Leukot Essent Fatty Acids 2008;79:147–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Clarke R. Quantitative effects on cardiovascular risk factors and coronary artery disease risk of replacing partially hydrogenated vegetable oils with other fats and oils. Eur J Clin Nutr 2009;63(suppl 2):S22–33 [DOI] [PubMed] [Google Scholar]
- 3.Mauger JF, Lichtenstein AH, Ausman LM, et al. Effect of different forms of dietary hydrogenated fats on LDL particle size. Am J Clin Nutr 2003;78:370–5 [DOI] [PubMed] [Google Scholar]
- 4.Baer DJ, Judd JT, Clevidence BA, Tracy RP. Dietary fatty acids affect plasma markers of inflammation in healthy men fed controlled diets: a randomized crossover study. Am J Clin Nutr 2004;79:969–73 [DOI] [PubMed] [Google Scholar]
- 5.Mozaffarian D, Pischon T, Hankinson SE, et al. Dietary intake of trans fatty acids and systemic inflammation in women. Am J Clin Nutr 2004;79:606–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez-Garcia E, Schulze MB, Meigs JB, et al. Consumption of trans fatty acids is related to plasma biomarkers of inflammation and endothelial dysfunction. J Nutr 2005;135:562–6 [DOI] [PubMed] [Google Scholar]
- 7.Koh-Banerjee P, Chu NF, Spiegelman D, et al. Prospective study of the association of changes in dietary intake, physical activity, alcohol consumption, and smoking with 9-y gain in waist circumference among 16 587 US men. Am J Clin Nutr 2003;78:719–27 [DOI] [PubMed] [Google Scholar]
- 8.Field AE, Willett WC, Lissner L, Colditz GA. Dietary fat and weight gain among women in the Nurses’ Health Study. Obesity (Silver Spring) 2007;15:967–76 [DOI] [PubMed] [Google Scholar]
- 9.Christiansen E, Schnider S, Palmvig B, Tauber-Lassen E, Pedersen O. Intake of a diet high in trans monounsaturated fatty acids or saturated fatty acids. Effects on postprandial insulinemia and glycemia in obese patients with NIDDM. Diabetes Care 1997;20:881–7 [DOI] [PubMed] [Google Scholar]
- 10.Vega-Lopez S, Ausman LM, Jalbert SM, Erkkila AT, Lichtenstein AH. Palm and partially hydrogenated soybean oils adversely alter lipoprotein profiles compared with soybean and canola oils in moderately hyperlipidemic subjects. Am J Clin Nutr 2006;84:54–62 [DOI] [PubMed] [Google Scholar]
- 11.Micha R, Mozaffarian D. Trans fatty acids: effects on metabolic syndrome, heart disease and diabetes. Nat Rev Endocrinol 2009;5:335–44 [DOI] [PubMed] [Google Scholar]
- 12.Lemaitre RN, King IB, Mozaffarian D, et al. Plasma phospholipid trans fatty acids, fatal ischemic heart disease, and sudden cardiac death in older adults: the cardiovascular health study. Circulation 2006;114:209–15 [DOI] [PubMed] [Google Scholar]
- 13.Sun Q, Ma J, Campos H, et al. A prospective study of trans fatty acids in erythrocytes and risk of coronary artery disease. Circulation 2007;115:1858–65 [DOI] [PubMed] [Google Scholar]
- 14.Mozaffarian D, Rimm EB, King IB, Lawler RL, McDonald GB, Levy WC. trans Fatty acids and systemic inflammation in heart failure. Am J Clin Nutr 2004;80:1521–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lemaitre RN, King IB, Raghunathan TE, et al. Cell membrane trans-fatty acids and the risk of primary cardiac arrest. Circulation 2002;105:697–701 [DOI] [PubMed] [Google Scholar]
- 16.Allison DB, Egan SK, Barraj LM, Caughman C, Infante M, Heimbach JT. Estimated intakes of trans fatty and other fatty acids in the US population. J Am Diet Assoc 1999;99:166–74 [DOI] [PubMed] [Google Scholar]
- 17.Mozaffarian D, Abdollahi M, Campos H, Houshiarrad A, Willett WC. Consumption of trans fats and estimated effects on coronary artery disease in Iran. Eur J Clin Nutr 2007;61:1004–10 [DOI] [PubMed] [Google Scholar]
- 18.Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Trans fatty acids and cardiovascular disease. N Engl J Med 2006;354:1601–13 [DOI] [PubMed] [Google Scholar]
- 19.Williams MA, King IB, Sorensen TK, et al. Risk of preeclampsia in relation to elaidic acid (trans fatty acid) in maternal erythrocytes. Gynecol Obstet Invest 1998;46:84–7 [DOI] [PubMed] [Google Scholar]
- 20.Mahomed K, Williams MA, King IB, Mudzamiri S, Woelk GB. Erythrocyte omega-3, omega-6 and trans fatty acids in relation to risk of preeclampsia among women delivering at Harare Maternity Hospital, Zimbabwe. Physiol Res 2007;56:37–50 [DOI] [PubMed] [Google Scholar]
- 21.Delmonte P, Rader JI. Evaluation of gas chromatographic methods for the determination of trans fat. Anal Bioanal Chem 2007;389:77–85 [DOI] [PubMed] [Google Scholar]
- 22.Mossoba MM, Moss J, Kramer JK. Trans fat labeling and levels in U.S. foods: assessment of gas chromatographic and infrared spectroscopic techniques for regulatory compliance. J AOAC Int 2009;92:1284–300 [PubMed] [Google Scholar]
- 23.Arab L. Biomarkers of fat and fatty acid intake. J Nutr 2003;133(suppl 3):925S–32S [DOI] [PubMed] [Google Scholar]
- 24.Arab L, Akbar J. Biomarkers and the measurement of fatty acids. Public Health Nutr 2002;5:865–71 [DOI] [PubMed] [Google Scholar]
- 25.Vidgren HM, Louheranta AM, Agren JJ, Schwab US, Uusitupa MI. Divergent incorporation of dietary trans fatty acids in different serum lipid fractions. Lipids 1998;33:955–62 [DOI] [PubMed] [Google Scholar]
- 26.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1991;1:263–76 [DOI] [PubMed] [Google Scholar]
- 27.Tell GS, Fried LP, Hermanson B, Manolio TA, Newman AB, Borhani NO. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol 1993;3:358–66 [DOI] [PubMed] [Google Scholar]
- 28.Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem 1995;41:264–70 [PubMed] [Google Scholar]
- 29.Stanford JL, King I, Kristal AR. Long-term storage of red blood cells and correlations between red cell and dietary fatty acids: results from a pilot study. Nutr Cancer 1991;16:183–8 [DOI] [PubMed] [Google Scholar]
- 30.Hodson L, Skeaff CM, Wallace AJ, Arribas GL. Stability of plasma and erythrocyte fatty acid composition during cold storage. Clin Chim Acta 2002;321:63–7 [DOI] [PubMed] [Google Scholar]
- 31.Zeleniuch-Jacquotte A, Chajes V, Van Kappel AL, Riboli E, Toniolo P. Reliability of fatty acid composition in human serum phospholipids. Eur J Clin Nutr 2000;54:367–72 [DOI] [PubMed] [Google Scholar]
- 32.Lemaitre RN, King IB, Mozaffarian D, Kuller LH, Tracy RP, Siscovick DS. n−3 Polyunsaturated fatty acids, fatal ischemic heart disease, and nonfatal myocardial infarction in older adults: the Cardiovascular Health Study. Am J Clin Nutr 2003;77:319–25 [DOI] [PubMed] [Google Scholar]
- 33.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 1957;226:497–509 [PubMed] [Google Scholar]
- 34.Lepage G, Roy CC. Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res 1986;27:114–20 [PubMed] [Google Scholar]
- 35.Ulberth F, Henninger M. Simplified method for the determination of trans monoenes in edible fats by TLC-GLC. JAOCS 1992;69:829–31 [Google Scholar]
- 36.Kumanyika S, Tell GS, Shemanski L, Polak J, Savage PJ. Eating patterns of community-dwelling older adults: the Cardiovascular Health Study. Ann Epidemiol 1994;4:404–15 [DOI] [PubMed] [Google Scholar]
- 37.Kumanyika SK, Tell GS, Shemanski L, Martel J, Chinchilli VM. Dietary assessment using a picture-sort approach. Am J Clin Nutr 1997;65:1123S–9S [DOI] [PubMed] [Google Scholar]
- 38.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–26, discussion 1127–36 [DOI] [PubMed] [Google Scholar]
- 39.Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc 1993;93:790–6 [DOI] [PubMed] [Google Scholar]
- 40.Kemény ZR, Hénon K, Kővári G, Zwobada KF. Deodorization of vegetable oils: Prediction of trans polyunsaturated fatty acid content. J AOAC 2001;78:973–9 [Google Scholar]
- 41.Willett WC. Nutritional epidemiology. 2nd ed.New York, NY: Oxford University Press, 1998 [Google Scholar]
- 42.Beaton GH, Milner J, Corey P, et al. Sources of variance in 24-hour dietary recall data: implications for nutrition study design and interpretation. Am J Clin Nutr 1979;32:2546–59 [DOI] [PubMed] [Google Scholar]
- 43.Salvini S, Hunter DJ, Sampson L, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol 1989;18:858–67 [DOI] [PubMed] [Google Scholar]
- 44.Mansour MP, Li D, Sinclair AJ. The occurrence of trans-18:1 isomers in plasma lipids classes in humans. Eur J Clin Nutr 2001;55:59–64 [DOI] [PubMed] [Google Scholar]
- 45.Shahin AM, McGuire MK, Anderson N, Williams J, McGuire MA. Effects of margarine and butter consumption on distribution of trans-18:1 fatty acid isomers and conjugated linoleic acid in major serum lipid classes in lactating women. Lipids 2006;41:141–7 [DOI] [PubMed] [Google Scholar]
- 46.Skeaff CM, Gowans S. Home use of margarine is an important determinant of plasma trans fatty acid status: a biomarker study. Br J Nutr 2006;96:377–83 [DOI] [PubMed] [Google Scholar]
- 47.Thiebaut AC, Rotival M, Gauthier E, et al. Correlation between serum phospholipid fatty acids and dietary intakes assessed a few years earlier. Nutr Cancer 2009;61:500–9 [DOI] [PubMed] [Google Scholar]
- 48.Saadatian-Elahi M, Slimani N, Chajes V, et al. Plasma phospholipid fatty acid profiles and their association with food intakes: results from a cross-sectional study within the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr 2009;89:331–46 [DOI] [PubMed] [Google Scholar]
- 49.Wolff RL, Combe NA, Destaillats F, et al. Follow-up of the delta4 to delta16 trans-18:1 isomer profile and content in French processed foods containing partially hydrogenated vegetable oils during the period 1995-1999. Analytical and nutritional implications. Lipids 2000;35:815–25 [DOI] [PubMed] [Google Scholar]
- 50.Mendis S, Cruz-Hernandez C, Ratnayake WM. Fatty acid profile of Canadian dairy products with special attention to the trans-octadecenoic acid and conjugated linoleic acid isomers. J AOAC Int 2008;91:811–9 [PubMed] [Google Scholar]
- 51.Aro A, Van Amelsvoort J, Becker W, et al. TransFatty acids in dietary fats and oils from 14 European countries: the TRANSFAIR study. J Food Compost Anal 1998;11:137–49 [Google Scholar]
- 52.Baylin A, Siles X, Donovan-Palmer A, Fernandez X, Campos H. Fatty acid composition of Costa Rican foods including trans fatty acid content. J Food Compost Anal 2007;20:182–92 [Google Scholar]
- 53.Food and Drug Administration Food labelling: trans fatty acids in nutrition labeling, nutrient content claims, and health claims (21 CFR Part 101). Fed Regist 2006;1–74 [PubMed] [Google Scholar]