Abstract
Background: Dietary recommendations for selenium differ between countries, mainly because of uncertainties over the definition of optimal selenium status.
Objective: The objective was to examine the dose-response relations for different forms of selenium.
Design: A randomized, double-blind, placebo-controlled dietary intervention was carried out in 119 healthy men and women aged 50–64 y living in the United Kingdom. Daily placebo or selenium-enriched yeast tablets containing 50, 100, or 200 μg Se (≈60% selenomethionine), selenium-enriched onion meals (≈66% γ-glutamyl-methylselenocysteine, providing the equivalent of 50 μg Se/d), or unenriched onion meals were consumed for 12 wk. Changes in platelet glutathione peroxidase activity and in plasma selenium and selenoprotein P concentrations were measured.
Results: The mean baseline plasma selenium concentration for all subjects was 95.7 ± 11.5 ng/mL, which increased significantly by 10 wk to steady state concentrations of 118.3 ± 13.1, 152.0 ± 24.3, and 177.4 ± 26.3 ng/mL in those who consumed 50, 100, or 200 μg Se-yeast/d, respectively. Platelet glutathione peroxidase activity did not change significantly in response to either dose or form of selenium. Selenoprotein P increased significantly in all selenium intervention groups from an overall baseline mean of 4.99 ± 0.80 μg/mL to 6.17 ± 0.85, 6.73 ± 1.01, 6.59 ± 0.64, and 5.72 ± 0.75 μg/mL in those who consumed 50, 100, or 200 μg Se-yeast/d and 50 μg Se-enriched onions/d, respectively.
Conclusions: Plasma selenoprotein P is a useful biomarker of status in populations with relatively low selenium intakes because it responds to different dietary forms of selenium. To optimize the plasma selenoprotein P concentration in this study, 50 μg Se/d was required in addition to the habitual intake of ≈55 μg/d. In the context of established relations between plasma selenium and risk of cancer and mortality, and recognizing the important functions of selenoprotein P, these results provide important evidence for deriving estimated average requirements for selenium in adults. This trial was registered at clinicaltrials.gov as NCT00279812.
INTRODUCTION
Intakes of selenium in the United Kingdom fell from a mean of 60 μg/d in 1991 to a minimum of 30 to 40 μg/d in 1995–2000; currently, the mean intake is 48–58 μg/d (1–3). This has resulted in a reduction in the mean plasma selenium concentration (4). Low serum or plasma selenium concentrations, as found in some populations in the United Kingdom and in other countries in Europe and worldwide, are associated with a significantly increased risk of mortality (5, 6). In addition, epidemiologic studies, animal tumor models, and prospective cohort studies show an inverse relation between selenium status and cancer incidence over a range from deficient to replete (7). Although recent estimated intakes of selenium in the United Kingdom have increased, presumably reflecting an influx of selenium into the food chain through animal supplements (8), inclusion of selenium in fertilizers (9), and consumption of selenium supplements and imported foods with high selenium contents, they are still below the UK recommended nutrient intake of 75 and 60 μg/d for men and women, respectively (1), and the US Recommended Dietary Allowance of 55 μg/d for adults (10). They are also below the intake associated with health benefits, including a reduction in risk of some cancers, improved fertility, and reduced mortality risk (5, 6, 11).
Dietary recommendations for selenium are currently based on intakes that maximize the activity of glutathione peroxidase in plasma (10), but there is debate about the appropriateness of this endpoint, and insufficient information on selenium biomarkers was the reason for the inability to derive estimated average requirements in the United Kingdom (1). In addition, dietary reference values are now focused on optimal health, not just on the prevention of deficiency disorders, and this requires a greater understanding of the reported effects of beneficial/optimal intakes of selenium, the relative merits of different forms of selenium, and the effect of marginal selenium status on health. The anticancer effects of selenium are dose-responsive and form-specific (7), and the levels of selenium intakes and status that correlate with a reduction in cancer risk (7) are greater than those required to optimize plasma glutathione peroxidase (12), which reaches a plateau at a plasma selenium concentration of >70 ng/mL (12). Selenoprotein P, a plasma selenium transport protein with antioxidant activity, has been identified as a functional biomarker of selenium status (13, 14) in populations in Europe (15), China (16), and New Zealand (17), and elevated plasma selenoprotein P concentrations have been associated with lower overall risk of cancers (18). However, the relation between intake of selenium and plasma concentration required for optimal concentrations of selenoprotein P are currently unknown. In a selenium-replete US cohort with an average plasma selenium concentration >125 ng/mL, no further increase of selenoprotein P was detected after selenium supplementation with 200 or 600 μg/d (19). At the other end of the spectrum, in a selenium-deficient population in China, the relation between plasma selenium and selenoprotein P was linear between plasma selenium concentrations of 20 and 90 ng/mL (20). In other studies, plasma selenium concentrations between 95 and 135 ng/mL showed a weak correlation with selenoprotein P, which indicated that this was the range at which selenoprotein P concentrations reached a plateau, but it was not possible to pinpoint the intake of selenium and the plasma selenium concentration that were associated with maximum expression of selenoprotein P. The amount and form of selenium required to increase selenium status to “optimal” concentrations are currently unknown for selenium-deficient populations in the United Kingdom and Europe. We therefore designed a human dietary intervention to determine the effects of consuming selenium-enriched food containing dietary relevant forms of selenium with putative anticancer activity, γ-glutamyl methylselenocysteine (21), and high selenium-yeast supplements on biomarkers of selenium status to determine the optimal range of selenium intake and status.
SUBJECTS AND METHODS
A randomized, double-blind, placebo-controlled trial was undertaken by using a parallel design in adults (n = 119) with a suboptimal selenium status, defined as a low (<110 ng/mL) plasma selenium concentration. Apparently healthy volunteers aged 50–64 y from Norfolk, United Kingdom, were randomly assigned to 1 of 6 groups. They were given selenium-enriched yeast supplements (50, 100, or 200 μg Se/d, containing 60% selenomethionine), consumed 3 meals/wk containing selenium-enriched onions (to provide an additional intake equivalent to 50 μg Se/d), consumed meals containing unenriched onions (to provide an additional intake of <4 μg Se/d), or underwent no dietary intervention (control placebo group) for a total period of 12 wk. Blood samples were collected at weeks 0, 6, and 10 for selenium-status biomarker quantification. Immediately after the blood sample was collected at week 10, as an additional part to the study, an influenza vaccine was administered to the subjects to study immune response (results to be reported separately). When possible, the biomarker data up to week 10 was used to examine the response to different doses and forms of selenium to avoid any potential confounding issues relating to the vaccine. Outcome measures included changes in plasma selenium and selenoprotein P concentrations and in platelet glutathione peroxidase activity after 6 and 10 wk of supplementation. For the 200 μg Se-yeast/d group, to verify that the plasma selenium concentrations had reached steady state, plasma selenium concentrations after supplementation at weeks 11 and 12 were measured. The weeks 11 and 12 data were not used in the joinpoint regression, and only selenium status biomarker data up to and including week 10 data were analyzed and are presented in the figures. All selenium-intervention groups had reached steady state selenium concentrations by 10 wk, and all data presented are from samples collected before the influenza vaccine was administered.
Volunteers were excluded if they were smokers, had a body mass index (BMI; in kg/m2) <18.5 or >35.0, had a plasma selenium concentration >110 ng/mL (ie, persons with relatively high habitual intakes of selenium who would not therefore be expected to respond to selenium supplementation), had a long-term illness requiring active treatment (eg, diabetes, cancer, cardiovascular disease, or gastrointestinal disease, excluding hiatus hernia unless symptomatic or study intervention/procedure was contraindicated), regularly took a prescribed medication, regularly used antacids and laxatives (at least once per week), regularly took medication for hay fever, had undergone a hysterectomy, were unwilling to discontinue dietary (other than vitamins and minerals) or herbal supplements >1 mo before the study started and for the duration of the study, had consumed selenium supplements more than once per week and were unwilling to discontinue occasional use for the duration of study, had donated blood within 16 wk of the first study sample and intended to donate blood <16 wk after the last study sample, had taken antibiotics within 4 wk before the study started, were participating in another study, or had asthma, requiring treatment within the past 2 y. Also, as an add-on to the study relating to administration of the influenza vaccine, the following exclusion criteria were used: anaphylactic hypersensitivity to egg products or a history of Guillain-Barre syndrome.
The selenium-yeast supplements and placebo tablets were supplied by Pharma Nord (Vejle, Denmark) as a single-batch delivery to ensure that the supplements used over the duration of the study were consistent and that the composition of the supplement doses was comparable except for the selenium content; the selenium-yeast supplements were provided with a certified selenomethionine content of 60% (22). Supplements were supplied coded as either A, B, C, or D according to strict quality control procedures so that the trial remained double-blind, and the scientists and subjects did not know which intervention they were receiving. Randomization data were kept strictly confidential in sealed envelopes and were not opened until the end of the study and after all of the data had been analyzed. The selenium-enriched and -unenriched onions were also supplied, coded as E and F. The United Kingdom's most popular variety of main crop onions (Allium cepa L., cv. Renate; a Rijnsburger type) were grown in a Cambridge-type glasshouse compartment at the University of Nottingham, Sutton Bonington, United Kingdom. To deliver a target selenium concentration of 70 to 90 μg/100 g (fresh weight) onion, an application rate equivalent to 300 g Se (sodium selenate solution) per hectare was applied. Each batch of onions was provided “blind” by the University of Nottingham and analyzed by inductively coupled mass spectrometry (ICP/MS) to determine the selenium content, and the correct weight of onion added to each meal to achieve 117 μg Se/meal (3 meals/wk were consumed to provide an average of 50 μg Se/d). Meals (6 variants) were prepared and batch-cooked, in accordance with Regulation (EC) 852/2004 on the hygiene of foodstuffs and ISO 9001. Portions were stored at −18°C until they were required for consumption, when they were defrosted and heated in an oven at a specified temperature and time for each type of meal.
Blood samples were taken from the subjects after an overnight fast at 0 (baseline), 6, and 10 wk. Samples for ICP/MS analysis were collected in trace element–free tubes (Becton Dickinson UK Limited, Oxford, United Kingdom), and samples for selenoprotein P analysis were collected in sterile tubes containing EDTA (Becton Dickinson UK Limited). The blood samples were processed within 30 min and centrifuged at 1500 × g for 10 min, and the plasma was carefully removed and stored at −80°C until required. Plasma selenium was measured by using an Agilent 7500ce ICP/MS (Agilent Technologies UK Ltd, Stockport, Cheshire, United Kingdom) fitted with an Octopole Reaction System operated in hydrogen mode (for removal of interferences from Ar-Ar dimers). Platelets were isolated by centrifugation from whole blood collected in sterile tubes containing citrate (Sarstedt Ltd, Leicester, United Kingdom) (23). Selenium-dependent platelet glutathione peroxidase activity was quantified as described previously (24), with tert-butyl hydroperoxide as the substrate (25). One unit (U) of glutathione peroxidase activity is defined as 1 μmol NADPH oxidized/min. Total protein concentrations were determined by using the method of Bradford (26). Selenoprotein P was quantified in plasma samples by using a sensitive and specific enzyme-linked immunoassay (20). The plate reference plasma values were 6.6 ± 0.7 and 6.3 ± 0.2 μg/mL. All volunteers provided written informed consent, and the study was approved by the Institute of Food Research Governance Committee, the East Norfolk and Waveney Research Governance Committee, and the Norwich Local Ethics Committee.
Calculation of sample size was determined with a significance level of 5% and power of 80% to detect a difference of 1 SD in plasma selenium concentration with the use of a population SD of 11.7 ng/mL derived from a UK cohort (27). A sample size of 144 (24 per group) would allow a statistically significant difference in plasma selenium to be determined between the groups, allowing for an expected dropout rate of 25%. Data analysis was completed by using SPSS version 16.0 and R data analysis software (28). The Joinpoint Regression Program (version 3.4.2) was downloaded from http://srab.cancer.gov/joinpoint/ and used as described (29). The program required that the data for the independent variable (plasma selenium concentration) be in discrete segments, and this required it to be “binned” in sections of ≈10 ng/mL. The (X,Y) data for the program was entered as the mean of each bin (X = plasma selenium concentration, Y = selenoprotein P concentration). Standard linear models were used to investigate the relation between age, sex, and BMI to baseline measures of interest. Mixed-effects models were used in R to analyze the effects of treatment and time on plasma selenium concentration, selenoprotein P concentration, and platelet glutathione peroxidase activity. Subject was entered in the mixed-effect models as a random factor to take account of the repeated sampling of the volunteers over time. Tukey's honestly significant difference test was used to investigate difference between groups or between time points. The results were considered significant if P < 0.05.
RESULTS
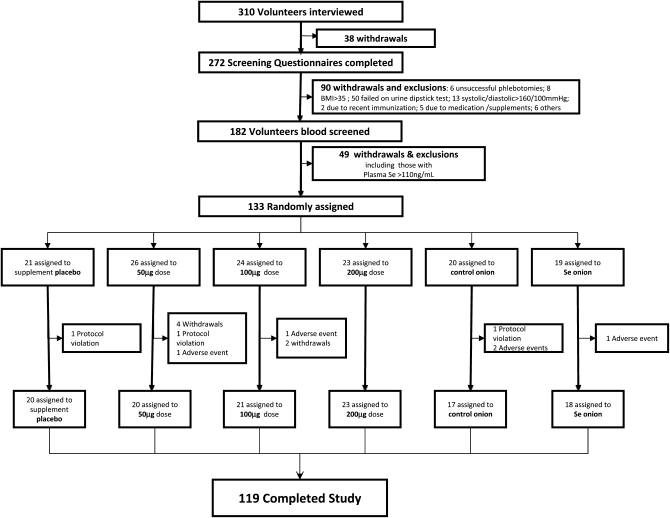
Recruitment ran from May until the following February in 2005, 2006, and 2007. The study was completed in June 2008. A total of 272 volunteers completed a screening questionnaire, after which 90 volunteers were excluded (Figure 1). A total of 182 volunteers had blood samples screened for red and white blood cell count and selenium status. This resulted in a total of 133 subjects being randomly assigned into each of 6 groups. Of these, 3 subjects were excluded for protocol violations, 5 were excluded because of adverse events (colds and influenza symptoms for >3 wk), and 6 withdrew from the study for personal reasons. Thus, a total of 119 subjects (89.5%) completed the study: 54 men (45.4%) and 65 women (54.6%). Data from a total of 117 subjects were used for further analyses because of 1) incomplete time-course samples for one volunteer and 2) a baseline plasma selenium concentration of >120 ng/mL for one subjects, which did not comply with the exclusion criteria. All 6 groups (n = 117) had similar demographic and baseline characteristics (Table 1).
FIGURE 1.
Trial profile. Numbers of volunteers who were screened and took part in the intervention trial.
TABLE 1.
Characteristics of the subjects at baseline1
| Supplement groups |
Food groups |
|||||
| Placebo (n = 20) | 50 μg Se/d (n = 18)2 | 100 μg Se/d (n = 21) | 200 μg Se/d (n = 23) | Unenriched onions (n = 17) | 50 μg Se-enriched onions/d (n = 18) | |
| Age (y) | 55.8 ± 3.9 (50–62)3 | 56.5 ± 4.6 (50–64) | 58.4 ± 3.9 (52–64) | 56.1 ± 3.9 (50–64) | 58.2 ± 5.1 (50–64) | 57.7 ± 4.2 (52–65) |
| Sex (men/women) | 10/10 | 9/9 | 11/10 | 11/12 | 6/11 | 6/12 |
| BMI (kg/m2) | 25.0 ± 2.7 (22.0–33.0) | 26.1 ± 3.0 (19.5–33.0) | 26.3 ± 3.9 (20.0–33.5) | 25.9 ± 3.8 (20.5–34.5) | 26.6 ± 4.1 (20.0–21.5) | 26.1 ± 2.4 (21.5–30.5) |
There were no significant differences between the groups at baseline.
n = 18 because of incomplete time-course samples for one subject and because of the exclusion of one subject from the analyses because the baseline data at week 0 did not meet the exclusion criteria (plasma selenium: >120 ng/mL).
Mean ± SD; range in parentheses (all such values).
The mean selenium content of the unenriched onion meals was 9.7 μg and that of the selenium-enriched onion meals was 119.2 μg, which provided an equivalent daily intake of ≈4 μg/d for the unenriched onion meals and 50 μg/d for the selenium-enriched meals. The mean (± SD) plasma selenium concentration of all 182 volunteers screened for inclusion in the study was 98.6 ± 20.1 ng/mL, and concentrations ranged from 61.5 to 212.7 ng/mL. Only 2% (n = 4) of the subjects screened had a plasma selenium concentration >150 ng/mL, and 16% (n = 30) had a plasma selenium concentration of >110 ng/mL. More than one-third of the subjects (40%, n = 8) with a plasma selenium concentration >120 ng/mL reported regular use of supplements or multivitamins containing selenium. The mean plasma selenium concentration of subjects at baseline (week 0) was 95.7 ± 11.5 ng/mL (n = 117). Plasma selenium was not significantly related to age, sex, or BMI. There were statistically significant increases (P < 0.005) in plasma selenium concentrations after daily supplementation with 50, 100, and 200 μg Se/d as selenium-enriched yeast (Table 2), whereas no increase in plasma selenium was observed after the placebo. The increase in plasma selenium concentration reached steady state by 6 wk in subjects who received the selenium-yeast supplements (50 and 100 μg Se/d) and by 10 wk in subjects who received supplements containing 200 μg Se/d. The steady state plateau at week 10 was determined for the 200 μg/d group by additionally analyzing plasma concentrations from the 200-μg/d group at week 11 (173.2 ± 31.1 ng/mL; n = 23) and week 12 (180.8 ± 26.8 ng/mL; n = 23), which were not statistically significant from the week 10 data (177.4 ± 26.3 ng/mL; n = 23). The plasma selenium concentration in the group that consumed the selenium-enriched onion meals increased slightly over the duration of the intervention compared with the group that consumed the unenriched onion meals. There was a small significant increase in plasma selenium in the selenium-enriched onion group at weeks 6 and 10 compared with baseline; however, the increase in plasma selenium was not statistically significant compared with the control unenriched onion group at weeks 6 and 10. A significant time effect (P < 0.001), treatment effect (P < 0.001), and time × treatment interaction (P < 0.001) was observed for plasma selenium concentration.
TABLE 2.
Plasma selenium concentrations, platelet glutathione peroxidase activity, and plasma selenoprotein P concentrations: mean change from baseline and comparison of selenium-yeast supplements with selenium-enriched food dietary intervention in the placebo and unenriched onion groups, respectively1
| Supplement groups |
Food groups |
|||||
| Placebo (n = 20) | 50 μg Se/d (n = 18) | 100 μg Se/d (n = 21) | 200 μg Se/d (n = 23) | Unenriched onions (n = 17) | 50 μg Se-enriched onions/d (n = 18) | |
| Plasma selenium (ng/mL) | ||||||
| 0 wk | 92.0 ± 11.9 (72.7–116.8) | 92.2 ± 13.3 (63.0–116.3) | 98.6 ± 10.5 (83.2–117.5) | 99.1 ± 9.3 (75.3–113.3) | 93.3 ± 11.5 (65.8–118.0) | 97.6 ± 11.5 (81.5–115.6) |
| 6 wk | 95.6 ± 12.2 (78.2–120.3) | 116.3 ± 12.923 (87.1–145.6) | 146.4 ± 26.434 (99.8–193.9) | 160.9 ± 19.434 (116.4–188.8) | 95.4 ± 13.2 (61.3–120.9) | 106.7 ± 9.23 (92.8–119.3) |
| 10 wk | 93.7 ± 16.5 (69.8–126.8) | 118.3 ± 13.134 (82.6–141.7) | 152.0 ± 24.334 (108.3–189.5) | 177.4 ± 26.33–5 (109.0–221.1) | 94.2 ± 15.0 (61.9–115.1) | 106.0 ± 11.93 (85.3–135.1) |
| Platelet glutathione peroxidase activity (U/mg protein) | ||||||
| 0 wk | 0.25 ± 0.08 (0.11–0.53) | 0.29 ± 0.14 (0.17–0.72) | 0.27 ± 0.10 (0.14–0.52) | 0.25 ± 0.10 (0.08–0.50) | 0.25 ± 0.06 (0.14–0.33) | 0.28 ± 0.07 (0.16–0.45) |
| 6 wk | 0.27 ± 0.08 (0.12–0.44) | 0.34 ± 0.13 (0.12–0.57) | 0.32 ± 0.12 (0.14–0.59) | 0.32 ± 0.08 (0.21–0.52) | 0.25 ± 0.08 (0.15–0.40) | 0.29 ± 0.11 (0.14–0.62) |
| 10 wk | 0.26 ± 0.07 (0.15–0.40) | 0.32 ± 0.10 (0.19–0.49) | 0.33 ± 0.14 (0.14–0.68) | 0.31 ± 0.10 (0.12–0.52) | 0.27 ± 0.06 (0.19–0.41) | 0.29 ± 0.10 (0.14–0.57) |
| Plasma selenoprotein P (μg/mL) | ||||||
| 0 wk | 4.82 ± 0.69 (3.73–6.07) | 4.98 ± 0.90 (3.28–6.54) | 4.90 ± 0.90 (3.43–7.05) | 5.29 ± 0.82 (3.84–7.01) | 4.85 ± 0.66 (3.65–6.28) | 5.01 ± 0.81 (3.47–6.35) |
| 6 wk | 4.71 ± 0.98 (3.30–6.91) | 6.25 ± 0.56334 (5.39–7.02) | 6.47 ± 1.1534 (4.27–8.53) | 6.58 ± 0.7734 (5.40–8.29) | 4.56 ± 0.89 (2.86–5.86) | 5.40 ± 0.576 (4.12–6.55) |
| 10 wk | 4.96 ± 0.86 (3.47–7.04) | 6.17 ± 0.8534 (4.45–7.90) | 6.73 ± 1.0134 (4.60–8.17) | 6.59 ± 0.6434 (5.61–7.68) | 4.80 ± 1.03 (2.77–6.16) | 5.72 ± 0.75357 (4.42–6.75) |
All values are means ± SDs; ranges in parentheses. P values were calculated by using Tukey's honestly significant difference test after analysis with a single mixed-effects model. Plasma selenium: time effect (P < 0.001), treatment effect (P < 0.001), and time × treatment interaction (P < 0.001). Platelet glutathione peroxidase: time effect (P < 0.001) and time × treatment interaction (NS). Selenoprotein P: time effect (P < 0.001), treatment effect (P < 0.001), and time × treatment interaction (P < 0.001).
Significantly different from placebo: 2P = 0.005, 4P < 0.001.
Significantly different from 0 wk, P < 0.01.
Significantly different from 6 wk, P < 0.05.
Significantly different from unenriched onions: 6P = 0.047, 7P = 0.027.
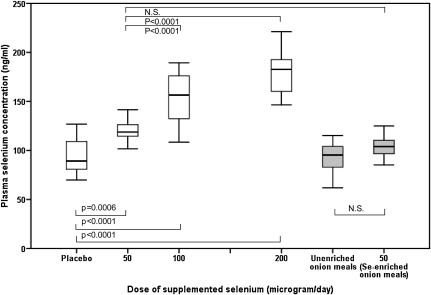
There was a dose-response increase in the plasma selenium concentration over the range of intakes investigated in the study, up to an additional intake of 200 μg Se-enriched yeast/d (Figure 2). The data show that an additional intake of 50 μg Se-yeast/d for 10 wk increases the plasma selenium concentration to ≈120 ng/mL. In a comparison of the response to the different forms of selenium, unlike the significant increase in plasma selenium at 10 wk with Se-yeast (50 μg/d), there was no similar significant increase in plasma selenium in the group that consumed 50 μg Se-enriched onion meals/d compared with the control unenriched group (Table 2). In a comparison of the two 50-μg/d groups and the change from baseline at week 10, plasma selenium increased by 28.3% in the 50-μg Se-enriched yeast/d group but increased less, by only 8.6%, in the 50 μg Se-enriched onions/d group.
FIGURE 2.
Dose response of plasma selenium concentrations over a range of selenium-enriched yeast supplement doses and comparison with the food intervention groups at week 10, when selenium status had reached steady state. The box plots show the median and interquartile range for the placebo (n = 20), 50 μg Se-yeast/d (n = 18), 100 μg Se-yeast/d (n = 20), 200 μg Se-yeast/d (n = 23), 0 μg Se/d unenriched onion meal (n = 16), and 50 μg Se-enriched onion meal/d (n = 17) groups. The significant changes over the dose range are shown, including the comparison between the two 50-μg/d groups (selenium-yeast compared with selenium-enriched onions). Data were analyzed by using a single mixed-effects model, and Tukey's honestly significant difference test was used to assess pairwise differences between groups. There was a significant effect of time (P < 0.001), treatment (P < 0.001), and time × treatment interaction (P < 0.001) on plasma selenium concentrations. The box plots displaying the food intervention group data are shaded gray to distinguish them from the supplement and food groups.
Glutathione peroxidase activity in platelet samples did not change significantly in the selenium-supplemented groups compared with the control groups at any of the doses (50–200 μg/d) investigated (Table 2), although a small significant increase over time (weeks 6 and 10 compared with week 0 for the 100- and 200-μg Se-enriched yeast/d groups) was observed. There was a significant time effect (P < 0.001) but the effect of treatment and the interaction of time and treatment were not significant for platelet glutathione peroxidase activity (P = 0.16 and P = 0.35, respectively).
There were statistically significant increases in plasma selenoprotein P concentrations at week 10 in the subjects who consumed 50 μg Se/d as selenium yeast (P < 0.001) and selenium-enriched onion meals (P = 0.019) and for the higher doses (100 and 200 μg/d) of selenium (P < 0.001) compared with the control groups (Table 2). As expected, there were no significant changes in selenoprotein P concentrations in the control groups (placebo supplement and unenriched onion meals) over time. There was an increase in plasma selenoprotein P concentrations in all 4 selenium intervention groups between weeks 0 and 6 and then the response reached a plateau (optimal expression of selenoprotein P) for the 3 selenium-yeast supplement groups (50, 100, and 200 μg/d) (Table 2). For the 50-μg/d group that consumed the selenium-enriched onion meals, the increase in selenoprotein P concentration was less than that of the 50-μg Se-yeast/d group at week 6 and was not statistically significant; however, by week 10 of the intervention, the mean concentration of selenoprotein P was comparable for both the 50-μg Se/d (6.17 ± 0.85 μg/mL) and the 50-μg Se-enriched onions/d (5.72 ± 0.75 μg/mL) groups. At week 10, the selenoprotein P concentration in the selenium-enriched onion group was significantly greater (P = 0.027) than that in the unenriched onion group. There was a significant effect of time (P < 0.001), treatment (P < 0.001), and time × treatment interaction (P < 0.001) observed for plasma selenoprotein P concentrations. The plasma selenoprotein P concentration was not significantly related to age, sex, or BMI.
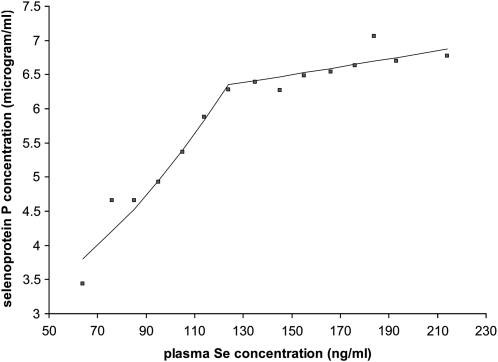
Joinpoint regression was performed to investigate the trend in the relation between plasma selenium and selenoprotein P. The data for all volunteers at time points 0, 6, and 10 wk were included in the analysis. The software fitted the simplest joinpoint model that the data allow. The program started with the minimum number of joinpoints (eg, 0 joinpoints, which is a straight line through the entire data set) and tested whether more joinpoints are statistically significant and must be added to the model to divide the data into separate linear segments. The program estimated that the data needed one joinpoint (ie, the data were divided into 2 separate linear segments) at a plasma selenium concentration of 124 ng/mL and a selenoprotein P concentration of 6.35 μg/mL (Figure 3). The first linear segment was constructed between plasma selenium concentration values of 64 and 124 ng/mL, and the second linear segment was constructed between plasma selenium concentration values of 124 and 214 ng/mL. The second segment of the data, from the mean plasma selenium concentration of 124 ng/mL, represents a plateau in the selenoprotein P concentration.
FIGURE 3.
Relation between plasma selenium and selenoprotein P concentrations from a joinpoint regression analysis (29) of data from weeks 0, 6, and 10 (when data were available for both biomarkers, n = 340). The mean values for each 10-ng/mL increase in plasma selenium concentration are shown for both biomarkers, and the 2 lines represent a joinpoint regression fit to the data. A single joinpoint gave the best fit to the data, with a linear slope from >50 ng/mL up to a mean plasma selenium concentration of 124 ng/mL (P < 0.001 for the test of the null hypothesis that the first slope was equal to zero), followed by a plateau, with the line >124 ng/mL displaying no significant change (P = 0.24 for the test of the null hypothesis that the second slope was equal to zero).
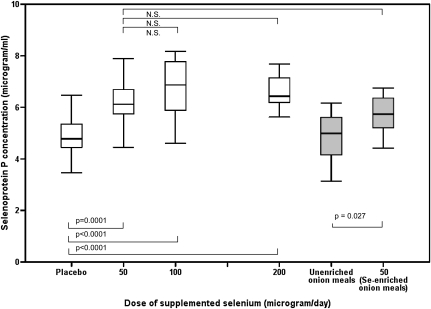
The dose-response relation between selenium supplement intake and selenoprotein P concentration (Figure 4) shows that the selenoprotein P concentration reached a plateau with a daily supplement of 50 μg Se from either the selenium-enriched onion meals or the selenium-yeast supplement. No further significant increase was observed with either 100 or 200 μg Se/d as selenium yeast compared with the 50 μg Se-yeast/d group (Figure 4). The results of this study show that an additional daily intake of 50 μg Se for 10 wk as selenium-enriched yeast or selenium-enriched onion meals increased plasma selenoprotein P concentration to the same degree as did higher doses (100 or 200 μg Se/d as yeast).
FIGURE 4.
Dose response of plasma selenoprotein P concentrations over a range of selenium-enriched yeast supplement doses and comparison with the food intervention groups. Data shown are from week 10 data when selenium status had reached steady state. The box plots show the median and interquartile range for the placebo (n = 20), 50 μg Se-yeast/d (n = 18), 100 μg Se-yeast/d (n = 21), 200 μg Se-yeast/d (n = 23), 0 μg Se/d unenriched onion meal (n = 17), and 50 μg Se-enriched onion meal/d (n = 18) groups. The significant changes over the dose range are shown, including the comparison between the two 50-μg/d groups (selenium-yeast compared with selenium-enriched onions). Data were analyzed by using a single mixed-effects model, and Tukey's honestly significant difference test was used to assess pairwise differences between groups. There was a significant effect of time (P < 0.001), treatment (P < 0.001), and time × treatment interaction (P < 0.001) on plasma selenoprotein P concentrations. The box plots displaying the food intervention group data are shaded gray to distinguish them from the supplement and food groups.
DISCUSSION
Globally, plasma selenium concentrations vary considerably (30) from an extremely low concentration (22 ng/mL) in a selenium-deficient area in China (20), to a mean of 122 ng/mL in the United States (19, 30), and to >1000 ng/mL in Inuit from Greenland (11). In our subjects, the mean baseline value (98.6 ng/mL for all subjects screened for inclusion into the study, n = 182) was comparable with data reported from other cohorts in the United Kingdom (23, 31, 32) and Europe (33), where mean values range from 76.0 (33) to 91.3 ng/mL (31). Plasma selenium significantly increased in response to selenium-enriched yeast supplements compared with placebo, but did not significantly change in the group given the equivalent of a daily intake of 50 μg Se from selenium-enriched onion meals compared with the control unenriched onion group. This was likely due to differences in the metabolism of the various forms of selenium in the meals compared with the yeast supplement. The predominant extractable form of selenium in uncooked onions was γ-glutamyl selenium-methylselenocysteine (66%); other identified species included selenomethionine (8.6%), selenocysteine (1.2%), and selenite/selenate (6%) (D Hart, unpublished observations, 2009), which is similar to the species profile reported by Kotrebai et al (34), whereas the main species in the Se-yeast supplement was selenomethionine (60% of total selenium) (22). Selenomethionine, unlike other forms of selenium (19, 30) can be nonspecifically incorporated into plasma proteins (35) and can result in an increase in plasma selenium concentration over a range of intakes (19, 20). Total plasma selenium reflects selenium in the form of selenocysteine, as in the 2 plasma selenoproteins (plasma glutathione peroxidase and selenoprotein P), and also as selenomethionine containing proteins, mainly albumin (35). Because dietary selenomethionine can increase plasma selenium through nonspecific incorporation into plasma proteins, including albumin, but other dietary forms of selenium, including selenocysteine, are metabolized by specific selenium metabolism processes and are not known to be nonspecifically incorporated into plasma proteins (35), this may explain the different response between the 2 groups given 50 μg Se/d from onions or yeast. The effect of selenium-enriched onions on biomarkers of selenium status has not been previously reported.
The 2 main selenoproteins in plasma, selenoprotein P and plasma (extracellular) glutathione peroxidase 3 (GPx3), can vary in concentration depending on selenium status: plasma glutathione peroxidase generally accounts for 10% to 30% of the selenium in plasma (35, 36). Because plasma GPx3 activity plateaus at a plasma selenium concentration of 70 to 90 ng/mL (12, 37) and is not a reliable sensitive marker for status in other cohorts with similar selenium intake (12, 23), we did not quantify plasma GPx3 activity in this study. Instead, we quantified platelet glutathione peroxidase activity 1 (GPx1), because platelet GPx1 is reported to be a more sensitive marker of selenium status (14, 23, 38–40) and can plateau at higher levels of activity, 80–120 ng/mL plasma selenium (12, 38), depending on the form of selenium (38, 41). However, the mean plasma selenium concentration at baseline for the volunteers was 95.7 ± 11.5 ng/mL, and, as predicted, we did not detect a significant change in platelet GPx1 activity. Platelet GPx1 activity was not a sensitive biomarker for selenium status within the range of this intervention study.
Selenoprotein P is the most abundant selenoprotein in plasma, accounting for ≈25–50% of plasma selenium, depending on selenium status and the forms of selenium consumed (35, 36, 42). In this cohort, plasma selenoprotein P concentrations reached a plateau before 10 wk of supplementation in individuals given 50 μg Se-enriched yeast/d. At week 10, the mean concentrations were 6.17 μg/mL for selenoprotein P and 118.3 ng/mL for plasma selenium in the group given 50 μg Se-enriched yeast/d. The 50-μg Se-enriched onion meals/d group had increased plasma selenoprotein P concentrations at week 10, similar to the group that received 50 μg Se-enriched yeast/d. Higher intakes (up to 200 μg Se-enriched yeast/d) did not significantly increase selenoprotein P concentration further. In other selenium-supplementation studies, selenoprotein P has been shown to reach a steady state after 2 to 4 wk (15), and a linear relation with plasma selenium has been reported up to values of 110 to 118 ng/mL (15, 16). However, selenium supplementation of a US cohort with high baseline plasma selenium concentrations (mean: 125 ng/mL) resulted in no change in selenoprotein P concentration (19). This indicates that the plasma selenium concentration associated with maximal selenoprotein P lies between 110 and 125 ng/mL. In agreement with other studies (16, 17), we showed that selenoprotein P is a sensitive biomarker for selenium status at low-to-moderate selenium intakes and reflects the intake of selenium present in selenium-enriched food containing various selenium species, unlike plasma selenium, which appears to reflect mainly the selenomethionine content of the food (19). However, in populations with relatively high selenium intakes, selenoprotein P is a less useful as a biomarker (19). Currently, there is debate regarding the recommended intake of selenium required to optimize selenoprotein P, and the data presented in this report provide evidence that the maximum concentration of selenoprotein P in blood in this UK cohort was achieved with an additional daily intake of 50 μg Se. Joinpoint regression analysis of the relation between plasma selenium and selenoprotein P showed a mean value of 124 ng/mL for the plasma selenium concentration at which selenoprotein P plateaus. This value is within the range of plasma selenium concentrations associated with a decreased risk of mortality (5, 6) and also concurs with the level that is purported to offer potential protection against cancer (5–7, 43–45): several large studies have shown a significant decrease in mortality risk at serum selenium concentrations of ≈135 ng/mL (5, 6).
On the basis of the selenoprotein P data, supplementation with either 50 or 100 μg Se/d as yeast or 50 μg Se-enriched onions/d resulted in improvements in selenium status in a UK cohort with suboptimal selenium status. Only 9% (n = 17) of all volunteers screened for inclusion (n = 182) had a plasma selenium concentration in the putative beneficial range (120–150 ng/mL), and many of these participants (n = 10) regularly took supplements containing selenium. There is a narrow range between deficiency and toxicity; hence, considerable care is required in relation to public health and agricultural policies (eg, entry of selenium into the food chain and use of supplements). The habitual selenium intake of the cohort was estimated to range from 48 to 58 μg/d based on data from the total diet study in the United Kingdom (3), which is similar to the results from a cohort study in Reading, United Kingdom (46). The estimated selenium intake from baseline plasma selenium concentrations (47) was 55 μg/d for the participants recruited into this study. This latter estimated intake, calculated from published data reporting the relation between intake and plasma selenium from a human study conducted in China (47), was in agreement with other recent estimates of selenium intake (3, 46). The overall range of estimated selenium intakes calculated as described (47) from the plasma selenium data of the population screened in this study gave estimated intake values ranging from 26 to 198 μg/d, with more than one-third (n = 62) of the volunteers having an estimated selenium intake ≤50 μg/d
For those volunteers in this UK cohort recruited into the study, initial plasma selenium concentrations varied considerably, from 63 to 118 ng/mL, presumably reflecting differences in dietary patterns and in histories of selenium supplementation. Strategies to improve selenium status should focus on dietary advice/modification, and supplements should be avoided by selenium-replete individuals (daily intake >100 μg/d and plasma selenium >130 ng/mL) because they probably provide no additional health benefits. Long-term use of high-dose supplements and high serum selenium concentrations have been associated with an increased risk of diabetes (48, 49) and other adverse effects (48, 50). Whereas no adverse or toxic effects have been noted with high doses of selenium (400, 1600, or 3200 μg/d) provided to increase plasma selenium to concentrations of 250 (51) and to 490 and 640 ng/mL (50), such increases in plasma selenium are unlikely to provide additional benefits, including cancer prevention (51, 52).
In the United Kingdom, the generally low selenium status of the population provides a good argument for introducing biofortification practices. Other countries, such as Finland, have used this approach successfully to increase the selenium status of the population (53). Selected food products that contain enriched levels of selenium can be introduced to improve the selenium intake of the population, for example, wheat used for bread in the United Kingdom (9). However, any public health intervention requires careful monitoring of end products for human consumption to ensure that average dietary intakes do not exceed the current upper safe limit of selenium: 400 μg/d (1, 10).
This study confirms that selenoprotein P is a reliable and sensitive biomarker of selenium status and provides, for the first time, dose-response data that can be used to estimate the selenium intake required to achieve a plateau in plasma selenoprotein P concentrations. Selenoprotein P is involved in the protection against oxidative damage, a reduction in mortality and morbidity from infection (in animal models), the homeostasis and transport of selenium (54) and is associated with a reduction in the risk of morbidity from certain types of cancer (18), all of which provide support for achieving the optimal expression of selenoprotein P. We estimate that an additional 50 μg Se/d is required in addition to the habitual intake (≈55 μg/d) to maximize selenoprotein P concentrations in this 50–64-y-old UK cohort. Considering the other potential health benefits associated with optimal selenium intake and status and the importance of selenoprotein P, the results support an increase in the recommended dietary intake of selenium in adults.
Acknowledgments
We thank Raymond Burk and Kristina Hill for their valuable advice and assistance regarding selenoprotein P. We also thank Pharma Nord, Denmark, for the selenium-yeast supplements used in this study and Robert Foxall for completing the power calculations at the design stage of the intervention trial.
The authors’ responsibilities were as follows—SJF-T, RH, and BT: conception, initiation, and design; CNA, MRB, DJH, and AJG: implementation of the study and sample collection; AKM and DJH: sample analysis; AKM, DJH, and CNA: collection and assembly of data; RH, JRD, and SJF-T: analysis and interpretation of the data; JRD: statistical analysis; CNA, MRB, DJH, and AJG: administrative, technical, and/or logistic support; and RH and SJF-T: drafting of the manuscript. All authors approved the final manuscript. None of the authors had a conflict of interest.
REFERENCES
- 1.Department of Health Dietary reference values for food, energy and nutrients for the UK. London, United Kingdom: HM Stationery Office, 1991 [Google Scholar]
- 2.Ysart G, Miller P, Crews H, et al. Dietary exposure estimates of 30 elements from the UK Total Diet Study. Food Addit Contam 1999;16:391–403 [DOI] [PubMed] [Google Scholar]
- 3.Food Standards Agency Survey on measurement of the concentrations of metals and other elements from the 2006 UK total diet study. Food Survey Information Sheet 01/09. London, United Kingdom: Food Standards Agency, 2009:16–17, 37–45 [Google Scholar]
- 4.Rayman MP. Dietary selenium: time to act. BMJ 1997;314:387–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akbaraly NT, Arnaud J, Hininger-Favier I, Gourlet V, Roussel AM, Berr C. Selenium and mortality in the elderly: results from the EVA study. Clin Chem 2005;51:2117–23 [DOI] [PubMed] [Google Scholar]
- 6.Bleys J, Navas-Acien A, Guallar E. Serum selenium levels and all-cause, cancer, and cardiovascular mortality among US adults. Arch Intern Med 2008;168:404–10 [DOI] [PubMed] [Google Scholar]
- 7.Raich PC, Lu J, Thompson HJ, Combs GF., Jr Selenium in cancer prevention: clinical issues and implications. Cancer Invest 2001;19:540–53 [DOI] [PubMed] [Google Scholar]
- 8.Juniper DT, Phipps RH, Givens DI, Jones AK, Green C, Bertin G. Tolerance of ruminant animals to high dose in-feed administration of a selenium-enriched yeast. J Anim Sci 2008;86:197–204 [DOI] [PubMed] [Google Scholar]
- 9.Broadley MR, White PJ, Bryson RJ, et al. Biofortification of UK food crops with selenium. Proc Nutr Soc 2006;65:169–81 [DOI] [PubMed] [Google Scholar]
- 10.Institute of Medicine Dietary Reference Intakes for vitamin C, vitamin E, selenium and carotenoids. Washington, DC: National Academy Press, 2000 [PubMed] [Google Scholar]
- 11.Rayman MP. Food-chain selenium and human health: emphasis on intake. Br J Nutr 2008;100:254–68 [DOI] [PubMed] [Google Scholar]
- 12.Neve J. Human selenium supplementation as assessed by changes in blood selenium concentration and glutathione peroxidase activity. J Trace Elem Med Biol 1995;9:65–73 [DOI] [PubMed] [Google Scholar]
- 13.Burk RF, Hill KE. Selenoprotein P: an extracellular protein with unique physical characteristics and a role in selenium homeostasis. Annu Rev Nutr 2005;25:215–35 [DOI] [PubMed] [Google Scholar]
- 14.Ashton K, Hooper L, Harvey LJ, Hurst R, Casgrain A, Fairweather-Tait SJ. Methods of assessment of selenium status in humans: a systematic review. Am J Clin Nutr 2009;89:2025S–39S [DOI] [PubMed] [Google Scholar]
- 15.Moschos MP. Selenoprotein P. Cell Mol Life Sci 2000;57:1836–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill KE, Xia Y, Akesson B, Boeglin ME, Burk RF. Selenoprotein P concentration in plasma is an index of selenium status in selenium-deficient and selenium-supplemented Chinese subjects. J Nutr 1996;126:138–45 [DOI] [PubMed] [Google Scholar]
- 17.Duffield AJ, Thomson CD, Hill KE, Williams S. An estimation of selenium requirements for New Zealanders. Am J Clin Nutr 1999;70:896–903 [DOI] [PubMed] [Google Scholar]
- 18.Persson-Moschos ME, Stavenow L, Akesson B, Lindgarde F. Selenoprotein P in plasma in relation to cancer morbidity in middle-aged Swedish men. Nutr Cancer 2000;36:19–26 [DOI] [PubMed] [Google Scholar]
- 19.Burk RF, Norsworthy BK, Hill KE, Motley AK, Byrne DW. Effects of chemical form of selenium on plasma biomarkers in a high-dose human supplementation trial. Cancer Epidemiol Biomarkers Prev 2006;15:804–10 [DOI] [PubMed] [Google Scholar]
- 20.Xia Y, Hill KE, Byrne DW, Xu J, Burk RF. Effectiveness of selenium supplements in a low-selenium area of China. Am J Clin Nutr 2005;81:829–34 [DOI] [PubMed] [Google Scholar]
- 21.Finley JW. Proposed criteria for assessing the efficacy of cancer reduction by plant foods enriched in carotenoids, glucosinolates, polyphenols and selenocompounds. Ann Bot 2005;95:1075–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larsen EH, Hansen M, Paulin H, Moesgaard S, Reid M, Rayman M. Speciation and bioavailability of selenium in yeast-based intervention agents used in cancer chemoprevention studies. J AOAC Int 2004;87:225–32 [PubMed] [Google Scholar]
- 23.Brown KM, Pickard K, Nicol F, Beckett GJ, Duthie GG, Arthur JR. Effects of organic and inorganic selenium supplementation on selenoenzyme activity in blood lymphocytes, granulocytes, platelets and erythrocytes. Clin Sci (Lond) 2000;98:593–9 [PubMed] [Google Scholar]
- 24.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 1967;70:158–69 [PubMed] [Google Scholar]
- 25.Thomson CD. Selenium-dependent and non-selenium-dependent glutathione peroxidase in human tissues of New Zealand residents. Biochem Int 1985;10:673–9 [PubMed] [Google Scholar]
- 26.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–54 [DOI] [PubMed] [Google Scholar]
- 27.Shortt CT, Duthie GG, Robertson JD, Morrice PC, Nicol F, Arthur JR. Selenium status of a group of Scottish adults. Eur J Clin Nutr 1997;51:400–4 [DOI] [PubMed] [Google Scholar]
- 28.R Development Core Team R: a language and environment for statistical computing. Vienna, Austria: R Foundation of Statistical Computing; 2008. ISBN 3-900051-07-0. Available from: http://www.R-project.org (cited 23 June 2008) [Google Scholar]
- 29.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 2000;19:335–51 [DOI] [PubMed] [Google Scholar]
- 30.Combs GF., Jr Selenium in global food systems. Br J Nutr 2001;85:517–47 [DOI] [PubMed] [Google Scholar]
- 31.Rayman MP, Thompson AJ, Bekaert B, et al. Randomized controlled trial of the effect of selenium supplementation on thyroid function in the elderly in the United Kingdom. Am J Clin Nutr 2008;87:370–8 [DOI] [PubMed] [Google Scholar]
- 32.Broome CS, McArdle F, Kyle JA, et al. An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status. Am J Clin Nutr 2004;80:154–62 [DOI] [PubMed] [Google Scholar]
- 33.Allen NE, Appleby PN, Roddam AW, et al. Plasma selenium concentration and prostate cancer risk: results from the European Prospective Investigation into Cancer and Nutrition (EPIC). Am J Clin Nutr 2008;88:1567–75 [DOI] [PubMed] [Google Scholar]
- 34.Kotrebai M, Tyson JF, Uden PC, Birringer M, Block E. Selenium speciation in enriched and natural samples by HPLC-ICP-MS and HPLC-ESI-MS with perfluorinated carboxylic acid ion-pairing agents. Analyst 2000;125:71–8 [DOI] [PubMed] [Google Scholar]
- 35.Burk RF, Hill KE, Motley AK. Plasma selenium in specific and non-specific forms. Biofactors 2001;14:107–14 [DOI] [PubMed] [Google Scholar]
- 36.Deagen JT, Butler JA, Zachara BA, Whanger PD. Determination of the distribution of selenium between glutathione peroxidase, selenoprotein P, and albumin in plasma. Anal Biochem 1993;208:176–81 [DOI] [PubMed] [Google Scholar]
- 37.Thomson CD. Assessment of requirements for selenium and adequacy of selenium status: a review. Eur J Clin Nutr 2004;58:391–402 [DOI] [PubMed] [Google Scholar]
- 38.Thomson CD, Robinson MF, Butler JA, Whanger PD. Long-term supplementation with selenate and selenomethionine: selenium and glutathione peroxidase (EC 1.11.1.9) in blood components of New Zealand women. Br J Nutr 1993;69:577–88 [DOI] [PubMed] [Google Scholar]
- 39.Hawkes WC, Richter BD, Alkan Z, et al. Response of selenium status indicators to supplementation of healthy North American men with high-selenium yeast. Biol Trace Elem Res 2008;122:107–21 [DOI] [PubMed] [Google Scholar]
- 40.Neve J, Vertongen F, Capel P. Selenium supplementation in healthy Belgian adults: response in platelet glutathione peroxidase activity and other blood indices. Am J Clin Nutr 1988;48:139–43 [DOI] [PubMed] [Google Scholar]
- 41.Levander OA, Alfthan G, Arvilommi H, et al. Bioavailability of selenium to Finnish men as assessed by platelet glutathione peroxidase activity and other blood parameters. Am J Clin Nutr 1983;37:887–97 [DOI] [PubMed] [Google Scholar]
- 42.Akesson B, Bellew T, Burk RF. Purification of selenoprotein P from human plasma. Biochim Biophys Acta 1994;1204:243–9 [DOI] [PubMed] [Google Scholar]
- 43.Duffield-Lillico AJ, Dalkin BL, Reid ME, et al. Selenium supplementation, baseline plasma selenium status and incidence of prostate cancer: an analysis of the complete treatment period of the Nutritional Prevention of Cancer Trial. BJU Int 2003;91:608–12 [DOI] [PubMed] [Google Scholar]
- 44.Combs GF, Jr, Clark LC, Turnbull BW. An analysis of cancer prevention by selenium. Biofactors 2001;14:153–9 [DOI] [PubMed] [Google Scholar]
- 45.World Cancer Research Fund/American Institute for Cancer Research Food, nutrition, physical activity and the prevention of cancer: a global perspective. Washington, DC: AICR, 2007:109–11 [Google Scholar]
- 46.Sunde RA, Paterson E, Evenson JK, Barnes KM, Lovegrove JA, Gordon MH. Longitudinal selenium status in healthy British adults: assessment using biochemical and molecular biomarkers. Br J Nutr 2008;99(suppl 3):S37–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang G, Zhou R, Yin S, et al. Studies of safe maximal daily dietary selenium intake in a seleniferous area in China. I. Selenium intake and tissue selenium levels of the inhabitants. J Trace Elem Electrolytes Health Dis 1989;3:77–87 [PubMed] [Google Scholar]
- 48.Stranges S, Marshall JR, Natarajan R, et al. Effects of long-term selenium supplementation on the incidence of type 2 diabetes: a randomized trial. Ann Intern Med 2007;147:217–23 [DOI] [PubMed] [Google Scholar]
- 49.Bleys J, Navas-Acien A, Guallar E. Serum selenium and diabetes in U.S. adults. Diabetes Care 2007;30:829–34 [DOI] [PubMed] [Google Scholar]
- 50.Reid ME, Stratton MS, Lillico AJ, et al. A report of high-dose selenium supplementation: response and toxicities. J Trace Elem Med Biol 2004;18:69–74 [DOI] [PubMed] [Google Scholar]
- 51.Reid ME, Duffield-Lillico AJ, Slate E, et al. The nutritional prevention of cancer: 400 mcg per day selenium treatment. Nutr Cancer 2008;60:155–63 [DOI] [PubMed] [Google Scholar]
- 52.Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2009;30:39–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang WC, Makela AL, Nanto V, Makela P, Lagstrom H. The serum selenium concentrations in children and young adults: a long-term study during the Finnish selenium fertilization programme. Eur J Clin Nutr 1998;52:529–35 [DOI] [PubMed] [Google Scholar]
- 54.Burk RF, Hill KE. Selenoprotein P-expression, functions, and roles in mammals. Biochim Biophys Acta 2009;1790:1441–7 [DOI] [PMC free article] [PubMed] [Google Scholar]