Abstract
Background: Emerging evidence suggests that docosahexaenoic acid (DHA, 22:6n−3), the principal omega-3 (n−3) fatty acid in brain gray matter, positively regulates cortical metabolic function and cognitive development. However, the effects of DHA supplementation on functional cortical activity in human subjects are unknown.
Objective: The objective was to determine the effects of DHA supplementation on functional cortical activity during sustained attention in human subjects.
Design: Healthy boys aged 8–10 y (n = 33) were randomly assigned to receive placebo or 1 of 2 doses of DHA (400 or 1200 mg/d) for 8 wk. Relative changes in cortical activation patterns during sustained attention at baseline and endpoint were determined by functional magnetic resonance imaging.
Results: At 8 wk, erythrocyte membrane DHA composition increased significantly from baseline in subjects who received low-dose (by 47%) or high-dose (by 70%) DHA but not in those who received placebo (−11%). During sustained attention, both DHA dose groups had significantly greater changes from baseline in activation of the dorsolateral prefrontal cortex than did the placebo group, and the low-dose and high-dose DHA groups had greater decreases in the occipital cortex and cerebellar cortex, respectively. Relative to low-dose DHA, high-dose DHA resulted in greater decreases in activation of bilateral cerebellum. The erythrocyte DHA composition was positively correlated with dorsolateral prefrontal cortex activation and was inversely correlated with reaction time, at baseline and endpoint.
Conclusion: Dietary DHA intake and associated elevations in erythrocyte DHA composition are associated with alterations in functional activity in cortical attention networks during sustained attention in healthy boys. This trial was registered at clinicaltrials.gov as NCT00662142.
INTRODUCTION
Docosahexaenoic acid (DHA, 22:6n−3) is the principal omega-3 (n−3) fatty acid in mammalian brain gray matter, representing ≈15–20% of the total fatty acid composition in the frontal cortex of adult humans (1) and nonhuman primates (2, 3). Because mammals cannot synthesize DHA de novo, cortical DHA composition is positively correlated with dietary omega-3 fatty acid intake (2, 3). There is now considerable preclinical evidence that DHA and/or its bioactive metabolites have neurotrophic properties during perinatal brain development (4–7) and are neuroprotective against a variety of insults associated with elevations in oxidative stress and lipid peroxidation (8–11). Moreover, preclinical studies have identified DHA as an important determinant of cortical astrocyte maturation and vascular coupling (12, 13) and cortical glucose uptake and metabolism (14–16). These findings suggest that the functional integrity and resilience of cortical neurons is mediated in part by cortical DHA composition.
In the human frontal cortex, DHA rapidly accumulates between birth and 20 y of age (1)—a period corresponding with rapid neuronal maturation, synaptogenesis, and gray matter expansion (17). There is mounting clinical evidence that the DHA status of human infants is positively associated with neurocognitive developmental trajectories, particularly on measures of attention and memory (18–20). Attention-deficit hyperactivity disorder (ADHD), which commonly emerges in childhood, is associated with deficits in erythrocyte and plasma DHA (21–24) and reductions in prefrontal cortex (PFC) blood flow (25, 26) and gray matter volume (27). Furthermore, patients with major depressive disorder also have erythrocyte (28) and PFC (29) DHA deficits and PFC astrocyte and neuronal pathology (30). Last, dietary omega-3 fatty acid intake has been shown to be positively correlated with corticolimbic gray matter volumes in healthy adults (31). Although these clinical studies suggest that dietary DHA intake may augment cortical maturation and functional integrity, the effect of increasing dietary DHA intake on functional cortical activity has not been directly investigated in human subjects.
In the present study, we determined the effects of 8 wk of supplementation with 1 of 2 doses of DHA (400 or 1200 mg/d), contrasted with placebo, on cortical activation patterns measured by changes in brain blood oxygen level–dependent activity and functional magnetic resonance imaging (fMRI) (32). Healthy boys (aged 8–10 y) were selected to obtain normative data and to increase sample homogeneity. Scans were acquired during the performance of a sustained attention task (identical-pairs continuous performance task; CPT-IP) previously found to increase activation of the dorsolateral PFC [DLPFC, Brodmann's area (BA) 9] in healthy subjects (33). On the basis of the reviewed evidence, our specific prediction was that DHA supplementation would dose-dependently increase functional activation of the DLPFC during sustained attention.
SUBJECTS AND METHODS
Subjects
Subjects were healthy boys aged 8–10 y who had no history of Axis I psychiatric disorders as determined by the Children's Interview for Psychiatric Syndromes (34). Written informed consent and assent were provided by a legal guardian and the subject, respectively. Subjects were screened to ensure that they were right-hand dominant by using the Crovitz test for handedness (35), could participate safely in an MRI scan (eg, had no ferromagnetic metal in their body and were not claustrophobic), had normal intelligence defined by the Kaufman Brief Intelligence Test (36), were not taking (current or lifetime) psychoactive medications, and did not have a history of seizures, major medical illness, or traumatic brain injury. Annual household income and breastfeeding duration were acquired during the baseline visit by questionnaire. A validated Omega-3 Dietary Intake Questionnaire was administered at baseline. The subjects were asked to maintain their normal diet throughout the trial. This trial was approved by the University of Cincinnati Institutional Review Board.
Treatments
Subjects were randomly assigned to receive algal triglyceride DHA (DHASCO; Martek Biosciences Corporation, Baltimore, MD) at doses of either 400 mg/d (200 mg twice daily) or 1200 mg/d (600 mg twice daily) or placebo (corn oil) in a double-blind manner by an investigational pharmacist. The corn oil placebo was selected because it did not contain omega-3 fatty acids, including DHA, and to minimally alter the fatty acid composition of the typical American diet. To maintain the blind condition, all subjects took 6 capsules daily (to match the number of capsules taken by the high-dose DHA group), and the placebo and DHA capsules were identical in color (orange) and size (500 mg capsules), and both contained orange flavoring. To facilitate accurate dosing and adherence, the capsules were provided to subjects in twice-a-day pill organizers with the day of the week and “am” and “pm” indicated on each compartment. To mitigate potential gastrointestinal side effects, the subjects were requested to take half of the capsules with breakfast and half with dinner. Independent analysis of the fatty acid composition of placebo and DHA capsule oils confirmed that placebo capsules did not contain DHA and that DHA capsules contained 41% (by wt of total fatty acids) DHA (≈200 mg/capsule) and no eicosapentaenoic acid (20:5n−3, EPA).
Safety and tolerability
A complete medical and treatment history, a physical examination including vital signs [blood pressure, pulse, weight, height, body mass index (in kg/m2), and temperature], and serum laboratory tests (including a renal/electrolyte profile, complete blood count, and liver function tests) were performed at baseline and at 8 wk. Safety and tolerability were also evaluated by using a structured side effect interview, the Side Effects Form for Children and Adolescents (37), performed at baseline, at the interim visit (4 wk), and at 8 wk. The Side Effects Form for Children and Adolescents measures the frequency and severity of specific cardiovascular, gastrointestinal, central nervous system (including questions regarding cognition, eg, concentration and speech), ocular, mouth and nasal, genitourinary, dermatologic, and musculoskeletal side effects.
Sustained attention task
During performance of the identical-pairs version of the continuous performance task (CPT-IP), subjects were presented with a series of one-digit numbers and asked to respond with a button press using their right index finger when they saw the same number twice sequentially. The one-digit CPT-IP was selected because we previously found that younger subjects perform consistently on this simple task (38). Numbers were presented at 750-ms intervals. Targets constituted 12.5% of the presentations (5 per block) and were randomly distributed. The attention task was alternated with a control task consisting of the number “1” presented at the same rate as with the CPT-IP. The control task required subjects to press the response button 5 times to control for finger movement. Both experimental and control tasks were presented in epochs of 30 s each for a total of 40 numbers per epoch. Five blocks consisting of one epoch each of the active and control tasks were obtained, as was an additional control epoch at the start of the session. Responses were electronically recorded to permit calculation of response parameters (ie, sensitivity, A′ and response bias, B′). Before all imaging sessions, the subjects underwent a training session during which they were required to demonstrate an understanding of the CPT-IP task. Performance on the CPT-IP task was evaluated on the basis of percentage correct selections, errors of commission, discriminability {0.5 + [(hit rate – false alarm rate)(1 + hit rate – false alarm rate)] / (4 × hit rate (1 – false alarm rate)}, and reaction time. The CPT-IP task was administered by using PsyScope software on a Macintosh computer and was viewed by subjects during the fMRI sessions using a nonferromagnetic audiovisual goggle system (Resonance Technologies Inc, Salem, MA). Performance parameters were evaluated by using a 2-factor ANOVA, with time (baseline and 8 wk) and dose (placebo, low-dose DHA, and high-dose DHA) as main factors.
fMRI acquisition
fMRI scans were performed at baseline and at 8 wk by using a 4.0 Tesla Varian Unity INOVA Whole Body MRI/MRS system (Varian Inc, Palo Alto, CA). The INOVA system is controlled by a SUN workstation running Varian's VNMR-J and SPIN-CAD image processing and pulse-sequence development software under a Unix-based operating system. During the scan session, subjects recline in a supine position on the scanner bed. Nonferromagnetic goggles are positioned to provide clear visualization of the stimuli, and a radiofrequency coil is placed over the subject's head. Padding was inserted around the subject's head to minimize movement. Headphones were provided to block background noise and to allow communication with subjects during scan acquisition. A microphone in the scanner permitted subjects to communicate with the MRI technician in case of concern or discomfort. After a 3-plane gradient echo scan for alignment and brain localization was performed, a shim procedure was performed to generate a homogeneous magnetic field. To provide anatomic localization for activation maps, a high-resolution, T1-weighted, 3-dimensional brain scan was obtained by using a modified driven equilibrium Fourier transform (MDEFT) sequence [TMD = 1.1 s, TR (repetition time) = 13 ms, TE (echo time) = 6 ms, FOV (field of view) = 25.6 × 19.2 × 19.2 cm, matrix 256 × 192 × 96 pixels, flip angle = 20°]. A midsagittal localizer scan was obtained to place 40 contiguous 4-mm axial slices that extend from the inferior cerebellum to encompass the entire brain. Subjects then completed an fMRI session in which scans were acquired while the CPT-IP task was being performed (described above) by using a T2*-weighted gradient-echo echoplanar imaging (EPI) pulse sequence (TR/TE = 2000/30 ms, FOV = 25.6 × 25.6 cm, matrix 64 × 64 pixels, slice thickness = 4 mm, flip angle = 75°).
fMRI analysis
Whole-brain activation patterns were determined by using a random-effects analysis with statistical parametric mapping software (Welcome Department of Cognitive Neurology, Institute of Neurology, London, United Kingdom) (39). Scans were normalized to Talairach space and smoothed with a 3-dimensional Gaussian kernel of 8 mm FWHM (full width at half maximum) (40). Significance was defined by a voxel-level P ≤ 0.01 and a cluster extent of T ≥ 233 contiguous voxels, as determined by Monte Carlo simulation, to control type I error and achieve a corrected P ≤ 0.05 (41). This level of significance was selected to ensure only very strong group differences. For exploratory analyses, significance was defined by a voxel-level P ≤ 0.01 and a voxel cluster-extent threshold of T ≥ 100 contiguous voxels. For between-group comparisons, one-factor analyses of covariance were used, with treatment group as a factor. Because breastfeeding (compared with DHA-free formula) is associated with greater postnatal cortical DHA composition in infants (42–44) and because we found that breastfeeding duration was associated with alterations in regional blood oxygen level–dependent activation during performance of the CPT-IP (RK McNamara, MP DelBello, CM Adler, unpublished observations, 2007), breastfeeding duration was a covariate. Regression analyses were also performed to determine the relation between erythrocyte DHA composition and cortical activation patterns during sustained attention at baseline and endpoint. Significant correlations were defined as an r value equivalent to P ≤ 0.01 and a cluster extent of T ≥ 233 contiguous voxels.
Gas chromatography
At baseline and 8 wk, whole blood (20 mL) was collected into EDTA-coated collection tubes and centrifuged for 20 min (3000 × g, 4°C). Plasma was removed and the erythrocytes were washed 3 times with 0.9% NaCl and then stored at −80°C. The total fatty acid composition of erythrocyte membranes was determined by using saponification and methylation methods described previously (45). The samples were analyzed with a Shimadzu GC-2010 equipped with an auto-injector (Shimadzu Scientific Instruments Inc, Columbia, MD). The column was a DB-23 (123-2332): 30 m in length, internal diameter of 0.32 mm (wide bore), and film thickness of 0.25 μm (J&W Scientific, Folsom CA). The carrier gas was helium with a column flow rate of 2.5 mL/min. Fatty acids were identified by using retention times of authenticated fatty acid methyl ester standards (Matreya LLC Inc, Pleasant Gap, PA). The analysis of fatty acid methyl esters is based on areas calculated with Shimadzu Class VP 4.3 software. All samples were processed by a technician blinded to treatment.
Statistical analysis
Statistical analyses were performed by using GB-STAT (version 10.0; Dynamic Microsystems Inc, Silver Spring, MD). Bonferroni-corrected post hoc tests (2-tailed) were used to evaluate individual group differences for the 3 fatty acids [DHA, arachidonic acid (AA), and linoleic acid] of interest (α = 0.05/3 fatty acids = 0.017).
RESULTS
Subject characteristics and attrition
A total of 48 subjects were screened, and 38 subjects met the entrance criteria and were randomly assigned to treatment (placebo, n = 12; low-dose DHA, n = 12; high-dose DHA, n = 14). A total of 33 subjects completed the study (placebo, n = 10; low-dose DHA, n = 10; high-dose DHA, n = 13). Three subjects (placebo, n = 1; low-dose DHA, n = 1; high-dose DHA, n = 1) were lost to follow-up, and 2 subjects (placebo, n = 1; low-dose DHA, n = 1) withdrew from the study because of family emergencies unrelated to the study. A comparison of subject characteristics at baseline is presented in Table 1. The mean annual household income, an index of socioeconomic status, was $73.8 ± 27 thousand dollars, and did not differ significantly between treatment groups (F2,32 = 0.01, P = 0.99). There were no baseline group differences in weekly fish consumption frequency or erythrocyte DHA composition (see below). On the basis of subject body weights, the low-dose and high-dose DHA groups received mean daily DHA doses of 11 and 33 mg ⋅ kg−1 ⋅ d−1, respectively.
TABLE 1.
Demographic characteristics of the subjects1
| Placebo | 400 mg DHA/d | 1200 mg DHA/d | P value2 | |
| Age (y) | 8.8 ± 0.83 | 9.2 ± 1.0 | 9.5 ± 0.7 | 0.12 |
| Race (n) | ||||
| White | 10 | 9 | 12 | — |
| African American | — | — | 1 | — |
| Hispanic | — | 1 | — | — |
| Siblings (n) | 1.4 ± 1.1 | 2.0 ± 0.7 | 2.0 ± 1.0 | 0.26 |
| Height (cm) | 137 ± 9.1 | 139 ± 8.9 | 141 ± 8.7 | 0.54 |
| Body weight (kg) | 36 ± 11 | 37 ± 12 | 36 ± 8 | 0.96 |
| BMI (kg/m2) | 19.4 ± 4.5 | 18.8 ± 4.2 | 17.2 ± 2.3 | 0.40 |
| Fish intake (times/wk) | 0.8 ± 0.5 | 1.1 ± 1.1 | 0.7 ± 0.8 | 0.44 |
| Breastfeeding duration (mo) | 7.4 ± 7.2 | 12.7 ± 11.7 | 8.8 ± 8.2 | 0.42 |
| KBIT | ||||
| Vocabulary | 85 ± 12 | 79 ± 24 | 77 ± 20 | 0.63 |
| Matrices | 81 ± 17 | 78 ± 29 | 88 ± 15 | 0.46 |
DHA, docosahexaenoic acid; KBIT, Kaufman Brief Intelligence Test (national percentile rank).
One-factor ANOVA.
Mean ± SD (all such values).
Safety and tolerability
There were no significant differences between baseline and 8 wk for any treatment group in laboratory results, including complete blood count, electrolyte, and liver function tests (see Supplemental Table 1 under “Supplemental data” in the online issue). There were no clinically significant treatment-emergent adverse events reported in any treatment group, and the most frequently reported side effects were rated as mild in severity (see Supplemental Table 2 under “Supplemental data” in the online issue). In addition, the time × dose interaction was not significant for pulse (bpm) (F2,65 = 0.71, P = 0.47), systolic blood pressure (mm Hg) (F2,65 = 0.43, P = 0.65), diastolic blood pressure (mm Hg) (F2,65 = 0.65, P = 0.52), body weight (kg) (F2,65 = 0.07, P = 0.93), height (m) (F2,65 = 0.1, P = 0.90), body mass index (kg/m2) (F2,65 = 0.11, P = 0.89), or body temperature (°C) (F2,65 = 1.58, P = 0.21).
Erythrocyte DHA composition
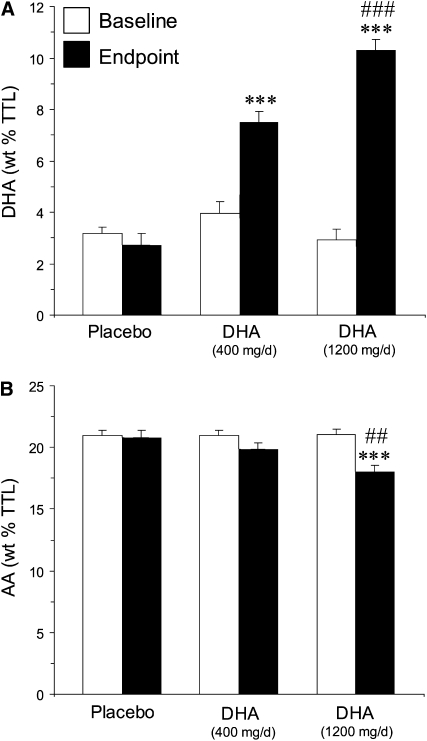
Consistent with a prior study (46), weekly fish consumption frequency was positively correlated with erythrocyte DHA composition at baseline (r = 0.60, P = 0.0002). Mean erythrocyte DHA composition at baseline was 3.3 ± 1.3% and did not differ between individual treatment groups (F2,32 = 1.8, P = 0.19). This baseline value is similar to that observed in healthy US adults (≈3.7%) (46) and ≈50% of the value observed in healthy Japanese adults (6.8%) (47). The time × dose interaction was significant (F2,65 = 26.7, P ≤ 0.0001), and the erythrocyte DHA composition increased significantly between baseline and 8 wk in subjects receiving low-dose DHA (by 47%; P ≤ 0.0001) and high-dose DHA (by 70%; P ≤ 0.0001), but not placebo (−11%; P = 0.45) (Figure 1A). At 8 wk, the mean erythrocyte DHA composition was 7.5 ± 1.3% of total fatty acids in the low-dose DHA group, 10.3 ± 1.5% in the high-dose DHA group, and 2.5 ± 1.6% in the placebo group. The erythrocyte linoleic acid (18:2n−6) composition did not differ between treatment groups at baseline (F2,32 = 1.46, P = 0.25), and the time × dose interaction was not significant (F2,65 = 0.57, P = 0.57). The erythrocyte AA (20:4n−6) composition did not differ between treatment groups at baseline (F2,32 = 0.03, P = 0.97), and the time × dose interaction was significant (F2,65 = 4.99, P = 0.009). Erythrocyte AA composition decreased significantly from baseline in subjects who received high-dose DHA (−14%, P ≤ 0.0001), but not in those who received low-dose DHA (−5%; P = 0.12) or placebo (−1%; P = 0.82). At 8 wk, subjects who received high-dose DHA (−13%; P = 0.001), but not low-dose DHA (−5%; P = 0.20), had a significantly lower erythrocyte AA composition than did the placebo group, and the erythrocyte AA composition in the high-dose DHA group was significantly lower than that in the low-dose DHA group (−9%, P = 0.01) (Figure 1B).
FIGURE 1.
Erythrocyte membrane docosahexaenoic acid (DHA) (A) and arachidonic acid (AA) (B) composition [% by wt of total fatty acids (wt % TTL)] at baseline and study endpoint (8 wk) in subjects treated with placebo, low-dose DHA (400 mg/d), or high-dose DHA (1200 mg/d). Values are expressed as group means ± SEMs. ***Significantly different frombaseline and placebo, P ≤ 0.001 (unpaired t test, 2-tailed). ##,###Significantly different fromlow-dose DHA: ##P < 0.01, ###P < 0.001 (unpaired t test, 2-tailed). The time × dose interactions for DHA (P ≤ 0.0001) and AA (P = 0.009) were significant.
Visual sustained attention performance
Indexes of CPT-IP performance are presented in Table 2. At baseline, there were no significant group differences for percentage correct, commission errors, discriminability, or reaction time. At 8 wk, there were no significant group differences in percentage correct, commission errors, discriminability, or reaction time. The time × dose interaction was not significant for percentage correct, commission errors, discriminability, or reaction time. Among all subjects (n = 33), erythrocyte DHA composition was inversely correlated with reaction time at baseline (r = −0.43, P = 0.01) and endpoint (r = −0.41, P = 0.02), but was not correlated with other performance measures.
TABLE 2.
Performance measures on the identical-pairs continuous performance task1
| Placebo | 400 mg DHA/d | 1200 mg DHA/d | P value2 | P value3 | |
| Baseline | |||||
| Percentage correct | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.9 ± 0.1 | 0.17 | — |
| Discriminability | 0.9 ± 0.0 | 0.9 ± 0.0 | 0.9 ± 0.0 | 0.18 | — |
| Commission errors | 2.1 ± 1.7 | 1.4 ± 1.3 | 1.7 ± 1.6 | 0.61 | — |
| Reaction time (ms) | 692 ± 83 | 652 ± 50 | 655 ± 48 | 0.29 | — |
| Endpoint | |||||
| Percentage correct | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.71 | 0.68 |
| Discriminability | 0.9 ± 0.0 | 0.9 ± 0.0 | 0.9 ± 0.0 | 0.73 | 0.18 |
| Commission errors | 2.3 ± 1.7 | 2.6 ± 1.7 | 2.1 ± 2.1 | 0.81 | 0.64 |
| Reaction time (ms) | 695 ± 94 | 641 ± 59 | 652 ± 39 | 0.18 | 0.64 |
All values are means ± SDs. DHA, docosahexaenoic acid.
One-factor ANOVA.
Two-factor ANOVA (treatment phase × dose interaction).
fMRI
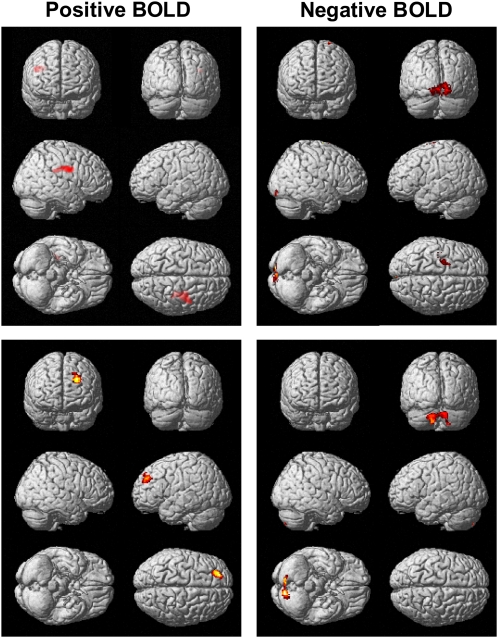
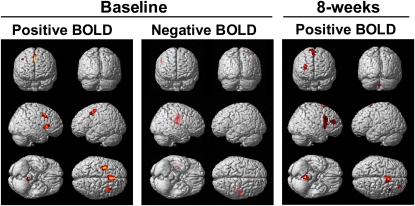
Between baseline and endpoint, the subjects treated with low-dose DHA had greater increases in activation of the right DLPFC (BA9) and precentral gyrus (BA6) and greater decreases in activation of bilateral occipital cortex (BA17) relative to the placebo group (Table 3, Figure 2). No additional regions were identified as having greater increases in activation based on a less-stringent voxel-extent threshold (T ≥ 100), whereas greater decreases in activation were observed in the left parahippocampal gyrus (BA35,36, T140), right temporal lobe (BA20, T137), and right cerebellum (anterior lobe, T176). Between baseline and endpoint, the subjects treated with high-dose DHA had greater increases in activation of the left DLPFC (BA9) and greater decreases in activation of bilateral cerebellum relative to placebo (Table 3, Figure 2). On the basis of a voxel-extent threshold of T ≥ 100, no additional regions were identified as having greater increases in activation, whereas greater decreases in activation were observed in the left middle frontal gyrus (BA6, T135) and left temporal lobe (BA20, T107). A contrast of the DHA dose groups found greater decreases in activation of bilateral cerebellum in the high-dose DHA group than in the low-dose DHA group (Table 3). Greater decreases in activation were observed in the left middle frontal gyrus (BA6 T167) of the high-dose DHA group on the basis of a less stringent voxel extent threshold (T ≥ 100). At baseline, among all subjects (n = 33), erythrocyte DHA composition was positively correlated with activation in the right (BA9,47) and left (BA6,8) frontal regions and bilateral cerebellum and was negatively correlated with the right frontal precentral gyrus (BA4) and right insula (BA13) (Table 4, Figure 3). At study endpoint, the erythrocyte DHA composition was positively correlated with activation in right frontal regions (BA6,8,9,10) and cingulate gyrus (BA23,24), and there were no significant negative correlations.
FIGURE 2.
Statistical parametric maps illustrating group differences from baseline to endpoint in regional activation [positive brain blood oxygen level–dependent activity (BOLD), docosahexaenoic acid (DHA) > placebo] and decreased activation (negative BOLD, placebo > DHA) during performance of the identical-pairs continuous performance task. The top row represents placebo compared with 400 mg DHA/d, and the bottom row represents placebo compared with 1200 mg DHA/d. Images are overlaid on a T1-weighted anatomic image, and the color gradient reflects increasing (red→yellow) statistical significances from a voxel intensity threshold of P ≤ 0.01 and cluster extent threshold of 233 voxels (P ≤ 0.05, corrected) relative to placebo.
FIGURE 3.
Statistical parametric maps illustrating the relations between erythrocyte docosahexaenoic acid (DHA) composition and regional activation patterns during performance of the identical-pairs continuous performance task at baseline and endpoint (8 wk) [positive brain blood oxygen level–dependent activity (BOLD), positive correlation with DHA; negative BOLD, negative correlation with DHA]. There were no significant negative correlations between BOLD and DHA at 8 wk.
TABLE 3.
Regions exhibiting differential activation during sustained attention1
| Talairach coordinates |
Cluster extent (voxels) | |||
| Brain region (Brodmann's area) | x | y | z | |
| mm | ||||
| Placebo versus low dose | ||||
| DHA > placebo | ||||
| Right inferior frontal gyrus (BA9) | 44 | −3 | 26 | 445 |
| Right precentral gyrus (BA6) | 36 | −10 | 30 | |
| Placebo > DHA | ||||
| Right occipital lobe, lingual gyrus (BA17) | 14 | −87 | 3 | 275 |
| 22 | −89 | −2 | ||
| Left occipital lobe, lingual gyrus (BA17) | −8 | −90 | −4 | |
| Placebo versus high dose | ||||
| DHA > placebo | ||||
| Left superior frontal gyrus (BA9) | −20 | 48 | 36 | 288 |
| −26 | 44 | 29 | ||
| Placebo > DHA | ||||
| Right cerebellum, posterior lobe | 8 | −81 | −21 | 382 |
| Left cerebellum, posterior lobe | −8 | −83 | −23 | |
| −10 | −82 | −35 | ||
| Low dose versus high dose | ||||
| High > low | ||||
| Left parietal lobe (BA43) | −61 | −9 | 17 | 358 |
| Left temporal lobe (BA42) | −59 | −9 | 10 | |
| −51 | −21 | 1 | ||
| Right occipital lobe (BA30) | 26 | −71 | 9 | 278 |
| Right posterior cingulate (BA31) | 26 | −61 | 14 | |
| Right cerebellum, posterior lobe | 8 | −77 | −20 | 1091 |
| 30 | −63 | −20 | ||
| Left cerebellum, posterior lobe | −22 | −69 | −20 | |
DHA, docosahexaenoic acid. Only comparisons that were significant at P < 0.05 (corrected) are included in the table. Larger voxel clusters required more than one set of coordinates.
TABLE 4.
Relation between erythrocyte docosahexaenoic acid (DHA) composition and regional brain activation during sustained attention1
| Talairach coordinates |
Cluster extent (voxels) | |||
| Brain region (Brodmann's area) | x | y | z | |
| mm | ||||
| Baseline | ||||
| Positively correlated with DHA | ||||
| Right middle frontal gyrus (BA9) | 36 | 15 | 27 | 532 |
| Right inferior frontal gyrus (BA47) | 40 | 21 | 1 | 377 |
| Left middle frontal gyrus (BA6) | −32 | 2 | 48 | 473 |
| Left medial frontal gyrus (BA8) | 0 | 27 | 37 | 596 |
| Left superior frontal gyrus (BA8) | 0 | 20 | 51 | |
| Right cerebellum, anterior lobe | 8 | −40 | −20 | 749 |
| Right cerebellum, posterior lobe | 4 | −47 | −44 | |
| Left cerebellum, anterior lobe | −4 | −38 | −28 | |
| Negatively correlated with DHA | ||||
| Right precentral gyrus (BA4) | 49 | −10 | 39 | 472 |
| Right sublobar insula (BA13) | 38 | −18 | 23 | |
| 42 | −15 | 14 | ||
| Endpoint (8 wk) | ||||
| Positively correlated with DHA | ||||
| Right inferior frontal gyrus (BA9) | 32 | 9 | 30 | 2312 |
| Right sublobar, insula (BA13) | 32 | 18 | 8 | |
| Right middle frontal gyrus (BA10) | 30 | 43 | 11 | |
| Right cingulate gyrus (BA23) | 8 | −16 | 27 | 521 |
| Right cingulate gyrus (BA24) | 2 | 7 | 24 | |
| Right superior frontal gyrus (BA8) | 4 | 16 | 51 | 424 |
| Right superior frontal gyrus (BA6) | 14 | 11 | 66 | |
| 6 | 13 | 64 | ||
| Left claustrum | −30 | 16 | 7 | 277 |
| Right cerebellum, anterior lobe | 0 | −50 | −19 | 1208 |
| 0 | −48 | −30 | ||
| Left cerebellum, posterior lobe | 4 | −43 | −35 | |
Only comparisons that were significant at P < 0.05 (corrected) are included in the table.
DISCUSSION
The main finding of the present study was that 8 wk of supplementation with either low- or high-dose DHA significantly increased functional activation in the DLPFC (BA9) during performance of an attention task compared with placebo. Additionally, lower activation was observed in the low-dose (occipital cortex) and high-dose (cerebellar cortex) DHA groups compared with placebo. Relative to low-dose DHA, high-dose DHA resulted in a greater decrease in activation of the bilateral cerebellum. Increased DLPFC activation after DHA supplementation could not be attributed to differences in performance on the CPT-IP task and were independent of breastfeeding duration. DHA supplementation dose-dependently increased erythrocyte DHA composition, with posttreatment erythrocyte DHA values increasing 2-fold in the low-dose DHA group and 3-fold in the high-dose DHA group. Erythrocyte DHA composition was positively correlated with DLPFC activation and inversely correlated with reaction time at baseline and the endpoint. To our knowledge, this is the first controlled neuroimaging study to show an effect of DHA supplementation on functional cortical activity in human subjects and suggests that DHA modulates functional activity in cortical attention networks.
DHA supplementation dose-dependently increased the erythrocyte membrane DHA composition, which was positively correlated with functional cortical activity and inversely correlated with reaction time during performance of the CPT. It is not known whether 8 wk of DHA supplementation was sufficient to increase cortical membrane DHA composition. However, previous nonhuman primate studies have found that DHA deficits in erythrocyte and cortical biopsy samples, resulting from perinatal omega-3 fatty acid insufficiency, increased substantially after ≥8 wk of dietary EPA+DHA fortification, albeit at different rates (erythrocyte > cortex) (3). Moreover, a postmortem study found that the frontal cortex and erythrocyte DHA composition increased rapidly during the pediatric and adolescent periods (1). Nevertheless, the present data suggest that dietary DHA has important to-be-defined modulatory effects on cortical neuron activity.
Supplementation with either low- or high-dose DHA resulted in a greater change from baseline in DLPFC (BA9) activation than did placebo. However, increased activity was found in the right DLPFC of the low-dose DHA group and in the left DLPFC of the high-dose DHA group. This lateralization effect is of interest because a prior study found that plasma DHA composition was positively correlated with glucose metabolism in right hemisphere cortical regions and negatively correlated with glucose metabolism in the left hemisphere cortical regions of adult patients with major depression (48). Furthermore, dietary-induced DHA deficits in the brain are associated with robust lateralization effects in adult rats (49). Although the mechanisms mediating lateralization in the DHA dose groups are not known, these data suggest that the brain DHA composition may be an important determinant of interhemisphere communication and functional connectivity.
Treatment with high-dose DHA also resulted in greater decreases in activation of bilateral cerebellar cortex during sustained attention relative to low-dose DHA and placebo. Cerebellar projections to the PFC have been identified in primates (50), and structural abnormalities (51) and blood flow deficits (25) have been observed in the cerebellar cortex of children with ADHD. Moreover, a prior fMRI study found that mediation-naive pediatric ADHD patients exhibited greater cerebellar cortex activation, in conjunction with reduced PFC activation, during sustained attention relative to healthy control subjects (52), a pattern opposite that observed in the present study. These findings suggest that DHA may modulate cerebellar-PFC attention networks.
This imaging trial had important limitations. First, we used a one-digit, rather than the more difficult 4-digit, version of the CPT, and the high level of performance exhibited by all groups (80–90% accuracy) may have prevented the detection of potential performance-enhancing effects of DHA supplementation. Second, scans were normalized to the Talairach space by using an adult template. However, the error associated with spatial normalization of pediatric brains to an adult template does not result in artifacts in the SPM analysis in older pediatric subjects (>6 y), and potential spatial effects are well within the area of smoothing used in the present study (53, 54). Third, the duration of DHA intervention was relatively short (8 wk), and greater changes in regional brain activation patterns may have been observed after a longer period of DHA supplementation. Fourth, the relatively small number of subjects randomly assigned to each treatment group may not be a representative sample of this age group. Therefore, a larger and longer controlled imaging study using a more difficult version of the CPT will be required to replicate and extend the present findings.
The present findings add to an emerging body of evidence from preclinical (16, 55) and clinical (48) imaging studies that suggest that dietary DHA intake is a robust modulator of functional cortical activity. Although the mechanisms mediating altered functional cortical activity after DHA supplementation cannot be discerned from the present results, augmentation of astrocyte-mediated neurovascular metabolic coupling (12–16, 48, 55), reductions in central inflammatory signaling cascades (56, 57), neurotrophic effects (4–7), and/or augmented dopamine receptor–mediated activity (58, 59) are candidates for future investigation. These findings further suggest that this imaging paradigm could be useful for elucidating neurobiological mechanisms underlying deficits in cortical activity in psychiatric disorders associated with DHA deficiencies, including ADHD and major depression.
Supplementary Material
Acknowledgments
The authors' responsibilities were as follows—RKM, MPD, and CMA: study concept and design; JA, JCE, KJ, DA, and WW: imaging data acquisition and analysis; RKM, TR, RJ, and PT: fatty acid composition analysis; RKM, SMS, MPD, and CMA: analysis and interpretation of data; RKM, SMS, MPD, and CMA: manuscript draft and critical revision; and RKM: obtained funding and overall study coordination. RKM received investigator-initiated research funding from Martek Biosciences Inc (the producer of the algal DHA used in this study), Janssen, the National Alliance for Research on Schizophrenia and Depression (NARSAD), the National Institute on Aging, the National Institute of Mental Health, and the Inflammation Research Foundation. SMS received research grant support from Eli Lilly, Janssen, AstraZeneca, Nutrition 21, Repligen, the National Institute on Drug Abuse, the National Institute on Alcohol Abuse and Alcoholism, NARSAD, and the Thrasher Foundation and is a consultant for Pfizer. MPD received research grant support from AstraZeneca, Eli Lilly, Johnson & Johnson, Shire, Janssen, Pfizer, Bristol-Myers Squibb, Repligen, Somerset, Sumitomo, the Thrasher Foundation, and GlaxoSmithKline and is a consultant for GlaxoSmithKline, Eli Lilly, France Foundation, Kappa Clinical, Pfizer, Medical Communications Media, and Shering-Plough. CMA received research grant support from Abbott Laboratories, AstraZeneca, Eli Lilly, Johnson & Johnson, Shire, Janssen (Johnson & Johnson), Pfizer, Bristol-Myers Squibb, Repligen, and Somerset, and is a consultant for AstraZeneca and Janssen. None of the other authors had a conflict of interest to disclose.
REFERENCES
- 1.Carver JD, Benford VJ, Han B, Cantor AB. The relationship between age and the fatty acid composition of cerebral cortex and erythrocytes in human subjects. Brain Res Bull 2001;56:79–85 [DOI] [PubMed] [Google Scholar]
- 2.Anderson GJ, Neuringer M, Lin DS, Connor WE. Can prenatal N-3 fatty acid deficiency be completely reversed after birth? Effects on retinal and brain biochemistry and visual function in rhesus monkeys. Pediatr Res 2005;58:865–72 [DOI] [PubMed] [Google Scholar]
- 3.Connor WE, Neuringer M, Lin DS. Dietary effects on brain fatty acid composition: the reversibility of n−3 fatty acid deficiency and turnover of docosahexaenoic acid in the brain, erythrocytes, and plasma of rhesus monkeys. J Lipid Res 1990;31:237–47 [PubMed] [Google Scholar]
- 4.Calderon F, Kim HY. Docosahexaenoic acid promotes neurite growth in hippocampal neurons. J Neurochem 2004;90:979–88 [DOI] [PubMed] [Google Scholar]
- 5.Coti Bertrand P, O'Kusky JR, Innis SM. Maternal dietary (n−3) fatty acid deficiency alters neurogenesis in the embryonic rat brain. J Nutr 2006;136:1570–5 [DOI] [PubMed] [Google Scholar]
- 6.Ikemoto A, Nitta A, Furukawa S, et al. Dietary n−3 fatty acid deficiency decreases nerve growth factor content in rat hippocampus. Neurosci Lett 2000;285:99–102 [DOI] [PubMed] [Google Scholar]
- 7.Kawakita E, Hashimoto M, Shido O. Docosahexaenoic acid promotes neurogenesis in vitro and in vivo. Neuroscience 2006;139:991–7 [DOI] [PubMed] [Google Scholar]
- 8.Bazan NG. Cellular and molecular events mediated by docosahexaenoic acid-derived neuroprotectin D1 signaling in photoreceptor cell survival and brain protection. Prostaglandins Leukot Essent Fatty Acids 2009;81:205–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belayev L, Khoutorova L, Atkins KD, Bazan NG. Robust docosahexaenoic acid-mediated neuroprotection in a rat model of transient, focal cerebral ischemia. Stroke 2009;40:3121–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blondeau N, Widmann C, Lazdunski M, Heurteaux C. Polyunsaturated fatty acids induce ischemic and epileptic tolerance. Neuroscience 2002;109:231–41 [DOI] [PubMed] [Google Scholar]
- 11.Ozyurt B, Sarsilmaz M, Akpolat N, et al. The protective effects of omega-3 fatty acids against MK-801-induced neurotoxicity in prefrontal cortex of rat. Neurochem Int 2007;50:196–202 [DOI] [PubMed] [Google Scholar]
- 12.Champeil-Potokar G, Chaumontet C, Guesnet P, Lavialle M, Denis I. Docosahexaenoic acid (22:6n−3) enrichment of membrane phospholipids increases gap junction coupling capacity in cultured astrocytes. Eur J Neurosci 2006;24:3084–90 [DOI] [PubMed] [Google Scholar]
- 13.Joardar A, Sen AK, Das S. Docosahexaenoic acid facilitates cell maturation and beta-adrenergic transmission in astrocytes. J Lipid Res 2006;47:571–81 [DOI] [PubMed] [Google Scholar]
- 14.Pifferi F, Roux F, Langelier B, et al. (n−3) polyunsaturated fatty acid deficiency reduces the expression of both isoforms of the brain glucose transporter GLUT1 in rats. J Nutr 2005;135:2241–6 [DOI] [PubMed] [Google Scholar]
- 15.Ximenes da Silva A, Lavialle F, Gendrot G, Guesnet P, Alessandri JM, Lavialle M. Glucose transport and utilization are altered in the brain of rats deficient in n−3 polyunsaturated fatty acids. J Neurochem 2002;81:1328–37 [DOI] [PubMed] [Google Scholar]
- 16.McNamara RK, Able J, Jandacek R, Rider T, Tso P, Lindquist DM. Perinatal n−3 fatty acid deficiency selectively reduces myo-inositol levels in the adult rat PFC: an in vivo (1)H-MRS study. J Lipid Res 2009;50:405–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci 1999;2:861–3 [DOI] [PubMed] [Google Scholar]
- 18.Agostoni C, Trojan S, Bellu R, Riva E, Giovannini M. Neurodevelopmental quotient of healthy term infants at 4 months and feeding practice: the role of long-chain polyunsaturated fatty acids. Pediatr Res 1995;38:262–6 [DOI] [PubMed] [Google Scholar]
- 19.Colombo J, Kannass KN, Shaddy DJ, et al. Maternal DHA and the development of attention in infancy and toddlerhood. Child Dev 2004;75:1254–67 [DOI] [PubMed] [Google Scholar]
- 20.Lauritzen L, Hansen HS, Jorgensen MH, Michaelsen KF. The essentiality of long chain n−3 fatty acids in relation to development and function of the brain and retina. Prog Lipid Res 2001;40:1–94 [DOI] [PubMed] [Google Scholar]
- 21.Chen JR, Hsu SF, Hsu CD, Hwang LH, Yang SC. Dietary patterns and blood fatty acid composition in children with attention-deficit hyperactivity disorder in Taiwan. J Nutr Biochem 2004;15:467–72 [DOI] [PubMed] [Google Scholar]
- 22.Mitchell EA, Aman MG, Turbott SH, Manku M. Clinical characteristics and serum essential fatty acid levels in hyperactive children. Clin Pediatr 1987;26:406–11 [DOI] [PubMed] [Google Scholar]
- 23.Stevens LJ, Zentall SS, Deck JL, et al. Essential fatty acid metabolism in boys with attention-deficit hyperactivity disorder. Am J Clin Nutr 1995;62:761–8 [DOI] [PubMed] [Google Scholar]
- 24.Young GS, Maharaj NJ, Conquer JA. Blood phospholipid fatty acid analysis of adults with and without attention deficit/hyperactivity disorder. Lipids 2004;39:117–23 [DOI] [PubMed] [Google Scholar]
- 25.Kim BN, Lee JS, Shin MS, Cho SC, Lee DS. Regional cerebral perfusion abnormalities in attention deficit/hyperactivity disorder. Statistical parametric mapping analysis. Eur Arch Psychiatry Clin Neurosci 2002;252:219–25 [DOI] [PubMed] [Google Scholar]
- 26.Spalletta G, Pasini A, Pau F, Guido G, Menghini L, Caltagirone C. Prefrontal blood flow dysregulation in drug naive ADHD children without structural abnormalities. J Neural Transm 2001;108:1203–16 [DOI] [PubMed] [Google Scholar]
- 27.Seidman LJ, Valera EM, Makris N. Structural brain imaging of attention-deficit/hyperactivity disorder. Biol Psychiatry 2005;57:1263–72 [DOI] [PubMed] [Google Scholar]
- 28.Peet M, Murphy B, Shay J, Horrobin D. Depletion of omega-3 fatty acid levels in red blood cell membranes of depressive patients. Biol Psychiatry 1998;43:315–9 [DOI] [PubMed] [Google Scholar]
- 29.McNamara RK, Hahn CG, Jandacek R, et al. Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Biol Psychiatry 2007;62:17–24 [DOI] [PubMed] [Google Scholar]
- 30.Rajkowska G. Cell pathology in mood disorders. Semin Clin Neuropsychiatry 2002;7:281–92 [DOI] [PubMed] [Google Scholar]
- 31.Conklin SM, Gianaros PJ, Brown SM, et al. Long-chain omega-3 fatty acid intake is associated positively with corticolimbic gray matter volume in healthy adults. Neurosci Lett 2007;421:209–12 [DOI] [PubMed] [Google Scholar]
- 32.Sutton BP, Ouyang C, Karampinos DC, Miller GA. Current trends and challenges in MRI acquisitions to investigate brain function. Int J Psychophysiol 2009;73:33–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adler CM, Sax KW, Holland SK, Schmithorst V, Rosenberg L, Strakowski SM. Changes in neuronal activation with increasing attention demand in healthy volunteers: an fMRI study. Synapse 2001;42:266–72 [DOI] [PubMed] [Google Scholar]
- 34.Fristad MA, Glickman AR, Verducci JS, et al. Children's Interview for Psychiatric Syndromes (ChIPS): psychometrics in two community samples. J Child Adolesc Psychopharmacol 1998;8:237–45 [DOI] [PubMed] [Google Scholar]
- 35.Crovitz HF, Zener K. A group-test for assessing hand- and eye-dominance. Am J Psychol 1962;75:271–6 [PubMed] [Google Scholar]
- 36.Kaufman AS, Kaufman NL. Kaufman brief intelligence test. 1st ed Circle Pines, MN: American Guidance Service, 1990 [Google Scholar]
- 37.Klein RG, Abikoff H, Barkley RA, et al. Clinical trials in children and adolescents Prien RF, Robinson DS. Clinical evaluation of psychotropic drugs: principles and guidelines New York, NY: Raven, 1994 [Google Scholar]
- 38.Adler CM, DelBello MP, Mills NP, Schmithorst V, Holland S, Strakowski SM. Comorbid ADHD is associated with altered patterns of neuronal activation in adolescents with bipolar disorder performing a simple attention task. Bipolar Disord 2005;7:577–8 [DOI] [PubMed] [Google Scholar]
- 39.Friston KJ, Holmes AP, Worsley KJ, Poline J-B, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general approach. Hum Brain Mapp 1995;2:189–210 [Google Scholar]
- 40.Talairach J, Tournoux PA. Co-planar stereotaxic atlas of the human brain. Stuttgart, Germany: Thieme Medical, 1988 [Google Scholar]
- 41.Bullmore ET, Suckling J, Overmeyer S, Rabe-Hesketh S, Taylor E, Brammer MJ. Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans Med Imaging 1999;18:32–42 [DOI] [PubMed] [Google Scholar]
- 42.Farquharson J, Cockburn F, Patrick WA, Jamieson EC, Logan RW. Infant cerebral cortex phospholipid fatty-acid composition and diet. Lancet 1992;340:810–3 [DOI] [PubMed] [Google Scholar]
- 43.Gibson RA, Neumann MA, Makrides M. Effect of dietary docosahexaenoic acid on brain composition and neural function in term infants. Lipids 1996;31S:177–81 [DOI] [PubMed] [Google Scholar]
- 44.Makrides M, Neumann MA, Byard RW, Simmer K, Gibson RA. Fatty acid composition of brain, retina, and erythrocytes in breast- and formula-fed infants. Am J Clin Nutr 1994;60:189–94 [DOI] [PubMed] [Google Scholar]
- 45.Metcalfe LD, Schmitz AA, Pelka JR. Rapid preparation of fatty acid esters from lipids for gas chromatographic analysis. Anal Chem 1966;38:514–5 [Google Scholar]
- 46.Sands SA, Reid KJ, Windsor SL, Harris WS. The impact of age, body mass index, and fish intake on the EPA and DHA content of human erythrocytes. Lipids 2005;40:343–7 [DOI] [PubMed] [Google Scholar]
- 47.Itomura M, Fujioka S, Hamazaki K, et al. Factors influencing EPA+DHA levels in red blood cells in Japan. In Vivo 2008;22:131–5 [PubMed] [Google Scholar]
- 48.Sublette ME, Milak MS, Hibbeln JR, et al. Plasma polyunsaturated fatty acids and regional cerebral glucose metabolism in major depression. Prostaglandins Leukot Essent Fatty Acids 2009;80:57–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vancassel S, Aïd S, Pifferi F, et al. Cerebral asymmetry and behavioral lateralization in rats chronically lacking n−3 polyunsaturated fatty acids. Biol Psychiatry 2005;58:805–11 [DOI] [PubMed] [Google Scholar]
- 50.Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. J Neurosci 2001;21:700–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berquin PC, Giedd JN, Jacobsen LK, et al. Cerebellum in attention-deficit hyperactivity disorder: a morphometric MRI study. Neurology 1998;50:1087–93 [DOI] [PubMed] [Google Scholar]
- 52.Rubia K, Smith AB, Halari R, et al. Disorder-specific dissociation of orbitofrontal dysfunction in boys with pure conduct disorder during reward and ventrolateral prefrontal dysfunction in boys with pure ADHD during sustained attention. Am J Psychiatry 2009;166:83–94 [DOI] [PubMed] [Google Scholar]
- 53.Muzik O, Chugani DC, Juhász C, Shen C, Chugani HT. Statistical parametric mapping: assessment of application in children. Neuroimage 2000;12:538–49 [DOI] [PubMed] [Google Scholar]
- 54.Wilke M, Schmithorst VJ, Holland SK. Assessment of spatial normalization of whole-brain magnetic resonance images in children. Hum Brain Mapp 2002;17:48–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsukada H, Kakiuchi T, Fukumoto D, Nishiyama S, Koga K. Docosahexaenoic acid (DHA) improves the age-related impairment of the coupling mechanism between neuronal activation and functional cerebral blood flow response: a PET study in conscious monkeys. Brain Res 2000;862:180–6 [DOI] [PubMed] [Google Scholar]
- 56.Phillis JW, Horrocks LA, Farooqui AA. Cyclooxygenases, lipoxygenases, and epoxygenases in CNS: their role and involvement in neurological disorders. Brain Res Rev 2006;52:201–43 [DOI] [PubMed] [Google Scholar]
- 57.Rao JS, Ertley RN, Demar JC, Jr, Rapoport SI, Bazinet RP, Lee HJ. Dietary n−3 PUFA deprivation alters expression of enzymes of the arachidonic and docosahexaenoic acid cascades in rat frontal cortex. Mol Psychiatry 2007;12:151–7 [DOI] [PubMed] [Google Scholar]
- 58.Zimmer L, Vancassel S, Cantagrel S, et al. The dopamine mesocorticolimbic pathway is affected by deficiency in n−3 polyunsaturated fatty acids. Am J Clin Nutr 2002;75:662–7 [DOI] [PubMed] [Google Scholar]
- 59.Chen YC, Galpern WR, Brownell AL, et al. Detection of dopaminergic neurotransmitter activity using pharmacologic MRI: correlation with PET, microdialysis, and behavioral data. Magn Reson Med 1997;38:389–98 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.