Abstract
The cystic fibrosis transmembrane conductance regulator (CFTR) is an ATP-binding cassette (ABC) transporter that functions as a cAMP-activated chloride channel. The recent model of CFTR gating predicts that the ATP binding to both nucleotide-binding domains (NBD1 and NBD2) of CFTR is required for the opening of the channel, while the ATP hydrolysis at NBD2 induces subsequent channel closing. In most ABC proteins, efficient hydrolysis of ATP requires the presence of the invariant histidine residue within the H-loop located in the C-terminal part of the NBD. However, the contribution of the corresponding region (H-loop) of NBD2 to the CFTR channel gating has not been examined so far. Here we report that the alanine substitution of the conserved dipeptide HR motif (HR→AA) in the H-loop of NBD2 leads to prolonged open states of CFTR channel, indicating that the H-loop is required for efficient channel closing. On the other hand, the HR→AA substitution lead to the substantial decrease of CFTR-mediated current density (pA/pF) in transfected HEK 293 cells, as recorded in the whole-cell patch-clamp analysis. These results suggest that the H-loop of NBD2, apart from being required for CFTR channel closing, may be involved in regulating CFTR trafficking to the cell surface.
Key Words: CFTR, Cystic fibrosis, ABC transporters, Nucleotide-binding domain, H-loop
Introduction
The ATP-binding cassette (ABC) transporters are a large group of transmembrane proteins that utilize the energy derived from ATP hydrolysis to transport various substrates across the membrane against the concentration gradient [1]. The cystic fibrosis transmembrane conductance regulator (CFTR) differs from other members of this superfamily in that it functions as an epithelial cAMP-activated anion channel that allows for passive transport of chloride ions down their electrochemical gradient [2, 3]. Additionally, CFTR may regulate other epithelial ion channels or transporters, mediating the transmembrane Cl−, HCO3−, Na+, K+ and Ca2+ transport, thereby significantly contributing to the maintaining of the overall ion balance in the epithelia [4]. Defective function of CFTR is responsible for several human diseases, including cystic fibrosis (CF), congenital bilateral absence of the vas deferens (CBAVD) and chronic pancreatitis [5], all associated with abnormal ion fluxes in epithelial tissues of different organs.
According to the current model of ABC transporter mechanism, the concerted action of two nucleotide-binding domains (NBDs), forming a so-called nucleotide-sandwich dimer, is essential for efficient transport activity [6, 7]. The NBD dimer, arranged in a head to tail orientation, is predicted to contain two ATP-binding sites, each formed by the Walker A and Walker B motifs of one nucleotide-binding domain and the ABC signature from the other NBD [8, 9]. It has been proposed that both the formation of an ATP-bound dimer and the subsequent hydrolysis and dissociation of the bound nucleotides are associated with specific conformational changes that are further transmitted to the transmembrane domains of the protein, thus facilitating the active transport of the substrate molecule [10, 11]. The application of the above model to explain the functioning of CFTR, a protein being an atypical ABC transporter, requires certain modifications that take into account the structural and functional differences between CFTR and other ABC transporters. Many details of this CFTR-specific model are still unknown and the mechanism of CFTR gating remains a subject of ongoing debate [12, 13, 14]. Nevertheless, there seems to be general agreement that ATP binding to both NBDs of CFTR is required for the formation of a tightly bound NBD1-NBD2 heterodimer that induces channel opening [15, 16]. The subsequent hydrolysis of the ATP molecule bound to the site formed by the Walker A and Walker B motifs of NBD2 has been suggested to promote channel closing [17, 18, 19]. Indeed, mutations predicted to affect the NBD2-mediated hydrolysis retard the closing of CFTR [15, 20, 21].
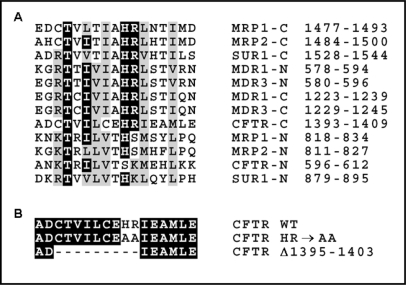
Among different amino acid motifs contributing to the NBD-mediated hydrolysis in ABC proteins, a special attention has been given to the so-called H-loop or switch motif, constituting a conserved structural element within the carboxyl terminal region of NBD in most ABC proteins [22]. This loop contains an invariant histidine residue that is required for both ATP hydrolysis and transport activity [23, 25, 27, 29], although not for ATP binding [23, 25, 27, 29, 30]. In about half of the known ABC transporters, this invariant histidine is accompanied by the arginine residue suggested to undergo a significant displacement following the conformational change induced by nucleotide binding or release [31]. Importantly, it has been noted that the region corresponding to the H-loop in NBD1 of CFTR lacks the conserved histidine residue (Fig. 1), which seems to be consistent with the limited ability of NBD1 to hydrolyze ATP [32, 33]. Since the analogous region in NBD2 retains the characteristic consensus sequence, including the conserved HR motif, it seems plausible that the H-loop of NBD2 plays an important role in the NBD2-mediated ATP hydrolysis. However, the exact function of this motif in CFTR has not been tested so far.
Fig. 1.
The H-loop region in nucleotide-binding domains of CFTR and other human ABC transporters. A. Multiple sequence alignment showing conserved amino acids within the H-loop region of the N-terminal (N) or C-terminal (C) NBDs of different human ABC proteins. MRP1 (ABCC1) and MRP2 (ABCC2) – multidrug resistance related proteins 1 and 2; MDR1 (ABCB1) and MDR3 (ABCB4) – multidrug resistance proteins 1 and 3; SUR1 (ABCC8) – sulfonylurea receptor 1. The position of the H-loop region in the amino acid sequence of the full-length proteins is indicated on the left. B. The sequence of the H-loop region in CFTR constructs with HR→AA and Δ1395–1403 mutations.
The C-terminal region of NBD2 in CFTR has been previously found to affect the folding of CFTR [34, 35] and of CFTR-derived peptides [36, 37, 38]. Specifically, the short nine-amino acid sequence, termed the “ag region”, has been found to induce the aggregation of the CFTR-derived C-terminal peptides [36, 37], even when inserted into a new amino-acid context [38]. The ag region contains amino acids 1395–1403 of the full-length human CFTR, including the His1402 residue that corresponds to the conserved histidine in the H-loop. Intriguingly, the potential of the ag region to induce protein aggregation seems to depend mostly on the presence of His1402 and the adjacent Arg 1403 residue, since substitution of these two residues with alanines prevents the aggregation of the CFTR-derived peptides [37]. This suggests that the conserved HR motif in H-loop may constitute an important element of the NBD2 structure.
To investigate whether the ag region is critical for the structural and functional integrity of the full-length CFTR protein, we examined the maturation process and the chloride channel function of two mutant CFTR proteins, devoid either of the entire ag region or of the conserved HR motif, predicted to constitute an essential part of the H-loop in NBD2.
Materials and Methods
Plasmid construction
The creation of pRSV-CFTR, a Rous sarcoma virus (RSV)-driven expression plasmid containing the full-length wild type (WT) CFTR sequence, was described elsewhere [39]. Also, the introduction of the ΔF508 mutation into this expression plasmid was previously described [40]. Mutations within the H-loop of NBD2, including the deletion of the entire ag region (Δ1395–1403) and the double alanine substitution H1402A, R1403A (HR→AA), were created in the CFTR-containing pBQ4.7 vector (a gift from J. Rommens and L.-C. Tsui) using the site-directed mutagenesis system “Transformer” (Becton Dickinson). The mutations were then shuttled into the pRSV-CFTR plasmid using the NcoI and SalI restriction sites common to both plasmids. The GFP-encoding eukaryotic expression plasmid pRK5-GFP was previously described [36].
Cell culture
The human embryonic kidney cell line HEK 293 was grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS), 2mM L-glutamine and 1mM penicillin and streptomycin. Cell cultures were incubated in a humid air atmosphere enriched with 5% CO2 in 37°C. All cell culture reagents were from ICN Biochemicals.
Cell transfection
To obtain transiently transfected HEK 293 cells for the immunoprecipitation analysis, the lipofectin reagent (Invitrogen) was used according to the manufacturer's protocol. For the patch clamp analysis, the transfection protocol was as follows: cells were plated at 100 mm culture dishes in the sufficient density to obtain 30–50% confluency following overnight growth. On the next day, the CFTR- and GFP-encoding plasmids (mixed in 1:1 ratio) were diluted in DMEM and incubated for 15 minutes at room temperature with Plus Reagent. The lipofectamine solution (in DMEM) was then added and the cells were incubated for 3 hours. After transfection the cells were kept in the standard medium and used for experiments 24–48 hours later. Only cells showing GFP expression under fluorescence microscope were used for subsequent patch-clamp experiments. Lipofectamine and PlusReagent were from Invitrogen.
CFTR immunoprecipitation
Transiently transfected HEK 293 cells were lysed in the lysis buffer (20 mM HEPES pH 7.0, 150 mM NaCl, 1 mM EDTA, and 1% NP-40) supplemented with aprotinin and phenylmethylsulfonyl fluoride (PMSF). Lysates were pre-cleared overnight at 4°C with protein A-Sepharose beads, and the protein concentration of the lysate was determined using a protein assay kit (Sigma). Four mg of protein was incubated with 1 μg of the C-terminus-specific monoclonal anti-CFTR antibody (Zymed) in 1 ml of RIPA buffer (20 mM Tris-HCl pH=7.5, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate and 0.1% SDS) for 90 min at 4°C. Then 20 μl of washed protein A-Sepharose beads was added and incubated for 30 min at 4°C. Samples were centrifuged at 10 krpm and the pellets were then washed five times (10 min each at 4°C) with RIPA buffer. After additional washing with TBS pH=8.0 the precipitate was resuspended in 50 mM Tris pH=7.5, 10 mM MgCl2, 0.1 mg/ml bovine serum albumin.
PKA labeling
Five units of protein kinase A (PKA, Sigma) and 10 μCi of [γ-32P]ATP (Dupont NEN) were added to the beads with immunoprecipitated CFTR, and the whole solution was incubated at 30°C for 1 h. The beads were then washed twice (10 min at 4°C) in RIPA buffer, and the labeled proteins were eluted in standard electrophoresis buffer by 5 min incubation at 37°C. The radiolabeled samples were electrophoresed on 6% SDS-PAGE gel at 150 V for 1–2 h. The gels were then fixed, dried and autoradiographed.
Patch-clamp analysis
The single-channel and whole-cell currents were measured using the Axopatch 200A amplifier (Axon Instruments). The classic patch clamp protocol was applied [41]. Shortly, cells grown on a Petri dish were placed under the inverted Nikon fluorescent microscope. The patch pipette tip was placed in the close proximity of a cell and gentle suction was applied to draw a small patch of the membrane into the pipette. A tight seal was formed between the pipette and the cell membrane, resulting in the so-called cell-attached configuration. The whole-cell configuration was obtained by a subsequent application of a short electric pulse that broke the membrane patch. The cell-attached configuration allows the measurement of single channel activity, while the whole-cell mode measures the current flowing through all the channels present in the plasma membrane. Patch pipettes were pulled from a thin-wall borosilicate glass with micropipette puller and fire-polished (Narishige). Pipettes resistances were 3–5 Mω for the whole-cell configuration and 10–12 Mω for single-channel measurements. All measurements were made at room temperature. The compositions of the bath and the pipette solutions were designed to have chloride as the main anion. In control experiments, the bath and the pipette solution contained 129 mM Tris-HCl and 16 mM TEA-Cl (tetraethylamine chloride) to block voltage-dependent potassium channels. Perfusion solutions (bath solution) were supplemented with 250 μM 8-CPT-cAMP (8-[4-chlorophentylthio]-adenosine-3':5'-cyclic monophosphate) or 250 μM 8-CPT-cAMP with either 500 μM DIDS (4,4'- diisothiocyanato-stilbene-2,2'-disulfonic acid) or 100 μM glibenclamide, pH=7.4. The holding potential was set to −30 mV. A voltage stepped from −100 mV to +100 mV, at 10 mV increments, for a duration of 345 ms (whole-cell) or 2–5 seconds (single-channel). The whole-cell data were recorded after filtration at 280 Hz, while the single channel measurements were recorded after 5 kHz filtration. For the analysis of single-channel data, the CFTR currents were filtered again at 50 Hz (the noise 0.006 pA), whereas the ORCC-like currents were filtered at 500 Hz (noise 0.02 pA). To determine the open and closed time constants, the 5 kHz filtered data were used. When testing the impact of channel blockers, the measurements were made 5 min after addition of DIDS and 10 min after addition of glibenclamide. Pulse generation, data collection and analyses were done with Clampex 7.0 software (Axon Instrument). The single channel conductance, mean open/closed state duration, open/closed probability, etc. were calculated from data obtained in multiple experiments (with exemplary traces, illustrating the general trend in the data, shown in the corresponding figures). The number of experiments (n) was five or greater, unless otherwise stated. Current density (pA/pF) was defined as the ratio of the current magnitude to the capacitance. Data points from a steady-state levels were taken to generate current-voltage (I-V) plots. Data were given as means ± S.E.M. To test whether a mean of a variables differs between the “non-expressing” and “expressing” cells, One-way Analysis of Variance (ANOVA) and Tuke-Kramer Multiple Comparisons Test were applied. Significant differences between results were assumed when P<0.05. To test linear correlation of the data points, Person test was performed.
Results
The region overlapping with the H-loop in NBD2 is required for proper folding of CFTR
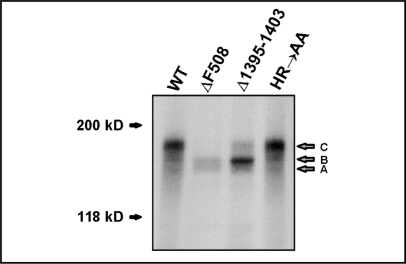
To examine whether mutations in the aggregation-prone region (ag region), that overlaps with the H-loop in NBD2 of CFTR, affect the maturation rate of the full-length CFTR protein, we created the CFTR expression plasmids containing two different mutations: 1) the deletion of the entire ag region (Δ1395–1403) or 2) the alanine substitution of the highly conserved HR motif (HR→AA) within the ag region (Fig. 1). To analyze the impact of these mutations on the processing of CFTR, we immunoprecipitated the mutant CFTR proteins from the transiently transfected HEK 293 cells. The proteins were then labeled using protein kinase A (PKA)-mediated 32P radiolabeling. Their maturation status was evaluated based on their migration rate in denaturing gel electrophoresis (Fig. 2). The wild-type (WT) CFTR and the ΔF508 mutant were used as controls for the properly processed (mature, fully glycosylated) and misprocessed (immature, core glycosylated) proteins, respectively. Most of the protein with the deletion of the entire ag region (Δ1395–1403) was misprocessed and retained in the endoplasmic reticulum, as can be judged from its incomplete glycosylation status. Only about 10–20% of this mutant protein underwent full glycosylation in the Golgi apparatus and was most likely further transported to the post-Golgi compartments, including the cell surface. On the other hand, the introduction of the HR→AA substitution did not interfere with the maturation process of CFTR. These results suggested that the NBD2 fragment encompassing the H-loop is required for proper folding and maturation of CFTR, although certain conserved amino acids within this region can be substituted without disrupting the protein processing.
Fig. 2.
Maturation analysis of the CFTR mutants. The autoradiograph shows wild-type and mutant CFTR proteins that were expressed in HEK 293 cells, immunoprecipitated using monoclonal anti-CFTR antibody, radiolabeled with 32P using protein kinase A (PKA) and electrophoretically separated by SDS-PAGE. Band C corresponds to the fully glycosylated mature CFTR protein, whereas the non-glycosylated or core-glycosylated proteins migrate as bands A and B, respectively [80].
The HR→AA mutation in NBD2 retards CFTR channel closing
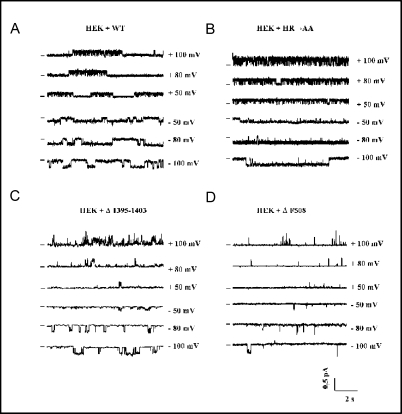
Since the HR→AA substitution did not affect the maturation of CFTR processing, we tested whether the mutant protein, once inserted into the plasma membrane, exhibits unaltered chloride channel properties. To this end, we used the single-channel patch-clamp analysis in the cell-attached configuration. The single channel currents, generated by the HR→AA mutant in response to 8-CPT-cAMP, resembled the currents produced by the wild-type protein (Fig. 3A and 3B). However, though the single channel conductances were almost identical in both cases (7.9±1.2 pS for HR→AA and 8.2±0.9 pS for WT CFTR), the channels formed by the HR→AA mutant showed significantly increased open probability (0.80±0.13 for HR→AA vs. 0.40±0.19 for WT CFTR at −80 mV; P<0.02). This corresponded to longer individual openings of the HR→AA CFTR channels (63.8±8.4 ms vs. 33.7±13.5 ms for WT CFTR), which clearly indicated that the mutation in the H-loop resulted in slowing down the channel's closing rate. The same mutation did not seem to substantially affect the channel's opening rate, as the difference between the mean closed interburst duration recorded for HR→AA (3.4±1.3 ms) and for WT CFTR (4.1±1.2 ms) was not statistically significant. This indicated that the HR→AA substitution did not prevent the NBD-mediated ATP binding, required for efficient channel opening. On the other hand, the recordings of the Δ1395–1403 and ΔF508 mutant channels (Fig. 3C and 3D) revealed prolonged duration of the closed (interburst) states, suggesting that these two folding mutants, even when reaching the plasma membrane, show decreased ability to acquire the open channel conformation.
Fig. 3.
Exemplary single channel traces from HEK 293 cells expressing WT (A), HR→AA (B), Δ1395–1403 (C) or Δ508 (D) CFTR. The horizontal bars, placed to the left of the individual records, indicate closed states of the channel (baseline level). The voltage was stepped from −100 to +100 mV at 10 mV increments for 2 seconds. Signal was filtered at 280 Hz. Both pipette and bath solutions contained 129 mM Tris-HCl and 16 mM TEA-Cl. Bath solution was supplemented with 250 μM 8-CPT-cAMP.
The small subconductance state of CFTR is not eliminated by mutations in the NBD2 H-loop
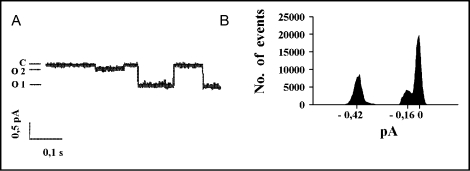
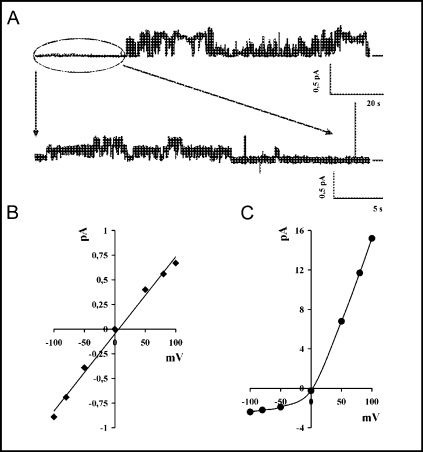
Apart from the most frequent 8 pS conductance state, additional small subconductances were occasionally seen in single-channel patch-clamp recordings following the stimulation with 8-CPT-cAMP (Fig. 4). These small subconductance states were produced by both the wildtype (2.0±0.3 pS) and mutant CFTR proteins (1.9±0.3 pS for HR→AA and 2.4±0.3 pS for Δ1395–1403 CFTR), although they were not observed in patches from mock-transfected cells (data not shown). The openings of the small conductance channels were very rare (Po ≈ 0.01) and seen only at high values (either negative or positive) of holding potentials, when the small amplitudes could be distinguished from the background signal. In contrast to a previous study reporting similar double-conductance behavior of human CFTR in HEK 293 cells [42], we observed no openings demonstrating cumulated conductance (8 pS + 2 pS), which suggested that the large (O1) and small (O2) subconductance states were mutually exclusive. Since the large subconductance openings for the HR→AA mutant were very long, the small subconductance openings of this mutant channel were seen even less frequently than the very rare O2 openings of WT CFTR, making the statistical comparison of their mean open times unfeasible. Both subconductances (O1 and O2) showed sensitivity to glibenclamide and insensitivity to DIDS (data not shown), which was consistent with the characteristic blocker profile of CFTR [43].
Fig. 4.
The subconductance states recorded in patches expressing the wild-type CFTR channel. Potential −80 mV, signal was filtered at 50 Hz. A. An exemplary single channel trace showing closed (C) state with large (O1) and small (O2) subconductance openings. B. A diagram showing the distribution of amplitudes for all openings recorded at potential −80 mV.
Mutations in the H-loop of NBD2 do not abolish the CFTR-mediated ORCC activation
In certain plasma membrane patches expressing the CFTR-specific currents, an additional chloride channel activity was seen (Fig. 5). This new channel was easily distinguished from CFTR based on its relatively large conductance (Fig. 5A) that reached about 120 pS at conductive potentials and 25 pS at rectifying ones. In contrast to CFTR, it showed apparent outward rectification (Fig. 5B and 5C, respectively) and sensitivity to DIDS (data not shown). The activity of this outwardly rectified chloride channel (ORCC) was seen in 5 of 22 patches expressing WT CFTR, in 1 of 8 patches expressing HR→AA CFTR, and in 2 of 6 patches expressing Δ1395–1403 CFTR, while it was never observed in control experiments, performed on patches from mock-transfected cells. This was consistent with previous studies, showing the ability of CFTR to regulate the activity of DIDS-sensitive outwardly rectified chloride channels [44, 45]. It also indicated that both H-loop CFTR mutants were able to induce the ORCC-specific currents. However, our results suggested that the endogenous ORCC channels, expressed in HEK 293 cells, are much less numerous than the exogenously expressed CFTR channels. Unfortunately, the limited number of patches showing ORCC activity did not allow for statistically reliable comparison of single-channel properties of these outwardly rectifying channels, expressed in the presence of different CFTR variants.
Fig. 5.
The outwardly rectified chloride channel (ORCC) activity in patches expressing the wild type CFTR channel. A. An exemplary single channel recording showing both CFTR and ORCC activity. The first part of the upper trace, enhanced in the lower trace, shows CFTR openings, while the remaining part of the upper trace corresponds to ORCC activity, characterized by large amplitude of channel openings. Potential was +50 mV and signal was filtered at 500 Hz (upper trace) or 50 Hz (lower trace). The horizontal bars, placed to the right of the individual records, indicate closed states of the channel (baseline level). B. The linear I-V relationship for the CFTR channel. C. The I-V relationship for the large conductance channel showing outward rectification (ORCC).
Mutations within the H-loop of NBD2 affect the density of CFTR- and ORCC-specific currents
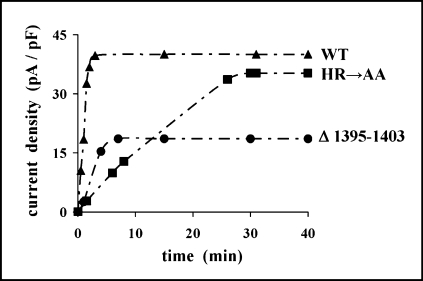
To examine the impact of mutations within the H-loop on macroscopic CFTR- and ORCC-mediated currents, we performed the whole-cell patch-clamp measurements in transfected HEK 293 cells. In contrast to the ΔF508 CFTR-expressing cells, showing practically no response to the 8-CPT-cAMP stimulation, the cells expressing the HR→AA and Δ1395–1403 CFTR mutants responded to such stimulation in a way similar to the wildtype CFTR-expressing cells. However, although 8-CPT-cAMP fully activated WT CFTR after about 3 minutes, the Δ1395–1403 and HR→AA CFTR mutants required approximately 7 and 30 minutes, respectively, to reach the maximal level of activation (Fig. 6).
Fig. 6.
The kinetics of a whole-cell currents in exemplary HEK 293 cells transfected with a wild-type (WT, triangles), HR→AA (squares) or Δ1395–1403 (circles) CFTR. At time zero 250 μM 8-CPT-cAMP was added to the bath. Holding potential was −30 mV. Once in 60 seconds the potential was changed to +100 mV and the current was recorded after 345 ms.
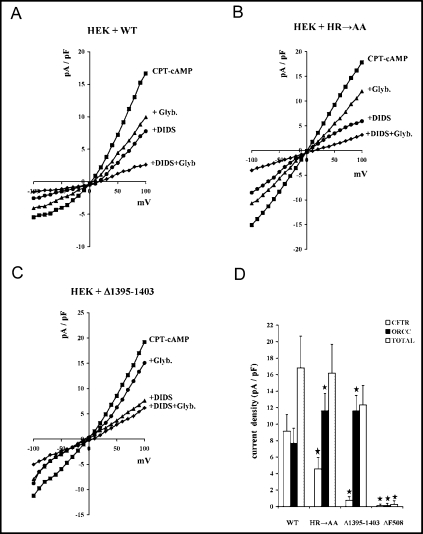
To distinguish between the CFTR- and ORCC-generated currents, we analyzed their sensitivity to DIDS, assuming that DIDS-sensitive currents should correspond to ORCC activity [46]. Since CFTR-mediated currents were reported to be sensitive to glibenclamide [47], we additionally tested the impact of this channel blocker on the recorded whole-cell currents. The current generated by cells expressing the wild-type CFTR protein showed apparent outward rectification (Fig. 7A), which indicated that the ORCC-mediated ion fluxes constituted a significant fraction of the overall chloride channel activity that was induced upon 8-CPT-cAMP stimulation. The outward rectification was much less evident in cells expressing the mutated HR→AA or Δ1395–1403 CFTR proteins (Fig. 7B and 7C, respectively). However, the ORCC-specific channel blocker DIDS was able to substantially decrease the currents generated in the presence of all above CFTR variants (Fig. 7A-C). The analysis of the relative contribution of the ORCC-specific (DIDS-sensitive) and CFTR-specific (DIDS-insensitive and glibenclamide-sensitive) currents (Fig. 7D) revealed that the density of the CFTR-specific currents in cells expressing HR→AA CFTR (4.6±1.4 pA/pF) or Δ1395–1403 CFTR (0.74±0.46 pA/pF) was significantly lower than in the case of cells expressing WT CFTR (9.2±2.0 pA/pF). Most likely, the reduced ability of the maturation-deficient Δ1395–1403 mutant to reach the plasma membrane was responsible for its low activity at the cell surface. However, such an explanation could not be satisfactory in the case of the HR→AA mutant that was previously shown to be properly processed (see Fig. 2) and active in the plasma membrane, where it additionally showed increased open channel probability (Fig. 3B). Thus, the decreased density of CFTR currents, generated by the HR→AA mutant, was most likely associated with much lower number of active channels present in the plasma membrane.
Fig. 7.
The CFTR- and ORCC-specific currents in HEK 293 cells expressing different variants of CFTR. A-C. The I-V relationship for currents recorded in whole-cell patch-clamp experiments performed on HEK 293 cells expressing WT (A, n=10), HR→AA (B, n=8), and Δ1395–1403 (C, n=5) CFTR channels. Currents were activated by 250 μM 8-CPT-cAMP and after reaching the maximum amplitude they were blocked with 500 μM DIDS, and then with 500 μM DIDS and 100 μM glibenclamide. In some experiments 5 minutes after activation 100 μM glibenclamide was added followed by 500 μM DIDS and 100 μM glibenclamide. Holding potential was −30 mV; voltage steps from −100 mV to +100 mV with 10 mV increments; duration of 345 ms; no leak subtraction. The outward rectification, seen in the 8-CPT-cAMP-stimulated currents generated by all three CFTR variants, lead to increased current densities at positive (+100 mV) voltages, as compared to the corresponding values at negative (−100 mV) voltages. Rectification was stronger for the wild-type CFTR (A, about three-fold increase) than for both mutants (B and C, less than two-fold increase). D. Average chloride current density (pA/pF) of CFTR (glibenclamide-sensitive), ORCC (DIDS-sensitive), and total chloride channels measured in HEK 293 cells expressing WT and mutant CFTR: HR→AA (n=8), Δ1395–1403 (n=5), Δ508 (n=7). Potential 80 mV. * denotes significant (p<0.01) change of current density, as compared with WT.
In contrast to the CFTR-specific currents, the ORCC activity seemed to be elevated in cells expressing the HR→AA and Δ1395–1403 mutants (Fig. 7D). The densities of DIDS-sensitive currents were significantly higher in cells expressing HR→AA CFTR (11.6±2.1 pA/pF) or Δ1395–1403 CFTR (11.6±1.9 pA/pF) than in cells expressing the wild-type protein (7.7±1.8 pA/pF). This suggested that mutations in the H-loop of NBD2 may affect the CFTR-mediated regulation of ORCC.
Discussion
A large body of evidence indicates that the transition from the open to closed state of CFTR is facilitated by the hydrolysis of ATP bound at NBD2 [15, 16, 19, 20, 21, 48, 49, 50]. According to the NBD dimer model, the NBD2 nucleotide-binding site is formed by the Walker A and B motifs of NBD2 and the ABC signature motif of NBD1 [3, 32, 33]. Based on the analogy with NBDs of other ABC transporters, it has been predicted that the H-loop of NBD2 participates in hydrolysis of ATP bound at this particular site [32, 33, 49], thereby possibly contributing to channel closing. Our finding that the mutation of the conserved HR motif within the H-loop of NBD2 prolongs the open time duration of individual CFTR channels provides the first evidence that this conserved element of NBD structure is indeed required for efficient closing of CFTR, the only ion channel among ABC transporters.
Previous studies on conserved amino acids in the H-loop of ABC transporters have focused on the invariant histidine residue that has been recently suggested to form a “catalytic dyad” with the conserved glutamate residue from the Walker B motif [51, 52]. This model assumes that both these residues are essential for ATP hydrolysis, with the H-loop histidine acting as a linchpin that holds together all the components required for this reaction. Specifically, the histidine residue is supposed to stabilize the transition state of hydrolysis by forming the hydrogen bonds with the ATP γ-phosphate and the catalytic water molecule that interacts with both residues of the catalytic dyad. In agreement with this model, the ability of the H-loop histidine to form the hydrogen bonds has been recently shown to be crucial for the proper functioning of the multidrug resistance protein MRP1 [53]. However, another recent study has demonstrated that alanine substitution of the H-loop histidine residue in NBD2 of the yeast multidrug transporter Pdr5 does not affect the steady ATPase activity of this protein, although it selectively abolishes the transport of rhodamine, while not affecting the transport of other substrates [54]. This clearly shows that ATP hydrolysis may be uncoupled from the transport activity. Furthermore, it indicates that the H-loop may have different functions in different ABC transporters. Thus, though our study indicates that the H-loop in NBD2 of CFTR is involved in chloride channel closing, it may also have an additional role related, for example, to the regulatory function of CFTR.
Several other amino acid residues in NBD2 of CFTR have previously been reported to affect the rate of CFTR channel closing. This includes Lys1250 [15, 19, 20, 55] and Asp1370 [15, 20, 21], both required for efficient ATP hydrolysis at NBD2, as well as Asn1303 in the putative γ-phosphate linker region, predicted to couple the nucleotide binding to a movement of an α-helical subdomain of NBD [56]. However, mutations at all above positions abrogate not only ATP hydrolysis but also ATP binding, thus affecting both the opening and closing rates of the channel. By contrast, the HR→AA substitution in the H-loop of NBD2, although slowing down the channel closing, did not decrease the rate of channel opening. This suggests that the conserved histidine and arginine residues in the H-loop are not required for ATP binding. Although additional biochemical studies are needed to support this conclusion, it is consistent with the observation that NBD1 of CFTR is able to bind ATP [57, 58], despite lacking the HR motif in its H-loop region. The only other conserved CFTR residue that shows similar effect on NBD function is Glu 1371, a putative partner of His1402 from the catalytic dyad in NBD2. Substitution of this conserved glutamate with either serine (E1371S) or glutamine (E1371Q) produces a channel that displays prolonged open times [3, 16, 21, 59]. Importantly, a recent study has demonstrated that the E1371Q substitution leads to significant decrease of ATPase activity in the full-length CFTR [50] and an earlier study has shown that the same mutation abolishes the ATPase activity of the NBD1-NBD2 heterodimer, while not affecting the nucleotide binding affinity of NBD2 [49]. Hence, although we have no data regarding the influence of E1371Q on the CFTR opening rate, it seems that mutations affecting either the H-loop or Glu 1371 have a very similar impact on ATP-dependent CFTR gating, which suggests close cooperation of both these elements, as predicted in the catalytic dyad model. As the arginine residue in H-loop of ABC transporters is less conserved than the adjacent invariant histidine, it seems likely that Arg 1403 is not required for NBD2-mediated ATP hydrolysis. However, it would be interesting to know whether, and to what extent, this particular residue contributes to CFTR channel closing, especially when suspecting that it may undergo a considerable displacement upon ATP release, as has been shown for the analogous arginine residue in the archeal MJ1267 transporter [31].
Although we have shown that the HR motif in NBD2 contributes to CFTR channel closing, it remains uncertain whether both subconductance states of CFTR are equally affected by mutations within the H-loop. While our data clearly indicate that the HR→AA mutation in NBD2 prolongs the large subconductance (8 pS) channel openings, no such unequivocal conclusion could be made for the relatively rare small (2 pS) subconductance states. It is worth noting that the exact nature of these small CFTR subconductance states remains enigmatic, as their relationship with the large subconductance state of CFTR is not clear and they show remarkable variation in the amplitude, with reported conductance values ranging from 0.4 to 6 pS [42, 60, 61, 62, 63, 64]. It has been proposed that the small subconductance pore is formed by the second half of the human CFTR protein [42]. Intriguingly, the small subconductance state seems to represent a major form of chloride channel activity in murine CFTR [64], which is in apparent contrast to the human protein where large subconductance constitutes the dominant state [65]. Importantly, the replacement of either of the human NBDs with its murine counterpart seems to be not sufficient to ensure high frequency of small subconductance openings [65], thus suggesting that the membrane-spanning domains (MSDs) of CFTR rather than NBDs are responsible for imposing a specific subconductance state.
The whole-cell patch-clamp analysis revealed that the macroscopic CFTR-mediated currents were significantly reduced in cells expressing the H-loop mutants when compared to cells expressing WT CFTR (Fig. 7D). Similar decrease in whole-cell currents has been very recently reported for another H-loop mutation E1401K, associated with CBAVD [66]. The above results may seem surprising in light of our finding that the HR→AA CFTR mutant shows increased open probability in single-channel analysis (Fig. 3A). However, this observation suggests that certain consequences of mutations in the H-loop of NBD2 are discernible only in the whole cell context. One possible explanation of this opposite effect of the HR→AA mutation on the CFTR function is that the H-loop may have a dual role in regulating the CFTR activity. Apart from being involved in ATP hydrolysis-dependent CFTR closing, it may also regulate other processes that could potentially lead to increased activity of CFTR on the cell surface. For example, the H-loop may be engaged in certain regulatory mechanisms that facilitate the surface-directed trafficking of intracellular CFTR, including the retrograde trafficking of endocytosed channel molecules. This kind of regulation would result in increased CFTR activity when measured at the whole-cell level, while not affecting the single-channel properties of CFTR. Importantly, this scenario is consistent with our observation that cells expressing the CFTR H-loop mutants respond very slowly to 8-CPT-cAMP stimulation (Fig. 6), which is what one would expect from mutations affecting the vesicular transport of intracellular CFTR to the cell surface.
Besides affecting the CFTR-specific currents, the H-loop seems to influence the activity of the outwardly rectified chloride channel (ORCC), previously reported to be regulated by CFTR [43, 44]. The exact mechanism of the CFTR-mediated regulation of ORCC remains unknown [67, 68], although it has been suggested that NBD1 and the regulatory (R) domain of CFTR may be essential for regulatory interaction with ORCC [69]. Our study, showing increased ORCC activity in cells expressing the CFTR protein with mutations within the H-loop of NBD2 (Fig. 7D), suggests that NBD2 may also play an important role in this process by restraining CFTR-stimulated ORCC activity. However, we have been unable to determine whether the mutations in H-loop of CFTR affect the rate of ORCC opening or closing, or whether they interfere with other steps of ORCC regulation, related for example to the subcellular trafficking of the channel. Further studies, including single-channel analysis performed on epithelial cells expressing ORCC at much higher level, should be undertaken to answer these questions. It is worth noting that the ORCC-specific macroscopic currents in cells expressing either HR→AA or Δ1395–1403 CFTR were increased to identical levels (Fig. 7D), although these two mutations in H-loop showed different effects on the CFTR single-channel behavior (Fig. 3B and 3C). This indicates that the H-loop-dependent regulation of ORCC may be uncoupled from the CFTR channel closing. Consequently, the CFTR-mediated regulation of ORCC may be uncoupled from the ATP hydrolysis, similarly to what has been previously observed for the Pdr5-mediated rhodamine transport in yeast [54].
It should be noted that the DIDS-sensitive ORCC activity has, to our knowledge, never been previously detected in HEK 293 cells, although several studies have used this cell line to measure the currents mediated by exogenous CFTR (for example see [42, 70, 71, 72, 73]). The channel described in this work exhibits relatively large single-channel conductance at depolarizing voltages (120 pS as compared to 40–80 pS reported for ORCC in other studies [45, 74, 75, 76, 77]) and shows no sensitivity to glibenclamide, which is in marked contrast to most previous reports [75, 78, 79], though in agreement with the studies on IB3-1 cells [39]. Since the molecular identity of ORCC has not been definitively established, one may speculate that different proteins/channels are responsible for ORCC-specific currents in different cell lines and under different conditions, thus contributing to the above discrepancies.
Acknowledgements
The work was supported by grants from the State Committee for Scientific Research (Poland) No. 3-P05A-06-923 and No. N401-056-31/1471, grant 504-10-06020011 from the President of WULS-SGGW (KD), as well as by the National Institutes of Health (United States) grant DK044003 (GRC).
References
- 1.Higgins CF. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 2.Riordan The cystic fibrosis transmembrane conductance regulator. Annu Rev Physiol. 1993;55:609–630. doi: 10.1146/annurev.ph.55.030193.003141. [DOI] [PubMed] [Google Scholar]
- 3.Gadsby DC, Vergani P, Csanady L. The ABC protein turned chloride channel whose failure causes cystic fibrosis. Nature. 2006;440:477–483. doi: 10.1038/nature04712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coakley RD, Stutts MJ. Function of CFTR Protein: Regulatory Functions. In: Bush A, Alton EWFW, Davies JC, Griesenbach U, Jaffe A, editors. Cystic Fibrosis in the 21st Century. vol 34. Basel: Karger; 2006. pp. 45–53. [Google Scholar]
- 5.Noone PG, Knowles MR. ‘CFTR-opathies’: disease phenotypes associated with cystic fibrosis transmembrane regulator gene mutations. Respir Res. 2001;2:328–332. doi: 10.1186/rr82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moody JE, Thomas PJ. Nucleotide binding domain interactions during the mechanochemical reaction cycle of ATP-binding cassette transporters. J Bioenerg Biomembr. 2005;37:475–479. doi: 10.1007/s10863-005-9494-8. [DOI] [PubMed] [Google Scholar]
- 7.Linton KJ. Structure and function of ABC transporters. Physiology (Bethesda) 2007;22:122–130. doi: 10.1152/physiol.00046.2006. [DOI] [PubMed] [Google Scholar]
- 8.Moody JE, Millen L, Binns D, Hunt JF, Thomas PJ. Cooperative: ATP-dependent association of the nucleotide binding cassettes during the catalytic cycle of ATP-binding cassette transporters. J Biol Chem. 2002;277:21111–21114. doi: 10.1074/jbc.C200228200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith PC, Karpowich N, Millen L, Moody JE, Rosen J, Thomas PJ, Hunt JF. ATP binding to the motor domain from an ABC transporter drives formation of a nucleotide sandwich dimer. Mol Cell. 2002;10:139–149. doi: 10.1016/s1097-2765(02)00576-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins CF, Linton KJ. The ATP switch model for ABC transporters. Nat Struct Mol Biol. 2004;11:918–926. doi: 10.1038/nsmb836. [DOI] [PubMed] [Google Scholar]
- 11.Linton KJ, Higgins CF. Structure and function of ABC transporters: the ATP switch provides flexible control. Pflugers Arch. 2007;453:555–567. doi: 10.1007/s00424-006-0126-x. [DOI] [PubMed] [Google Scholar]
- 12.Vergani P, Basso C, Mense M, Nairn AC, Gadsby DC. Control of the CFTR channel's gates. Biochem Soc Trans. 2005;33:1003–1007. doi: 10.1042/BST20051003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aleksandrov AA, Aleksandrov LA, Riordan CFTR (ABCC7) is a hydrolyzable-ligand-gated channel. Pflugers Arch. 2007;453:693–702. doi: 10.1007/s00424-006-0140-z. [DOI] [PubMed] [Google Scholar]
- 14.Cheung JC, Kim CP, Pasyk S, Bear CE. Molecular basis for the ATPase activity of CFTR. Arch Biochem Biophys. 2008;476:95–100. doi: 10.1016/j.abb.2008.03.033. [DOI] [PubMed] [Google Scholar]
- 15.Berger AL, Ikuma M, Welsh MJ. Normal gating of CFTR requires ATP binding to both nucleotide-binding domains and hydrolysis at the second nucleotide-binding domain. Proc Natl Acad Sci U S A. 2005;102:455–460. doi: 10.1073/pnas.0408575102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vergani P, Lockless SW, Nairn AC, Gadsby DC. CFTR channel opening by ATP-driven tight dimerization of its nucleotide-binding domains. Nature. 2005;433:876–880. doi: 10.1038/nature03313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang TC, Nagel G, Nairn AC, Gadsby DC. Regulation of the gating of cystic fibrosis transmembrane conductance regulator C1 channels by phosphorylation and ATP hydrolysis. Proc Natl Acad Sci U S A. 1994;91:4698–4702. doi: 10.1073/pnas.91.11.4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baukrowitz T, Hwang TC, Nairn AC, Gadsby DC. Coupling of CFTR Cl-channel gating to an ATP hydrolysis cycle. Neuron. 1994;12:473–482. doi: 10.1016/0896-6273(94)90206-2. [DOI] [PubMed] [Google Scholar]
- 19.Carson MR, Travis SM, Welsh MJ. The two nucleotide-binding domains of cystic fibrosis transmembrane conductance regulator (CFTR) have distinct functions in controlling channel activity. J Biol Chem. 1995;270:1711–1717. doi: 10.1074/jbc.270.4.1711. [DOI] [PubMed] [Google Scholar]
- 20.Gunderson KL, Kopito RR. Conformational states of CFTR associated with channel gating: the role ATP binding and hydrolysis. Cell. 1995;82:231–239. doi: 10.1016/0092-8674(95)90310-0. [DOI] [PubMed] [Google Scholar]
- 21.Vergani P, Nairn AC, Gadsby DC. On the mechanism of MgATP-dependent gating of CFTR Cl-channels. J Gen Physiol. 2003;121:17–36. doi: 10.1085/jgp.20028673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneider E, Hunke S. ATP-binding-cassette (ABC) transport systems: functional and structural aspects of the ATP-hydrolyzing subunits/domains. FEMS Microbiol Rev. 1998;22:1–20. doi: 10.1111/j.1574-6976.1998.tb00358.x. [DOI] [PubMed] [Google Scholar]
- 23.Shyamala V, Baichwal V, Beall E, Ames GF. Structure-function analysis of the histidine permease and comparison with cystic fibrosis mutations. J Biol Chem. 1991;266:18714–18719. [PubMed] [Google Scholar]
- 24.Bliss JM, Garon CF, Silver RP. Polysialic acid export in Escherichia coli K1: the role of KpsT, the ATP-binding component of an ABC transporter, in chain translocation. Glycobiology. 1996;6:445–452. doi: 10.1093/glycob/6.4.445. [DOI] [PubMed] [Google Scholar]
- 25.Davidson AL, Sharma S. Mutation of a single MalK subunit severely impairs maltose transport activity in Escherichia coli. J Bacteriol. 1997;179:5458–5464. doi: 10.1128/jb.179.17.5458-5464.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nikaido K, Ames GF. One intact ATP-binding subunit is sufficient to support ATP hydrolysis and translocation in an ABC transporter, the histidine permease. J Biol Chem. 1999;274:26727–26735. doi: 10.1074/jbc.274.38.26727. [DOI] [PubMed] [Google Scholar]
- 27.Benabdelhak H, Schmitt L, Horn C, Jumel K, Blight MA, Holland IB. Positive cooperative activity and dimerization of the isolated ABC ATPase domain of HlyB from Escherichia coli. Biochem J. 2005;386:489–495. doi: 10.1042/BJ20041282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perria CL, Rajamanickam V, Lapinski PE, Raghavan M. Catalytic site modifications of TAP1 and TAP2 and their functional consequences. J Biol Chem. 2006;281:39839–39851. doi: 10.1074/jbc.M605492200. [DOI] [PubMed] [Google Scholar]
- 29.Hofacker M, Gompf S, Zutz A, Presenti C, Haase W, van der Does C, Model K, Tampe R. Structural and functional fingerprint of the mitochondrial ATP-binding cassette transporter Mdl1 from Saccharomyces cerevisiae. J Biol Chem. 2007;282:3951–3961. doi: 10.1074/jbc.M609899200. [DOI] [PubMed] [Google Scholar]
- 30.Walter C, Wilken S, Schneider E. Characterization of site-directed mutations in conserved domains of MalK, a bacterial member of the ATP-binding cassette (ABC) family [corrected] FEBS Lett. 1992;303:41–44. doi: 10.1016/0014-5793(92)80473-t. [DOI] [PubMed] [Google Scholar]
- 31.Karpowich N, Martsinkevich O, Millen L, Yuan YR, Dai PL, MacVey K, Thomas PJ, Hunt JF. Crystal structures of the MJ1267 ATP binding cassette reveal an induced-fit effect at the ATPase active site of an ABC transporter. Structure. 2001;9:571–586. doi: 10.1016/s0969-2126(01)00617-7. [DOI] [PubMed] [Google Scholar]
- 32.Lewis HA, Buchanan SG, Burley SK, Conners K, Dickey M, Dorwart M, Fowler R, Gao X, Guggino WB, Hendrickson WA, Hunt JF, Kearins M, Lorimer D, Maloney PC, Post KW, Rajashankar KR, Rutter ME, Sauder JM, Shriver S, Thibodeau PH, Thomas PJ, Zhang M, Zhao X, Emtage S. Structure of nucleotide-binding domain 1 of the cystic fibrosis transmembrane conductance regulator. EMBO J. 2004;23:282–293. doi: 10.1038/sj.emboj.7600040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kidd JF, Ramjeesingh M, Stratford F, Huan LJ, Bear CE. A heteromeric complex of the two nucleotide binding domains of cystic fibrosis transmembrane conductance regulator (CFTR) mediates ATPase activity. J Biol Chem. 2004;279:41664–41669. doi: 10.1074/jbc.M407666200. [DOI] [PubMed] [Google Scholar]
- 34.Rich DP, Gregory RJ, Cheng SH, Smith AE, Welsh MJ. Effect of deletion mutations on the function of CFTR chloride channels. Receptors Channels. 1993;1:221–232. [PubMed] [Google Scholar]
- 35.Gentzsch M, Riordan Localization of sequences within the C-terminal domain of the cystic fibrosis transmembrane conductance regulator which impact maturation and stability. J Biol Chem. 2001;276:1291–1298. doi: 10.1074/jbc.M003672200. [DOI] [PubMed] [Google Scholar]
- 36.Milewski MI, Mickle JE, Forrest JK, Stafford DM, Moyer BD, Cheng J, Guggino WB, Stanton BA, Cutting GR. A PDZ-binding motif is essential but not sufficient to localize the C terminus of CFTR to the apical membrane. J Cell Sci. 2001;114:719–726. doi: 10.1242/jcs.114.4.719. [DOI] [PubMed] [Google Scholar]
- 37.Milewski MI, Mickle JE, Forrest JK, Stanton BA, Cutting GR. Aggregation of misfolded proteins can be a selective process dependent upon peptide composition. J Biol Chem. 2002;277:34462–34470. doi: 10.1074/jbc.M205420200. [DOI] [PubMed] [Google Scholar]
- 38.Bak D, Cutting GR, Milewski M. The CFTR-derived peptides as a model of sequence-specific protein aggregation. Cell Mol Biol Lett. 2007;12:435–447. doi: 10.2478/s11658-007-0014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fulmer SB, Schwiebert EM, Morales MM, Guggino WB. Cutting GR Two cystic fibrosis transmembrane conductance regulator mutations have different effects on both pulmonary phenotype and regulation of outwardly rectified chloride currents. Proc Natl Acad Sci U S A. 1995;92:6832–6836. doi: 10.1073/pnas.92.15.6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mickle JE, Milewski MI, Macek, M, Jr, Cutting GR. Effects of cystic fibrosis and congenital bilateral absence of the vas deferens-associated mutations on cystic fibrosis transmembrane conductance regulator-mediated regulation of separate channels. Am J Hum Genet. 2000;66:1485–1495. doi: 10.1086/302893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakmann B, Neher E, editors. Single-Channel Recording. Second Edition. New York: Kluwer Academic/Plenum Publishers; 1995. [Google Scholar]
- 42.Yue H, Devidas S, Guggino WB. The two halves of CFTR form a dual-pore ion channel. J Biol Chem. 2000;275:10030–10034. doi: 10.1074/jbc.275.14.10030. [DOI] [PubMed] [Google Scholar]
- 43.Schultz BD, Singh AK, Devor DC, Bridges RJ. Pharmacology of CFTR chloride channel activity. Physiol Rev. 1999;79:S109–S144. doi: 10.1152/physrev.1999.79.1.S109. [DOI] [PubMed] [Google Scholar]
- 44.Gabriel SE, Clarke LL, Boucher RC, Stutts MJ. CFTR and outward rectifying chloride channels are distinct proteins with a regulatory relationship. Nature. 1993;363:263–268. doi: 10.1038/363263a0. [DOI] [PubMed] [Google Scholar]
- 45.Jovov B, Ismailov, Benos DJ. Cystic fibrosis transmembrane conductance regulator is required for protein kinase A activation of an outwardly rectified anion channel purified from bovine tracheal epithelia. J Biol Chem. 1995;270:1521–1528. doi: 10.1074/jbc.270.4.1521. [DOI] [PubMed] [Google Scholar]
- 46.Schwiebert EM, Flotte T, Cutting GR, Guggino WB. Both CFTR and outwardly rectifying chloride channels contribute to cAMP-stimulated whole cell chloride currents. Am J Physiol. 1994;266:C1464–C1477. doi: 10.1152/ajpcell.1994.266.5.C1464. [DOI] [PubMed] [Google Scholar]
- 47.Sheppard DN, Welsh MJ. Effect of ATP-sensitive K+ channel regulators on cystic fibrosis transmembrane conductance regulator chloride currents. J Gen Physiol. 1992;100:573–591. doi: 10.1085/jgp.100.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Basso C, Vergani P, Nairn AC, Gadsby DC. Prolonged nonhydrolytic interaction of nucleotide with CFTR's NH2-terminal nucleotide binding domain and its role in channel gating. J Gen Physiol. 2003;122:333–348. doi: 10.1085/jgp.200308798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stratford FL, Ramjeesingh M, Cheung JC, Huan LJ, Bear CE. The Walker B motif of the second nucleotide-binding domain (NBD2) of CFTR plays a key role in ATPase activity by the NBD1-NBD2 heterodimer. Biochem J. 2007;401:581–586. doi: 10.1042/BJ20060968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramjeesingh M, Ugwu F, Stratford FL, Huan LJ, Li C, Bear CE. The intact CFTR protein mediates ATPase rather than adenylate kinase activity. Biochem J. 2008;412:315–321. doi: 10.1042/BJ20071719. [DOI] [PubMed] [Google Scholar]
- 51.Zaitseva J, Jenewein S, Jumpertz T, Holland IB, Schmitt L. H662 is the linchpin of ATP hydrolysis in the nucleotide-binding domain of the ABC transporter HlyB. EMBO J. 2005;24:1901–1910. doi: 10.1038/sj.emboj.7600657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zaitseva J, Jenewein S, Oswald C, Jumpertz T, Holland IB, Schmitt L. A molecular understanding of the catalytic cycle of the nucleotide-binding domain of the ABC transporter HlyB. Biochem Soc Trans. 2005;33:990–995. doi: 10.1042/BST20050990. [DOI] [PubMed] [Google Scholar]
- 53.Yang R, Chang XB. Hydrogen-bond formation of the residue in H-loop of the nucleotide binding domain 2 with the ATP in this site and/or other residues of multidrug resistance protein MRP1 plays a crucial role during ATP-dependent solute transport. Biochim Biophys Acta. 2007;1768:324–335. doi: 10.1016/j.bbamem.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ernst R, Kueppers P, Klein CM, Schwarzmueller T, Kuchler K, Schmitt L. A mutation of the H-loop selectively affects rhodamine transport by the yeast multidrug ABC transporter Pdr5. Proc Natl Acad Sci U S A. 2008;105:5069–5074. doi: 10.1073/pnas.0800191105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramjeesingh M, Li C, Garami E, Huan LJ, Galley K, Wang Y, Bear CE. Walker mutations reveal loose relationship between catalytic and channel-gating activities of purified CFTR (cystic fibrosis transmembrane conductance regulator) Biochemistry. 1999;38:1463–1468. doi: 10.1021/bi982243y. [DOI] [PubMed] [Google Scholar]
- 56.Berger AL, Ikuma M, Hunt JF, Thomas PJ, Welsh MJ. Mutations that change the position of the putative gamma-phosphate linker in the nucleotide binding domains of CFTR alter channel gating. J Biol Chem. 2002;277:2125–2131. doi: 10.1074/jbc.m109539200. [DOI] [PubMed] [Google Scholar]
- 57.Szabo K, Szakacs G, Hegeds T, Sarkadi B. Nucleotide occlusion in the human cystic fibrosis transmembrane conductance regulator. Different patterns in the two nucleotide binding domains. J Biol Chem. 1999;274:12209–12212. doi: 10.1074/jbc.274.18.12209. [DOI] [PubMed] [Google Scholar]
- 58.Aleksandrov L, Mengos A, Chang X, Aleksandrov A, Riordan Differential interactions of nucleotides at the two nucleotide binding domains of the cystic fibrosis transmembrane conductance regulator. J Biol Chem. 2001;276:12918–12923. doi: 10.1074/jbc.M100515200. [DOI] [PubMed] [Google Scholar]
- 59.Bompadre SG, Ai T, Cho JH, Wang X, Sohma Y, Li M, Hwang TC. CFTR gating I: Characterization of the ATP-dependent gating of a phosphorylation-independent CFTR channel (DeltaR-CFTR) J Gen Physiol. 2005;125:361–375. doi: 10.1085/jgp.200409227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xie J, Drumm ML, Ma J, Davis PB. Intracellular loop between transmembrane segments IV and V of cystic fibrosis transmembrane conductance regulator is involved in regulation of chloride channel conductance state. J Biol Chem. 1995;270:28084–28091. doi: 10.1074/jbc.270.47.28084. [DOI] [PubMed] [Google Scholar]
- 61.Xie J, Drumm ML, Zhao J, Ma J, Davis PB. Human epithelial cystic fibrosis transmembrane conductance regulator without exon 5 maintains partial chloride channel function in intracellular membranes. Biophys J. 1996;71:3148–3156. doi: 10.1016/S0006-3495(96)79508-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tao T, Xie J, Drumm ML, Zhao J, Davis PB, Ma J. Slow conversions among subconductance states of cystic fibrosis transmembrane conductance regulator chloride channel. Biophys J. 1996;70:743–753. doi: 10.1016/S0006-3495(96)79614-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harrington MA, Kopito RR. Cysteine residues in the nucleotide binding domains regulate the conductance state of CFTR channels. Biophys J. 2002;82:1278–1292. doi: 10.1016/S0006-3495(02)75484-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lansdell KA, Kidd JF, Delaney SJ, Wainwright BJ, Sheppard DN. Regulation of murine cystic fibrosis transmembrane conductance regulator Cl-channels expressed in Chinese hamster ovary cells. J Physiol. 1998;512:751–764. doi: 10.1111/j.1469-7793.1998.751bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scott-Ward TS, Cai Z, Dawson ES, Doherty A, Da Paula AC, Davidson H, Porteous DJ, Wainwright BJ, Amaral MD, Sheppard DN, Boyd AC. Chimeric constructs endow the human CFTR Cl-channel with the gating behavior of murine CFTR. Proc Natl Acad Sci U S A. 2007;104:16365–16370. doi: 10.1073/pnas.0701562104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grangeia A, Barro-Soria R, Carvalho F, Damas AM, Mauricio AC, Kunzelmann K, Barros A, Sousa M. Molecular and functional characterization of CBAVD-causing mutations located in CFTR nucleotide-binding domains. Cell Physiol Biochem. 2008;22:79–92. doi: 10.1159/000149785. [DOI] [PubMed] [Google Scholar]
- 67.Schwiebert EM, Benos DJ, Egan ME, Stutts MJ, Guggino WB. CFTR is a conductance regulator as well as a chloride channel. Physiol Rev. 1999;79:S145–S166. doi: 10.1152/physrev.1999.79.1.S145. [DOI] [PubMed] [Google Scholar]
- 68.Hryciw DH, Guggino WB. Cystic fibrosis transmembrane conductance regulator and the outwardly rectifying chloride channel: a relationship between two chloride channels expressed in epithelial cells. Clin Exp Pharmacol Physiol. 2000;27:892–895. doi: 10.1046/j.1440-1681.2000.03356.x. [DOI] [PubMed] [Google Scholar]
- 69.Schwiebert EM, Morales MM, Devidas S, Egan ME, Guggino WB. Chloride channel and chloride conductance regulator domains of CFTR, the cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci U S A. 1998;95:2674–2679. doi: 10.1073/pnas.95.5.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang S, Yue H, Derin RB, Guggino WB, Li M. Accessory protein facilitated CFTR-CFTR interaction, a molecular mechanism to potentiate the chloride channel activity. Cell. 2000;103:169–179. doi: 10.1016/s0092-8674(00)00096-9. [DOI] [PubMed] [Google Scholar]
- 71.Cormet-Boyaka E, Di A, Chang SY, Naren AP, Tousson A, Nelson DJ, Kirk KL. CFTR chloride channels are regulated by a SNAP-23/syntaxin 1A complex. Proc Natl Acad Sci U S A. 2002;99:12477–12482. doi: 10.1073/pnas.192203899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ko SB, Zeng W, Dorwart MR, Luo X, Kim KH, Millen L, Goto H, Naruse S, Soyombo A, Thomas PJ, Muallem S. Gating of CFTR by the STAS domain of SLC26 transporters. Nat Cell Biol. 2004;6:343–350. doi: 10.1038/ncb1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ando-Akatsuka Y, Abdullaev IF, Lee EL, Okada Y, Sabirov RZ. Down-regulation of volume-sensitive Cl− channels by CFTR is mediated by the second nucleotide-binding domain. Pflugers Arch. 2002;445:177–186. doi: 10.1007/s00424-002-0920-z. [DOI] [PubMed] [Google Scholar]
- 74.Halm DR, Rechkemmer GR, Schoumacher RA, Frizzell RA. Apical membrane chloride channels in a colonic cell line activated by secretory agonists. Am J Physiol. 1988;254:C505–C511. doi: 10.1152/ajpcell.1988.254.4.C505. [DOI] [PubMed] [Google Scholar]
- 75.Julien M, Verrier B, Cerutti M, Chappe V, Gola M, Devauchelle G, Becq F. Cystic fibrosis transmembrane conductance regulator (CFTR) confers glibenclamide sensitivity to outwardly rectifying chloride channel (ORCC) in Hi-5 insect cells. J Membr Biol. 1999;168:229–239. doi: 10.1007/s002329900512. [DOI] [PubMed] [Google Scholar]
- 76.Jeulin C, Fournier J, Marano F, Dazy AC. Effects of hydroxyl radicals on outwardly rectifying chloride channels in a cultured human bronchial cell line (16HBE14o-) Pflugers Arch. 2000;439:331–338. doi: 10.1007/s004249900191. [DOI] [PubMed] [Google Scholar]
- 77.Demion M, Guinamard R, El Chemaly A, Rahmati M, Bois P. An outwardly rectifying chloride channel in human atrial cardiomyocytes. J Cardiovasc Electrophysiol. 2006;17:60–68. doi: 10.1111/j.1540-8167.2005.00255.x. [DOI] [PubMed] [Google Scholar]
- 78.Volk T, Rabe A, Korbmacher C. Glibenclamide inhibits an outwardly rectifying chloride channel in M-1 mouse cortical collecting duct cells. Cell Physiol Biochem. 1995;5:222–231. [Google Scholar]
- 79.Rabe A, Disser J, Fromter E. Cl- channel inhibition by glibenclamide is not specific for the CFTR-type Cl− channel. Pflugers Arch. 1995;429:659–662. doi: 10.1007/BF00373986. [DOI] [PubMed] [Google Scholar]
- 80.Cheng SH, Gregory RJ, Marshall J, Paul S, Souza DW, White GA, O'Riordan CR, Smith AE. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell. 1990;63:827–834. doi: 10.1016/0092-8674(90)90148-8. [DOI] [PubMed] [Google Scholar]