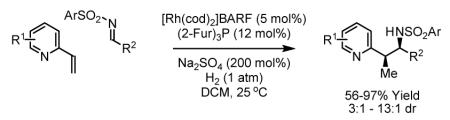
Nitrogen-bearing heterocycles are among the most prevalent substructures found in approved therapeutic agents.1 Among all heterocycles, pyridines most often appear in pharmaceutically active compounds.2 Modular methods for the catalytic coupling of pyridines and higher azines are largely limited to metal catalyzed cross-coupling processes (Suzuki, Stille, Kumada-Corriu, Negishi and Hiyama coupling reactions) and ortho-C-H activation initiated biaryl couplings3 and insertions of olefins or alkynes.4 A notable exception involves the rhodium catalyzed coupling of 2-vinyl azines5a and 2-alkynyl azines5b,c to organoboron reagents, which result in C-C coupling at the β-position of the vinyl or alkynyl moiety, respectively. Vinylpyridines also participate in rhodium catalyzed couplings to olefins, typically initiated via C-H insertion, again resulting in functionalization at the β-position of the vinyl moiety.6 Despite the significance of azine substructures, there are remarkably few methods available for catalytic C-C coupling of azine-containing building blocks.7,8,9
We have found that diverse π-unsaturated reactants engage in C-C coupling under the conditions of catalytic hydrogenation.10 For example, rhodium catalyzed hydrogenation of vinyl arenes in the presence of anhydrides was found to deliver formal products of acyl substitution with complete branched regioselectivity.11 Additionally, activated olefins in the form of conjugated enones engage in highly diastereo- and enantioselective reductive aldol and Mannich couplings when hydrogenated in the presence of aldehydes and imines.12,13 Here, we report the first catalytic reductive C-C couplings of vinyl azines. Specifically, we find that catalytic hydrogenation of 2-vinyl azines in the presence of N-arylsulfonyl imines results in regio- and diastereoselective reductive coupling to furnish branched products of imine addition.
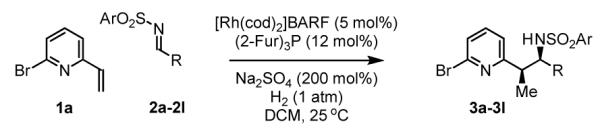
Initial studies focused on the hydrogenative coupling of 6-bromo-2-vinylpyridine 1a and N-ortho-toluenesulfonyl aldimine 2a. After extensive optimization, it was found that hydrogenation of 6-bromo-2-vinylpyridine 1a and imine 2a at ambient temperature and pressure employing a cationic rhodium catalyst ligated by (2-Fur)3P14 leads to formation of the reductive coupling product 3a in 97% isolated yield with complete branched regioselectivity and modest syn-diastereoselectivity (3:1 dr). Added Na2SO4 was found to suppress imine hydrolysis. To evaluate scope, these conditions were applied to aromatic imines 2a-2d, heteroaromatic imines 2e and 2f, α,β-unsaturated imine 2g, and aliphatic imines 2h-2l, which were all found to couple efficiently to provide adducts 3a-3l, respectively, in good to excellent yield with complete branched regioselectivity and modest to good levels of syn-diastereoselectivity (3:1 – 13:1 dr).15 The stereochemical assignment of adducts 3a and 3f were confirmed by single crystal X-ray diffraction analysis of diastereomerically pure samples. The stereochemical assignment of the remaining adducts are made in analogy to 3a and 3f (Table 1).
Table 1.
Hydrogenative coupling of 6-bromo-2-vinylpyridine 1a to N-arylsulfonyl aldimines 2a-2l.
 | ||
|---|---|---|
|
2a, R = p-NO2Ph 2d, R = Piperonyl 2g, R = Cinnamyl 2j, R = CH2OBn |
2b, R = Ph 2e, R = 2-Furyl 2h, R = n-Propyl 2k, R = c-C3H5 |
2c, R = p-MeOPh 2f, R = 2-Thienyl 2i, R = i-Butyl 2l, R = Me |
 97% Yield 3:1 dr, 3a |
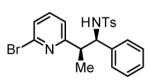 80% Yield 3:1 dr, 3b |
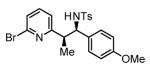 72% Yield 5:1 dr, 3c |
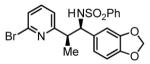 91% Yield 5:1 dr, 3d |
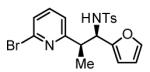 99% Yield 4:1 dr, 3e |
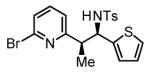 72% Yield 7:1 dr, 3f |
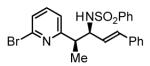 67% Yield 4:1 dr, 3g |
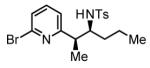 63% Yieldb,c 8:1 dr, 3h |
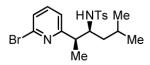 74% Yieldc 6:1 dr, 3i |
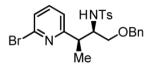 69% Yield 5:1 dr, 3j |
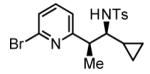 72% Yieldb,c 6:1 dr, 3k |
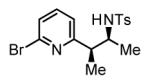 64% Yieldc 6:1 dr, 3l |
Cited yields are of isolated diastereomeric mixtures. Standard conditions employ 3 equiv. of 1a and 1 equiv. of imines 2a-2l. See Supporting Information for details.
Reaction was performed at 35 °C.
Reaction was performed using 7.5 mol% [Rh(cod)2]BARF and 18 mol% (2-Fur)3P.
The scope of the vinyl azine partner was evaluated next. Whereas the parent 2-vinylpyridine does not participate in the coupling, presumably due to strong coordination at nitrogen, 6-substituted-2-vinyl pyridines 1b-1d couple efficiently to (hetero)aromatic and aliphatic imines 2f, 2i and 2k (Table 2). Higher azines, for example 2,3-diphenyl-5-vinylpyrazine couple in diminished yield under standard conditions.16 However, as exemplified by the coupling of 8-benzyloxy-2-vinylquinoline 1e to imines 2e, 2f, 2i and 2k, fused vinyl azines are effective coupling partners.
Table 2.
Hydrogenative coupling of vinyl azines 1b-1d to N-toluenesulfonyl aldimines 2f, 2i and 2k.
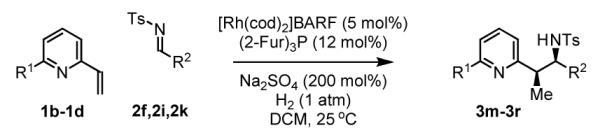 | ||
|---|---|---|
| 1b, R = Me | 1c, R = Ph | 1d, R = CH2OTBS |
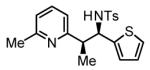 70% Yieldb 5:1 dr, 3m |
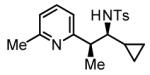 56% Yieldb 10:1 dr, 3n |
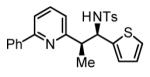 77% Yield 5:1 dr, 3o |
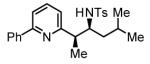 80% Yield 4:1 dr, 3p |
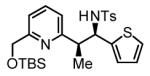 71% Yield 5:1 dr, 3q |
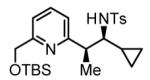 67% Yield 6:1 dr, 3r |
Cited yields are of isolated diastereomeric mixtures. Standard conditions employ 3 equiv. of 1b-1d and 1 equiv. of imines 2f, 2i and 2k. See Supporting Information for further details.
Reaction was performed using 7.5 mol% [Rh(cod)2]BARF and 18 mol% (2-Fur)3P.
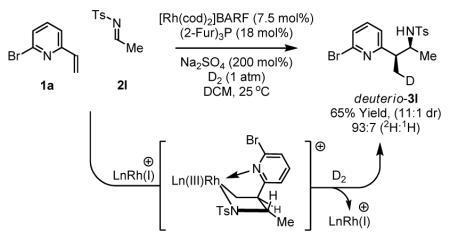
To gain insight into the catalytic mechanism, the reductive coupling of 6-bromo-2-vinylpyridine 1a to imine 2l was performed under an atmosphere of elemental deuterium (99.6% purity). As corroborated by 1H and 2H NMR spectroscopy, the branched adduct deuterio-3l incorporates deuterium exclusively at the former β-position of the vinyl moiety (93:7, 2H:1H). Deuterium incorporated at nitrogen is lost through exchange during chromatographic isolation. The results of isotopic labeling are consistent with a mechanism in which oxidative coupling of vinyl azine 1a and imine 2l delivers the indicated cationic aza-rhodacyclopentane, which upon deuteriolytic cleavage of the metallacycle17 releases deuterio-3l and regenerates cationic rhodium(I) to close the catalytic cycle. Mechanisms involving vinyl azine hydrometallation to form nucleophilic benzylrhodium intermediates cannot be excluded on the basis of this experiment.
In summary, we report the first metal catalyzed reductive C-C coupling of vinyl azines. By simply hydrogenating vinyl azines 1a-1e in the presence of N-arylsulfonyl aldimines 2a-2l, one gains access to the branched products of reductive coupling 3a-3v, which appear as single regioisomers. Using a rhodium catalyst ligated by tri-2-furylphosphine, modest to high levels of syn-diastereoselectivity may be achieved. Future studies will focus on the development of enantioselective variants of this process and related vinyl azine-carbonyl reductive couplings. Ultimately, through hydrogenative C-C coupling, byproduct-free protocols for the coupling of diverse unsaturated feedstocks will be achieved.
Supplementary Material
Table 3.
Hydrogenative coupling of vinyl azine 1e to N-toluenesulfonyl aldimines 2e, 2f, 2i and 2k.a
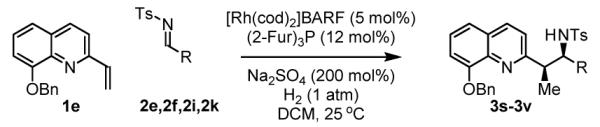 | |
|---|---|
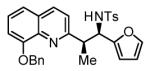 94% Yield 3:1 dr, 3s |
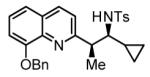 81% Yield 6:1 dr, 3t |
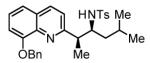 68% Yield 8:1 dr, 3u |
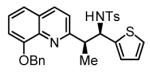 79% Yield 6:1 dr, 3v |
Cited yields are of isolated material. Standard conditions employ 3 equivalents of 6-bromo-2-vinylpyridine and 1 equivalent of imine. See Supporting Information for further details.
Acknowledgments
Acknowledgment is made to Merck, the Robert A. Welch Foundation, the ACS-GCI Pharmaceutical Roundtable, the NIH-NIGMS (RO1-GM069445) for partial support of this research. Dr. Oliver Briel of Umicore is thanked for the generous donation of [Rh(cod)2]BARF.
Footnotes
Supporting information available: Experimental procedures and spectral data for new compounds. Single crystal X-ray diffraction data for compounds 3a and 3f. This material is available free of charge via the internet at http://pubs.acs.org.
References
- (1).For an excellent review, see: Carey JS, Laffan D, Thomson C, Williams MT. Org. Biomol. Chem. 2006;4:2337. doi: 10.1039/b602413k.
- (2).Bonnet V, Mongin F, Trécourt F, Breton G, Marsais F, Knochel P, Quéguiner G. Synlett. 2002:1008. As state in reference 1 of the preceding article, “according to the MDL Drug Data Report, the most widespread heterocycles in pharmaceutically active compounds are pyridine (out of 15000 structures), imidazole (out of 11000), indole (out of 6700) and pyrimidine (out of 4500).”
- (3).Campeau L-C, Fagnou K. Chem. Soc. Rev. 2007;36:1058. doi: 10.1039/b616082d. [DOI] [PubMed] [Google Scholar]
- (4)(a).Lewis JC, Bergman RG, Ellman JA. J. Am. Chem. Soc. 2007;129:5332. doi: 10.1021/ja070388z. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Nakao Y, Kanyiva KS, Hiyama T. J. Am. Chem. Soc. 2008;130:2448. doi: 10.1021/ja710766j. [DOI] [PubMed] [Google Scholar]
- (5)(a).Lautens M, Roy A, Fukuoka K, Fagnou K, Martín-Matute B. J. Am. Chem. Soc. 2001;123:5358. doi: 10.1021/ja010402m. [DOI] [PubMed] [Google Scholar]; (b) Lautens M, Yoshida M. Org. Lett. 2002;4:123. doi: 10.1021/ol010261t. [DOI] [PubMed] [Google Scholar]; (c) Lautens M, Yoshida M. J. Org. Chem. 2003;68:762. doi: 10.1021/jo0205255. [DOI] [PubMed] [Google Scholar]
- (6)(a).Selimov FA, Ptashko OA, Fatykhov AA, Khalikova NR, Dzhemilev UM. Russ. Chem. Bull. 1993;42:913. [Google Scholar]; (b) Lim Y-G, Kang J-B, Kim Y-H. Chem. Commun. 1996:585. [Google Scholar]; (c) Lim Y-G, Kang J-B, Kim YH. J. Chem. Soc., Perkin Trans. 1998:699. [Google Scholar]; (d) Lim Y-G, Han J-S, Kang J-B. Bull. Kor. Chem. Soc. 1998;19:1143. [Google Scholar]; (e) Lim Y-G, Kang J-B, Koo BT. Tetrahedron Lett. 1999;40:7691. [Google Scholar]; (f) Lim Y-G, Han Jong-Soo, Koo Bon Tak, Kang Jung-Bu. Bull. Kor. Chem. Soc. 1999;20:1097. [Google Scholar]; (g) Lim Y-G, Kang J-B, Lee K, Kim YH. Heteroatom Chem. 2002;13:346. [Google Scholar]; (h) Aïssa C, Fürstner A. J. Am. Chem. Soc. 2007;129:14836. doi: 10.1021/ja0746316. [DOI] [PubMed] [Google Scholar]
- (7).Vinylpyridines engage in highly branch-selective hydroformylation: Settambolo R, Pucci S, Bertozzi S, Lazzaroni R. J. Organomet. Chem. 1995;489:C50. Botteghi C, Marchetti M, Paganelli S, Sechi B. J. Mol. Catal. 1997;118:173.
- (8).For Heck reactions of 2-vinylpyridine, see: Kasahara A, Izumi T, Takeda T, Imamura H. Bull. Chem. Soc. Jpn. 1974;47:183. Berthiol F, Doucet H, Santelli M. Syn. Lett. 2003:841. doi: 10.1039/b306428j. Narahashi H, Yamamoto A, Shimizu I. Chem. Lett. 2004;33:348.
- (9).For ruthenium catalyzed cross-metathesis reactions of vinylpyridine, see: Chatterjee AK, Toste FD, Choi T-L, Grubbs RH. Adv. Synth. Catal. 2002;344:634.
- (10).For selected reviews of hydrogenative C-C coupling, see: Ngai M-Y, Kong J-R, Krische MJ. J. Org. Chem. 2007;72:1063. doi: 10.1021/jo061895m. Iida H, Krische MJ. Top. Curr. Chem. 2007;279:77. Skucas E, Ngai M-Y, Komanduri V, Krische MJ. Acc. Chem. Res. 2007;40:1394. doi: 10.1021/ar7001123.
- (11).Hong Y-T, Barchuk A, Krische MJ. Angew. Chem. Int. Ed. 2006;128:6885. doi: 10.1002/anie.200602377. See also, Kokubo K, Miura M, Nomura M. Organometallics. 1995;14:4521.
- (12).For hydrogen-mediated reductive aldol addition, see: Jang HY, Huddleston RR, Krische MJ. J. Am. Chem. Soc. 2002;124:15156. doi: 10.1021/ja021163l. Huddleston RR, Krische MJ. Org. Lett. 2003;5:1143. doi: 10.1021/ol0300219. Koech PK, Krische MJ. Org. Lett. 2004;6:691. doi: 10.1021/ol030136c. Marriner GA, Garner SA, Jang HY, Krische MJ. J. Org. Chem. 2004;69:1380. doi: 10.1021/jo030310a. Jung CK, Garner SA, Krische MJ. Org. Lett. 2006;8:519. doi: 10.1021/ol052859x. Han SB, Krische MJ. Org. Lett. 2006;8:5657. doi: 10.1021/ol0624023. Jung CK, Krische M,J. J. Am. Chem. Soc. 2006;128:17051. doi: 10.1021/ja066198q. Bee C, Han SB, Hassan A, Iida H, Krische MJ. J. Am. Chem. Soc. 2008;130:2747. doi: 10.1021/ja710862u.
- (13).For hydrogen-mediated reductive Mannich addition, see: Garner SA, Krische MJ. J. Org. Chem. 2007;72:5843. doi: 10.1021/jo070779w.
- (14).For tri-2-furylphosphine and triphenylarsine effects in metal catalyzed reactions, see: Farina V, Krishnan B. J. Am. Chem. Soc. 1991;113:9585. Farina V. Pure Appl. Chem. 1996;68:73. Anderson NG, Keay BA. Chem. Rev. 2001;101:997. doi: 10.1021/cr000024o.
- (15).In the rhodium catalyzed hydrogenative coupling of vinyl ketones to aldehydes and imines (references 12e-h, 13), Fur3P enforces high levels of syn-diastereoselectivity. Given the structural homology of vinyl ketones and 2-vinyl azines, it is not surprising that analogous ligand effects are observed.
- (16).Coupling of 2,3-diphenyl-5-vinylpyrazine to imine 2f under standard conditions provides the branched adduct in 35% yield and 5:1 dr.
- (17).The stoichiometric reaction of isolated rhodacyclopentadienes with elemental hydrogen delivers the product of hydrogenolysis: Müller E, Thomas R, Zountsas G. Liebigs Ann. Chem. 1972;758:16.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.