Abstract
The neuropeptide calcitonin gene-related peptide (CGRP) plays a key role in migraine. However, a major challenge for studying CGRP actions is the lack of animal models for migraine. Clinical studies suggested that migraineurs are more sensitive to CGRP than people who do not suffer from migraine. We therefore generated a transgenic mouse that is sensitized to CGRP (nestin/hRAMP1 mice). The mice have elevated expression of a subunit of the CGRP receptor, human receptor activity-modifying protein 1 (hRAMP1). Nestin/hRAMP1 mice have two symptoms of migraine: photophobia and mechanical allodynia. The light aversion was greatly enhanced by intracerebroventricular administration of CGRP. CGRP had little effect on motility in the light zone, but once in the dark, the mice moved less than controls. The CGRP-induced light aversion was attenuated by co-administration of the CGRP receptor antagonist olcegepant. These findings suggest that CGRP acts as a neuromodulator to increase sensory responses and that regulation of a single gene, hRAMP1, could potentially contribute to migraine susceptibility.
Keywords: Calcitonin gene-related peptide, Migraine, Photophobia, Transgenic mouse, Receptor activity-modifying protein 1
The starting point for this PharmSight is the emerging concept that the neuropeptide CGRP is a peptide that is a regulator of the cardiovascular system, a mediator of neurogenic inflammation, and a modulator of nociceptive input (1–3). While the mechanisms underlying migraine remain elusive, there is growing agreement that it involves the trigeminal system and CGRP (4–7).
The first hint of a connection between CGRP and migraine was from pioneering studies by Edvinsson and Goadsby in the early 1990’s. They reported that CGRP was elevated in the jugular outflow during severe migraine attacks and that treatment with sumatriptan restored the levels to normal, co-incident with alleviation of symptoms (8, 9). While that finding has recently been questioned (10, 11), other studies have confirmed that CGRP is elevated in migraineurs (12–14).
Clinical studies have now firmly established the connection between CGRP and migraine. In a study by Olesen’s group, CGRP was injected into the cubital vein of migraineurs (15). Remarkably, CGRP was sufficient to cause a delayed headache in all the patients of an admittedly small study. Furthermore, three of the nine individuals had symptoms that met the criteria for migraine. In contrast, injection of CGRP into nonmigraineurs yielded only a mild headache (16). In 2004, a key proof of principle study was done using a small molecule inhibitor of the CGRP receptor developed by Doods at Boehringer Ingelheim (17). This compound, now called olcegepant, demonstrated that a CGRP receptor antagonist alleviates migraine headache and associated symptoms. This finding has now been extended with a second, oral CGRP receptor antagonist, telcagepant, developed by Merck. Telcagepant has successfully reduced the symptoms of migraine in two Phase III clinical trials (18, 19). Importantly, telcegepant appears to be as effective as the currently used triptan drugs, with fewer adverse side effects and without causing vasoconstriction, which contraindicates the use of triptans in patients with cardiovascular disease.
To study the role of CGRP in migraine, we generated a CGRP-sensitized mouse. The rationale was that migraineurs, but not non-migraineurs, are sensitive to CGRP-induced headache (15, 16). This suggested to us that migraineurs are somehow sensitized to CGRP. One mechanism that could account for this increased sensitivity is elevated CGRP receptors. We therefore reasoned that elevating CGRP receptor expression might sensitize a mouse to CGRP actions.
However, it was not immediately obvious which subunit of the receptor should be elevated. CGRP acts at an unusual G protein coupled receptor called CLR that has an obligate requirement for a subunit called receptor activity-modifying protein 1 (RAMP1) (Figure 1). CLR also binds an intracellular protein (RCP) that increases G protein coupling. RAMP1 is necessary for trafficking of CLR to the cell surface and CGRP binding specificity (20). Interaction of CLR with the related RAMP 2 and 3 subunits generates adrenomedullin receptors that have lower affinity for CGRP. Importantly, RAMP1 can also bind other G protein coupled receptors. Most notably, RAMP1 converts a calcitonin receptor to an amylin receptor (21), which may contribute to the phenotype of the mice described below. RAMP1 is also responsible for the species selectivity of CGRP receptor antagonists being used in clinical studies (22). For this reason we chose to use human RAMP1 (hRAMP1).
Figure 1. CGRP receptor.
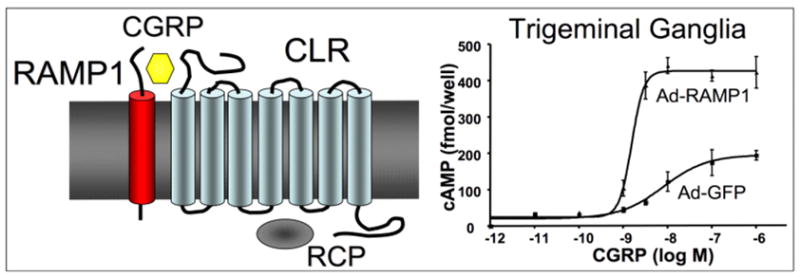
Left, schematic of CGRP receptor complex containing RAMP1, CLR, and RCP. Right, Increased sensitivity to CGRP following expression of RAMP1 from an adenoviral vector in cultured trigeminal ganglia neurons. Ad-GFP is a control vector. Figure modified from (24).
As a starting point, since RAMP1 and RCP proteins can couple with multiple receptors, we figured that RAMP1 or RCP was most likely limiting. Studies with adenoviral vectors encoding hRAMP1 with cultured trigeminal ganglia neurons and vascular smooth muscle revealed that RAMP1 is functionally rate limiting for CGRP-mediated actions (Figure 1) (23, 24). In contrast, overexpression of human RCP had no detectable effect in either system (unpublished data). These observations provided the rationale for engineering CGRP-sensitized mice by transgenic overexpression of hRAMP1.
Not knowing where we would need to elevate RAMP1, we chose a flexible expression strategy that relies on Cre recombinase to activate the RAMP1 transgene. As a starting point, we focused on the nervous system. The first studies were done with RAMP1 expressed throughout the peripheral and central nervous system (CNS), in both glia and neurons. These mice, referred to as nestin/hRAMP1, are double transgenics that express hRAMP1 only after removal of an upstream stop sequence in neurons and glia by Cre recombinase under control of the nestin promoter (24). Nestin/hRAMP1 mice have 1.5–2-fold greater levels of total mouse and human RAMP1 in peripheral ganglia and the CNS and increased CGRP-induced neurogenic inflammation (24).
Having established the transgenic mouse, we faced the question of how do you measure migraine in a mouse? Of course, we cannot fully recapitulate migraine in a mouse, but we reasoned that the associated symptoms might be measurable parameters. One such symptom we examined was mechanical allodynia (25). Mechanical allodynia is when normally nonpainful stimuli, such as a light touch, are painful. While not one of the diagnostic criteria of migraine, mechanical allodynia is reported by over half of migraineurs in the facial region, and in many cases, it can spread to other parts of the body (26–30). Nestin/hRAMP1 mice exhibited CGRP-induced mechanical allodynia due to central sensitization (25). These observations demonstrated that the mice are sensitized to CGRP actions and exhibit at least one symptom similar to some migraineurs.
A more characteristic symptom of migraine that we examined was photophobia, which is one of the diagnostic criteria of migraine (31). Photophobia reflects an allodynic response where ordinarily non-painful light is noxious. Photophobia is one of the most common migraine symptoms, affecting 66%-88% of migraineurs (32) and enhanced sensitivity to light is also reported to a lesser degree between attacks (32). In addition to migraine, photophobia also accompanies other less common, but likewise disabling conditions, such as mild traumatic brain injury, cluster headache, trigeminal autonomic cephalalgias, blepharospasm, meningitis, and subarachnoid hemorrrhage (33–38). An emerging problem is that a large number of veterans suffering from mild traumatic brain injury have an unexpectedly high incidence of migraine with photophobia compared to civilians with similar brain injuries (39, 40). In addition, approximately 50% have light sensitivity independent of migraine symptoms (34, 41).
The approach we took was to study light aversive behavior as a surrogate for photophobia. To do this we used the light/dark box developed by Crawley that has primarily been used to study anxiety behavior in rodents (42–44). Along with our studies, another group has also recently used this relatively straightforward paradigm as an indicator of photophobia (45–47). We have used a custom-made and a commercially available light/dark chamber (Med Associates Inc.), with similar results in both chambers (46). Advantages of the new unit are that it is more automated, is fully enclosed in a sound-proof cabinet, can be scaled up to analyze multiple mice simultaneously, and allows measurement of more parameters in both the light and dark zones, including control measurements with both zones kept dark.
Nestin/hRAMP1 mice display more light aversion than littermate controls. We have proposed that this light aversion is an indicator of photophobia (45). Untreated nestin/hRAMP1 mice spent 30% less time in the light than their littermates (45). While there were no significant differences based on sex or control genotypes, it is intriguing that female mice showed a trend towards greater light aversion. Further studies with changes in the experimental parameters may possibly reveal a sex-bias.
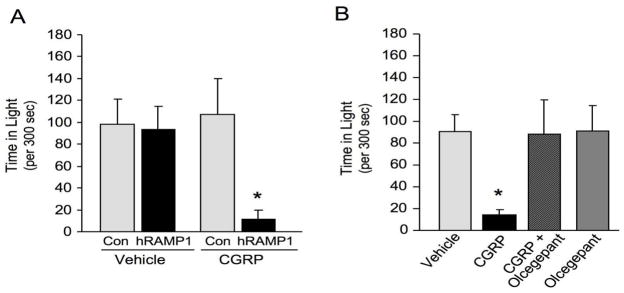
Since CGRP levels are reported to be elevated during migraine, we tested the effect of exogenously delivered CGRP on light aversion. The light aversion was greatly enhanced by icv injection of CGRP. After CGRP treatment, nestin/hRAMP1 mice spent less time in the light compared with vehicle or control mice given CGRP (Figure 2) 45. CGRP-induced light aversion was seen in both the first and second 5 min testing intervals, with the greatest effect in the second interval. Nestin/hRAMP1 mice spent 77% less time in the light after icv CGRP compared with vehicle and 85% less time than control mice given icv CGRP.
Figure 2. CGRP-induced light aversion by nestin/hRAMP1 mice.
A. The nestin/hRAMP1 mice spent less time in the light after icv CGRP (0.5 nmol/2 ul) compared to vehicle (PBS) or control mice given CGRP or vehicle. Data are shown from the second 5 min monitoring interval. B. The antagonist olcegepant (0.5 nmol) prevented the CGRP-induced light aversion seen in the nestin/hRAMP1 mice. For both panels, * P<0.05 (Student’s two tailed t test) compared to vehicle. Error bars are standard error of the mean. Figure modified from (45).
The specificity of CGRP-induced light aversion for the CGRP receptor was confirmed by co-injecting the CGRP receptor antagonist olcegepant (BIBN4096BS) (Figure 2). This drug was selected because of its relatively high affinity for the CLR/hRAMP1 CGRP receptor and its efficacy in migraine clinical trials (17). Olcegepant on its own had no effect on the mice, yet it was able to block CGRP induced light aversion. These results establish that the light aversion is due to CGRP actions at the CLR/hRAMP1 receptor complex. This is important because the amylin receptor (RAMP1 with calcitonin receptor, AMY1(a)) has a relatively high affinity for CGRP (21). Olcegepant has ~150-fold higher affinity for the CGRP receptor than for the AMY1(a) receptor (48). However, a caveat is that the drug concentrations at the relevant sites are not known; thus, we cannot exclude a combination of CGRP and AMY1(a) receptor actions contributing to the light-aversive phenotype. Further studies with olcegepant and the amylin antagonist AC187 will be needed to establish whether amylin also contributes to the light aversive phenotype.
A potential confounder of the light/dark exploratory assay is motor activity. For example, with a significant motor loss, a mouse would have difficulty moving within the chamber. We assessed motor activity as rearings, distance traveled, ambulatory time, mean ambulatory velocity, and resting time. In general our findings with control and nestin/hRAMP1 mice agree with previous reports that CGRP decreases motor activity (45, 46). An intriguing paradox from our initial studies was that while CGRP reduced motility in the lit open field (used for anxiety tests described below), there was no apparent decrease in the light zone of the light/dark chamber (45). This paradox was resolved by measuring motility in the dark zone using infra-red beams.
We found that after CGRP treatment there was a striking difference between movement in the light and dark zones. CGRP-treated nestin/hRAMP1 and control mice had similar behavior in the light zone, while in the dark zone, nestin/hRAMP1 moved less than control mice (46). We interpret this light-dependent difference to indicate that when the mice have reached a non-aversive environment (the dark), they prefer to not move as much. In the open field chamber they do not have an option to escape to the dark, hence reduced motility was observed. This may reflect the clinical state in which pain is aggravated upon movement during migraine. Thus, CGRP induced motility changes in both nestin/hRAMP1 and control mice that were consistently more noticeable in the dark compartment.
As mentioned earlier, the light/dark assay is often used to predict effects of anxiolytic drugs. Hence, the increased light aversion in the nestin/hRAMP1 mice could be due to increased anxiety/fear behavior. We ruled out a major contribution from anxiety by using two different anxiety or fear-related behavioral paradigms (45). There were no significant differences in an open field anxiety test both at baseline and after icv treatment with CGRP. Likewise, the differences in the predator odor freezing behavior assay would not explain the greater light aversion of hRAMP1 (e.g. hRAMP1 mice froze less than littermates at baseline). Future experiments with other paradigms, such as the elevated plus maze, should further elucidate behavioral contributions of hRAMP1 and CGRP.
It is interesting to speculate on the sites of CGRP action. Surprisingly, there is remarkably little known about the mechanisms underlying photophobia, although evidence points to involvement of the trigeminal pathway (49–51). The ophthalmic branch of the trigeminal nerve heavily innervates the eye and conveys nociceptive signals to second order neurons of the trigeminal nucleus that project to the thalamus and higher cortical regions. In a recent case study, activation of the trigeminal ganglion and trigeminal nucleus caudalis was observed by fMRI in a photophobic patient exposed to bright light (52). In a rat model, Bereiter and colleagues demonstrated that bright light activates the trigeminal nucleus caudalis (49). The possibility of direct input from melanopsin retinal ganglion cells that project to the thalamus has recently been raised by activation and tracing studies by Burstein (personal communication). Photophobia in patients blind due to rods and cones degeneration, but who have intact, functioning melanopsin retinal ganglion cells, further suggests contributions from the non-visual pathway mediated by the intrinsically light sensitive melanopsin retinal ganglion cells (53). Furthermore, the intrinsic light activation of these photosensitive ganglion cells is maximal in the blue wavelength range and blue-blocking lenses have been shown to reduce photophobia (54–56).
How exactly the trigeminal nerve is activated by light is unknown. Models have been proposed involving the convergence of afferent pathways of the trigeminal and visual systems, possibly from the melanopsin ganglion cells (53) as previously mentioned, or trigeminal activation by changes in vascular tone (49) or mechanosensitivity (52). A common theme of these models is release of CGRP. CGRP is in trigeminal ganglion neurons that innervate the anterior portion of the eye and CGRP binding sites are in ocular structures, which support a possible contributory role. As a major neuropeptide in the trigeminal system, CGRP is likely to be involved in the pathophysiology of photophobia, which is supported by our mouse studies (45, 46).
What does this all mean? We predict that CGRP is a neuromodulator at glutamatergic synapses that convey sensory information (Figure 3). CGRP is co-localized with glutamate in neurons of the trigeminal ganglion and presumably in the CNS. Hence, CGRP may act by sensitizing synapses in the CNS, and that over-sensitization leads to migraine. This modulation could be both presynaptic and postsynaptic. A presynaptic role is supported by our studies showing that CGRP increases cAMP levels and substance P release from cultured trigeminal ganglia neurons (24). Likewise, a postsynaptic function could involve cAMP or calcium signals from the CGRP receptor that could activate glutamate receptors. In addition, CGRP actions on nearby satellite glia or on dural mast cells in the periphery could influence synaptic signaling (57, 58). It will be interesting to test the effect of specific glutamate receptor antagonists on CGRP-induced light aversion. As a result of either presynaptic or postsynaptic enhancement, increased RAMP1 is predicted to increase CGRP modulation of sensory perception. This suggests that while migraine is clearly a multigenic disorder, genetic or epigenetic regulation of a single gene, hRAMP1, may contribute to migraine susceptibility.
Figure 3. Model of CGRP as a neuromodulator of glutamatergic synapses.
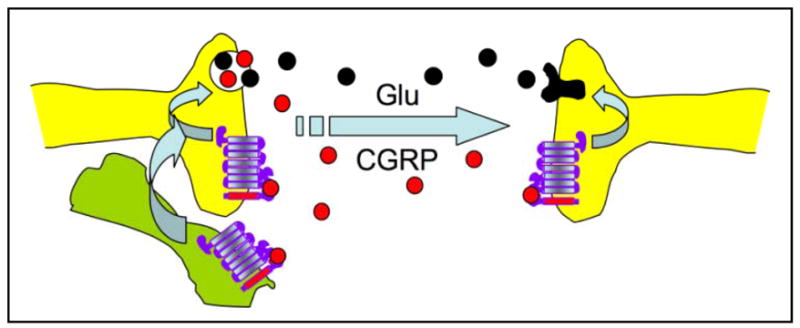
Co-release of CGRP with glutamate from presynaptic neurons (for example, a peripheral nociceptor) could act presynaptically to further increase neurotransmitter release and/or postsynaptically to increase glutamate receptor activity. In addition, CGRP could act in a paracrine fashion on nearby glia (lower left cell) and/or dural mast cells (not shown) to indirectly act on the neurons.
Acknowledgments
We appreciate helpful advice and suggestions from Drs. Donna Hammond and Kim Johnson. Supported by NIH DE016511, National Headache Foundation, and the University of Iowa Institute for Clinical and Translational Science.
Footnotes
Conflicts of Interest
No potential conflicts of interest to disclose.
References
- 1.Rosenfeld MG, Mermod JJ, Amara SG, et al. Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature. 1983;304:129–35. doi: 10.1038/304129a0. [DOI] [PubMed] [Google Scholar]
- 2.Russo AF, Dickerson IM. CGRP: A multifunctional neuropeptide. In: Lajtha A, editor. Handboook of Neurochemistry and Molecular Neurobiology. 3. New York, NY: Springer; 2006. pp. 391–426. [Google Scholar]
- 3.Recober A, Russo AF. Calcitonin gene-related peptide: an update on the biology. Curr Opin Neurol. 2009;22:241–6. doi: 10.1097/wco.0b013e32832b2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arulmani U, Maassenvandenbrink A, Villalon CM, Saxena PR. Calcitonin gene-related peptide and its role in migraine pathophysiology. Eur J Pharmacol. 2004;500:315–30. doi: 10.1016/j.ejphar.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 5.Durham PL. Inhibition of calcitonin gene-related peptide function: a promising strategy for treating migraine. Headache. 2008;48:1269–75. doi: 10.1111/j.1526-4610.2008.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edvinsson L. Novel migraine therapy with calcitonin gene-regulated peptide receptor antagonists. Expert Opin Ther Targets. 2007;11:1179–88. doi: 10.1517/14728222.11.9.1179. [DOI] [PubMed] [Google Scholar]
- 7.Doods H, Arndt K, Rudolf K, Just S. CGRP antagonists: unravelling the role of CGRP in migraine. Trends Pharmacol Sci. 2007;28:580–7. doi: 10.1016/j.tips.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurology. 1990;28:183–7. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- 9.Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol. 1993;33:48–56. doi: 10.1002/ana.410330109. [DOI] [PubMed] [Google Scholar]
- 10.Tvedskov JF, Lipka K, Ashina M, Iversen HK, Schifter S, Olesen J. No increase of calcitonin gene-related peptide in jugular blood during migraine. Ann Neurol. 2005;58:561–8. doi: 10.1002/ana.20605. [DOI] [PubMed] [Google Scholar]
- 11.Tfelt-Hansen P, Le H. Calcitonin gene-related peptide in blood: is it increased in the external jugular vein during migraine and cluster headache? A review. J Headache Pain. 2009;10:137–43. doi: 10.1007/s10194-009-0112-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Juhasz G, Zsombok T, Jakab B, Nemeth J, Szolcsanyi J, Bagdy G. Sumatriptan causes parallel decrease in plasma calcitonin gene-related peptide (CGRP) concentration and migraine headache during nitroglycerin induced migraine attack. Cephalalgia. 2005;25:179–83. doi: 10.1111/j.1468-2982.2005.00836.x. [DOI] [PubMed] [Google Scholar]
- 13.Bellamy JL, Cady RK, Durham PL. Salivary levels of CGRP and VIP in rhinosinusitis and migraine patients. Headache. 2006;46:24–33. doi: 10.1111/j.1526-4610.2006.00294.x. [DOI] [PubMed] [Google Scholar]
- 14.Cady RK, Vause CV, Ho TW, Bigal ME, Durham PL. Elevated saliva calcitonin gene-related peptide levels during acute migraine predict therapeutic response to rizatriptan. Headache. 2009;49:1258–66. doi: 10.1111/j.1526-4610.2009.01523.x. [DOI] [PubMed] [Google Scholar]
- 15.Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J. CGRP may play a causative role in migraine. Cephalalgia. 2002;22:54–61. doi: 10.1046/j.1468-2982.2002.00310.x. [DOI] [PubMed] [Google Scholar]
- 16.Petersen KA, Lassen LH, Birk S, Lesko L, Olesen J. BIBN4096BS antagonizes human alpha-calcitonin gene related peptide-induced headache and extracerebral artery dilatation. Clin Pharmacol Ther. 2005;77:202–13. doi: 10.1016/j.clpt.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Olesen J, Diener HC, Husstedt IW, et al. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med. 2004;350:1104–10. doi: 10.1056/NEJMoa030505. [DOI] [PubMed] [Google Scholar]
- 18.Ho TW, Ferrari MD, Dodick DW, et al. Efficacy and tolerability of MK-0974 (telcagepant), a new oral antagonist of calcitonin gene-related peptide receptor, compared with zolmitriptan for acute migraine: a randomised, placebo-controlled, parallel-treatment trial. Lancet. 2008;372:2115–23. doi: 10.1016/S0140-6736(08)61626-8. [DOI] [PubMed] [Google Scholar]
- 19.Connor KM, Shapiro RE, Diener HC, et al. Randomized, controlled trial of telcagepant for the acute treatment of migraine. Neurology. 2009;73:970–7. doi: 10.1212/WNL.0b013e3181b87942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McLatchie LM, Fraser NJ, Main MJ, et al. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–9. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- 21.Hay DL, Poyner DR, Sexton PM. GPCR modulation by RAMPs. Pharmacol Ther. 2006;109:173–97. doi: 10.1016/j.pharmthera.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 22.Mallee JJ, Salvatore CA, LeBourdelles B, et al. Receptor activity-modifying protein 1 determines the species selectivity of non-peptide CGRP receptor antagonists. J Biol Chem. 2002;277:14294–8. doi: 10.1074/jbc.M109661200. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z, Dickerson IM, Russo AF. Calcitonin gene-related peptide receptor activation by receptor activity-modifying protein-1 gene transfer to vascular smooth muscle cells. Endocrinology. 2006;147:1932–40. doi: 10.1210/en.2005-0918. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z, Winborn CS, Marquez de Prado B, Russo AF. Sensitization of calcitonin gene-related peptide receptors by receptor activity-modifying protein-1 in the trigeminal ganglion. J Neurosci. 2007;27:2693–703. doi: 10.1523/JNEUROSCI.4542-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marquez de Prado B, Hammond DL, Russo AF. Genetic enhancement of calcitonin gene-related Peptide-induced central sensitization to mechanical stimuli in mice. J Pain. 2009;10:992–1000. doi: 10.1016/j.jpain.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burstein R, Cutrer MF, Yarnitsky D. The development of cutaneous allodynia during a migraine attack clinical evidence for the sequential recruitment of spinal and supraspinal nociceptive neurons in migraine. Brain. 2000;123 ( Pt 8):1703–9. doi: 10.1093/brain/123.8.1703. [DOI] [PubMed] [Google Scholar]
- 27.LoPinto C, Young WB, Ashkenazi A. Comparison of dynamic (brush) and static (pressure) mechanical allodynia in migraine. Cephalalgia. 2006;26:852–6. doi: 10.1111/j.1468-2982.2006.01121.x. [DOI] [PubMed] [Google Scholar]
- 28.Burstein R. Deconstructing migraine headache into peripheral and central sensitization. Pain. 2001;89:107–10. doi: 10.1016/s0304-3959(00)00478-4. [DOI] [PubMed] [Google Scholar]
- 29.Burstein R, Yarnitsky D, Goor-Aryeh I, Ransil BJ, Bajwa ZH. An association between migraine and cutaneous allodynia. Ann Neurol. 2000;47:614–24. [PubMed] [Google Scholar]
- 30.Landy S, Rice K, Lobo B. Central sensitisation and cutaneous allodynia in migraine: implications for treatment. CNS Drugs. 2004;18:337–42. doi: 10.2165/00023210-200418060-00001. [DOI] [PubMed] [Google Scholar]
- 31.Goadsby PJ, Lipton RB, Ferrari MD. Migraine--current understanding and treatment. N Engl J Med. 2002;346:257–70. doi: 10.1056/NEJMra010917. [DOI] [PubMed] [Google Scholar]
- 32.Mulleners WM, Aurora SK, Chronicle EP, Stewart R, Gopal S, Koehler PJ. Self-reported photophobic symptoms in migraineurs and controls are reliable and predict diagnostic category accurately. Headache. 2001;41:31–9. doi: 10.1046/j.1526-4610.2001.111006031.x. [DOI] [PubMed] [Google Scholar]
- 33.Bohnen N, Twijnstra A, Wijnen G, Jolles J. Recovery from visual and acoustic hyperaesthesia after mild head injury in relation to patterns of behavioural dysfunction. J Neurol Neurosurg Psychiatry. 1992;55:222–4. doi: 10.1136/jnnp.55.3.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Du T, Ciuffreda KJ, Kapoor N. Elevated dark adaptation thresholds in traumatic brain injury. Brain Inj. 2005;19:1125–38. doi: 10.1080/02699050500149817. [DOI] [PubMed] [Google Scholar]
- 35.Irimia P, Cittadini E, Paemeleire K, Cohen AS, Goadsby PJ. Unilateral photophobia or phonophobia in migraine compared with trigeminal autonomic cephalalgias. Cephalalgia. 2008;28:626–30. doi: 10.1111/j.1468-2982.2008.01565.x. [DOI] [PubMed] [Google Scholar]
- 36.Bahra A, May A, Goadsby PJ. Cluster headache: a prospective clinical study with diagnostic implications. Neurology. 2002;58:354–61. doi: 10.1212/wnl.58.3.354. [DOI] [PubMed] [Google Scholar]
- 37.Goadsby PJ, Edvinsson L. Human in vivo evidence for trigeminovascular activation in cluster headache. Neuropeptide changes and effects of acute attacks therapies. Brain. 1994;117 ( Pt 3):427–34. doi: 10.1093/brain/117.3.427. [DOI] [PubMed] [Google Scholar]
- 38.Hallett M, Evinger C, Jankovic J, Stacy M. Update on blepharospasm: report from the BEBRF International Workshop. Neurology. 2008;71:1275–82. doi: 10.1212/01.wnl.0000327601.46315.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Theeler BJ, Erickson JC. Mild head trauma and chronic headaches in returning US soldiers. Headache. 2009;49:529–34. doi: 10.1111/j.1526-4610.2009.01345.x. [DOI] [PubMed] [Google Scholar]
- 40.Neely ET, Midgette LA, Scher AI. Clinical review and epidemiology of headache disorders in US service members: with emphasis on post-traumatic headache. Headache. 2009;49:1089–96. doi: 10.1111/j.1526-4610.2009.001460.x. [DOI] [PubMed] [Google Scholar]
- 41.Lew HL, Poole JH, Vanderploeg RD, et al. Program development and defining characteristics of returning military in a VA Polytrauma Network Site. J Rehabil Res Dev. 2007;44:1027–34. [PubMed] [Google Scholar]
- 42.Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1980;13:167–70. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- 43.Blumstein LK, Crawley JN. Further characterization of a simple, automated exploratory model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1983;18:37–40. doi: 10.1016/0091-3057(83)90247-2. [DOI] [PubMed] [Google Scholar]
- 44.Bourin M, Hascoet M. The mouse light/dark box test. Eur J Pharmacol. 2003;463:55–65. doi: 10.1016/s0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- 45.Recober A, Kuburas A, Zhang Z, Wemmie JA, Anderson MG, Russo AF. Role of calcitonin gene-related peptide in light-aversive behavior: implications for migraine. J Neurosci. 2009;29:8798–804. doi: 10.1523/JNEUROSCI.1727-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Recober A, Kaiser EA, Kuburas A, Russo AF. Induction of multiple photophobic behaviors in a transgenic mouse sensitized to CGRP. Neuropharmacology. 2009 doi: 10.1016/j.neuropharm.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thiels E, Hoffman EK, Gorin MB. A reliable behavioral assay for the assessment of sustained photophobia in mice. Curr Eye Res. 2008;33:483–91. doi: 10.1080/02713680802130347. [DOI] [PubMed] [Google Scholar]
- 48.Hay DL, Christopoulos G, Christopoulos A, Sexton PM. Determinants of BIBN4096BS affinity for CGRP and amylin receptors; the role of RAMP1. Mol Pharmacol. 2006 doi: 10.1124/mol.106.027953. [DOI] [PubMed] [Google Scholar]
- 49.Okamoto K, Thompson R, Tashiro A, Chang Z, Bereiter DA. Bright light produces Fos-positive neurons in caudal trigeminal brainstem. Neuroscience. 2009;160:858–64. doi: 10.1016/j.neuroscience.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 50.Drummond PD, Woodhouse A. Painful stimulation of the forehead increases photophobia in migraine sufferers. Cephalalgia. 1993;13:321–4. doi: 10.1046/j.1468-2982.1993.1305321.x. [DOI] [PubMed] [Google Scholar]
- 51.Kowacs PA, Piovesan EJ, Werneck LC, et al. Influence of intense light stimulation on trigeminal and cervical pain perception thresholds. Cephalalgia. 2001;21:184–8. doi: 10.1046/j.1468-2982.2001.00178.x. [DOI] [PubMed] [Google Scholar]
- 52.Moulton EA, Becerra L, Borsook D. An fMRI case report of photophobia: activation of the trigeminal nociceptive pathway. Pain. 2009;145:358–63. doi: 10.1016/j.pain.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Amini A, Digre K, Couldwell WT. Photophobia in a blind patient: An alternate visual pathway. Case report. J Neurosurg. 2006;105:765–8. doi: 10.3171/jns.2006.105.5.765. [DOI] [PubMed] [Google Scholar]
- 54.Herz NL, Yen MT. Modulation of sensory photophobia in essential blepharospasm with chromatic lenses. Ophthalmology. 2005;112:2208–11. doi: 10.1016/j.ophtha.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 55.Adams WH, Digre KB, Patel BC, Anderson RL, Warner JE, Katz BJ. The evaluation of light sensitivity in benign essential blepharospasm. Am J Ophthalmol. 2006;142:82–7. doi: 10.1016/j.ajo.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 56.Blackburn MK, Lamb RD, Digre KB, et al. FL-41 tint improves blink frequency, light sensitivity, and functional limitations in patients with benign essential blepharospasm. Ophthalmology. 2009;116:997–1001. doi: 10.1016/j.ophtha.2008.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li J, Vause CV, Durham PL. Calcitonin gene-related peptide stimulation of nitric oxide synthesis and release from trigeminal ganglion glial cells. Brain Res. 2008;1196:22–32. doi: 10.1016/j.brainres.2007.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Capuano A, De Corato A, Lisi L, Tringali G, Navarra P, Dello Russo C. Proinflammatory-activated trigeminal satellite cells promote neuronal sensitization: relevance for migraine pathology. Mol Pain. 2009;5:43. doi: 10.1186/1744-8069-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]