Abstract
Akt is a Ser–Thr kinase with pleiotropic effects on cell survival, growth and metabolism. Recent evidence from gene-deletion studies in mice, and analysis of human platelets treated with Akt inhibitors, suggest that Akt regulates platelet activation, with potential consequences for thrombosis. Akt activation is regulated by the level of phosphoinositide 3-phosphates, and proteins that regulate concentrations of this lipid also regulate Akt activation and platelet function. Although the effectors through which Akt contributes to platelet activation are not definitively known, several candidates are discussed, including endothelial nitric oxide synthase, glycogen synthase kinase 3β, phosphodiesterase 3A and the integrin β3 tail. Selective inhibitors of Akt isoforms or of proteins that contribute to its activation, such as individual PI3K isoforms, may make attractive targets for antithrombotic therapy. This review summarizes the current literature describing Akt activity and its regulation in platelets, including speculation regarding the future of Akt or its regulatory pathways as targets for the development of antithrombotic therapies.
Keywords: Akt, glycogen synthase kinase3, PI3K, platelet, thrombosis
Akt (also termed protein kinase B [PKB]) is a serine–threonine kinase that contributes to signaling and activation responses of human platelets, and has been shown to support thrombus formation in mouse models. These results have fueled speculation that Akt activation may be a contributing factor to thrombosis in humans. The recent identification of a single-nucleotide polymorphism (SNP) in the gene encoding Akt2 associated with reactivity of human platelets lends considerable weight to this idea [1], although direct association with prothrombotic consequences has yet to be demonstrated. Although no drugs targeting Akt are currently in trials for the management of thrombotic sequellae in at-risk patients, these studies suggest that some features of Akt signaling pathways might make attractive targets for antithrombotic therapy. However, none of the three Akt isoforms are entirely platelet specific, and all have diverse functions, so the direct targeting of individual isoforms as antithrombotic therapy raises significant concern regarding off-target effects. This review addresses our current understanding of the contribution of Akt isoforms to platelet function based upon studies of human platelets ex vivo and gene-targeting studies in mouse models of platelet activity and thrombosis.
Akt isoforms & function in platelets
There are three mammalian Akt isoforms: Akt1, 2 and 3 [2]. mRNA for Akt1 and Akt2 has been detected in human platelets [3,4], and expression has been verified by a proteomic approach for Akt1 [4]. Mice express the protein for both Akt1 and Akt2 in platelets [5–7]; Akt3 has not been detected in platelets to date. None of the iso-forms platelet specific. Akt1 is widely expressed and are associated with organismal growth, cell survival and carcinogenesis [8]. Akt2 is expressed in fat and skeletal muscle cells, among other cells. The targeted deletion of Akt2 in mice leads to a diabetic phenotype as mice reach adulthood [9]. Akt3 is expressed in the brain [10], testes [11] and melanocytes, and has been implicated in VEGF-dependent mitochondrial biogenesis [12], brain development [13,14] and in the development of malignant melanoma [15].
Our understanding of the function of individual Akt isoforms in platelets primarily relies on the results of studies of mice with targeted deletions of the individual isoforms. Deletion of Akt1 in a mixed 129R1/C57Bl/6 background of mice resulted in reduced platelet responses to thrombin and collagen [6,16]. Impaired aggregation, fibrinogen binding, ATP secretion and mobilization of calcium release were seen in response to thrombin; the latter suggesting a defect in an event proximal to receptor activation in Akt1−/− platelets [6].Akt1−/− mice exhibited a prolonged tail-bleeding time compared with wild-type control mice, suggesting that the defect in platelet function was sufficient to impair hemostasis [6]. In C57Bl/6 mice, deletion of Akt2 also resulted in impaired platelet function, characterized by reduced sensitivity to thrombin receptor activated peptide (TRAP)-dependent fibrinogen binding, reduced secretion of dense and α-granule contents and a relative resistance to an arterial injury model of thrombosis [5]. Additional deletion of a single allele of Akt1 with absence of Akt2 exacerbated the platelet defect in vitro. However, no defect in collagen-mediated aggregation was observed, nor was there any defect noted in tail-bleeding time in Akt2−/− mice of the C57Bl/6 background. Defects in GPIb-mediated signaling were also noted in mice lacking Akt1 or Akt2 [7]. These studies suggest that the two isoforms have similar overlapping functions in platelet activation, although it is possible that Akt1 activation is preferentially activated by collagen signaling pathways and contributes more directly to hemostasis. It is also possible that expression of Akt1 or Akt2 in the vasculature contributes to the role of these isoforms in hemostasis or thrombosis, although this would seem counter intuitive, given that Akt expression in vascular beds contributes to nitric oxide (NO) production [17], which has been shown to limit platelet activation and thrombosis [18–20].
Studies using Akt inhibitors in human platelets generally support a similar role for Akt in activation of human platelets [7,21,22]. Several inhibitors of Akt have been reported to reduce aggregation of human platelets in vitro. However, treatment of human platelets with Akt1/2i has been shown to increase SFLLRN-mediated platelet aggregation in a PI3K-independent manner, presumably owing to off-target mechanisms [23]. The specificity of putative Akt inhibitors is still being evaluated and, thus, the relative roles of individual Akt isoforms in human platelets remains undefined. Of notable significance, a SNP in the gene encoding Akt2 was recently identified and found to be associated with platelet activity, suggesting that there is a role for Akt2 in the function of human platelets [1].
Akt & the mechanism of platelet activation
Despite years of study, the precise mechanism by which Akt supports platelet activation remains poorly characterized. The fact that the platelet function defects seen at low agonist concentrations in the absence of Akt1 or Akt2 are overcome at higher agonist concentrations may suggest a primary defect in secretion, given the amplifying nature of platelet secretion on aggregation and fibrinogen binding. Consistent with this idea is the observation that platelets lacking Akt1 or Akt2 have defects in secretion of dense and α-granule contents [5,6]. Studies from the laboratory of Du provide more evidence suggesting that Akt contributes to platelet secretory pathways. The laboratory identified a second wave of secretion in platelets stimulated with the thromboxane A2 mimetic U46619 [24]. Second-wave secretion was dependent on PI3Kγ and outside in signaling by the fibrinogen receptor, αIIbβ3. As an effector of PI3K, Akt was a possible candidate to mediate this second-wave signal. Consistent with this, collagen and thrombin-induced ATP secretion are both reduced in the absence of Akt1 or in the presence of the Akt inhibitor SH-6 [16]. 8Br cGMP partially restores secretion and aggregation in response to either of these agonists, suggesting that Akt may regulate secretion in part by regulating a NO− and cGMP− dependent pathway. However, the role of NO in platelet secretion is controversial, as studies have shown for years that endothelial- or platelet-derived NO, as well as NO donors, suppress platelet aggregation, rather than support it [18–20,25]. Specifically, Morrell et al. have shown that NO donors inhibit, rather than stimulate secretion of platelet granules [26]. Thus, the specific mechanism by which Akt regulates platelet secretory function remains to be resolved and would benefit from the definitive identification of Akt substrates involved in platelet secretory pathways.
In addition to influencing platelet secretory function, it has been hypothesized that Akt may regulate the function of integrin αIIbβ3. This platelet-specific integrin is subject to two modes of conformation-dependent regulation, termed inside-out and outside-in signaling [27]. Integrin inside-out signaling refers to signaling mechanisms, such as those induced by agonists for G-protein-coupled receptors, that induce conformational changes in the integrin structure that facilitate binding of soluble fibrinogen to the extracellular integrin domains. Outside in signaling refers to signaling that occurs subsequent to fibrinogen binding and results in such platelet phenotypic changes as retraction of thrombin-dependent platelet–fibrin clots and platelet spreading on fibrinogen-coated surfaces. Given that Gi-dependent activation of PI3Ks promotes activation of both Akt and Rap1, it has been considered that activation of Akt may contribute to activation of Rap1 as a downstream consequence [22]. Evidence in support of this view is that Gai-dependent GTP loading of Rap1 has recently been noted to be reduced in the presence of an Akt inhibitor [22]. However, it has been noted that application of phosphatidylinositiol-3,4-bisphophate (PI[3,4]P2) to platelets can facilitate Akt phosphorylation, but not Rap1 activation [28], suggesting that the two events occur through differentially regulated pathways. By contrast, a recent study in endothelial cells demonstrated reductions in Akt phosphorylation following silencing of Rap1A or Rap1B [29]. An increase in Akt phosphorylation had also been demonstrated in neuronal cells stimulated to increase Rap1 activation with the cAMP-dependent GEF Epac. A similar cAMP- and Epac-dependent phosphorylation of Akt phosphorylation was ablated by knockdown of Rap2 [30]. These studies suggest that, in some cells, Akt activation may be downstream, rather than upstream of Rap1. However, this possibility remains to be demonstrated in platelets.
Akt effectors in platelets
More than 100 substrates of Akt have been reported in a variety of cell types [31]. Many Akt substrates have roles in the regulation of gene transcription but Akt clearly modulates platelet aggregation and secretion through mechanisms that occur within a time frame of seconds to minutes to acutely regulate the function of this anucleate cell. A number of candidate substrates exist in platelets. The first relates to the reported effects of NO on platelet secretion described earlier. Endothelial NO synthase (eNOS) is an Akt substrate in endothelial cells and its phosphorylation induces activation of the enzyme, which catalyzes the synthesis of NO from l-arginine [32,33]. There are three mammalian isoforms of NOS (NOS1, 2 and 3, also termed NOS, inducible NOS [iNOS] and eNOS, respectively). Their expression in human platelets is a subject of some controversy, with one laboratory suggesting that both iNOS and eNOS are expressed [34,35], while others suggest the presence of only eNOS [36]. Du and colleagues propose a model in which Akt-mediated phosphorylation of eNOS induces platelet NO production, in turn leading to cGMP production and cGMP-induced secretion [16]. An increase in platelet cGMP production upon platelet activation has been reported by other laboratories [37,38]; however, these recent studies suggest that an eNOS-independent mechanism of soluble guanylyl cyclase activation may be responsible [37]. As noted previously, other studies conclude that eNOS is present in platelets, but plays an inhibitory role in platelet granule secretion and aggregation [26,36]. A recent study has found that none of the three NOS isoforms are present in platelets ,and that previous studies were confounded by the presence of contaminating leukocytes [37]. At this point, the preponderance of evidence would suggest that the majority of NO encountered by the platelet in the vasculature is not platelet derived and has an inhibitory function on platelet activation. Certainly, a role for Akt in endothelium-derived NO is supported by independent laboratories [32,33], but the majority of studies suggest a suppressive effect of NO on platelet function and thrombosis [20,26,36,39], rather than the positive effect noted by the Du laboratory [7,16,35]. Thus, the role of Akt in regulating platelet aggregation and thrombosis probably occurs through phosphorylation of platelet substrates other than, or in addition to, eNOS.
As in insulin-responsive cells, glucose uptake is active in platelets to maintain stores for ATP generation, which supports active processes, such as secretion. In platelets, glucose uptake can be stimulated by thrombin or insulin and is primarily mediated by the glucose transporter, GLUT3 [40]. Glucose uptake stimulated by thrombin or insulin is reduced in the presence of the Akt inhibitor, ML-9, suggesting that Akt contributes to glucose uptake in platelets, as it does in skeletal muscle or adipocytes [40]. However, the role of Akt in glucose uptake is not responsible for its role in platelet aggregation, since insulin induces Akt phosphorylation in platelets, but not platelet aggregation.
Another candidate effector of Akt is the β3 integrin itself. A peptide based upon the cytoplasmic sequence of β3 integrin becomes phosphorylated in vitro in the presence of Akt [41]. The phosphorylated residue was found to correspond to Thr753 in the β3 tail, and was found to inhibit binding of Shc to β3 tail phosphorylated on Tyr759 [41]. Phosphorylation of Thr753 is increased in the presence of phosphatase inhibitors, such as calyculin A, which also reduce platelet adhesion [42]. Thus, phosphorylation of the residue is associated with the negative regulation of platelet outside–in signaling. However, Akt inhibitors were not found to block phosphorylation of Thr 753 [42], and Akt has generally been found to positively regulate platelet activation. At present, it seems unlikely that Akt is the kinase directly responsible for phosphorylation of β3 Thr753 in platelets. Furthermore, the relevance of β3 Thr753 to regulation of platelet integrin function in vivo is still unclear. However, it is interesting to note that Thr753 occurs within a sequence that approximates the consensus sequence for recognition by a well-described Akt target in many cells, glycogen synthase kinase (GSK)3β (746-LFKEATSTFTNITYR). The potential regulation of Thr753 by GSK3β remains to be determined.
Glycogen synthase kinase 3β is a substrate for Akt in a variety of cell types and is present in platelets from humans and mice [43]. Li et al. showed that human platelets treated with GSK3 inhibitors display enhanced aggregation in vitro and that mouse platelets lacking a single allele of GSK3β are hypersensitive to agonist-induced aggregation and secretion [43]. Haploinsufficiency of GSK3β also results in hypersensitivity to arterial thrombosis in ferric chloride injury models in mice. These data suggest that GSK3β plays a negative role in regulating platelet function, but the substrates through which GSK3β regulates secretion and fibrinogen binding remain to be definitively identified. There is also some controversy regarding the role of GSK3β in platelet function. A previous report noted that platelets treated for short time periods with GSK3 inhibitors had reduced aggregation relative to untreated controls [44]. In our work noting enhancements of platelet function in the presence of GSK3 inhibitors, platelets were allowed to equilibrate with LiCl or other GSK3 inhibitors for 2 h, since a previous report had quantified the time-dependent accumulation of intracellular LiCl in platelets and noted that 2 h was required to reach equilibrium concentrations [45]. The gene-deletion studies, taken together with known roles of Akt and GSK3 in other cell systems, suggest that Akt-mediated phosphorylation of GSK3β inhibits the constitutive kinase activity of GSK3β, thus reducing phosphorylation of GSK3 substrate. Agonist-dependent dephosphorylation of the putative GSK3 substrate, microtubule-associated protein MAP τ, is seen in platelets [43] and its phosphorylation is sensitive to GSK3 inhibitors and reduced in GSK3 heterozygous or knockout platelets. Nevertheless, the functional relevance of phosphorylation of any GSK3 substrate to platelet activity remains to be defined.
Akt has also been reported to play a role in regulation of the cAMP phosphodiesterase (PDE)3A [46]. This enzyme regulates the concentration of cAMP in the platelet by hydrolyzing 3′,5′-cAMP to its inactive metabolite, 5′AMP. High concentrations of cAMP within the platelet is known to impede multiple steps in platelet activation, such as agonist-stimulated calcium mobilization and, ultimately, activation of integrin αIIbβ3. An increase in PDE3A phosphorylation was detected upon platelet stimulation with thrombin using an antibody developed to detect the phosphorylated Akt substrate consensus sequence (RXRXXS/T). The increase in PDE3A phosphorylation correlates with an increase in platelet PDE3A activity detected upon thrombin stimulation. A role for Akt in the regulation of cAMP levels was proposed, since inhibition of Akt blocked the suppression of cAMP concentrations induced by thrombin in this study. However, a more recent study demonstrated that thrombin or SFLLRN-mediated phosphorylation of PDE3A is reduced by inhibitors of PKC and not by the PI3K inhibitor, wortmannin, which inhibits Akt activation [47]. In this study, IGF-1-mediated activation of platelet Akt was insufficient to cause PDE3A phosphorylation or cAMP hydrolysis, but was sufficient for stimulation of platelets with the PKC-activating phorbol ester PMA-induced PDE3A phosphorylation and hydrolysis of cAMP. Thus, a role for Akt in cAMP modulation remains unconfirmed.
While a role for Akt in regulating platelet activation is not disputed, the precise mechanism by which it does so remains poorly defined. The mechanism is probably multifactorial, but the relative roles of each of these putative substrates in contributing to the potentiation of platelet activation by Akt requires further study.
Regulation of Akt activation in platelets
Given that Akt undoubtedly plays a supporting role in platelet function in vivo, the regulation of Akt activity is also the subject of intense study. The Akt kinase is comprised of three domains: a pleckstrin homology domain (PH), a catalytic domain and a regulatory domain [2]. The consensus model for its activation is that the binding of 3-phosphorylated polyphosphoinositides to the PH domain localizes Akt to the membrane, where it becomes exposed to kinases that catalyze its phosphorylation at two sites: Thr308 in the kinase domain and Ser473 in the regulatory domain (reviewed in [48,49]). Akt is, thus, critically regulated by the concentrations of PI(3,4)P2 and phosphatidyl inositol-3,4,5-trisphospohate (PI[3,4,5]P3) and by the kinases that phosphory-[3,4,5]P3) and by the kinases that phosphory-P3) and by the kinases that phosphorylate these two sites. Thr308 in the catalytic domain is phosphorylated by a 3-phosphoinositide-dependent kinase, PDK1 [50], so termed because of its regulation by phosphoinositide binding. The identity of the kinase responsible for phosphorylation of Ser473 was generically termed PDK2 before the protein responsible for the activity was identified. Currently, there appear to be a number of kinases mediating this activity in different cell lines. Phosphorylation of Ser473 is catalyzed by a complex of rictor and mTOR in various cell lines [51,52] or by DNA-dependent protein kinase in the nucleus [53], but there have been reports that PKCβ may regulate phosphorylation of the Akt residue in mast cells [54] and that PAK1 may do so in cardiomyocytes [55]. Platelets express each of these proteins (although they express the b1 form of PKC, whereas the b2 form regulated mast cell Akt) and it is unclear which is responsible for the activation of Akt in platelets.
Upon agonist binding to platelets, D3-phosphorylated phosphoinositides are generated in a PI3K-dependent fashion [56]. PI(3,4)P2 and PI(3,4,5)P3 both bind to the PH domain of Akt and have been shown to result in Akt activation. There is evidence that platelets stimulated with thrombin generate an initial wave of PI(3,4,5)P3, inducing Akt activation, which is then sustained by an increase in PI(3,4)P2 levels following integrin engagement [57,58].
A number of PI3Ks are expressed in platelets and the effects of individual isoforms of PI3K are somewhat agonist dependent. Akt becomes activated upon platelet stimulation with diverse agonists, such as collagen [6], ristocetin/vWF [7], thrombin [3,58], ADP [59], IGF-1 [60] and Gas6 [61]. In each case, stimulation of Akt phosphorylation is dependent upon PI3K. However, the roles of each of the PI3K isoforms in the regulation of Akt phosphorylation by different agonists is still the subject of much study. The best-studied class of PI3Ks is designated class I and, thus far, these seem to have most relevance to platelet signaling. This class of PI3Ks is composed of two poly peptide subunits: a regulatory subunit and a catalytic subunit (for a review see [62]). Platelets contain class IA regulatory subunits p85α, p85β and class IB p101γ. These may associate with the catalytic subunits p110α or β for the class IA enzymes, or with p110γ for the class IB. Genetic deletion of the catalytic subunit, for which the PI3K isoform is generally named, has yielded some insight into which isoforms regulate platelet function; however, results of studies conducted using subtype-selective inhibitors, gene deletion in mice or knockin of mutant forms of the PI3Ks have in some cases yielded contradictory results and suggest that the contribution of individual PI3Ks are agonist specific and, in some cases, cooperative.
In the first report addressing the role of PI3Kγ on Akt and platelet function, platelets from mice with genetic deletion of PI3Kγ displayed reduced aggregation and reduced Akt phosphorylation when stimulated with ADP; however, responses to other agonists were normal [63]. Although two additional studies confirmed defects in ADP-dependent aggregation in platelets from PI3Kγ−/− mice [64,65], one of these studies detected no difference in ADP-dependent phosphorylation of Akt-Ser473 accompanying the defect in ADP-dependent aggregation [65]. This result, combined with the observation that PI3Kγ-selective kinase inhibitors had no effect on ADP-induced platelet aggregation, led the authors to conclude that PI3Kγ plays a role in ADP-dependent platelet function, and this role is not dependent on its catalytic activity or on Akt activation [65]. However, a recent study examining the platelets of mice expressing a kinase-dead mutant of PI3Kγ found that ADP-dependent Akt phosphorylation is, indeed, selectively inhibited in platelets lacking PI3Kγ enzymatic activity [66]. Loss of PI3Kγ expression was associated with resistance to thrombosis in an arterial injury model [64] and a disseminated thrombosis model [63] but the contribution of Akt signaling to PI3Kγ-dependent platelet responses in vivo remains difficult to interpret.
While PI3Kγ signaling in platelets appears to be restricted to ADP-dependent pathways, a broader role for PI3Kβ is seen in Akt activation downstream of a number of platelet agonists. Studies using isoform-selective inhibitors suggest that PI3Kβ is required for Akt phosphorylation induced by the collagen receptor glycoprotein (GP)VI [67], and also contributes to Akt activation induced by several agonists for G-protein-coupled receptors [68]. Platelets from mice expressing a kinase-dead form of PI3Kβ exhibit near-complete loss of Akt phosphorylation in response to the GPVI agonist, convulxin, the thromboxane mimetic U46619, and ADP; aggregation in these platelets is impaired by approximately 30% to U46619, by approximately 50% to ADP, and is nearly blocked in response to convulxin [66]. That ADP-dependent Akt phosphorylation is ablated in the presence of kinase-dead forms of either PI3Kβ or γ suggests a possible cooperative relationship between the two isoforms and demonstrates that Akt activation is not strictly required for aggregation, since aggregation is reduced, but not ablated, by loss of activity of either isoform. There is also some evidence for unique roles of β and γ isoforms in stabilization of thrombi formed under arterial flow conditions. Mouse platelets lacking PI3Kγ failed to form thrombi of normal height on collagen and vWF-coated surface and the platelets detached with greater frequency [69]. As with platelets lacking PI3Kγ, mouse platelets treated with PI3Kβ inhibitor TGX-221 also detach more frequently from collagen-coated surfaces, but loss of PI3Kγ in combination with TGX-221 treatment did not yield additive effects [69]. Thus, both β and γ isoforms appear to be required for optimal thrombus formation and for optimal Akt activation. This, coupled with the defects in platelet activation and thrombosis seen in mice lacking Akt isoforms, suggests that at least part of the contribution of PI3K to platelet activity is due to Akt activation.
PI3Kα is also expressed in platelets, although its role in platelet function is not as well defined as that of PI3Kγ or β. Akt phosphorylation in platelets can be stimulated by IGF-1, and this mode of phosphorylation is predominantly sensitive to inhibitors of PI3Kα and is partially inhibited by an inhibitor of PI3Kβ [60,70]. IGF-1 potentiates platelet aggregation responses to PAR1 agonist peptide, and its potentiating effect is blocked by inhibitors of PI3Kα [60]. PI3Kα inhibitors also block the effect of IGF-1 on Akt phosphorylation, suggesting that PI3Kα-dependent Akt activation may enhance aggregation to PAR1 agonist. In summary, agonists stimulate unique pathways leading to activation of individual PI3K isoforms and the α, β and γ isoforms all play some role in activation of Akt by various agonists. The role of PI3Kα in thrombus formation has not been evaluated directly. Table 1 provides a summary of the functional effects of genetic deletion or inhibition of Akt-regulatory proteins in platelets.
Table 1.
Effects of genetic deletion/modification of Akt isoforms or its regulatory proteins in platelets.
| Mouse model | Defect in sensitivity to agonists | Effect on Akt phosphorylation | Effect on platelet function | Ref. |
|---|---|---|---|---|
|
Akt1
−/−
|
Collagen, thrombin |
|
Reduces calcium release, aggregation and hemostasis (increases tail-bleeding time) |
[6] |
|
Akt2
−/−
|
PAR4 agonist, ADP |
|
Reduces aggregation, secretion and thrombosis in carotid artery injury model |
[5] |
| PI3Kβ KD |
ADP, thromboxane A2 (U46619), glycoprotein VI agonist |
Decrease |
Reduces platelet aggregation, adhesion and spreading on fibrinogen |
[66] |
| PI3K γ −/− | ADP | Decrease | Reduces maximal ADP aggregation | [63,64] |
| ADP | No change | Reduces maximal ADP aggregation | [65] | |
| |
|
|
Reduces response to arterial injury model of thrombosis, thromboembolism |
[63,64] |
| PI3Kγ KD | ADP, thromboxane A2 (U46619) | Decrease | Reduces aggregation | [66] |
The role of PI(3,4,5)P3 versus PI(3,4)P2 in Akt activation in platelets is still not entirely clear. Lova et al. reported that exogenous application of PI(3,4)P2 was as effective in mediating Akt phosphorylation as PI(3,4,5)P3 [28]. It has been reported that, following thrombin activation of platelets, an initial wave of PI(3,4,5) P3 is generated, followed by a later wave of PI(3,4)P3 generated upon integrin activation [57]. While this latTer wave was initially reported to be associated with a second wave of Akt phosphorylation [58], a more recent study detected no difference in thrombin-induced Akt phosphorylation following integrin blockade with RGDS [21]. Ma et al. recently reported that in B cells, levels of PI(3,4)P2 correlate with phosphorylation of Ser473, while PI(3,4,5) P3 levels regulate Thr308 phosphorylation, implying that both phosphoinositides may play important roles in regulating Akt activity [71]. In any case, enzymes that regulate the concentration of either of these two phosphoinositides may effect activation of Akt. The best-characterized phosphoinositide phosphatase in platelets to date is Src homology 2-containing inositol phosphatase-1 (SHIP1), which dephosphorylates PI(3,4,5)P3 at the D5 position and may, therefore, be expected to regulate both basal and agonist-mediated PI(3,4,5)P3 levels. The effects of SHIP1 deletion on platelet function are controversial [72–74]. In one report, deletion of SHIP1 was accompanied by an increase in adhesion under physiological flow conditions, suggesting a negative role for SHIP1 in platelet adhesion [73]. By contrast, Severin et al. reported reduced outside in signaling of integrin αIIbβ3 in SHIP1−/− platelets and a decrease in arterial thrombus formation in vivo, suggesting a positive role for SHIP1 in platelet function and thrombosis [74]. Akt phosphorylation was not directly measured in either study, so, presently, it is not clear whether the functional results of SHIP1 deletion were in part, due to effects on Akt activation. Another phosphatase, PTEN, catalyzes the hydrolysis of the inositide D3 phosphate in many cells, and its expression in platelets has been detected [4]. The effects of PTEN deletion in platelets have not yet been reported.
There are some reports of PI3K-independent pathways to Akt activation. Kroner et al. reported that thrombin-dependent phosphorylation of Akt-Ser473 in platelets was not completely inhibited by the PI3K inhibitor LY294002 but was substantially inhibited by inhibitors of PKC isoforms α or β; however, PKC activity was insufficient to induce Akt phosphorylation in vitro, suggesting that another cofactor in the platelet lysate was required [3]. Resendiz et al. have also noted that during early time points after PAR1 or PAR4 stimulation (1 min and earlier), some platelet Akt phosphorylation is insensitive to PI3K inhibitors [21]. These PI3K-independent pathways are sensitive to inhibitors of phospholipase C, PKC, calcium and calmodulin but the details of these pathways remain to be fully described.
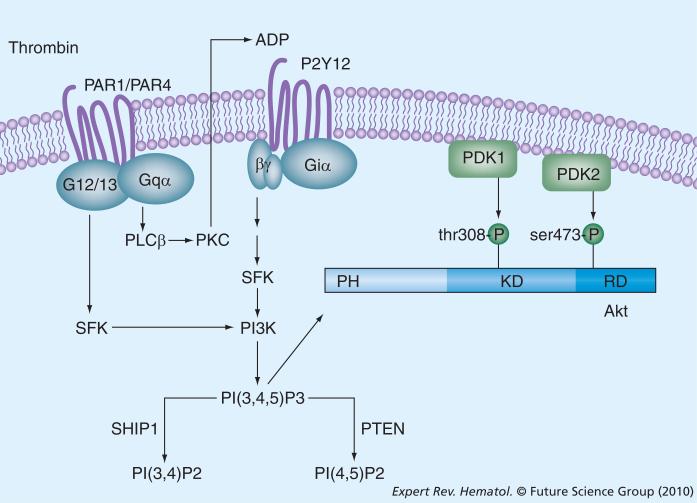
As mentioned previously, the pathways to Akt phosphorylation are somewhat agonist specific; however, Akt phosphorylation induced by a number of agonists, including thrombin, collagen, convulxin and ADP, are all dependent on the P2Y12 receptor for ADP [59,67,75,76]. ADP-stimulated Akt phosphorylation is entirely dependent on the Gi-coupled P2Y12 receptor, rather than the P2Y1 receptor. Thrombin-dependent signaling to Akt is also dependent on P2Y12 and is dramatically inhibited by inhibitors of P2Y12 or PKC inhibitors that block ADP secretion following thrombin exposure [59]. This is owing to an apparent requirement for activation of the Gi-subunit coupled to P2Y12 [75]. Further studies by the Kunapuli laboratory suggest that selective stimulation of G12/13 by PARs potentiates Gi-dependent signaling to Akt via a Src family kinase-dependent pathway [76]. The mechanism by which stimulation of Gi subunits leads to Akt activation is also dependent on Src family kinases, but the precise pathway remains to be elucidated. Figure 1 provides a summary of thrombin-mediated regulatory pathways of Akt activation in platelets.
Figure 1. Mechanisms of thrombin-mediated Akt activation in platelets.
Soluble platelet agonists, such as thrombin, ADP and thromboxane A2, induce PI3K-dependent production of PI(3,4,5)P3. Akt is activated by binding of this 3-phosphorylated phosphoinositide to the pleckstrin homology domain, which then colocalizes at the membrane with PDK1 and PDK2, which phosphorylate Akt on Thr308 and Ser473, respectively. The molecular identity of PDK2 in platelets is still unclear. Akt activation is limited by hydrolysis of PI(3,4,5)P3 by two phosphatases: SHIP1 and PTEN.
PLC: Phospholipase C; SFK: Src family protein kinase; SHIP: Src homology 2-containing inositol phosphatase.
Taken together, studies from platelets and other cells suggest that D3 phosphorylated phosphoinositides bind to the PH domain of Akt, allowing colocalization of Akt with the PDKs mediating its phosphorylation and activation. However, there remain many questions regarding how Akt becomes activated by different agonists in platelets, and the relative roles of PI3K family members and other kinases, such as PKC, in its activation.
Akt pathways in human platelets & disease
Despite compelling evidence from numerous animal models that Akt regulates platelet activation and thrombosis, evidence for a direct causal role of the dysregulation of Akt signaling in either human thrombosis or bleeding disorders is sparse. Much of the evidence for the contribution of Akt-dependent pathways to human cardiovascular disease comes from analysis of genome-wide association studies of SNPs in the genes coding for these proteins with particular human disease or functional effects. Variant TRIB3, a protein known to regulate insulin-dependent signaling to Akt, is associated with early-onset myocardial infarction (MI) in patients with Type 2 diabetes [77]. This TRIB3 variant was recently shown to reduce Akt and eNOS activation in endothelial cells, with consequent reduction in vascular NO concentrations [78]. It is unknown whether any effects on platelet activity result from expression of the variant. A polymorphism in the gene for soluble CD40 ligand (sCD40L) has been reported to regulate plasma levels of the protein and the concentration of sCD40L in plasma independently associated with the risk of acute coronary syndrome [79]. sCD40L has been shown to affect Akt activation in platelets [80], suggesting a potential role for platelet Akt in contributing to this effect. A stronger case can be made for a role for Akt2 in regulating general platelet activity, since expression of a polymorphic variant of Akt2 was associated with platelet function in a screen of 500 healthy European individuals [1]. Also supporting a role for a PI3K family member in human platelet derivation and function, a SNP associated with expression of PI3Kγ was recently found to associate with changes in mean platelet volume and annexin V binding, although these changes may not be Akt dependent [81]. These studies suggest that Akt and its regulatory pathways contribute to human platelet function and may contribute to cardiovascular disease, but the multifactorial nature of cardio vascular disorders may make it difficult to identify components directly attributable to variants in Akt isoforms. The redundancy of platelet pathways may make a unique contribution of any individual Akt isoform to cardio vascular disease rather rare. This may parallel the relative contribution of Akt iso-forms to diabetes in humans. Although the loss of Akt2 clearly leads to a diabetic phenotype in mice, mutation of Akt2 is rare (although documented) as a causal factor in insulin resistance and diabetes [82].
Akt pathways & drug development
Although data from mouse models suggest that Akt inhibitors may be beneficial to reduce prothrombotic effects in platelets, no studies to evaluate Akt inhibitors for prophylaxis of thrombotic sequellae in human patients have been undertaken yet. There are good reasons for remaining cautious in exploring Akt inhibitors for this indication. Akt isoforms are widely expressed and, of particular relevance for cardiovascular indications, have been shown to mediate recovery from ischemia-reperfusion injury following MI [83,84]. As such, Akt inhibitors may be counterindicated in patients suffering prior MI. Akt activation is also broadly associated with enhanced neuronal survival in animal models of stroke – an endogenous inhibitor of Akt was recently found to be required for ischemia-induced neuronal death in a mouse stroke model [85]. Akt1 is important for phosphorylation of endothelial eNOS, and the loss of Akt1 was found to reduce NO release and angiogenesis [86]. Endothelial NO production is important for maintaining vasorelaxation, as well as limiting platelet activation, suggesting that inhibition of Akt1 might interfere with these positive aspects of cardiovascular regulation [17,18]. In addition, although mTOR complexes contribute to Akt activation in some cells [51,52], the mTOR inhibitor rapamycin enhances arterial thrombus formation in a mouse model, probably through enhancing endothelial tissue-factor expression, rather than through a direct effect on platelets [87].
Nevertheless, proteins that target the activation of Akt in platelets have been promoted as attractive targets for development of antithrombotic therapeutics; perhaps chief among these is PI3Kβ. A PI3Kβ-selective inhibitor, TGX-221 was found to reduce adhesion of human platelets to fibrinogen under arterial flow conditions and to reduce thrombosis in a rat Folts-type stenosis injury model [68]. However, administration of TGX-221 to the rat did not impair hemostasis, as measured using tail-bleeding time. TGX-221 was also not associated with hypotension or carotid vasodilatation, as was seen for other nonselective PI3K isoform inhibitors [88]. The efficacy of Akt inhibitors for this indication has yet to be tested in vivo. Several Akt inhibitors have been found to inhibit PAR1-mediated aggregation, but others have shown unexpected non-specific effects on platelets, such as inducing P-selectin exposure [21] or enhancing PAR1-mediated aggregation [23]. Most inhibitors identified to date are nonselective among individual isoforms. A new compound was recently reported for serving as a basis for structure–activity-based design of Akt2-selective compounds [89]. The development of more potent and selective competitive small-molecule inhibitors for Akt isoforms may, thus, help to determine whether any of the individual Akt isoforms are a viable target for thrombosis. In addition, continued development of selective inhibitors for individual PI3K isoforms looks to be a promising avenue for antithrombotic therapy [90]. Increasing our understanding of the PI3K-Akt pathway and its regulation will probably continue to yield promising targets for therapeutic development.
Expert commentary
The dual observations that thrombin induced production of PI(3,4,5)P3 [57], and treatment of platelets with PI3K inhibitors reduced the stability of platelet aggregates [91,92], raised suspicion that Akt was a functional target for PI3K products in platelets. The development of gene-targeting techniques in mice facilitated confirmation of this suspicion, since deletion of Akt1 resulted in reduced hemostatic responses [6], and mice lacking Akt2 had reduced sensitivity to thrombotic insult [5]. The presence of both of these isoforms in human platelets, combined with the recent identification of a variant SNP in the gene for Akt2 associated with platelet functional response [1], suggest that Akt also regulates human platelet activation. Analyses of additional mouse models have allowed the identification of a number of proteins regulating the activity of Akt and platelet function, among them, PI3Ks α, β and γ. The Akt effectors contributing to platelet function in vivo are still being determined but promising candidates include GSK3, eNOS, PDE or the β3 tail. The identification of platelet regulatory pathways in mice presents us with a challenge to determine whether defects in any of these pathways are associated with disorders of hemostasis or contribute to platelet hyperactivity and thrombosis. These types of studies are increasingly feasible. The identification of new isoform-selective inhibitors of Akt or its regulating PI3Ks will allow additional basic analyses of the role of individual isoforms in human platelets and also serve as platforms for studies evaluating the feasibility of Akt and its regulatory proteins as drug targets for managing thrombosis.
Five-year view
The next 5 years promises to reveal more about the complexities of Akt activation in platelets and how Akt pathways may be dysregulated in disease states. In particular, whether aberrant signaling due to variant forms of Akt, PI3Ks or their regulators contribute to thrombophilia or hemostatic defects is a question only beginning to be addressed using genome-wide association studies in large patient populations. New research is also expected to address whether activation of Akt pathways contributes to platelet hyperactivity and thrombophilia associated with conditions such as diabetes. Whether any of the three Akt isoforms is a preferential target for antithrombotic therapeutics and whether they play any unique roles in signaling of human platelets remains to be determined. Both the list of upstream Akt regulatory pathways and downstream effectors in platelets remain incomplete. Of note, several investigators have noted that there is evidence for PI3K-independent signaling to Akt in platelets [3,5,21]. This pathway is, to date, uncharacterized. It is also unclear to what extent the possible effectors GSK3, eNOS, PDE or the β3 tail contribute to the positive role of Akt in platelet aggregation and thrombosis, or whether other currently uncharacterized effectors may play important roles. Clearer understanding of these pathways regulating Akt activation and its downstream targets will allow us to develop better inhibitors for therapeutic intervention in patients suffering from acute coronary syndrome and other prothrombotic complications, and to limit bleeding episodes and other side effects.
Key issues.
Human platelets express three Akt isoforms. Deletion of Akt1 in mice reduces platelet responses to collagen and thrombin in vitro and increases tail-bleeding time. Loss of Akt2 results in impaired responses to PAR4 agonist or ADP, but not collagen, and reduces thrombosis in arterial injury models.
Association of Akt2 variants with platelet function in humans suggests that Akt isoforms also contribute to human platelet activity, but any contribution of Akt signaling to thrombosis in humans is, as yet, unclear.
Akt regulates platelet secretion, probably subsequent to initial aggregation (second-wave secretion).
Akt contributes to phosphorylation of PDE3A, GSK3β and possibly endothelial nitric oxide synthase and the β3 integrin tail in platelets. The relative roles of these substrates in regulating platelet activation are still undefined.
Akt activation is regulated by PI3 k α, β and γ, and is also probably regulated by phosphoinositide phosphatases, SHIP1 and, possibly, PTEN, as these phosphatases regulate PI(3,4,5)P3 levels. There are PI3K-independent mechanisms of Akt activation that remain to be identified.
Akt activation induced by ADP, thrombin or collagen is dependent on activation of Gi signaling by the P2Y12 receptor and on activation of Src family kinases.
Proteins contributing to Akt activation, particularly PI3Kβ, may make attractive targets for development of antithrombotic therapeutics.
Individual isoforms of Akt may also be potential targets for antithrombotic therapy but would probably require development of more isoform-selective inhibitors.
Footnotes
Financial & competing interests disclosure
This work was supported in part by NIH grant R01HL081241. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1 ••.Jones CI, Bray S, Garner SF, et al. A functional genomics approach reveals novel quantitative trait loci associated with platelet signalling pathways. Blood. 2009;114(7):1405–1416. doi: 10.1182/blood-2009-02-202614. [Resequencing of candidate genes encoding signaling proteins in platelets yielded 1327 single-nucleotide polymorphisms (SNPs), which were then genotyped in 500 European subjects with known platelet phenotype. A total of 35 SNPs were found to associate with altered platelet function, among them, two in the gene encoding for Akt2] [DOI] [PubMed] [Google Scholar]
- 2.Hanada M, Feng J, Hemmings BA. Structure, regulation and function of PKB/AKT – a major therapeutic target. Biochim. Biophys. Acta. 2004;1697:3–16. doi: 10.1016/j.bbapap.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Kroner C, Eybrechts K, Akkerman JW. Dual regulation of platelet protein kinase B. J. Biol. Chem. 2000;275:27790–27798. doi: 10.1074/jbc.M000540200. [DOI] [PubMed] [Google Scholar]
- 4.Dittrich M, Birschmann I, Mietner S, et al. Platelet protein interactions: map, signaling components, and phosphorylation groundstate. Arterioscler. Thromb. Vasc. Biol. 2008;28:1326–1331. doi: 10.1161/ATVBAHA.107.161000. [DOI] [PubMed] [Google Scholar]
- 5 ••.Woulfe D, Jiang H, Morgans A, et al. Defects in secretion, aggregation, and thrombus formation in platelets from mice lacking Akt2. J. Clin. Invest. 2004;113:441–450. doi: 10.1172/JCI20267. [Describes reduced platelet function in vitro (fibrinogen binding, aggregation, and secretion) and in vivo (reduced thrombosis and pulmonary embolism for deletion of Akt2, an increase in bleeding time due to loss of Akt1) owing to genetic deletion of either of two isoforms of Akt] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6 ••.Chen J, De S, Damron DS, et al. Impaired platelet responses to thrombin and collagen in AKT-1-deficient mice. Blood. 2004;104:1703–1710. doi: 10.1182/blood-2003-10-3428. [Describes reduced platelet function in vitro (fibrinogen binding, aggregation, and secretion) and in vivo (reduced thrombosis and pulmonary embolism for deletion of Akt2, an increase in bleeding time due to loss of Akt1) owing to genetic deletion of either of two isoforms of Akt] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yin H, Stojanovic A, Hay N, et al. The role of Akt in the signaling pathway of the glycoprotein Ib-IX induced platelet activation. Blood. 2008;111:658–665. doi: 10.1182/blood-2007-04-085514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho H, Thorvaldsen JL, Chu Q, et al. Akt1/PKBα is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J. Biol. Chem. 2001;276:38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- 9.Cho H, Mu J, Kim JK, et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB β). Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 10.Boland E, Clayton-Smith J, Woo VG, et al. Mapping of deletion and translocation breakpoints in 1q44 implicates the serine/threonine kinase AKT3 in postnatal microcephaly and agenesis of the corpus callosum. Am. J. Hum. Genet. 2007;81:292–303. doi: 10.1086/519999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dummler B, Tschopp O, Hynx D, et al. Life with a single isoform of Akt: mice lacking Akt2 and Akt3 are viable but display impaired glucose homeostasis and growth deficiencies. Mol. Cell. Biol. 2006;26:8042–8051. doi: 10.1128/MCB.00722-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wright GL, Maroulakou IG, Eldridge J, et al. VEGF stimulation of mitochondrial biogenesis: requirement of AKT3 kinase. FASEB J. 2008;22:3264–3275. doi: 10.1096/fj.08-106468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tschopp O, Yang ZZ, Brodbeck D, et al. Essential role of protein kinase B γ (PKB γ/Akt3) in postnatal brain development but not in glucose homeostasis. Development. 2005;132:2943–2954. doi: 10.1242/dev.01864. [DOI] [PubMed] [Google Scholar]
- 14.Easton RM, Cho H, Roovers K, et al. Role for Akt3/protein kinase Bγ in attainment of normal brain size. Mol. Cell. Biol. 2005;25:1869–1878. doi: 10.1128/MCB.25.5.1869-1878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stahl JM, Sharma A, Cheung M, et al. Deregulated Akt3 activity promotes development of malignant melanoma. Cancer Res. 2004;64:7002–7010. doi: 10.1158/0008-5472.CAN-04-1399. [DOI] [PubMed] [Google Scholar]
- 16 •.Stojanovic A, Marjanovic JA, Brovkovych VM, et al. A phosphoinositide 3-kinase-AKT nitric oxide cGMP signaling pathway in stimulating platelet secretion and aggregation. J. Biol. Chem. 2006;281:16333–16339. doi: 10.1074/jbc.M512378200. [This study invokes a nitric oxide (NO) cGMP-dependent pathway to explain the role of Akt in platelet secretion, based upon the observation that reduced secretion observed in Akt1-/- platelets can be reconstituted in the presence of 8Br-cGMP. Note, however, that many of the studies describe a negative rather than positive role for NO in platelet aggregation and secretion] [DOI] [PubMed] [Google Scholar]
- 17.Iwakiri Y, Tsai MH, McCabe TJ, et al. Phosphorylation of eNOS initiates excessive NO production in early phases of portal hypertension. Am. J. Physiol. Heart Circ. Physiol. 2002;282:H2084–H2090. doi: 10.1152/ajpheart.00675.2001. [DOI] [PubMed] [Google Scholar]
- 18.Furlong B, Henderson AH, Lewis MJ, et al. Endothelium-derived relaxing factor inhibits in vitro platelet aggregation. Br. J. Pharmacol. 1987;90:687–692. doi: 10.1111/j.1476-5381.1987.tb11221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radomski MW, Palmer RM, Moncada S. An l-arginine/nitric oxide pathway present in human platelets regulates aggregation. Proc. Natl Acad. Sci. USA. 1990;87:5193–5197. doi: 10.1073/pnas.87.13.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radomski MW, Rees DD, Dutra A, et al. S-nitroso-glutathione inhibits platelet activation in vitro and in vivo. Br. J. Pharmacol. 1992;107:745–749. doi: 10.1111/j.1476-5381.1992.tb14517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Resendiz JC, Kroll MH, Lassila R. Protease-activated receptor-induced Akt activation – regulation and possible function. J. Thromb. Haemost. 2007;5:2484–2493. doi: 10.1111/j.1538-7836.2007.02769.x. [DOI] [PubMed] [Google Scholar]
- 22.Holinstat M, Preininger AM, Milne SB, et al. Irreversible platelet activation requires protease-activated receptor 1-mediated signaling to phosphatidylinositol phosphates. Mol. Pharmacol. 2009;76:301–313. doi: 10.1124/mol.109.056622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hunter RW, Harper MT, Hers I. The PKB inhibitor Akti-1/2 potentiates PAR-1-mediated platelet function independently of its ability to block PKB. J. Thromb. Haemost. 2008;6:1923–1932. doi: 10.1111/j.1538-7836.2008.03140.x. [DOI] [PubMed] [Google Scholar]
- 24.Li Z, Zhang G, Le Breton GC, et al. Two waves of platelet secretion induced by thromboxane A2 receptor and a critical role for phosphoinositide 3-kinases. J. Biol. Chem. 2003;278:30725–30731. doi: 10.1074/jbc.M301838200. [DOI] [PubMed] [Google Scholar]
- 25.Freedman JE, Loscalzo J, Benoit SE, et al. Decreased platelet inhibition by nitric oxide in two brothers with a history of arterial thrombosis. J. Clin. Invest. 1996;97:979–987. doi: 10.1172/JCI118522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morrell CN, Matsushita K, Chiles K, et al. Regulation of platelet granule exocytosis by S-nitrosylation. Proc. Natl Acad. Sci. USA. 2005;102:3782–3787. doi: 10.1073/pnas.0408310102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shattil SJ, Newman PJ. Integrins: dynamic scaffolds for adhesion and signaling in platelets. Blood. 2004;104:1606–1615. doi: 10.1182/blood-2004-04-1257. [DOI] [PubMed] [Google Scholar]
- 28.Lova P, Paganini S, Hirsch E, et al. A selective role for phosphatidylinositol 3,4,5-trisphosphate in the Gi-dependent activation of platelet Rap1B. J. Biol. Chem. 2003;278:131–138. doi: 10.1074/jbc.M204821200. [DOI] [PubMed] [Google Scholar]
- 29.Carmona G, Gottig S, Orlandi A, et al. Role of the small GTPase Rap1 for integrin activity regulation in endothelial cells and angiogenesis. Blood. 2009;113:488–497. doi: 10.1182/blood-2008-02-138438. [DOI] [PubMed] [Google Scholar]
- 30.Huston E, Lynch MJ, Mohamed A, et al. EPAC, PKA allow cAMP dual control over DNA-PK nuclear translocation. Proc. Natl Acad. Sci. USA. 2008;105:12791–12796. doi: 10.1073/pnas.0805167105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dimmeler S, Fleming I, Fisslthaler B, et al. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 33.Fulton D, Gratton JP, McCabe TJ, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marjanovic JA, Stojanovic A, Brovkovych VM, et al. Signaling-mediated functional activation of inducible nitric-oxide synthase and its role in stimulating platelet activation. J. Biol. Chem. 2008;283:28827–28834. doi: 10.1074/jbc.M801646200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marjanovic JA, Li Z, Stojanovic A, et al. Stimulatory roles of nitric-oxide synthase 3 and guanylyl cyclase in platelet activation. J. Biol. Chem. 2005;280:37430–37438. doi: 10.1074/jbc.M506518200. [DOI] [PubMed] [Google Scholar]
- 36.Freedman JE, Sauter R, Battinelli EM, et al. Deficient platelet-derived nitric oxide and enhanced hemostasis in mice lacking the NOSIII gene. Circ. Res. 1999;84:1416–1421. doi: 10.1161/01.res.84.12.1416. [DOI] [PubMed] [Google Scholar]
- 37.Gambaryan S, Kobsar A, Hartmann S, et al. NO-synthase-/NO-independent regulation of human and murine platelet soluble guanylyl cyclase activity. J. Thromb. Haemost. 2008;6:1376–1384. doi: 10.1111/j.1538-7836.2008.03014.x. [DOI] [PubMed] [Google Scholar]
- 38.Riba R, Patel B, Aburima A, et al. Globular adiponectin increases cGMP formation in blood platelets independently of nitric oxide. J. Thromb. Haemost. 2008;6:2121–2131. doi: 10.1111/j.1538-7836.2008.03179.x. [DOI] [PubMed] [Google Scholar]
- 39.Roberts W, Riba R, Homer-Vanniasinkam S, et al. Nitric oxide specifically inhibits integrin-mediated platelet adhesion and spreading on collagen. J. Thromb. Haemost. 2008;6:2175–2185. doi: 10.1111/j.1538-7836.2008.03190.x. [DOI] [PubMed] [Google Scholar]
- 40.Ferreira IA, Mocking AI, Urbanus RT, et al. Glucose uptake via glucose transporter 3 by human platelets is regulated by protein kinase B. J. Biol. Chem. 2005;280:32625–32633. doi: 10.1074/jbc.M507221200. [DOI] [PubMed] [Google Scholar]
- 41.Kirk RI, Sanderson MR, Lerea KM. Threonine phosphorylation of the β3 integrin cytoplasmic tail, at a site recognized by PDK1 and Akt/PKB in vitro, regulates Shc binding. J. Biol. Chem. 2000;275:30901–30906. doi: 10.1074/jbc.M001908200. [DOI] [PubMed] [Google Scholar]
- 42.Lerea KM, Venjara AY, Olson SC, et al. Threonine phosphorylation of integrin β3 in calyculin A-treated platelets is selectively sensitive to 5′-iodotubercidin. Biochim. Biophys. Acta. 2007;1773:185–191. doi: 10.1016/j.bbamcr.2006.08.053. [DOI] [PubMed] [Google Scholar]
- 43 •.Li D, August S, Woulfe DS. GSK3β is a negative regulator of platelet function and thrombosis. Blood. 2008;111:3522–3530. doi: 10.1182/blood-2007-09-111518. [Provides evidence that GSK3β is a functional substrate of Akt in platelets, as loss of a single allele of GSK3β or treatment of platelets with GSK3β inhibitors enhances platelet aggregation and secretion. Loss of single GSK3β allele also sensitizes mice to arterial thrombosis. The results suggest that GSK3β plays a suppressive role in platelet activation] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barry FA, Graham GJ, Fry MJ, et al. Regulation of glycogen synthase kinase 3 in human platelets: a possible role in platelet function? FEBS Lett. 2003;553:173–178. doi: 10.1016/s0014-5793(03)01015-9. [DOI] [PubMed] [Google Scholar]
- 45.Imandt L, Genders T, Wessels H, et al. The effect of lithium on platelet aggregation and platelet release reaction. Thromb. Res. 1977;11:297–308. doi: 10.1016/0049-3848(77)90183-9. [DOI] [PubMed] [Google Scholar]
- 46.Zhang W, Colman RW. Thrombin regulates intracellular cAMP concentration in human platelets through phosphorylation/activation of PDE3A. Blood. 2007;110(5):1475–1482. doi: 10.1182/blood-2006-10-052522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hunter R, Hers I. Insulin/IGF-1 hybrid receptor expression on human platelets; consequences for the effect of insulin on platelet function. J. Thromb. Haemost. 2009;7(12):2123–2130. doi: 10.1111/j.1538-7836.2009.03637.x. [DOI] [PubMed] [Google Scholar]
- 48.Chan TO, Rittenhouse SE, Tsichlis PN. AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annu. Rev. Biochem. 1999;68:965–1014. doi: 10.1146/annurev.biochem.68.1.965. [DOI] [PubMed] [Google Scholar]
- 49.Bozulic L, Hemmings BA. PIKKing on PKB: regulation of PKB activity by phosphorylation. Curr. Opin. Cell Biol. 2009;21:256–261. doi: 10.1016/j.ceb.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 50.Wick MJ, Dong LQ, Riojas RA, et al. Mechanism of phosphorylation of protein kinase B/Akt by a constitutively active 3-phosphoinositide-dependent protein kinase-1. J. Biol. Chem. 2000;275:40400–40406. doi: 10.1074/jbc.M003937200. [DOI] [PubMed] [Google Scholar]
- 51.Hresko RC, Mueckler M. mTOR RICTOR is the Ser473 kinase for Akt/protein kinase B in 3T3-L1 adipocytes. J. Biol. Chem. 2005;280:40406–40416. doi: 10.1074/jbc.M508361200. [DOI] [PubMed] [Google Scholar]
- 52.Sarbassov DD, Guertin DA, Ali SM, et al. Phosphorylation and regulation of Akt/PKB by the rictor–mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 53.Bozulic L, Surucu B, Hynx D, et al. PKBα/Akt1 acts downstream of DNA-PK in the DNA double-strand break response and promotes survival. Mol. Cell. 2008;30:203–213. doi: 10.1016/j.molcel.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 54.Kawakami Y, Nishimoto H, Kitaura J, et al. Protein kinase C βII regulates Akt phosphorylation on Ser-473 in a cell type- and stimulus-specific fashion. J. Biol. Chem. 2004;279:47720–47725. doi: 10.1074/jbc.M408797200. [DOI] [PubMed] [Google Scholar]
- 55.Mao K, Kobayashi S, Jaffer ZM, et al. Regulation of Akt/PKB activity by P21-activated kinase in cardiomyocytes. J. Mol. Cell. Cardiol. 2008;44:429–434. doi: 10.1016/j.yjmcc.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kucera GL, Rittenhouse SE. Human platelets form 3-phosphorylated phosphoinositides in response to α-thrombin, U46619, or GTP γ S. J. Biol. Chem. 1990;265:5345–5348. [PubMed] [Google Scholar]
- 57.Sorisky A, King WG, Rittenhouse SE. Accumulation of PtdIns(3, 4)P2 and PtdIns(3, 4,5)P3 in thrombin-stimulated platelets. Different sensitivities to Ca2+ or functional integrin. Biochem. J. 1992;286(Pt 2):581–584. doi: 10.1042/bj2860581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Banfic H, Downes CP, Rittenhouse SE. Biphasic activation of PKBα/Akt in platelets. Evidence for stimulation both by phosphatidylinositol 3, 4-bisphosphate, produced via a novel pathway, and by phosphatidylinositol 3, 4,5-trisphosphate. J. Biol. Chem. 1998;273:11630–11637. doi: 10.1074/jbc.273.19.11630. [DOI] [PubMed] [Google Scholar]
- 59.Kim S, Foster C, Lecchi A, et al. Protease-activated receptors 1 and 4 do not stimulate G(i) signaling pathways in the absence of secreted ADP and cause human platelet aggregation independently of G(i) signaling. Blood. 2002;99:3629–3636. doi: 10.1182/blood.v99.10.3629. [DOI] [PubMed] [Google Scholar]
- 60.Hers I. Insulin-like growth factor-1 potentiates platelet activation via the IRS/PI3Kα pathway. Blood. 2007;110:4243–4252. doi: 10.1182/blood-2006-10-050633. [DOI] [PubMed] [Google Scholar]
- 61.Angelillo-Scherrer A, Burnier L, Flores N, et al. Role of Gas6 receptors in platelet signaling during thrombus stabilization and implications for antithrombotic therapy. J. Clin. Invest. 2005;115:237–246. doi: 10.1172/JCI22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 63.Hirsch E, Bosco O, Tropel P, et al. Resistance to thromboembolism in PI3Kγ-deficient mice. FASEB J. 2001;15:2019–2021. doi: 10.1096/fj.00-0810fje. [DOI] [PubMed] [Google Scholar]
- 64.Lian L, Wang Y, Draznin J, et al. The relative role of PLCβ and PI3Kγ in platelet activation. Blood. 2005;106:110–117. doi: 10.1182/blood-2004-05-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schoenwaelder SM, Ono A, Sturgeon S, et al. Identification of a unique cooperative phosphoinositide 3-kinase signaling mechanism regulating integrin α IIb β 3 adhesive function in platelets. J. Biol. Chem. 2007;282:28648–28658. doi: 10.1074/jbc.M704358200. [DOI] [PubMed] [Google Scholar]
- 66.Canobbio I, Stefanini L, Cipolla L, et al. Genetic evidence for a predominant role of PI3Kβ catalytic activity in ITAM- and integrin-mediated signaling in platelets. Blood. 2009;114:2193–2196. doi: 10.1182/blood-2009-03-208074. [DOI] [PubMed] [Google Scholar]
- 67.Kim S, Mangin P, Dangelmaier C, et al. The role of PI 3-Kβ in glycoprotein VI-mediated akt activation in platelets. J. Biol. Chem. 2009;284(49):33763–33772. doi: 10.1074/jbc.M109.048553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68 ••.Jackson SP, Schoenwaelder SM, Goncalves I, et al. PI 3-kinase p110β: a new target for antithrombotic therapy. Nat. Med. 2005;11:507–514. doi: 10.1038/nm1232. [An isoform-selective inhibitor for the catalytic PI3K subunit p110β reduces platelet adhesion to fibrinogen under flow, platelet aggregation to ADP, and thrombosis after a stenosis injury model in the rat. Inhibitors selective for the PI3Kβ isoform may make desirable targets for development of antithrombotic therapeutics] [DOI] [PubMed] [Google Scholar]
- 69 ••.Cosemans JM, Munnix IC, Wetzker R, et al. Continuous signaling via PI3K isoforms β and γ is required for platelet ADP receptor function in dynamic thrombus stabilization. Blood. 2006;108:3045–3052. doi: 10.1182/blood-2006-03-006338. [Reveals that cooperative P2Y12 and αIIbβ3-dependent signaling are required to maintain the stability of platelet thrombi under arterial flow conditions and that PI3Ks β and γ contribute to these stabilizing signals] [DOI] [PubMed] [Google Scholar]
- 70.Kim S, Garcia A, Jackson SP, et al. Insulin-like growth factor-1 regulates platelet activation through PI3-Kα isoform. Blood. 2007;110:4206–4213. doi: 10.1182/blood-2007-03-080804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ma K, Cheung SM, Marshall AJ, et al. PI(3,4,5)P3 and PI(3, 4)P2 levels correlate with PKB/akt phosphorylation at Thr308 and Ser473, respectively; PI(3,4)P2 levels determine PKB activity. Cell Signal. 2008;20:684–694. doi: 10.1016/j.cellsig.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 72.Giuriato S, Pesesse X, Bodin S, et al. SH2-containing inositol 5-phosphatases 1 and 2 in blood platelets: their interactions and roles in the control of phosphatidylinositol 3,4,5-trisphosphate levels. Biochem. J. 2003;376:199–207. doi: 10.1042/BJ20030581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maxwell MJ, Yuan Y, Anderson KE, et al. SHIP1 and Lyn kinase negatively rRegulate integrin α IIb β 3 signaling in platelets. J. Biol. Chem. 2004;279:32196–32204. doi: 10.1074/jbc.M400746200. [DOI] [PubMed] [Google Scholar]
- 74.Severin S, Gratacap MP, Lenain N, et al. Deficiency of Src homology 2 domain-containing inositol 5-phosphatase 1 affects platelet responses and thrombus growth. J. Clin. Invest. 2007;117:944–952. doi: 10.1172/JCI29967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75 ••.Kim S, Jin J, Kunapuli SP. Akt activation in platelets depends on Gi signaling pathways. J. Biol. Chem. 2004;279:4186–4195. doi: 10.1074/jbc.M306162200. [Details the importance of P2Y12 and Gi-coupled signaling pathways for Akt phosphorylation by protease activated receptors] [DOI] [PubMed] [Google Scholar]
- 76.Kim S, Jin J, Kunapuli SP. Relative contribution of G-protein-coupled pathways to protease-activated receptor-mediated Akt phosphorylation in platelets. Blood. 2006;107:947–954. doi: 10.1182/blood-2005-07-3040. [DOI] [PubMed] [Google Scholar]
- 77.Prudente S, Hribal ML, Flex E, et al. The functional Q84R polymorphism of mammalian Tribbles homolog TRB3 is associated with insulin resistance and related cardiovascular risk in Caucasians from Italy. Diabetes. 2005;54:2807–2811. doi: 10.2337/diabetes.54.9.2807. [DOI] [PubMed] [Google Scholar]
- 78.Andreozzi F, Formoso G, Prudente S, et al. TRIB3 R84 variant is associated with impaired insulin-mediated nitric oxide production in human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2008;28:1355–1360. doi: 10.1161/ATVBAHA.108.162883. [DOI] [PubMed] [Google Scholar]
- 79.Malarstig A, Lindahl B, Wallentin L, et al. Soluble CD40L levels are regulated by the -3459 A>G polymorphism and predict myocardial infarction and the efficacy of antithrombotic treatment in non-ST elevation acute coronary syndrome. Arterioscler. Thromb. Vasc. Biol. 2006;26:1667–1673. doi: 10.1161/01.ATV.0000222908.78873.36. [DOI] [PubMed] [Google Scholar]
- 80.Chakrabarti S, Varghese S, Vitseva O, et al. CD40 ligand influences platelet release of reactive oxygen intermediates. Arterioscler. Thromb. Vasc. Biol. 2005;25:2428–2434. doi: 10.1161/01.ATV.0000184765.59207.f3. [DOI] [PubMed] [Google Scholar]
- 81.Soranzo N, Rendon A, Gieger C, et al. A novel variant on chromosome 7q22.3 associated with mean platelet volume, counts, and function. Blood. 2009;113:3831–3837. doi: 10.1182/blood-2008-10-184234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.George S, Rochford JJ, Wolfrum C, et al. A family with severe insulin resistance and diabetes due to a mutation in AKT2. Science. 2004;304:1325–1328. doi: 10.1126/science.1096706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Matsui T, Tao J, del Monte F, et al. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation. 2001;104:330–335. doi: 10.1161/01.cir.104.3.330. [DOI] [PubMed] [Google Scholar]
- 84.Fujio Y, Nguyen T, Wencker D, et al. Akt promotes survival of cardiomyocytes in vitro and protects against ischemiareperfusion injury in mouse heart. Circulation. 2000;101:660–667. doi: 10.1161/01.cir.101.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Miyawaki T, Ofengeim D, Noh KM, et al. The endogenous inhibitor of Akt, CTMP, is critical to ischemia-induced neuronal death. Nat. Neurosci. 2009;12:618–626. doi: 10.1038/nn.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ackah E, Yu J, Zoellner S, et al. Akt1/protein kinase Bα is critical for ischemic and VEGF-mediated angiogenesis. J. Clin. Invest. 2005;115:2119–2127. doi: 10.1172/JCI24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Camici GG, Steffel J, Amanovic I, et al. Rapamycin promotes arterial thrombosis in vivo: implications for everolimus and zotarolimus eluting stents. Eur. Heart J. 2009 doi: 10.1093/eurheartj/ehp259. DOI:10.1093/eurheartj/ehp259. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 88.Sturgeon SA, Jones C, Angus JA, et al. Advantages of a selective β-isoform phosphoinositide 3-kinase antagonist, an antithrombotic agent devoid of other cardiovascular actions in the rat. Eur. J. Pharmacol. 2008;587:209–215. doi: 10.1016/j.ejphar.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 89.Medina-Franco JL, Giulianotti MA, Yu Y, et al. Discovery of a novel protein kinase B inhibitor by structure-based virtual screening. Bioorg. Med. Chem. Lett. 2009;19:4634–4638. doi: 10.1016/j.bmcl.2009.06.078. [DOI] [PubMed] [Google Scholar]
- 90.Carnero A. Novel inhibitors of the PI3K family. Expert Opin. Investig. Drugs. 2009;18(9):1265–1277. doi: 10.1517/13543780903066798. [DOI] [PubMed] [Google Scholar]
- 91.Yatomi Y, Hazeki O, Kume S, et al. Suppression by wortmannin of platelet responses to stimuli due to inhibition of pleckstrin phosphorylation. Biochem. J. 1992;285(Pt 3):745–751. doi: 10.1042/bj2850745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kovacsovics TJ, Bachelot C, Toker A, et al. Phosphoinositide 3-kinase inhibition spares actin assembly in activating platelets but reverses platelet aggregation. J. Biol. Chem. 1995;270:11358–11366. doi: 10.1074/jbc.270.19.11358. [DOI] [PubMed] [Google Scholar]