Abstract
Motor proteins are involved in a wide range of cellular and subcellular movements. Recent studies have implicated two motor proteins in particular, myosin II and cytoplasmic dynein, in diverse aspects of cell migration. This review focuses on emerging roles for these proteins in the nervous system, with particular emphasis on migrating neurons and neuronal growth cones. The former cells exhibit unusual features of centrosome and nuclear movement, whereas growth cones offer an opportunity to evaluate motor protein function in a region of cytoplasm free of these organelles.
Introduction
Work over the past two decades has revealed that each of the three classes of cytoskeleton-associated motor proteins – the myosins, kinesins and dyneins – are represented in the higher eukaryotic genome by large gene families (Box 1). A major function of these proteins is in intracellular transport of membranous vesicles and macromolecular complexes. However, these proteins are also involved in basic aspects of cell movement and morphogenesis. Myosin II has long been understood to have a contractile role in muscle cells and in most other cell types but, until recently, there was little evidence of a role for microtubule-associated motors in cell translocation and other large scale cellular movements. Cytoplasmic dynein in particular was well known for its roles in subcellular transport. However, it has recently been implicated in several aspects of directed cell movement as well. Myosin II has been extensively studied for its role in fibroblast migration and growth cone extension. More recently, this motor protein has also been found to participate in neuronal migration. Cytoplasmic dynein and several of its regulatory factors, especially LIS1, NudE and NudEL (gene names Nde1 and Ndel1), have been implicated in neuronal migration from studies of brain developmental disease. A role for these proteins in growth-cone behavior and non-neuronal cell migration has also been identified. This article reviews recent evidence for a predominant role for myosin II and the major form of cytoplasmic dynein (MAP1C; dynein I) in neuronal migration and growth-cone function, with reference to studies in non-neuronal cells (Box 2). Despite the differential emphasis given to each of the two motor proteins in the several cellular systems that have been investigated, this review argues that myosin II and cytoplasmic dynein commonly act in concert to effect cell movement.
Role of motor proteins in migrating neurons
Behavior of migrating neurons
During development, neurons are generated within the germinal layers of the nervous system by the proliferation of progenitor cells. In the developing cerebrum (neocortex), pyramidal neurons are generated from radial glial progenitor cells (Box 3). These cells exhibit an extraordinary form of ‘interkinetic’ nuclear oscillations that are coordinated with cell-cycle progression. LIS1 RNAi in developing rat neo-cortex [1] and a dynactin mutation in zebra fish retina [2] have been found to interfere with this movement, but little additional information is available regarding the detailed underlying mechanisms for this aspect of neuronal progenitor motility. Once generated from radial glial cell divisions, neuronal precursors ascend to the subventricular zone and then migrate over substantial distances along the radial glial fibers to their final destinations, where they mature (Box 3). The migrating neurons have a characteristic bipolar shape, with a single or branched ‘migratory’ process extending in the direction of movement and a trailing axon.
As the leading process advances, the cell body catches up in a series of discontinuous movements (http://www.nature.com/neuro/journal/v10/n8/extref/nn1934-S2.mov). This overall behavior is observed for a variety of migrating neuronal cell types in other brain regions, including inter-neurons involved in tangential migration from the ganglionic eminence [3–5] and descending cerebellar granule cells [6]. Discontinuous cell body movement behind an extending migratory process is also characteristic of glial precursors [7] and even rodent and human glioma cells [8].
Migrating cells tend to contain a single centrosome [4–6,9], which is typically situated ahead of the nucleus, although the nucleus has recently been reported to switch positions with the centrosome in granule cells migrating within live cerebellar slices [10]. In cerebellar neurons in dissociated culture, the centrosome was seen to advance by 1–2 μm, followed by the nucleus [6] in a ‘two-stroke’ process, suggesting an involvement of the centrosome in directing nuclear movement. Independent behavior of the centrosome and nucleus was considerably more exaggerated in embryonic rat brain neocortical slices, in which case the centrosome moved ahead of the nucleus by as much as 20 μm [9]. Whereas centrosome movement was continuous [9], the nucleus exhibited extended periods of immobility and seemed to experience considerable resistance, either from other cells within the closely packed brain tissue or from the narrow migratory process into which it was attempting to move. Related aspects of centrosome movement have also been observed in subsequent studies [11,12].
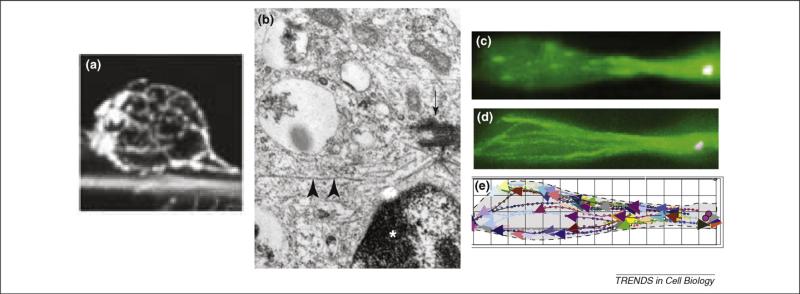
Understanding microtubule organization within migrating neurons has been challenging owing to the high density of microtubules within the cell processes and the thin layer of cytoplasm surrounding the nucleus. It has been known for some time that microtubules in the cell body region course around the nucleus as a cage-like structure [13] (Figure 1a). In freely migrating neocortical neurons, microtubules were observed to emanate from the centrosomal region [5] (Figure 1b). Microtubules have also been reported to be aligned parallel to the long axis of both the migratory and trailing process of descending cerebellar granule cells in situ [14]. The microtubules were found to be oriented with their plus ends toward the distal end of the migratory process, although in the trailing process the microtubules were of mixed polarity. Imaging of the micro-tubule plus-end-associated protein EB3 has more recently been used to evaluate microtubule organization in migrating rat brain neurons [9]. Microtubules were observed to emanate directly from the centrosome and extend outward in both the migratory and trailing axonal process (Figure 1c–e). This pattern persisted even when the centrosome had advanced well ahead of the nucleus. This observation leads to the surprising conclusion that the entire microtubule cytoskeleton must move along with the centrosome in advance of the nucleus, a remarkable feat for a cell in which the cytoplasm is restricted to long, narrow processes. The basis for the difference in microtubule orientation in the trailing processes of cerebellar granule cells versus radially migrating neocortical neurons is uncertain but could relate to the use of different methods of analysis or differences in the fate or migratory behavior of cells in the two regions. For example, microtubule organization within the descending process of the cerebellar granule cell could affect or be affected by the reported bidirectional nuclear movements [10].
Box 1. Motor protein families.
The myosins interact with actin filaments through a common 90-kDa motor domain. At least 17 myosin subfamilies have been defined on the basis of primary sequence and secondary structure. Myosin II is the first known member of the family, originally purified from skeletal muscle. Additional myosin II isoforms are expressed in non-muscle cells. Neuronal cells express the myosin II A, B and C isoforms. The kinesins interact with microtubules through a common 35-kDa motor domain and have been sorted into at least 14 subfamilies. The dyneins arose from an ancestral member of the AAA ATPase superfamily, a common feature of which is a multimeric ring of 35–40-kDa AAA modules. The dynein heavy chain contains six identifiable AAA units as part of a single, very large motor domain, from which a microtubule-binding ‘stalk’ and a cargo-binding tail extend. All dynein heavy chains have the same domain organization but two functional subfamilies are known, the axonemal dyneins, which power ciliary and flagellar movement, and the cytoplasmic dyneins. Curiously, of only two cytoplasmic dyneins, one (dynein II) is exclusively devoted to transport within cilia and flagella. This leaves a single major form of cytoplasmic dynein, referred to as dynein I and a focus of this review, to carry out a very wide range of cellular functions. This form of dynein is regulated by a large group of accessory proteins and protein complexes, including dynactin, Bicaudal D (BicD1 and BicD2 in vertebrates) and ZW10. LIS1, NudE and NudEL are additional factors that are of particular interest as regulators of cytoplasmic dynein in neuronal and non-neuronal motility. Sporadic mutations in the human gene encoding LIS1 in particular cause classical lissencephaly (smooth brain) [68], which results from defects in neuronal migration and division, and Nde1- and Ndel1-null mice exhibit lissencephalic and microcephalic defects [62,69,70]. Both microtubules and actin filaments are polar polymers, with ‘plus’ and ‘minus’ ends. All myosins but one, myosin VI, produce force toward the plus ends of actin filaments. Plus, minus and depolymerizing kinesins have been identified, although interphase cells express the plus-end forms almost exclusively. Dyneins are all microtubule minus-end-directed.
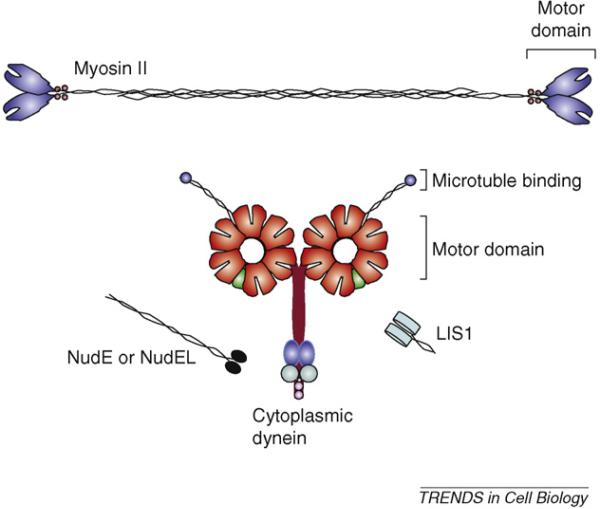
Figure I shows the structural organization of the motor proteins and regulators discussed in this review that are involved in neuronal migration and growth-cone motility. Myosin II is a dimer of 200-kDa heavy chains, each with an N-terminal 90-kDa motor domain and two light chains. Dimerization of the heavy chains is mediated through a highly elongated C-terminal α-helical coiled-coil, which is shown interacting with another myosin II molecule to form a nascent antiparallel thick filament. Cytoplasmic dynein is also dimeric. Each of the 350-kDa motor domains is comprised of six AAA ATPase modules and a C-terminal regulatory domain. From each motor domain extends a coiled-coil stalk, which binds to microtubules at its tip, and a 200-kDa tail for dimerization and interaction with accessory cargo-binding intermediate chains, light chains and light intermediate chains. NudE and NudEL each have a globular C terminus that binds to the dynein intermediate and light chains [49] and contains phosphorylation sites for cdk2, cdk5, Erk1 and Aurora kinases [71,72]. LIS1 binds to the elongated coiled-coil tail of NudEL and NudE, and to the tail and motor domain of the dynein molecule [73,74]. LIS1 and NudE or NudEL illustrations adapted from structural information available elsewhere [75,76]. Molecular dimensions are shown approximately to scale.
Figure 1.
Substructure of migratory bipolar migrating neurons. (a) Anti-tubulin staining shows a cage of microtubules coursing around the nucleus of a cerebellar granule cell as it migrates along the process of an underlying radial glial cell [6]. (b) An electron micrograph of a neuronal precursor cell showing the centrosome (arrow) with radiating microtubules (arrowheads) located near the nucleus (indicated by asterisk) [5]. (c) Microtubule plus ends decorated with GFP–EB3 in live bipolar neuronal precursor cell located within embryonic rat brain slice [9]. (d) Superposition of movie frames during 2 minute imaging period and (e) tracings showing path and orientation of microtubules [9].Part(a) reproduced, with permission, from Ref. [6]; part (b) reproduced, with permission, from Ref. [5]; and part (c) reproduced, with permission, from Ref. [9].
The mechanisms controlling and coordinating the movements of the nucleus, centrosome and microtubules in these cells are incompletely understood. One newly appreciated feature of migrating neural cells is the formation of one or more transient ‘swellings’ or ‘dilations’ within the migratory process. It has been speculated that these structures represent sites of attachment to the underlying radial glial cell, although they are also evident in dissociated cultures of bipolar cells grown in Matrigel and in other cases of non-glial guided migration [4,5]. Recent work has localized a particular form of connexin, the polypeptide constituent of gap junctions, to these sites [15]. Connexins could serve a role in adhesion between junctions of adjacent cells and were proposed to serve this function with underlying radial glial fibers.
During neuronal migration, the centrosome advances towards the swelling [9]. It occasionally reaches the swelling [4,5,9], at which location it stops [9]. If, however, the swelling disappears before this can happen, the centrosome then seems to be guided by swellings appearing further ahead within the migratory process [9]. This behavior suggested that the swelling is a locus for motor protein activity.
Roles of myosin II and dynein
To test whether migrating neural precursor cells use myosin II for migration as in non-neuronal cells, blebbistatin, a small molecule inhibitor of the A and B forms of myosin II [16], was used [4,5]. Blebbistatin at normal inhibitory levels blocked the forward advance of tangentially migrating neural precursors in dissociated culture [4,5]. To test where within the cell the myosin II might be acting, immunofluorescence microscopy was performed. Myosin II [4] and the activated form of its regulatory light chain [5] were concentrated at the rear of the nucleus, suggesting that myosin-II-mediated actin contractility might serve to push the nucleus forward from behind. Blebbistatin and vector-based RNAi of myosin IIb were also used to test for effects on migrating cells in live neocortical embryonic rat brain slices, and the advance of the soma was blocked by each treatment [9].
The effects of LIS1 and dynein HC RNAi were also evaluated in the latter experiments [1,9] and were each found to block somal translocation. Surprisingly, extension of the leading process was completely unaffected, as judged by live measurement of the rate of process extension relative to migrating neurons in control brain tissue. The result was arrested somata with hyper-elongated migratory processes. Related behavior has been observed in tangentially migrating interneurons in live slices from heterozygous Lis1+/ mice, in which neural precursors exhibited slower than normal migration, associated with elongated migratory processes [3]. We note that, conversely, LIS1 inhibition potently interferes with axon elongation and growth-cone advance [9,17]. These results strongly support a basic underlying difference in the organization and function of the migratory process and axon [13]. Furthermore, they indicate that neither the swellings within the migratory process nor the extension of the process are under LIS1 and cytoplasmic dynein control. Which factors might control the formation and growth of these structures remains largely unknown.
Box 2. Role of motor proteins in non-neuronal migration.
Non-neuronal cells make use of myosin II and cytoplasmic dynein for migration. Myosin II functions in contraction of actin arrays in two general cellular regions – in advance of the nucleus towards the leading cell edge and behind the nucleus towards the cell rear [77] (Figure 2). As for skeletal muscle myosin II, the non-muscle forms of this protein assemble into bipolar thick filaments, which are very disordered relative to the strict hexagonal packing seen in muscle [78]. In migrating non-neuronal cells, myosin II is present within the lamellum and becomes more concentrated away from the leading edge within the ‘transition’ zone where it pulls on actin filaments assembling from the leading cell edge [77,79]. Myosin II also clearly serves in retraction of the cell rear. Actin stress fibers, oriented along the nucleus in the direction of migration, terminate in focal adhesions near the trailing edge, where myosin II is enriched [80]. Regulation of myosin II contraction at these sites creates tension on stress fibers and is crucial for efficient migration [81,82]. Cytoplasmic dynein and its regulators dynactin, LIS1, NudE, NudEL and ZW10 have each been found to accumulate at the leading edge of migrating fibroblasts and inhibition of dynein or its cofactors inhibits forward cell migration [47,49,50].
Box 3. Neuronal migration in the developing neocortex.
The vertebrate neocortex begins as a neural tube comprising a pseudostratified neuroepithelium, depicted in Figure I, which indicates successive stages in genesis and migration of neurons. The highly elongated radial glial cells which are shown extend from the ventricular surface at bottom to the outer, pial surface of the developing brain. These cells serve as neuronal and glial progenitors and, subsequently, as guides for neuronal migration. The overall process of neurogenesis and migration involves a complex sequence of motile and morphogenetic events, beginning with cell-cycle-dependent oscillations of the radial glial cell nuclei at left  .
.  Cell division occurs when the nuclei reach the ventricular surface, and S-phase occurs at the apex of their oscillatory excursions. ‘Symmetric’ cell divisions repeat this cycle, whereas ‘asymmetric’ divisions generate post-mitotic neurons (darker green), which migrate to the subventricular zone (SVZ), where they take on a multipolar morphology
Cell division occurs when the nuclei reach the ventricular surface, and S-phase occurs at the apex of their oscillatory excursions. ‘Symmetric’ cell divisions repeat this cycle, whereas ‘asymmetric’ divisions generate post-mitotic neurons (darker green), which migrate to the subventricular zone (SVZ), where they take on a multipolar morphology  [83,84]. These cells subsequently convert to a bipolar migratory morphology with a major migratory process in the lead
[83,84]. These cells subsequently convert to a bipolar migratory morphology with a major migratory process in the lead  and an axon
and an axon  elongating behind. The migrating neurons then ascend along the radial glial fibers to their final destination in the developing neuronal layers of the cerebral cortex [85]. Inhibition of cytoplasmic dynein or LIS1 blocks steps
elongating behind. The migrating neurons then ascend along the radial glial fibers to their final destination in the developing neuronal layers of the cerebral cortex [85]. Inhibition of cytoplasmic dynein or LIS1 blocks steps 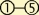 . [1,86].
. [1,86].  Inhibition of myosin II also blocks advance of the bipolar migratory neurons [4,5,9]. Drawing modified, with permission, from Refs [1,86]. See main text for further details.
Inhibition of myosin II also blocks advance of the bipolar migratory neurons [4,5,9]. Drawing modified, with permission, from Refs [1,86]. See main text for further details.
To gain a more detailed understanding of the cellular mechanism underlying the effects of inhibiting LIS1 and dynein, RNAi was performed in neural progenitor cells cotransfected with markers for centrosomes and nuclei [9]. RNAi against LIS1 blocked the advance of both structures, although it had no apparent effect on the formation and distribution of the swellings located in the migratory process. Under conditions of partial LIS1 or dynein heavy chain knockdown, centrosomes could be seen to advance, albeit reversibly and at overall slower rates; nonetheless, the nucleus remained immobile.* Cytoplasmic dynein immunoreactivity was found to be enriched within the transient swellings located within the migratory process, supporting the possibility that force is generated from these sites. Dynein in this region would be positioned to pull on microtubules extending from the centrosomal region, thereby serving to draw the microtubule cytoskeleton, including the centrosome, forward. Blebbistatin and myosin IIb RNAi each inhibited nuclear behavior, but had no effect on centrosomes [9], arguing that centrosome movement is solely under the control of LIS1 and cytoplasmic dynein.
The partial independence of nuclear from centrosome movement in LIS1 and dynein knockdown cells suggests two loci for dynein force production. The relative disposition of microtubules and nuclei revealed that nuclear movement is directed towards the minus ends of the perinuclear microtubules. This observation suggests that dynein and LIS1 might act from the nuclear surface. Dynein immunoreactivity was, indeed, enriched in the cell body [9]. Dynein, LIS1 and their cofactors have been reported to decorate the nuclear envelope during mitotic prophase [18–20]. Thus, it seems reasonable to speculate that a related dynein recruitment mechanism could operate in migrating neurons, although there is still no direct evidence for this possibility.
Role of motor proteins in growth-cone extension
Structural organization of the growth cone
The growth cone is a specialized structure involved in axonal elongation and pathfinding [21,22] (Figure 2). It shares properties with other cellular domains such as lamellipodia and filopodia but has unique features as well. Although growth cones are morphologically dynamic, basic features of their organization are well established. Growth cones of neurons plated on artificial polyamine substrates are slow-advancing and tend to be well spread with a well-defined substructure. Actin filaments predominate in the peripheral domain (P-domain), which typically consists of a flat, lamellar region of parallel actin filaments and filopo-dial actin bundles which are rooted in this region but protrude through the leading growth-cone edge. Microtubules emerging from the axon shaft predominate in the central (C)-domain, along with associated vesicular organelles. Microtubule growth occurs preferentially along filopodia and is counteracted by retrograde actin flow [23,24]. A transition zone exists between the P- and C-domains, which is comparable to the transition zone of fibroblasts and contains as its most prominent feature actin bundles (arcs) arrayed parallel to the leading edge of the growth cone [23,25]. In slow-advancing growth cones, the microtubules make only limited incursions into the P-domain. A shift to rapid growth occurs in response to permissive extracellular matrix proteins, such as laminin [26]. In this state, microtubules can protrude through the actin-rich P-domain, approaching the leading growth cone boundary and entering the filopodia [27]. However, even under these conditions, growth cones can pause and spread out, in which case they tend to exhibit the underlying organization observed on artificial polyamine substrates.
Figure 2.
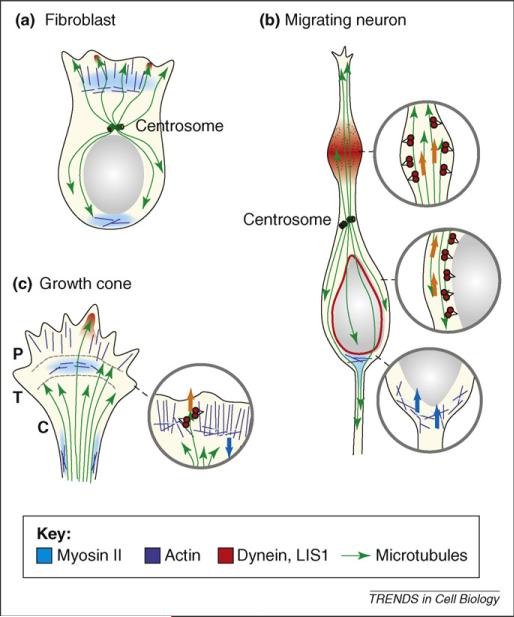
Diagrammatic representation of myosin II and cytoplasmic dynein roles in cell migration. Regions of (a) fibroblast, (b) migrating neuron and (c) growth cone in which myosin II and dynein are concentrated during migration are shown, along with actin filaments and microtubules. Microtubule plus ends are indicated by arrows. Actin filaments in lamellipodia and growth cone are primarily oriented with their plus ends toward the leading cell edge. In the expanded section, myosin II thick filaments are shown as light blue bars, dynein molecules in red. In fibroblasts and growth cones, myosin II concentrates in the transition zone (T) and produces a contractile force on growing filaments in the peripheral zone (P) extending from the leading cell edge. Myosin II also concentrates at the rear of the migrating fibroblast and at the growth cone neck. Cytoplasmic dynein and LIS1 (not shown) become concentrated within the leading lamellipodium and growth cone peripheral domain along with invading microtubules. In migrating neurons, myosin II acts behind the nucleus, which it seems to push forward. Cytoplasmic dynein acts from swellings that appear transiently within the migratory process and probably also from the nuclear surface. The centrosome is situated ahead of the nucleus in migrating fibroblast and neuron, but experiences force from dynein and LIS1 acting on microtubules well in advance. Arrows indicate direction of force production by dynein (orange) and myosin II (blue).
Interference with actin or microtubule assembly or behavior has long been known to have pronounced effects on growth-cone morphology and motility. Treatments that disrupt microtubule dynamics (taxol or nocodazole) reduce or block growth-cone extension [28]. Conversely, F-actin disruption using cytochalasin-B or -D reduces the extent of the P-domain but enhances the rate of growth-cone advance [29]. Growth cones subjected to treatment that inhibits the dynamics of either cytoskeletal network exhibit little directional sensitivity to guidance factors and show defects in axonal pathfinding [28,30]. These observations reveal the importance of the two filament systems in growth-cone behavior. Diverse regulatory factors have been identified that influence actin and microtubule assembly within the growth cone and are important in regulating its motile behavior [21]. A prominent role for motor proteins in controlling actin and microtubule behavior in the growth cone has emerged only recently.
A variety of forms of myosin have been detected in growth cones, including myosin I, myosin II, myosin V, myosin VI and myosin X [31]. Myosin X has been clearly shown to participate in filopodial formation in cells in general, including neurons [32]. Of the other myosin forms, myosin II has been studied most extensively and seems to have a major role in the organization and behavior of the growth cone actin cytoskeleton. Myosin II has been localized to the transition zone near the proximal ends of filopodial F-actin bundles [33,34], although some isoforms of myosin II might be active elsewhere within the growth cone – at the neck, for example [35,36].
Myosin-II-mediated actin behavior
Growth cones deficient in or inhibited for myosin II exhibit a reduced area, especially in the actin-rich P-domain [37,38]. Growth-cone turning at artificial laminin borders is also inhibited in neurons from myosin-IIb-null mice, an effect that could be rescued by expression of myosin IIb– GFP [39].
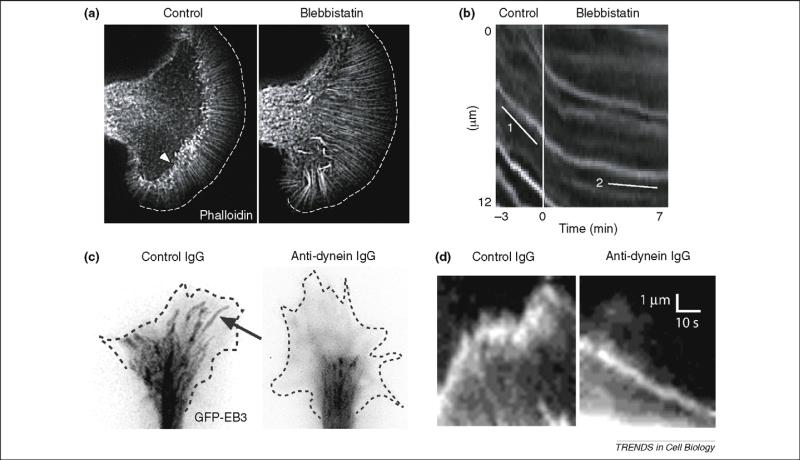
To determine how myosin modulates the behavior of the actin cytoskeleton, the effects of myosin perturbation on retrograde flow at the leading edge of the growth cone were investigated. In one study, retrograde movement of beads applied to the growth-cone surface was unaffected by chromophore-assisted LASER inactivation (CALI) of myosin II [40]. Conversely, it was stimulated in a myosin IIB null mouse [38], conceivably owing to compensatory activity of the other myosin II isoforms. More recently, blebbistatin was used to monitor retrograde actin flow more directly using fluorescence speckle microscopy (FSM) of Alexa594-labeled phalloidin [34]. Using growth cones from the marine gastropod Aplysia, actin speckles were observed to flow rearward within the P-domain, but exhibited extensive re-arrangement and breakage as they entered the transition zone (Figure 3). Blebbistatin reduced the rate of retrograde actin flow within the P-domain and more substantially within the transition zone, where actin arc formation was almost abolished. These and related findings suggest that myosin II located near or within thetransitionzoneisrequiredtoguide andreorganize actin filaments as they extend from the leading growth-cone margin (Figure 3b).
Figure 3.
Effect of myosin II and dynein inhibition on growth-cone actin and microtubule behavior. (a) Slow-advancing Aplysia growth cone injected with Alexa594-phalloidin to image actin filaments. The myosin II inhibitor blebbistatin causes distortion of actin bundles and loss of actin arcs in the transition zone (indicated by arrowhead in control image). (b) Kymographs from live recording of the transition zone showing retrograde movement of phalloidin speckles (slope = line 1), which declines upon blebbistatin treatment (line 2). These data provide direct support for a permissive role for myosin II in retrograde actin flow. Parts (a) and (b) reproduced, with permission, from Ref. [34]. (c) Fast-advancing chicken dorsal root ganglia (DRG) growth cones plated on laminin and expressing GFP–EB3, which associates with the plus ends of growing microtubules. Images from video (30 frames/min) are overlaid and shown in negative contrast. Control microtubules (arrow) grow through the peripheral (P)-domain towards the leading growth-cone edge, behavior that is blocked by injected monoclonal antibody against dynein. (d) Kymographs show individual microtubule ends, revealed to be growing by the presence of GFP–EB3. The control microtubule advances and pauses as it continues to assemble as judged by the persistence of EB3 at the microtubule end. The microtubule from the dynein-inhibited growth cone moves in a retrograde direction. Persistence of EB3 at microtubule end again reveals that the microtubule continues to assemble. The rate of forward EB3 movement is that expected for microtubule assembly and is much slower than that for anterograde microtubule translocation. The rearward movement of microtubules in the dynein-inhibited cell provides direct evidence that dynein is responsible for restraining microtubules against rearward forces, such as retrograde actin flow. Parts (c) and (d) reproduced, with permission, from Ref. [17].
Dynein-mediated microtubule behavior
Within axons, microtubules are arrayed in parallel, with their plus ends directed distally. This pattern continues into the C-domain of the growth cone. The microtubule ends keep up with the growth cone as it advances, an ability that is, at least in part, a result of microtubule assembly [41]. Microtubules are also transported within the axon proper, as indicated by live imaging of GFP-labeled microtubules in primary rat sympathetic neurons [42]. At least part of this activity has been ascribed to cytoplasmic dynein based on expression of the dominant-negative ‘dynamitin’ subunit of the dynein regulatory complex dynactin and RNAi against the cytoplasmic dynein heavy chain [43].
Dynein and LIS1 are found at elevated levels in axonal growth cones of polarized primary hippocampal neurons [17,44]. In well-spread, slow-advancing growth cones of dorsal root ganglia (DRG), diffuse staining of these proteins could be detected within the C-domain [17].However, upon addition of laminin to the medium, rapid, coordinated induction of narrow, fast-growing processes with small growth cones occurred. Shortly after laminin exposure, bright, punctate immunostaining for dynein, dynactin and LIS1 appeared towards the leading growth-cone edge, including the bottom surface as assayed using total internal reflectionfluorescence (TIRF) microscopy. Particularly intense staining was observed at sites of microtubule convergence at the leading edge of the growth cone and at the tips of fast-growing processes, by which stage discrete P- and C-domains could no longer be discerned. Acute inhibition of dynein and LIS1 activity by antibody injection potently inhibited the morphogenetic and growth-enhancing effects of laminin and blocked the invasion of microtubules from the C-domain into the P-domain. Live-cell analysis of microtubule plus ends decorated with GFP revealed that the microtubules continued to assemble (Figure 3), but many shifted rearward at rates consistent with retrograde actin flow. Similar results have been obtained using RNAi of dynein heavy chain [45]. Together, these results reveal that cytoplasmic dynein and its regulatory factors LIS1 and dynactin are required to retain growing microtubules at the leading edge of the advancing growth cone, but by exerting tension on the microtubules rather than by microtubule translocation.
In addition to these effects, both myosin II and cytoplasmic dynein participate in ‘consolidation’ of the growth-cone neck and distal axon shaft. Myosin II has been reported to compress actin arcs at the sides of the growth-cone neck and microtubules in the C-domain toward the central axis of the growth cone [36]. Blebbistatin interfered with this effect. Inhibition of dynein and LIS1 also interfered with neck consolidation [17], although the underlying effects on cytoskeletal elements have not yet been examined in detail.
Molecular models for myosin- and dynein-mediated cell movement
The data reviewed here indicate that myosin II and cytoplasmic dynein function in concert to carry out a variety of motile cell functions (Figure 2). Although the two motor proteins act in association with distinct cytoskeletal filaments, they do so in an interdependent fashion. Current data enable us to address in detail the sites at which the motor proteins function and reveal that their activities can be either antagonistic or cooperative in different neuronal cell contexts.
Growth-cone advance
The cytoskeletal substructure in the growth cone has been defined in sufficient detail to permit precise models for motor protein activity. We now envision dynein and myosin II to act at different sites within the growth cone. Myosin II is concentrated in the transition zone and in the growth-cone neck (Figure 2). In stationary or slow-advancing growth cones, cytoplasmic dynein is weakly concentrated within the central domain, where we assume it associates with vesicular cargoes destined for retrograde transport through the axon. In actively extending growth cones, dynein relocalizes to the P-domain, where it seems to pull on microtubules (Figure 2). In this capacity, it serves to resist the rearward flux of actin generated by the combined effects of actin assembly at the leading growth cone edge and the contractile forces of myosin II on the proximal ends of the actin filaments. Cytoplasmic dynein could be anchored to the cortical portion of the growth cone. This conclusion is suggested in part by TIRF imaging, which revealed dynein staining within the bottom 100–200 nm of the cell in contact with the underlying coverslip [17], and by evidence for a cortical dynein pool during mitosis [46] and fibroblast migration [47]. Although it is well established that dynein moves vesicles and other particles towards the minus ends of microtubules, cortically anchored dynein is presumably immobile. In this case, dynein-generated forces should, instead, affect the behavior of the microtubules, as is observed in the growth cone (Figure 3). A similar mechanism has been proposed to account for anterograde transport of free microtubules within axons [48].
Myosin II and dynein each curiously affect growth-cone advance in an indirect manner. Myosin II pulls rearward (Figure 2), opposite to the direction of growth. Its function within the transition domain seems to be in compacting and clearing growing actin filaments, perhaps to prevent jam-ups as the actin filaments extend rearward as a result of polymerization. In stationary growth cones, myosin II is permissive for rearward actin flow. Growth-cone protrusion seems primarily to result from actin assembly, whereas myosin II persists in its housekeeping role.
Conversely, cytoplasmic dynein pulls microtubules anterogradely toward the advancing growth-cone edge (Figure 2). In contrast to the axon shaft [48], most if not all microtubules seem to be immobile in the growth cone [17], but do extend into the P-domain by polymerization. Dynein-generated force serves to exert tension on the microtubules, resisting myosin-generated forces on the actin cytoskeleton. As a result of dynein activity, micro-tubules remain capable of extending into and through the peripheral domain and into filopodia [17,45]. Thus, the main function of dynein seems to be to counter the effects of an actin motor, which itself generates force opposite to the direction of growth-cone advance.
Why dynein inhibition and the resulting microtubule retraction should interfere with forward growth-cone extension is uncertain. Dynein, dynactin, LIS1, ZW10, NudE and NudEL inhibition produce a related effect on lamellipodial advance in migrating fibroblasts [47,49,50]. These results suggest that dynein and its cofactors serve to retain microtubules in the actin-rich leading regions of both neuronal and non-neuronal cells, in which microtubules are known to stimulate actin assembly [51,52]. This effect occurs through activation of the small GTPase Rac1 [53,54], which is also activated in rapidly extending growth cones exposed to laminin [55]. The small GTPase Cdc42 has also been implicated as a further upstream regulator of dynein activity during centrosome reorientation [56]. NudEL has recently been found to sequester the GTPase-activating protein Cdc42GAP, a protein that turns off Cdc42 by promoting GTP hydrolysis at the leading edge of migrating fibroblasts, thereby locally enabling activation of Cdc42 [50]. An additional potential candidate for mediating crosstalk between the actin and microtubule networks in growth cones is the Rac1 and Cdc42 effector IQGAP1 [57–59] which, interestingly, binds to active Rac1 and Cdc42 and LIS1 in migrating neurons [60].
Nucleus and centrosome transport in neural precursors
Nuclear and centrosome movements in migrating bipolar neurons seem to be driven by interdependent but discrete mechanisms. This situation contrasts with that in nonneuronal cells, such as fibroblasts, in which the centrosome and nucleus are tightly linked. In the latter case, dynein pulls the microtubule network forward [61] and the centrosome and associated nucleus act as passengers. In bipolar neuronal progenitors, the nucleus also follows the centrosome and cytoplasmic microtubules, but often with a substantial delay and physical separation [6,9]. This unusual behavior could indicate that the molecular links between the nucleus and centrosome are weaker in migrating neurons than in other cell types. Whether this is the case or not, the ability of the nucleus to catch up with the centrosome is LIS1- and dynein-dependent [9,62]). This observation suggests that, in these cells, the centrosome and nucleus are linked through dynein to microtubules. Nuclear movement, unlike in other cells examined, seems to be effected by direct transport along microtubules in the minus direction.
Myosin II also has a crucial role in nuclear movement in the bipolar migrating neurons, apparently acting from behind the nucleus [4,5]. Thus, in these cells, dynein and myosin II function cooperatively. This could be an adaptation required to overcome an extreme resistance to nuclear movement. An alternative hypothesis is that myosin functions in constricting the newly forming proximal axon as the neuron migrates ahead. Perhaps the contribution of myosin to nuclear movement is an indirect consequence of this function. We note that the direction of myosin-II-mediated nuclear movement in these cells is opposite to that in fibroblasts [63], suggesting that the regions of greatest actomyosin contractility differ between these cell types. Inhibition of myosin II does not affect centrosome movement in migrating neurons [9], leaving cytoplasmic dynein as the sole candidate for this behavior. In further contrast to lamellipodia and growth cones, cytoplasmic dynein acts from a site within the migratory process, rather than at its tip.
Concluding remarks
Myosin II and cytoplasmic dynein have emerged as major players in non-neuronal cell migration, neuronal migration and growth-cone motility (Figure 2). Myosin II has an expected contractile role in these cellular contexts, although at different cellular sites in each case and in a far less highly organized manner than in striated muscle cells. Cytoplasmic dynein exerts its effects on cell movement not through retrograde cargo transport but, rather, through exertion of equal and opposite forces on its microtubule tracks. Dynein-mediated effects on cell movement in these systems are consistent with an association of the motor protein and its multiple regulatory factors with the nuclear surface and the cell cortex. The mechanism for recruitment to these sites is almost completely unknown. How factors such as LIS1, NudE and NudEL regulate cytoplasmic dynein function also remains a major frontier to be addressed in future research.
Myosin II, acting downstream of the RhoA–ROCK pathway, is thought to be an important target of growth-cone guidance factors [25,35]. We suspect that cytoplasmic dynein could have a complementary role. Mutations in one of the subunits of cytoplasmic dynein have been reported to produce apparent axon pathfinding defects in Drosophila [64,65]. Dynein-mediated tension in the leading edge of wounded fibroblast monolayers is activated by lysophosphatidic acid in a cdc42-dependent manner [56,66] and in DRG growth cones by laminin [17]. The extent to which these and other factors regulate micro-tubule behavior during neuronal pathfinding remains to be explored. However, the opposing forces generated by dynein and myosin II in growth cones seem to provide an elegant mechanism for fine-tuning the rate and direction of growth-cone movement.
Nuclear migration in neuronal precursors is also under coordinate myosin II and dynein control. Dynein seems to drive the nucleus directly. Whether such a role is also responsible for the interkinetic nuclear oscillation in the radial glial cells during the initial stages of neurogenetic and migration pathway (Box 3) remains to be explored in detail. Nuclear movement is bidirectional in this system, but whether this behavior involves minor or major modifications of the mechanisms operative in migrating bipolar neurons is a fascinating question for future study.
Finally, it should be remembered that several forms of myosin in addition to myosin II are already known to function in growth cones [31]. Furthermore, at least one form of kinesin is active in these structures [67]. It will be of great interest to determine the complete extent to which additional motor proteins contribute to growth-cone behavior and neuronal migration.
Figure I.
Structural organization of motor and regulatory proteins involved in neuronal migration and growth-cone motility. Color code: red, AAA ATPase modules; green, C-terminal regulatory domain; aqua, accessory cargo-binding intermediate chains; light purple, light chains; blue, light intermediate chains.
Figure I.
Stages in neurogenesis and migration in the developing neocortex. Abbreviations: CP, cortical plate; IZ, intermediate zone; SVZ, subventricular zone; VZ, ventricular zone.
Acknowledgements
We thank Mary Beth Hatten, Bruce Schaar, Susan McConnell and Paul Forscher for providing images for this article, and Silvia Cappello, Wei-Nan Lian, Timothy Petros, Richard McKenney and Kassandra Ori for critical reading of the manuscript and helpful suggestions. This work was supported by NIH Grants HD40182 and GM47434 and the March of Dimes Birth Defects Foundation.
Footnotes
The differential effects of LIS1 and dynein RNAi on centrosome versus nuclear movement can, in part, explain the extreme disparity in centrosome–nucleus spacing reported in some studies [6,62,87]. A very small increase in spacing in some studies either indicates a very weak or a very strong inhibition of dynein activity, whereas increased spacing probably indicates partial inhibition of dynein activity. Continuous live imaging has been useful for resolving these issues [9].
References
- 1.Tsai JW, et al. LIS1 RNA interference blocks neural stem cell division, morphogenesis, and motility at multiple stages. J. Cell Biol. 2005;170:935–945. doi: 10.1083/jcb.200505166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Del Bene F, et al. Regulation of neurogenesis by interkinetic nuclear migration through an apical-basal notch gradient. Cell. 2008;134:1055–1065. doi: 10.1016/j.cell.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McManus MF, et al. Lis1 is necessary for normal non-radial migration of inhibitory interneurons. Am. J. Pathol. 2004;165:775–784. doi: 10.1016/S0002-9440(10)63340-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellion A, et al. Nucleokinesis in tangentially migrating neurons comprises two alternating phases: forward migration of the Golgi/centrosome associated with centrosome splitting and myosin contraction at the rear. J. Neurosci. 2005;25:5691–5699. doi: 10.1523/JNEUROSCI.1030-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schaar BT, McConnell SK. Cytoskeletal coordination during neuronal migration. Proc. Natl. Acad. Sci. U. S. A. 2005;102:13652–13657. doi: 10.1073/pnas.0506008102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solecki DJ, et al. Par6α signaling controls glial-guided neuronal migration. Nat. Neurosci. 2004;7:1195–1203. doi: 10.1038/nn1332. [DOI] [PubMed] [Google Scholar]
- 7.Kakita A, Goldman JE. Patterns and dynamics of SVZ cell migration in the postnatal forebrain: monitoring living progenitors in slice preparations. Neuron. 1999;23:461–472. doi: 10.1016/s0896-6273(00)80800-4. [DOI] [PubMed] [Google Scholar]
- 8.Farin A, et al. Transplanted glioma cells migrate and proliferate on host brain vasculature: a dynamic analysis. Glia. 2006;53:799–808. doi: 10.1002/glia.20334. [DOI] [PubMed] [Google Scholar]
- 9.Tsai JW, et al. Dual subcellular roles for LIS1 and dynein in radial neuronal migration in live brain tissue. Nat. Neurosci. 2007;10:970–979. doi: 10.1038/nn1934. [DOI] [PubMed] [Google Scholar]
- 10.Umeshima H, et al. Microtubule-based nuclear movement occurs independently of centrosome positioning in migrating neurons. Proc. Natl. Acad. Sci. U. S. A. 2007;104:16182–16187. doi: 10.1073/pnas.0708047104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sapir T, et al. Antagonistic effects of doublecortin and MARK2/Par-1 in the developing cerebral cortex. J. Neurosci. 2008;28:13008–13013. doi: 10.1523/JNEUROSCI.2363-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sapir T, et al. Accurate balance of the polarity kinase MARK2/Par-1 is required for proper cortical neuronal migration. J. Neurosci. 2008;28:5710–5720. doi: 10.1523/JNEUROSCI.0911-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rivas RJ, Hatten ME. Motility and cytoskeletal organization of migrating cerebellar granule neurons. J. Neurosci. 1995;15:981–989. doi: 10.1523/JNEUROSCI.15-02-00981.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rakic P, et al. Polarity of microtubule assemblies during neuronal cell migration. Proc. Natl. Acad. Sci. U. S. A. 1996;93:9218–9222. doi: 10.1073/pnas.93.17.9218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elias LA, et al. Gap junction adhesion is necessary for radial migration in the neocortex. Nature. 2007;448:901–907. doi: 10.1038/nature06063. [DOI] [PubMed] [Google Scholar]
- 16.Limouze J, et al. Specificity of blebbistatin, an inhibitor of myosin II. J. Muscle Res. Cell Motil. 2004;25:337–341. doi: 10.1007/s10974-004-6060-7. [DOI] [PubMed] [Google Scholar]
- 17.Grabham PW, et al. Cytoplasmic dynein and LIS1 are required for microtubule advance during growth cone remodeling and fast axonal outgrowth. J. Neurosci. 2007;27:5823–5834. doi: 10.1523/JNEUROSCI.1135-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hebbar S, et al. Lis1 and Ndel1 influence the timing of nuclear envelope breakdown in neural stem cells. J. Cell Biol. 2008;182:1063–1071. doi: 10.1083/jcb.200803071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beaudouin J, et al. Nuclear envelope breakdown proceeds by microtubule-induced tearing of the lamina. Cell. 2002;108:83–96. doi: 10.1016/s0092-8674(01)00627-4. [DOI] [PubMed] [Google Scholar]
- 20.Salina D, et al. Cytoplasmic dynein as a facilitator of nuclear envelope breakdown. Cell. 2002;108:97–107. doi: 10.1016/s0092-8674(01)00628-6. [DOI] [PubMed] [Google Scholar]
- 21.Dent EW, Gertler FB. Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron. 2003;40:209–227. doi: 10.1016/s0896-6273(03)00633-0. [DOI] [PubMed] [Google Scholar]
- 22.Pak CW, et al. Actin-binding proteins take the reins in growth cones. Nat. Rev. Neurosci. 2008;9:136–147. doi: 10.1038/nrn2236. [DOI] [PubMed] [Google Scholar]
- 23.Schaefer AW, et al. Filopodia and actin arcs guide the assembly and transport of two populations of microtubules with unique dynamic parameters in neuronal growth cones. J. Cell Biol. 2002;158:139–152. doi: 10.1083/jcb.200203038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burnette DT, et al. Filopodial actin bundles are not necessary for microtubule advance into the peripheral domain of Aplysia neuronal growth cones. Nat Cell Biol. 2007;12:1360–1369. doi: 10.1038/ncb1655. [DOI] [PubMed] [Google Scholar]
- 25.Zhang XF, et al. Rho-dependent contractile responses in the neuronal growth cone are independent of classical peripheral retrograde actin flow. Neuron. 2003;40:931–944. doi: 10.1016/s0896-6273(03)00754-2. [DOI] [PubMed] [Google Scholar]
- 26.Rivas RJ, et al. Rapid effects of laminin on the growth cone. Neuron. 1992;8:107–115. doi: 10.1016/0896-6273(92)90112-q. [DOI] [PubMed] [Google Scholar]
- 27.Tang D, Goldberg DJ. Bundling of microtubules in the growth cone induced by laminin. Mol. Cell. Neurosci. 2000;15:303–313. doi: 10.1006/mcne.1999.0820. [DOI] [PubMed] [Google Scholar]
- 28.Buck KB, Zheng JQ. Growth cone turning induced by direct local modification of microtubule dynamics. J. Neurosci. 2002;22:9358–9367. doi: 10.1523/JNEUROSCI.22-21-09358.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marsh L, Letourneau PC. Growth of neurites without filopodial or lamellipodial activity in the presence of cytochalasin B. J. Cell Biol. 1984;99:2041–2047. doi: 10.1083/jcb.99.6.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Challacombe JF, et al. Dynamic microtubule ends are required for growth cone turning to avoid an inhibitory guidance cue. J. Neurosci. 1997;17:3085–3095. doi: 10.1523/JNEUROSCI.17-09-03085.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown ME, Bridgman PC. Myosin function in nervous and sensory systems. J. Neurobiol. 2004;58:118–130. doi: 10.1002/neu.10285. [DOI] [PubMed] [Google Scholar]
- 32.Zhu XJ, et al. Myosin X regulates netrin receptors and functions in axonal path-finding. Nat. Cell Biol. 2007;9:184–192. doi: 10.1038/ncb1535. [DOI] [PubMed] [Google Scholar]
- 33.Rochlin MW, et al. Localization of myosin II A and B isoforms in cultured neurons. J. Cell Sci. 1995;108:3661–3670. doi: 10.1242/jcs.108.12.3661. [DOI] [PubMed] [Google Scholar]
- 34.Medeiros NA, et al. Myosin II functions in actin-bundle turnover in neuronal growth cones. Nat. Cell Biol. 2006;8:215–226. doi: 10.1038/ncb1367. [DOI] [PubMed] [Google Scholar]
- 35.Loudon RP, et al. RhoA-kinase and myosin II are required for the maintenance of growth cone polarity and guidance by nerve growth factor. J. Neurobiol. 2006;66:847–867. doi: 10.1002/neu.20258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burnette DT, et al. Myosin II activity facilitates microtubule bundling in the neuronal growth cone neck. Dev. Cell. 2008;15:163–169. doi: 10.1016/j.devcel.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown ME, Bridgman PC. Retrograde flow rate is increased in growth cones from myosin IIB knockout mice. J. Cell Sci. 2003;116:1087–1094. doi: 10.1242/jcs.00335. [DOI] [PubMed] [Google Scholar]
- 38.Bridgman PC, et al. Myosin IIB is required for growth cone motility. J. Neurosci. 2001;21:6159–6169. doi: 10.1523/JNEUROSCI.21-16-06159.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turney SG, Bridgman PC. Laminin stimulates and guides axonal outgrowth via growth cone myosin II activity. Nat. Neurosci. 2005;8:717–719. doi: 10.1038/nn1466. [DOI] [PubMed] [Google Scholar]
- 40.Diefenbach TJ, et al. Myosin 1c and myosin IIB serve opposing roles in lamellipodial dynamics of the neuronal growth cone. J. Cell Biol. 2002;158:1207–1217. doi: 10.1083/jcb.200202028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bamburg JR, et al. Assembly of microtubules at the tip of growing axons. Nature. 1986;321:788–790. doi: 10.1038/321788a0. [DOI] [PubMed] [Google Scholar]
- 42.Wang L, Brown A. Rapid movement of microtubules in axons. Curr. Biol. 2002;12:1496–1501. doi: 10.1016/s0960-9822(02)01078-3. [DOI] [PubMed] [Google Scholar]
- 43.Ahmad FJ, et al. Effects of dynactin disruption and Dynein depletion on axonal microtubules. Traffic. 2006;7:524–537. doi: 10.1111/j.1600-0854.2006.00403.x. [DOI] [PubMed] [Google Scholar]
- 44.Mesngon MT, et al. Regulation of cytoplasmic dynein ATPase by Lis1. J. Neurosci. 2006;26:2132–2139. doi: 10.1523/JNEUROSCI.5095-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Myers KA, et al. Antagonistic forces generated by cytoplasmic dynein and myosin-II during growth cone turning and axonal retraction. Traffic. 2006;7:1333–1351. doi: 10.1111/j.1600-0854.2006.00476.x. [DOI] [PubMed] [Google Scholar]
- 46.Busson S, et al. Dynein and dynactin are localized to astral microtubules and at cortical sites in mitotic epithelial cells. Curr. Biol. 1998;8:541–544. doi: 10.1016/s0960-9822(98)70208-8. [DOI] [PubMed] [Google Scholar]
- 47.Dujardin DL, et al. A role for cytoplasmic dynein and LIS1 in directed cell movement. J. Cell Biol. 2003;163:1205–1211. doi: 10.1083/jcb.200310097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He Y, et al. Role of cytoplasmic dynein in the axonal transport of microtubules and neurofilaments. J. Cell Biol. 2005;168:697–703. doi: 10.1083/jcb.200407191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stehman SA, et al. NudE and NudEL are required for mitotic progression and are involved in dynein recruitment to kinetochores. J. Cell Biol. 2007;178:583–594. doi: 10.1083/jcb.200610112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shen Y, et al. Nudel Binds Cdc42GAP to modulate Cdc42 activity at the leading edge of migrating cells. Dev. Cell. 2008;14:342–353. doi: 10.1016/j.devcel.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 51.Rochlin MW, et al. Polymerizing microtubules activate site-directed F-actin assembly in nerve growth cones. Mol. Biol. Cell. 1999;10:2309–2327. doi: 10.1091/mbc.10.7.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Waterman-Storer CM, Salmon E. Positive feedback interactions between microtubule and actin dynamics during cell motility. Curr. Opin. Cell Biol. 1999;11:61–67. doi: 10.1016/s0955-0674(99)80008-8. [DOI] [PubMed] [Google Scholar]
- 53.Waterman-Storer CM, et al. Microtubule growth activates Rac1 to promote lamellipodial protrusion in fibroblasts. Nat. Cell Biol. 1999;1:45–50. doi: 10.1038/9018. [DOI] [PubMed] [Google Scholar]
- 54.Wittmann T, et al. Regulation of leading edge microtubule and actin dynamics downstream of Rac1. J. Cell Biol. 2003;161:845–851. doi: 10.1083/jcb.200303082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grabham PW, et al. Microtubule and Rac 1-dependent F-actin in growth cones. J. Cell Sci. 2003;116:3739–3748. doi: 10.1242/jcs.00686. [DOI] [PubMed] [Google Scholar]
- 56.Palazzo AF, et al. Cdc42, dynein, and dynactin regulate MTOC reorientation independent of Rho-regulated microtubule stabilization. Curr. Biol. 2001;11:1536–1541. doi: 10.1016/s0960-9822(01)00475-4. [DOI] [PubMed] [Google Scholar]
- 57.Balenci L, et al. IQGAP1 regulates adult neural progenitors in vivo and vascular endothelial growth factor-triggered neural progenitor migration in vitro. J. Neurosci. 2007;27:4716–4724. doi: 10.1523/JNEUROSCI.0830-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang S, et al. IQGAP3, a novel effector of Rac1 and Cdc42, regulates neurite outgrowth. J. Cell Sci. 2007;120:567–577. doi: 10.1242/jcs.03356. [DOI] [PubMed] [Google Scholar]
- 59.Li Z, et al. IQGAP1 promotes neurite outgrowth in a phosphorylation-dependent manner. J. Biol. Chem. 2005;280:13871–13878. doi: 10.1074/jbc.M413482200. [DOI] [PubMed] [Google Scholar]
- 60.Kholmanskikh SS, et al. Calcium-dependent interaction of Lis1 with IQGAP1 and Cdc42 promotes neuronal motility. Nat. Neurosci. 2006;9:50–57. doi: 10.1038/nn1619. [DOI] [PubMed] [Google Scholar]
- 61.Vallee RB, Stehman SA. How dynein helps the cell find its center: a servomechanical model. Trends Cell Biol. 2005;15:288–294. doi: 10.1016/j.tcb.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 62.Shu T, et al. Ndel1 operates in a common pathway with LIS1 and cytoplasmic Dynein to regulate cortical neuronal positioning. Neuron. 2004;44:263–277. doi: 10.1016/j.neuron.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 63.Gomes ER, et al. Nuclear movement regulated by Cdc42, MRCK, myosin, and actin flow establishes MTOC polarization in migrating cells. Cell. 2005;121:451–463. doi: 10.1016/j.cell.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 64.Reddy S, et al. Mutant molecular motors disrupt neural circuits in Drosophila. J. Neurobiol. 1997;33:711–723. doi: 10.1002/(sici)1097-4695(19971120)33:6<711::aid-neu1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 65.Phillis R, et al. Mutations in the 8 kDa dynein light chain gene disrupt sensory axon projections in the Drosophila imaginal CNS. Development. 1996;122:2955–2963. doi: 10.1242/dev.122.10.2955. [DOI] [PubMed] [Google Scholar]
- 66.Etienne-Manneville S, Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCζ. Cell. 2001;106:489–498. doi: 10.1016/s0092-8674(01)00471-8. [DOI] [PubMed] [Google Scholar]
- 67.Nadar VC, et al. Kinesin-5 is essential for growth-cone turning. Curr. Biol. 2008;18:1972–1977. doi: 10.1016/j.cub.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reiner O, et al. Isolation of a Miller-Dieker lissencephaly gene containing G protein β-subunit-like repeats. Nature. 1993;364:717–721. doi: 10.1038/364717a0. [DOI] [PubMed] [Google Scholar]
- 69.Feng Y, et al. LIS1 regulates CNS lamination by interacting with mNudE, a central component of the centrosome. Neuron. 2000;28:665–679. doi: 10.1016/s0896-6273(00)00145-8. [DOI] [PubMed] [Google Scholar]
- 70.Sasaki S, et al. Complete loss of Ndel1 results in neuronal migration defects and early embryonic lethality. Mol. Cell. Biol. 2005;25:7812–7827. doi: 10.1128/MCB.25.17.7812-7827.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Niethammer M, et al. NUDEL is a novel Cdk5 substrate that associates with LIS1 and cytoplasmic dynein. Neuron. 2000;28:697–711. doi: 10.1016/s0896-6273(00)00147-1. [DOI] [PubMed] [Google Scholar]
- 72.Mori D, et al. NDEL1 phosphorylation by Aurora-A kinase is essential for centrosomal maturation, separation, and TACC3 recruitment. Mol. Cell. Biol. 2007;27:352–367. doi: 10.1128/MCB.00878-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tai CY, et al. Role of dynein, dynactin, and CLIP-170 interactions in LIS1 kinetochore function. J. Cell Biol. 2002;156:959–968. doi: 10.1083/jcb.200109046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sasaki S, et al. A LIS1/NUDEL/cytoplasmic dynein heavy chain complex in the developing and adult nervous system. Neuron. 2000;28:681–696. doi: 10.1016/s0896-6273(00)00146-x. [DOI] [PubMed] [Google Scholar]
- 75.Tarricone C, et al. Coupling PAF signaling to dynein regulation: structure of LIS1 in complex with PAF-acetylhydrolase. Neuron. 2004;44:809–821. doi: 10.1016/j.neuron.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 76.Derewenda U, et al. The structure of the coiled-coil domain of Ndel1 and the basis of its interaction with Lis1, the causal protein of Miller-Dieker lissencephaly. Structure. 2007;15:1467–1481. doi: 10.1016/j.str.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 77.Verkhovsky AB, et al. Myosin II filament assemblies in the active lamella of fibroblasts: their morphogenesis and role in the formation of actin filament bundles. J. Cell Biol. 1995;131:989–1002. doi: 10.1083/jcb.131.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Svitkina TM, et al. Analysis of the actin-myosin II system in fish epidermal keratocytes: mechanism of cell body translocation. J. Cell Biol. 1997;139:397–415. doi: 10.1083/jcb.139.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gupton SL, Waterman-Storer CM. Spatiotemporal feedback between actomyosin and focal-adhesion systems optimizes rapid cell migration. Cell. 2006;125:1361–1374. doi: 10.1016/j.cell.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 80.Lo CM, et al. Nonmuscle myosin IIb is involved in the guidance of fibroblast migration. Mol. Biol. Cell. 2004;15:982–989. doi: 10.1091/mbc.E03-06-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mizutani T, et al. Diphosphorylation of the myosin regulatory light chain enhances the tension acting on stress fibers in fibroblasts. J. Cell. Physiol. 2006;209:726–731. doi: 10.1002/jcp.20773. [DOI] [PubMed] [Google Scholar]
- 82.Vicente-Manzanares M, et al. Regulation of protrusion, adhesion dynamics, and polarity by myosins IIA and IIB in migrating cells. J. Cell Biol. 2007;176:573–580. doi: 10.1083/jcb.200612043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tabata H, Nakajima K. Multipolar migration: the third mode of radial neuronal migration in the developing cerebral cortex. J. Neurosci. 2003;23:9996–10001. doi: 10.1523/JNEUROSCI.23-31-09996.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Noctor SC, et al. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat. Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- 85.Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J. Comp. Neurol. 1972;145:61–83. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- 86.Vallee RB, Tsai JW. The cellular roles of the lissencephaly gene LIS1, and what they tell us about brain development. Genes Dev. 2006;20:1384–1393. doi: 10.1101/gad.1417206. [DOI] [PubMed] [Google Scholar]
- 87.Tanaka T, et al. Lis1 and doublecortin function with dynein to mediate coupling of the nucleus to the centrosome in neuronal migration. J. Cell Biol. 2004;165:709–721. doi: 10.1083/jcb.200309025. [DOI] [PMC free article] [PubMed] [Google Scholar]