Abstract
Aims
Cardiac resynchronization therapy (CRT) improves echocardiographic parameters, symptoms, hospitalizations, and mortality in patients with New York Heart Association (NYHA) Class III or IV symptoms with left ventricular systolic dysfunction, sinus rhythm, and a prolonged QRS duration. The effectiveness of CRT in patients with mild heart failure symptoms has not been systematically reviewed.
Methods and results
Randomized controlled trials of CRT in patients with NYHA Class I or II heart failure were identified from MEDLINE and EMBASE. The effects of CRT on left ventricular remodelling at 1 year were systematically reviewed, and the effects of CRT on clinical outcomes at 1 year were meta-analysed. Two studies met the pre-specified search criteria, with a total of 2430 patients (REVERSE n = 610 and MADIT-CRT n = 1820). CRT was associated with a reduction in heart failure events in both trials [combined OR 0.57, 95% confidence interval (CI) 0.46–0.70], but not mortality (combined OR 0.96, 95% CI 0.67–1.36). The effect of CRT on the combined endpoint of heart failure events or death favoured CRT (OR 0.63, 95% CI 0.51–0.77). CRT was also associated with improvement in left ventricular remodelling parameters in both studies, including a greater increase in left ventricular ejection fraction in the CRT group than in the control group, at 1 year after randomization. Serious adverse events were rare with CRT.
Conclusion
CRT reduces heart failure events in patients with mild heart failure symptoms, left ventricular dysfunction, sinus rhythm, and prolonged QRS duration.
Keywords: Artificial cardiac pacemaker, Artificial pacemaker, Heart failure, Mortality, Cardiac resynchronization therapy
Introduction
Advanced heart failure poses a substantial clinical and economic public health burden.1,2 In many patients, the clinical syndrome of congestion arises from electro-mechanical dyssynchrony, leading to inefficient ventricular contraction, mitral regurgitation, and worsening ventricular dilation.3 Studies of cardiac resynchronization therapy (CRT) using a left ventricular lead implanted via the coronary sinus have demonstrated improvements in echocardiographic parameters, symptoms, hospitalizations, and mortality in individuals with systolic left ventricular dysfunction, sinus rhythm, prolonged QRS durations, and New York Heart Association (NYHA) Class III or IV heart failure symptoms.4–8 Current guidelines support the use of CRT for individuals with left ventricular ejection fractions (LVEF) ≤35%, QRS durations ≥120 ms, and advanced heart failure symptoms similar to those in clinical trials, despite optimal medical therapy.1,2
Although improvements in left ventricular remodelling and clinical outcomes with CRT have been demonstrated in individuals with advanced heart failure, evidence of improved left ventricular remodelling has also been demonstrated in two short-term studies of individuals with NYHA Class II symptoms9,10 as well as in one observational registry.11 Moreover, recently published data suggest improved heart failure and survival outcomes associated with the use of CRT in either asymptomatic or only mildly symptomatic heart failure patients.12,13 Small elevations in brain natriuretic peptide levels are likely a surrogate for mild heart failure symptoms. Interestingly, in a post hoc analysis of the Cardiac Resynchronization in Heart Failure trial, subjects with brain natriuretic peptide levels above or below the median had similar survival outcomes with CRT.14 We performed a systematic review and meta-analysis to determine the effects of CRT on long-term left ventricular remodelling parameters and clinical outcomes in patients with mildly symptomatic heart failure.
Methods
Search strategy
An electronic search of EMBASE and MEDLINE was performed for all English articles of human studies through July 2009 using the search terms ‘CRT’ OR ‘CRT’ OR ‘biventricular pacing’ OR ‘biventricular pacer’ OR ‘BiV’ OR ‘biventricular pacemaker’. Bibliographies from published meta-analyses and review articles were hand-searched and experts in the field were consulted to ensure inclusion of all pertinent studies for the preliminary review.
Article selection and eligibility criteria
The search strategy focused on randomized controlled trials in which an experimental arm included CRT and the control arm did not. Our analysis was restricted to those trials that included subjects with NYHA Class I or II symptoms, and reported specified outcomes of interest (see below) at 1 year of follow-up.
Data abstraction and quality assessment
Two investigators (P.L. and N.F.) independently extracted data on study and patient characteristics, outcomes, and study quality using a standardized extraction form. Disagreements were resolved by consensus with all four investigators. Study quality was assessed using the Jadad scale, which ranges from 0 to 5 with higher values indicating better study quality.15
Data collected included left ventricular remodelling parameters, QRS duration, and ejection fraction, as well as the clinical outcomes of heart failure events or death from any cause at 1 year. Complications associated with CRT were also ascertained.
Data analysis
Agreement between the two data extractors were assessed with the Kappa statistic. Odds ratios for the outcome of heart failure events, mortality, or heart failure events or mortality were calculated using the DerSimonian and Laird random effects method.16 Heterogeneity was quantified using the I2 statistic.17 Statistical analyses were performed with Stata™ v10.1 (College Station, TX, USA).
Results
Literature search
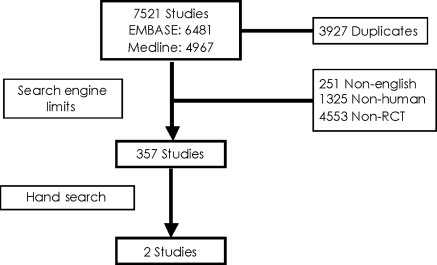
The initial search identified 6481 citations from EMBASE and 4967 from MEDLINE. Of these 3927 were duplicates, leaving 7521 unique citations. Electronic filtering of non-English, followed by non-human, non-randomized controlled trial left 357 studies. These 357 studies were hand searched by two independent investigators to yield two randomized controlled trials. These included the REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction (REVERSE) trial by Linde et al.12 and the Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (MADIT-CRT) trial by Moss et al.13 Two sub-studies of the REVERSE trial were also identified and were not included in the main analysis.18,19 Details of the study flow are displayed in Figure 1. Agreement between the two reviewers was 0.99 and the Cohen–Kappa statistic was 0.875 [95% confidence interval (CI] 0.735, 1.000]. Hand-searching of bibliographies and consultation with experts did not contribute any additional articles that met the pre-specified inclusion criteria.
Figure 1.
Study flow.
Characteristics of included studies
Both the REVERSE and MADIT-CRT trials were randomized controlled trials involving patients with poor LV systolic function and a history of symptomatic heart failure (Table 1). REVERSE included patients with an LVEF of 40% or less, a QRS duration of at least 120 ms, a left ventricular end diastolic diameter of 55 mm or greater, and NYHA functional Class I or II, irrespective of ischaemic or non-ischaemic heart failure aetiology classification. MADIT-CRT included individuals with an LVEF of 30% or less, a QRS duration of at least 130 ms, and patients with ischaemic cardiomyopathy with NYHA functional Class I or II, or non-ischaemic cardiomyopathy with NYHA functional Class II. Both trials required that patients were treated with optimal medical therapy.
Table 1.
Characteristics of included trials
| Study | Design | Patients | Primary endpoint | Secondary endpoints | Blinding strategy | Location | Sponsor | Jadad score |
|---|---|---|---|---|---|---|---|---|
| REVERSE12 | RCT of CRT on or off (2:1); ICD based on proper indications | NYHA I-II; LVEF ≤ 40%; QRS ≥ 120 ms; LVEDD ≥ 55 mm | Death, HF hospitalization, worsening heart failure class | LVESVI; cardiac hospitalization | Double-blind | Multi-centre, Canada, Europe, and USA | Not stated | 5 |
| MADIT-CRT13 | RCT of CRT or no CRT (3:2); all subjects received ICDs | NYHA I-II; LVEF ≤ 30; QRS ≥ 130 ms | Death or nonfatal HF event | LVESV, LVEDV, rate of multiple HF events | None | Multi-centre, Canada, Europe, and USA | Boston Scientific | 3 |
CRT, cardiac resynchronization therapy; ICD, implantable cardioverter-defibrillator; LVEDD, left ventricular end diastolic dimension; LVESV, left ventricular end systolic volume; LVEDV, left ventricular end diastolic volume; LVESVI, left ventricular end systolic volume index; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association functional class; RCT, randomized controlled trial.
In the REVERSE trial, all subjects received a CRT device, with or without an implantable cardioverter defibrillator (ICD) in accordance with practice guidelines. The patients were then randomized to have their CRT devices turned on (CRT-ON) or off (CRT-OFF) in a 2:1 fashion. In MADIT-CRT, patients were randomized to receive an ICD with or without CRT in a 3:2 fashion.
The primary outcome in REVERSE was a clinical composite of worsening heart failure, which included mortality, and in MADIT-CRT the primary outcome was a composite endpoint of all-cause mortality and heart failure events.
Both studies were of high quality, with a Jadad Score of 5/5 for REVERSE and 3/5 for MADIT-CRT. Points were lost in MADIT-CRT due to lack of blinding in the patients or physicians, as CRT implantation was only performed in patients randomized to intervention. However, a blinded committee adjudicated events in both of these trials. Follow-up was excellent in both studies with primary endpoint data available for 100% of patients in REVERSE and 95% in MADIT-CRT.
The characteristics of the patients included in both studies are detailed in Table 2. The studies included a combined total of 2430 patients (REVERSE n = 610 and MADIT-CRT n = 1820). In each study, 55% of the subjects were classified as having ischaemic cardiomyopathy. The median age was 62 years in REVERSE and 65 years in MADIT-CRT. The majority of subjects were male (75–78%). Medical therapy for cardiac dysfunction with angiotensin converting enzyme inhibitors or angiotensin receptor blockers, beta-blockers, and diuretics was consistent with clinical practice guidelines.1 In the REVERSE trial, 163 (85%) of subjects in the CRT-OFF arm, and 345 (82%) of subjects in the CRT-ON arm, received ICDs. In MADIT-CRT, by protocol all subjects were treated with ICDs.
Table 2.
Baseline characteristics of patients included in the REVERSE and MADIT-CRT studies
| Study | Number of subjects | Ischaemic, % | Median age | Male, % | Mean QRS, ms | ACE-I, % | ARB, % | Beta-blocker, % | Diuretic, % |
|---|---|---|---|---|---|---|---|---|---|
| REVERSE12 | 610 | 55 | 62 | 78 | 153 | 79 | 21 | 96 | 81 |
| MADIT-CRT13 | 1820 | 55 | 65 | 75 | NRa | 77 | 21 | 93 | 75 |
ACE-I, angiotensin converting enzyme-inhibitor; ARB, angiotensin receptor blocker; NR, not reported.
aMean QRS not reported in MADIT-CRT; ∼65% of subjects had QRS durations ≥150 ms in each group.
Heart failure events and mortality
The definition of heart failure events is based on that used in each of the two clinical trials.12,13 In the REVERSE trial, heart failure events were defined as either hospitalization due to or associated with worsening heart failure, crossover to the CRT therapy arm due to worsening heart failure, and worsened patient global assessment or NYHA functional class. In MADIT-CRT, heart failure events were defined as those signs and symptoms consistent with heart failure and the requirement of decongestive therapy on an outpatient basis, or an augmented decongestive regimen as an inpatient.
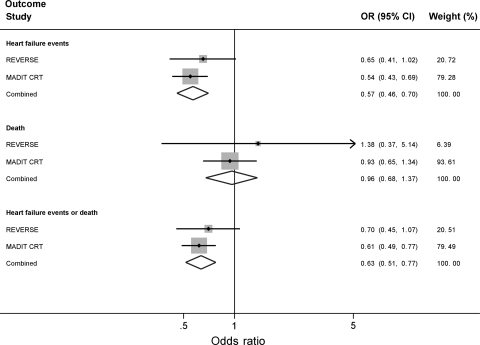
In the combined meta-analysis of the REVERSE and MADIT-CRT trials, CRT was associated with a reduction in heart failure events (combined OR 0.57, 95% CI 0.46–0.70, Figure 2). In contrast, CRT was not associated with reductions in mortality (combined OR 0.96, 95% CI 0.67–1.36, Figure 2). The overall benefit of CRT on the combined endpoint of heart failure events or death (OR 0.63, 95% CI 0.51–0.77, Figure 2) was primarily attributable to the effects of CRT on reducing heart failure events. There was no evidence of statistical heterogeneity for each of the analysed outcomes (I2 = 0).
Figure 2.
Forest plot demonstrating the effect of CRT on heart failure events, death, or the combined endpoint of heart failure events or death.
A sub-study from the REVERSE trial reported 24-month clinical and left ventricular remodelling outcomes in the European arm of the trial.19 The European arm was randomized throughout the 24-month period as opposed to the North American arm, which was randomized only to 12 months. CRT-ON was associated with a reduction in the odds of heart failure events or death relative to those in the CRT-OFF group (OR = 0.45, 95% CI 0.25–0.81). Notably, when compared with those patients in the North American arm of the study, patients in the European arm were younger (61 ± 10 vs. 63 ± 11 years, P = 0.02), less likely to have ischaemic cardiomyopathy (44 vs. 63%, P < 0.001), had a longer QRS duration (156 ± 23 vs. 151 ± 21 ms, P = 0.008), and had fewer comorbidities. Patients in the European arm were also less likely to have received ICDs (68 vs. 95%, P < 0.001). Due to the smaller study sample, we used the 12-month outcomes reported for both the North American and European arms in our meta-analysis.
Reverse left ventricular remodelling
Both trials evaluated the impact of CRT therapy on left ventricular remodelling as assessed by baseline and 12-month echocardiographic parameters. The two trials differed in their approach to CRT programming status during follow-up echocardiography. In REVERSE, echocardiographic measurements were made with CRT turned off, irrespective of treatment assignment. For CRT-ON subjects these measurements were recorded after waiting for a 10 min period. In MADIT-CRT, echocardiography was initially performed with CRT turned off for subjects who received CRT–ICD, as required by the Food and Drug Administration; however, this requirement was later reversed, and the 1-year echocardiograms were subsequently performed with CRT turned on for the duration of the study. The initial 201 CRT–ICD subjects in whom CRT was turned off were excluded from the final analysis of LV remodelling parameters reported in MADIT-CRT.
CRT significantly improved LV remodelling parameters in both studies (Table 3). In REVERSE, CRT-ON subjects experienced a significantly greater reduction in LV end systolic volume index when compared with CRT-OFF subjects (−18.4 ± 29.5 vs. −1.3 ± 23.4 mL/m2, respectively, P = <0.001). The difference in left ventricular end systolic volume index significantly favoured CRT-ON for all subgroups assessed. A similar improvement in LV end systolic volume was observed with CRT in MADIT-CRT (−57 and −18 mL, respectively, P < 0.001). Significant improvement in LVEF was observed with CRT in both studies, with a greater benefit demonstrated in MADIT-CRT (Table 3). Superior improvements in left ventricular end diastolic volume index, left ventricular end systolic and diastolic diameter, and interventricular conduction delay were also demonstrated with CRT in REVERSE, and in left ventricular end diastolic volume in MADIT-CRT (Table 3).
Table 3.
Assessment of left ventricular remodelling after 1-year follow-up in REVERSE and MADIT-CRT
| Study group | No. subjects | LVESVI (mL/m2) |
IVCD (ms) |
LVEDV (mL) |
LVESV (mL) |
LVEF (%) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Change | P-value | Change | P-value | Change | P-value | Change | P-value | Change | P-value | ||
| REVERSE | |||||||||||
| CRT-ON | 324 | −18.4 ± 29.5 | <0.001 | −13.0 ± 43.2 | <0.001 | 3.8 | <0.001 | ||||
| CRT-OFF | 163 | −1.3 ± 23.4 | −0.2 ± 34.0 | 0.6 | |||||||
| MADIT-CRT | |||||||||||
| CRT-ICD | 746 | −52 | <0.001 | −57 | <0.001 | 11 | <0.001 | ||||
| ICD-only | 620 | −15 | −18 | 3 | |||||||
Data presented as mean ± standard deviation, if reported.
IVCD, interventricular conduction delay; LVEDV, left ventricular end diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end systolic volume; LVESVI, left ventricular end systolic volume index.
Two sub-studies of REVERSE also reported on the left ventricular remodelling parameters.18,19 In a sub-study analysis including 487 of the 610 patients in the trial, improvements in left ventricular remodelling parameters with CRT immediately after implantation did not correlate with long-term improvement in parameters (left ventricular end systolic volume index r = 0.11, P = 0.31, left ventricular end diastolic volume index r = 0.10 P = 0.38, LVEF r = 0.07, P = 0.72). Favourable effects of CRT on remodelling were greatest in subjects with more prolonged interventricular mechanical delay (>40 ms), longer QRS duration (>160 ms), and for those with a non-ischaemic HF aetiology.18 Additionally, no differences in changes of diastolic function measurements were noted between CRT-ON and CRT-OFF groups. In a separate sub-study of the 262 patients in the European arm of the REVERSE study, improvement in left ventricular remodelling parameters was also greater for patients in the CRT-ON rather than CRT-OFF arm at the 24-month follow-up period.19
Detailed results of left ventricular remodelling according to subgroup in MADIT-CRT have not yet been released.
Complications
The success rate of CRT implantation was 97% in the REVERSE trial and 99% in MADIT-CRT. One death was reported during the peri-implantation period in a patient receiving CRT in the MADIT-CRT trial as a result of a pulmonary embolism. Peri-implantation mechanical complications, including pneumothorax, coronary dissection, and pericardial tamponade occurred with a 1% frequency in the REVERSE trial, and 2% frequency in MADIT-CRT. Left ventricular lead problems following implantation were reported in ∼7% of participants in the REVERSE trial during the 12-month follow-up period, and 4% in MADIT-CRT during a reported 30-day period. Device related infections occurred in 1% of subjects with CRT in the MADIT-CRT trial within 30 days of implantation.
No significant difference in the rate of complications was detected during the 12 months of follow-up between the CRT-ON and CRT-OFF groups in the REVERSE trial. During follow-up in the REVERSE trial, one case of heart failure occurred that resolved after turning CRT off. During follow-up beyond 30 days in the MADIT-CRT trial, adverse events defined as serious device-related events were reported with an incidence of 4.5 per 100 device-months in the CRT–ICD group, when compared with 5.2 per 100 device-months in the ICD-only group.
Discussion
This meta-analysis of prospective randomized controlled trials comparing CRT in patients with Class I or II heart failure symptoms, left ventricular systolic dysfunction, and a prolonged QRS interval in sinus rhythm demonstrated that CRT is associated with an ∼40% reduction in the odds of heart failure events, but no differences in all-cause mortality at 1 year following implantation. CRT also reverses the negative remodelling effects of heart failure in patients with mild HF symptoms receiving optimal medical therapy.
Neither REVERSE nor MADIT-CRT demonstrated a reduction in mortality with CRT. This may be related to the fact that ICD therapy was widely used in both trials. In REVERSE, over 80% of individuals in both arms received ICDs. In MADIT-CRT, all of the patients received ICD therapy. ICDs have been shown to reduce mortality in primary prevention trials of individuals with left ventricular systolic dysfunction either of ischaemic,20–23 or non-ischaemic aetiology.24 Furthermore, the benefit of ICD therapy has been observed in patients with mild or moderate heart failure.22,25,26 When examined in individuals with moderate or severe heart failure symptoms, CRT alone has been associated with improved survival in one randomized controlled trial,7 a finding further supported in a previously published meta-analysis of randomized controlled trials of CRT.27
CRT has been previously demonstrated to cause reversal of left ventricular structural and functional remodelling changes that occur in chronic heart failure for patients with NYHA functional Class III and IV symptoms.4,5,7 Improvements in LV structure and function with CRT have also been observed in smaller studies of patients with NYHA Class II symptoms with 6 months of follow-up9,10 and long-term in an observational registry.11 Our findings extend these observations to 1 year of follow-up, and suggest that CRT may prevent the natural progression and clinical consequences of left ventricular dysfunction observed in patients with heart failure.28
These data demonstrate that patients with mild heart failure symptoms despite optimal medical therapy, severe left ventricular dysfunction, and QRS prolongation may benefit from CRT. Observations that the benefit of CRT also applies to those with only mild heart failure symptoms may result in an expansion of existing guideline recommendations for the use of CRT. It is difficult to assess the potential clinical and economic impact of any such change in indications for CRT, however, as it has been estimated that up to 22% of patients currently receiving CRT have NYHA Class I or II symptoms and up to 17% have LVEF above 35%.29 Moreover, substantial differences across countries have been noted in the prescription of CRT.30 Future studies are necessary to understand the reasons underlying disparities in the application of CRT, as well as the cost-effectiveness of CRT in patients with mild heart failure symptoms.
Limitations
The analysis is limited in that only two randomized trials evaluated the long-term effects of CRT on left ventricular remodelling or death in patients with mild heart failure symptoms. In both of these trials, the vast majority of subjects were also treated with ICDs, thereby perhaps masking any potential benefit of CRT on mortality reduction. Additionally, the absence of patient-level data limits our ability to assess subgroup effects of CRT on clinical or functional outcomes.
Conclusions
CRT is associated with an improvement in left ventricular remodelling parameters and a substantial reduction in heart failure events among individuals with NYHA Class I or II heart failure symptoms, left ventricular systolic dysfunction, and a prolonged QRS duration in sinus rhythm. These findings add to the array of therapies available for improving clinical outcomes among patients with mild heart failure symptoms.
Funding
S.A.L. is supported by an NIH training grant in the Epidemiology of Cardiovascular Disease (T32HL007575). P.T.E. is supported by grants from the NIH (HL092577 and DA027021).
Conflicts of interest: J.S. reports the following disclosures: Biotronik—lecture honoraria, consulting, and research grants; Boston Scientific—lecture honoraria, consulting, and research grants; Medtronic—lecture honoraria, consulting, and research grants; St. Jude—lecture honoraria, consulting, and research grants; Sorin—lecture honoraria and consulting; Sanofi Aventis: consulting.
References
- 1.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1–e90. doi: 10.1016/j.jacc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 2.Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, Stromberg A, van Veldhuisen DJ, Atar D, Hoes AW, Keren A, Mebazaa A, Nieminen M, Priori SG, Swedberg K, Vahanian A, Camm J, De Caterina R, Dean V, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Auricchio A, Bax J, Bohm M, Corra U, della Bella P, Elliott PM, Follath F, Gheorghiade M, Hasin Y, Hernborg A, Jaarsma T, Komajda M, Kornowski R, Piepoli M, Prendergast B, Tavazzi L, Vachiery JL, Verheugt FW, Zannad F. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur J Heart Fail. 2008;10:933–989. doi: 10.1016/j.ejheart.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Bilchick KC, Helm RH, Kass DA. Physiology of biventricular pacing. Curr Cardiol Rep. 2007;9:358–365. doi: 10.1007/BF02938362. [DOI] [PubMed] [Google Scholar]
- 4.Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, Kocovic DZ, Packer M, Clavell AL, Hayes DL, Ellestad M, Trupp RJ, Underwood J, Pickering F, Truex C, McAtee P, Messenger J. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–1853. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 5.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 6.Cazeau S, Leclercq C, Lavergne T, Walker S, Varma C, Linde C, Garrigue S, Kappenberger L, Haywood GA, Santini M, Bailleul C, Daubert JC. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med. 2001;344:873–880. doi: 10.1056/NEJM200103223441202. [DOI] [PubMed] [Google Scholar]
- 7.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 8.Young JB, Abraham WT, Smith AL, Leon AR, Lieberman R, Wilkoff B, Canby RC, Schroeder JS, Liem LB, Hall S, Wheelan K. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. J Am Med Assoc. 2003;289:2685–2694. doi: 10.1001/jama.289.20.2685. [DOI] [PubMed] [Google Scholar]
- 9.Abraham WT, Young JB, Leon AR, Adler S, Bank AJ, Hall SA, Lieberman R, Liem LB, O'Connell JB, Schroeder JS, Wheelan KR. Effects of cardiac resynchronization on disease progression in patients with left ventricular systolic dysfunction, an indication for an implantable cardioverter-defibrillator, and mildly symptomatic chronic heart failure. Circulation. 2004;110:2864–2868. doi: 10.1161/01.CIR.0000146336.92331.D1. [DOI] [PubMed] [Google Scholar]
- 10.Higgins SL, Hummel JD, Niazi IK, Giudici MC, Worley SJ, Saxon LA, Boehmer JP, Higginbotham MB, De Marco T, Foster E, Yong PG. Cardiac resynchronization therapy for the treatment of heart failure in patients with intraventricular conduction delay and malignant ventricular tachyarrhythmias. J Am Coll Cardiol. 2003;42:1454–1459. doi: 10.1016/s0735-1097(03)01042-8. [DOI] [PubMed] [Google Scholar]
- 11.Landolina M, Lunati M, Gasparini M, Santini M, Padeletti L, Achilli A, Bianchi S, Laurenzi F, Curnis A, Vincenti A, Valsecchi S, Denaro A. Comparison of the effects of cardiac resynchronization therapy in patients with class II versus class III and IV heart failure (from the InSync/InSync ICD Italian Registry) Am J Cardiol. 2007;100:1007–1012. doi: 10.1016/j.amjcard.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 12.Linde C, Abraham WT, Gold MR, St John Sutton M, Ghio S, Daubert C. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol. 2008;52:1834–1843. doi: 10.1016/j.jacc.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 13.Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, Estes NA, 3rd, Foster E, Greenberg H, Higgins SL, Pfeffer MA, Solomon SD, Wilber D, Zareba W. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–1338. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 14.Berger R, Shankar A, Fruhwald F, Fahrleitner-Pammer A, Freemantle N, Tavazzi L, Cleland JG, Pacher R. Relationships between cardiac resynchronization therapy and N-terminal pro-brain natriuretic peptide in patients with heart failure and markers of cardiac dyssynchrony: an analysis from the Cardiac Resynchronization in Heart Failure (CARE-HF) study. Eur Heart J. 2009;30:2109–2116. doi: 10.1093/eurheartj/ehp210. [DOI] [PubMed] [Google Scholar]
- 15.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 16.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.St John Sutton M, Ghio S, Plappert T, Tavazzi L, Scelsi L, Daubert C, Abraham WT, Gold MR, Hassager C, Herre JM, Linde C. Cardiac resynchronization induces major structural and functional reverse remodeling in patients with New York Heart Association class I/II heart failure. Circulation. 2009;120:1858–1865. doi: 10.1161/CIRCULATIONAHA.108.818724. [DOI] [PubMed] [Google Scholar]
- 19.Daubert C, Gold MR, Abraham WT, Ghio S, Hassager C, Goode G, Szili-Torok T, Linde C. Prevention of disease progression by cardiac resynchronization therapy in patients with asymptomatic or mildly symptomatic left ventricular dysfunction: insights from the european cohort of the reverse (Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction) trial. J Am Coll Cardiol. 2009;54:1837–1846. doi: 10.1016/j.jacc.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, Brown MW, Heo M. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335:1933–1940. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 21.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 22.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 23.Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999;341:1882–1890. doi: 10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- 24.Desai AS, Fang JC, Maisel WH, Baughman KL. Implantable defibrillators for the prevention of mortality in patients with nonischemic cardiomyopathy: a meta-analysis of randomized controlled trials. J Am Med Assoc. 2004;292:2874–2879. doi: 10.1001/jama.292.23.2874. [DOI] [PubMed] [Google Scholar]
- 25.Kadish A, Dyer A, Daubert JP, Quigg R, Estes NA, Anderson KP, Calkins H, Hoch D, Goldberger J, Shalaby A, Sanders WE, Schaechter A, Levine JH. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151–2158. doi: 10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 26.Zareba W, Piotrowicz K, McNitt S, Moss AJ. Implantable cardioverter-defibrillator efficacy in patients with heart failure and left ventricular dysfunction (from the MADIT II population) Am J Cardiol. 2005;95:1487–1491. doi: 10.1016/j.amjcard.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 27.McAlister FA, Ezekowitz J, Hooton N, Vandermeer B, Spooner C, Dryden DM, Page RL, Hlatky MA, Rowe BH. Cardiac resynchronization therapy for patients with left ventricular systolic dysfunction: a systematic review. J Am Med Assoc. 2007;297:2502–2514. doi: 10.1001/jama.297.22.2502. [DOI] [PubMed] [Google Scholar]
- 28.Wang TJ, Evans JC, Benjamin EJ, Levy D, LeRoy EC, Vasan RS. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation. 2003;108:977–982. doi: 10.1161/01.CIR.0000085166.44904.79. [DOI] [PubMed] [Google Scholar]
- 29.Dickstein K, Bogale N, Priori S, Auricchio A, Cleland JG, Gitt A, Limbourg T, Linde C, van Veldhuisen DJ, Brugada J. The European cardiac resynchronization therapy survey. Eur Heart J. 2009;30:2450–2460. doi: 10.1093/eurheartj/ehp359. [DOI] [PubMed] [Google Scholar]
- 30.van Veldhuisen DJ, Maass AH, Priori SG, Stolt P, van Gelder IC, Dickstein K, Swedberg K. Implementation of device therapy (cardiac resynchronization therapy and implantable cardioverter defibrillator) for patients with heart failure in Europe: changes from 2004 to 2008. Eur J Heart Fail. 2009;11:1143–1151. doi: 10.1093/eurjhf/hfp149. [DOI] [PubMed] [Google Scholar]