Abstract
Methionine aminopeptidase (MetAP) is a promising target for the development of novel antibiotics. However, many potent inhibitors of the purified enzyme failed to show significant antibacterial activity. It is uncertain which divalent metal MetAP uses as its native cofactor in bacterial cells. Herein, we describe a cell-based assay that monitors the hydrolysis of a fluorogenic substrate by overexpressed MetAP in permeabilized E. coli cells and its validation with a set of MetAP inhibitors. This cell-based assay is applicable to those cellular targets with poorly defined native cofactor, increasing the chances of identifying inhibitors that can inhibit the cellular target.
Cell-based assays have become powerful tools in identifying biologically active small molecules. Unlike biochemical assays with well-defined molecular targets, most of the current cell-based assays focus on pathways by monitoring reporter genes or phenotypic variations.1 We describe here a unique cell-based assay with Escherichia coli methionine aminopeptidase (MetAP) as the specific cellular target and its validation with a set of MetAP inhibitors by comparing the inhibition of the purified enzyme, the same enzyme in a cellular environment, and bacterial growth. This type of cell-based assay is critically important for molecular targets, such as MetAP, whose native cofactor is not clearly defined.
MetAP plays a critical role in protein maturation by catalyzing the removal of the N-terminal initiator methionine from nascent proteins.2 MetAP is a ubiquitous enzyme found in both prokaryotes and eukaryotes, and the lethal effect of MetAP gene deletion has been reported for E. coli,3 Salmonella typhimurium,4 and Saccharomyces cerevisiae.5 Therefore, MetAP is an appealing target for the development of broad-spectrum antibacterial and antifungal drugs with a novel mechanism of action.6
Divalent metal ions directly participate in the MetAP-catalyzed N-terminal methionine excision of proteins and peptides. The purified MetAP apoenzyme can be reproducibly activated by a number of divalent metal ions, such as Co(II), Mn(II), Ni(II), and Fe(II).7, 8 Several X-ray structures of MetAP depict the catalytic site as a shallow and mostly hydrophobic pocket with two metal ions situated at the bottom in a dinuclear arrangement.9 The structures also indicate that most of the inhibitors reported to date interact directly with the catalytic metal cofactor, taking advantage of strong interactions with the metal ions.10-13 However, many MetAP inhibitors with high potency on the purified enzyme failed to show significant antibacterial activity.12, 14, 15 This discrepancy is likely due to the difference in the metal cofactor utilized in biochemical assays for inhibitor screening and characterization and that used by MetAP in bacterial cells. Most in vitro MetAP assays make use of Co(II) or Mn(II) as the cofactor, as they are relatively stable and are consistently among the best activators of the enzyme. However, MetAP inhibitors with high potency against the Co(II)-form or Mn(II)-form may not inhibit MetAP in other metalloforms.10 We have recently reported that Fe(II) is the physiologically relevant metal used by MetAP in E. coli cells, and probably in other bacteria such as Bacillus.16, 17
In situations when the native cofactor is not well defined, the best way is to evaluate the inhibitory effect of compounds on the target in a cellular environment where the native cofactor is functioning. We established the cellular MetAP activity assay by overexpressing E. coli MetAP in E. coli cells and making the E. coli cells permeable to substrates and inhibitors by Ca(II) treatment.16 E. coli MetAP was overexpressed in E. coli BL21(DE3) cells.8 With the enzymatically active MetAP inside live E. coli cells, we monitored the hydrolysis of a fluorogenic substrate, methionyl aminomethylcoumarin (Met-AMC), by fluorescence (ex/em 360/460 nm) as the indication of cell permeability, when we varied the concentration of Ca(II) in Tris buffer. We found that the rate of hydrolysis reached maximum at around 200 μM of CaCl2 and maintained at that level to 10 mM of CaCl2. Therefore, we choose the concentration of Ca(II) to be 5 mM for optimal cell permeability in our cellular MetAP activity assay.
The cell-based assay was carried out on 384-well plates containing 12 serial concentrations for each inhibitor, and the highest final concentration in the assay was 1 mM. The freshly prepared suspension of cells containing the recombinant MetAP enzyme and the substrate Met-AMC were dispensed to the microplate by a Multidrop Combi reagent dispenser (Thermo Scientific, Waltham, MA). The final assay volume was 80 μl with 150 μM Met-AMC, 5 mM CaCl2, and 50 mM Tris (pH 7.5). The plates were sealed, and production of the fluorescent aminomethylcoumarin was monitored via fluorescence using a SpectraMax Gemini XPS plate reader (Molecular Devices, Sunnyvale, CA). Readings were taken at 2 min intervals for several hours at room temperature. IC50 values were calculated from the rate of substrate hydrolysis in the 2-4 h time range by non-linear regression curve fitting of percent inhibitions as a function of inhibitor concentrations.
To validate the cell-based MetAP assay, we tested a set of 31 MetAP inhibitors for their inhibition of the cellular MetAP activity and correlated the inhibition with the previously published inhibition of purified MetAP enzyme in the Fe(II)-form and inhibition of bacterial growth.10, 11 Using the Ca(II) treated cells, IC50 values were obtained for all the compounds as presented in Table S1 (supplementary material) and illustrated in Fig 1A. The results from the cellular assay underscore the importance of the catechol group (such as in 1), as decreased potency was observed for compounds with blocked dihydroxyls by methylation, or when the hydroxyl moieties are replaced by other functional groups such as amino, nitro, acetylamido, and aminocarbonyl groups (2, 5-8). The presence of the contiguous hydroxyl pair also plays a significant role in inhibition, as exclusion of one of the hydroxyl groups or their spatial separation resulted in no inhibition (3-4). While all the thiazole derivatives with intact catechol moiety (1, 9-12) displayed inhibition of intracellular Met-AMC hydrolysis, most thiophene derivatives (13-27) showed improved potency. Compounds 22 and 23 are exceptions, presumably due to the presence of bulkier groups or the amide moiety.
Figure 1.
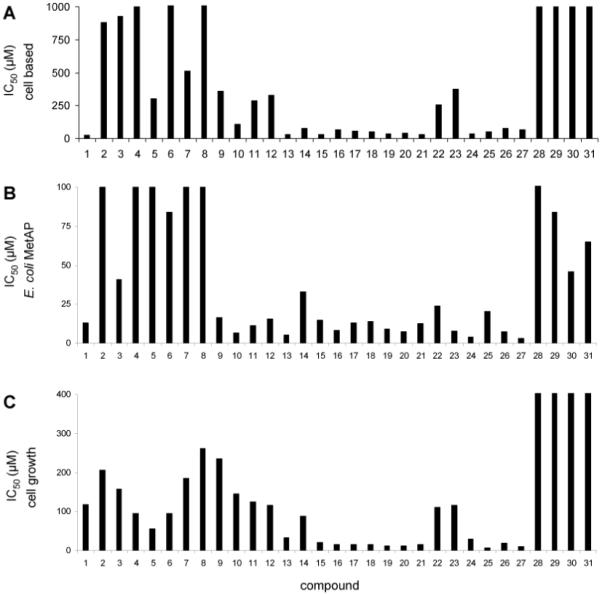
Comparison of IC50 values from the cell-based MetAP assay (A), the biochemical assay using the purified E. coli MetAP in the Fe(II)-form (B), and the cell growth assay using E. coli AS19 strain (C). Data from C were scaled to a maximum value of 400 μM, although 28-31 showed no inhibition up to 1 mM. Data from B and C obtained from references 11 and 16.
As an added advantage of the cell-based assay, these time-course plots can provide information on the time-dependent permeability of the inhibitors. Upon incubation of the Ca(II) treated cells with Met-AMC, the hydrolytic reaction continued steadily for over 8 h. (Fig. 2A). Most of the compounds tested gained access into the cells without noticeable delay, as the Met-AMC hydrolysis under different inhibitor concentrations resulted in linear product formation (Figure 2A). When the cellular assay was allowed to proceed for several hours, we noticed that compound 1 inhibited in a time-dependent fashion (Fig. 2B). This effect was more noticeable when the IC50 values were calculated from the slope of the curve at every 20 min intervals (Fig. 2C). The inhibitory potency increased over time, leveling off after 400 min, at which point the IC50 for 1 was 23 μM. If 1 were to be calculate in the same manner as with the other compounds (using the 2-4 h time range), the IC50 value for 1 would be 100 μM instead.16
Figure 2.
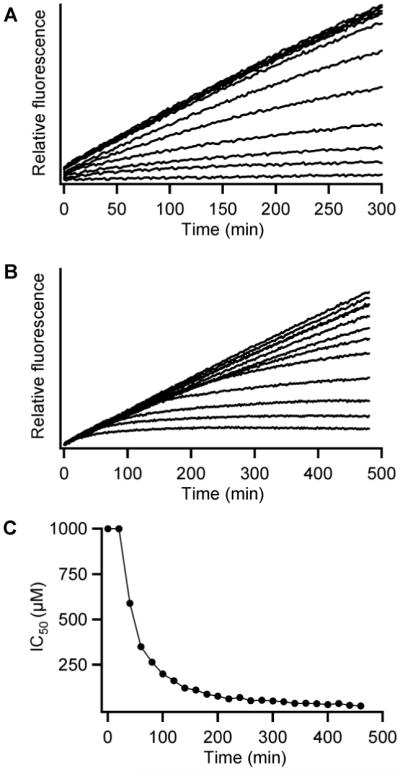
(A) Intracellular hydrolysis of the fluorogenic substrate Met-AMC in the presence of various concentrations of 16. (B) Time-dependent inhibition of intracellular Met-AMC hydrolysis at several concentrations of 1. (C) IC50 values corresponding to 1 obtained at various time points using data from Fig. 1B.
The calculated IC50 values obtained by the cell-based assay were compared with the IC50 values previously obtained from purified enzyme preparations and bacterial growth assays (Fig. 1).11 In general, there is correlation between the data from the cell-based assay and that from purified E. coli MetAP. Comparable among all three different types of assays, derivatives containing a thiophene coupled to the catechol (13-27) generally display the highest potency. Values from the cell-based assay correlate with those from the assay using the purified enzyme, because Fe(II) was used in the purified enzyme assay and Fe(II) is the likely the native cofactor in the cell-based assay.16, 17 It is interesting that compounds 2-8 did not inhibit either the purified enzyme or the cellular enzyme potently, but they affected cell growth considerably. This may indicate that these compounds hamper cell growth by a different mechanism other than MetAP inhibition. Inhibitors that are highly potent and selective towards the purified MetAP in the Mn(II)-form (28-29) or in the Co(II)-form (30-31) displayed no inhibition of the Met-AMC hydrolysis in the cellular assay. Consistently, they showed weak inhibition of the purified MetAP in the Fe(II)-form and they did not halt bacterial growth.
It is critically important that appropriate assay conditions are used during the screening process in order to obtain inhibitors that can act on the cellular target. The biochemical assay using the purified MetAP enzyme in the Fe(II)-form is predicative for inhibition of the cellular MetAP activity and inhibition of bacterial growth, because the native metal has been correctly assigned.16 However, the native cofactor for a cellular target is often not well defined. In those situations, the cell-based assay, exemplified by the cell-based MetAP assay, places the target protein with its native cofactor in a cellular environment, increasing the chances of identifying inhibitors that can inhibit the cellular target.
Supplementary Material
Chart 1.
MetAP inhibitors used for the assay validation
Acknowledgement
This work was supported by National Institutes of Health Grant R01 AI065898 (to Q.-Z. Y.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.An WF, Tolliday NJ. In: Cell-based assays for high-throughput screening. Clemons PA, Tolliday NJ, Wagner BK, editors. Humana Press; New York: 2009. [Google Scholar]
- 2.Bradshaw RA, Brickey WW, Walker KW. Trends Biochem Sci. 1998;23:263. doi: 10.1016/s0968-0004(98)01227-4. [DOI] [PubMed] [Google Scholar]
- 3.Chang SY, McGary EC, Chang S. J Bacteriol. 1989;171:4071. doi: 10.1128/jb.171.7.4071-4072.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller CG, Kukral AM, Miller JL, Movva NR. J Bacteriol. 1989;171:5215. doi: 10.1128/jb.171.9.5215-5217.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X, Chang YH. Proc Natl Acad Sci U S A. 1995;92:12357. doi: 10.1073/pnas.92.26.12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaughan MD, Sampson PB, Honek JF. Curr Med Chem. 2002;9:385. doi: 10.2174/0929867023371102. [DOI] [PubMed] [Google Scholar]
- 7.D’Souza VM, Holz RC. Biochemistry. 1999;38:11079. doi: 10.1021/bi990872h. [DOI] [PubMed] [Google Scholar]
- 8.Li JY, Chen LL, Cui YM, Luo QL, Li J, Nan FJ, Ye QZ. Biochem Biophys Res Commun. 2003;307:172. doi: 10.1016/s0006-291x(03)01144-6. [DOI] [PubMed] [Google Scholar]
- 9.Lowther WT, Matthews BW. Biochim Biophys Acta. 2000;1477:157. doi: 10.1016/s0167-4838(99)00271-x. [DOI] [PubMed] [Google Scholar]
- 10.Ye QZ, Xie SX, Huang M, Huang WJ, Lu JP, Ma ZQ. J Am Chem Soc. 2004;126:13940. doi: 10.1021/ja045864p. [DOI] [PubMed] [Google Scholar]
- 11.Wang WL, Chai SC, Huang M, He HZ, Hurley TD, Ye QZ. J Med Chem. 2008;51:6110. doi: 10.1021/jm8005788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oefner C, Douangamath A, D’Arcy A, Hafeli S, Mareque D, Mac Sweeney A, Padilla J, Pierau S, Schulz H, Thormann M, Wadman S, Dale GE. J Mol Biol. 2003;332:13. doi: 10.1016/s0022-2836(03)00862-3. [DOI] [PubMed] [Google Scholar]
- 13.Douangamath A, Dale GE, D’Arcy A, Almstetter M, Eckl R, Frutos-Hoener A, Henkel B, Illgen K, Nerdinger S, Schulz H, Mac Sweeney A, Thormann M, Treml A, Pierau S, Wadman S, Oefner C. J Med Chem. 2004;47:1325. doi: 10.1021/jm034188j. [DOI] [PubMed] [Google Scholar]
- 14.Luo QL, Li JY, Liu ZY, Chen LL, Li J, Qian Z, Shen Q, Li Y, Lushington GH, Ye QZ, Nan FJ. J Med Chem. 2003;46:2631. doi: 10.1021/jm0300532. [DOI] [PubMed] [Google Scholar]
- 15.Schiffmann R, Heine A, Klebe G, Klein CD. Angew Chem Int Ed Engl. 2005;44:3620. doi: 10.1002/anie.200500592. [DOI] [PubMed] [Google Scholar]
- 16.Chai SC, Wang WL, Ye QZ. J Biol Chem. 2008;283:26879. doi: 10.1074/jbc.M804345200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang WL, Chai SC, Ye QZ. Bioorg Med Chem Lett. 2009;19:1080. doi: 10.1016/j.bmcl.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.