Abstract
Objective
To determine if metabolite ratios at near-term age predict outcome in very low birth weight preterm infants at 18 to 24 months adjusted age.
Study Design
Thirty-six infants (birth weight ≤1510 g, gestational age ≤32 weeks) were scanned at a postmenstrual age (PMA) of 35 to 43 weeks from July 2001 to September 2003. Multivoxel proton spectroscopic data were acquired and metabolite ratios were calculated in regions of the thalamus and basal ganglia. Bayley Scales of Infant Development were assessed between 18 and 24 months corrected age.
Result
Metabolic ratios showed no significant correlation with developmental outcome. A correlation was seen between N-acetylaspartate (NAA)/choline (Ch) and PMA in thalamus and basal ganglia.
Conclusion
Metabolite ratios from near-term proton magnetic resonance spectroscopy (MRS) were not predictive of Bayley scores at 18 to 24 months adjusted age. There was a positive correlation between NAA/Ch and PMA, which supports previous work by others for the importance of developmental changes in the MRS with age.
Keywords: very low birth weight preterm infant, proton magnetic resonance spectroscopy, neurodevelopmental outcome
Introduction
Very low birth weight (VLBW, ≤1500 g) preterm infants are at an increased risk of neurological abnormalities, including poor cognitive function, decreased academic achievement, visual disability, hearing impairments and cerebral palsy (CP).1-6 At present, clinical evaluation of these infants does not provide adequate diagnostic or prognostic information. Neuroimaging has been proposed to diagnose brain injury so that appropriate medical management can be provided and to detect lesions associated with long-term neurodevelopmental disability.7 Serial ultrasound is commonly used in neonatal intensive care practices to detect intraventricular hemorrhages, ventriculomegaly, periventricular leukomalacia and other white matter abnormalities. Recently, magnetic resonance imaging (MRI) has been shown to be more sensitive than ultrasound in detecting subtle white matter abnormalities seen in preterm infants.8-10
Proton magnetic resonance spectroscopy (MRS) is a technique that acquires signals from certain detectable metabolites in selected regions of the brain. Whereas common MRI scans provide high-resolution detail of brain anatomy by using signals from water and lipids, MRS probes the inherently weaker signals of low-concentration chemicals involved in physiological processes. Because of this connection to biochemical activity, MRS has become a valuable research tool for investigating age-related development and pathogenesis of the pediatric brain, as well as an important clinical tool for the diagnosis and prognosis of trauma and disease.11-15 For in vivo brain tissue, the strongest metabolite signals are from choline (Ch), creatine (Cr) and N-acetylaspartate (NAA); the resonance for Cr also contains some contribution from phosphocreatine. As the brain develops, concentrations of these neurochemicals change, with the most rapid variations occurring during the first 2 years of life.15-18 A number of studies have found that MRS can predict the long-term neurodevelopmental outcome in term, asphyxiated newborns scanned at term age;19-25 specifically, these studies found increased lactate and decreased NAA correlated with poor developmental outcome. Few studies, however, have explored the potential of proton MRS in predicting the outcome of preterm infants. The purpose of this study was to primarily examine changes in MRS ratios of brain metabolite levels in VLBW preterm infants at near-term age and, by comparing with clinical test results, to evaluate the efficacy of these methods for predicting long-term neurodevelopmental outcome.
Methods
Patient population
At Lucile Packard Children’s Hospital at Stanford, MRI is routinely performed to assess the brain status of preterm infants at term-equivalent age and will be presented as ancillary information. The main criteria for inclusion were birth weight ≤1500 g and/or gestational age ≤32 weeks. Fifty-eight patients scanned between July 2001 and September 2003 met the inclusion criteria: birth weight ≤1510 g or gestational age ≤32 weeks, scan age between 35 and 43 postmenstrual weeks and absence of congenital brain malformations, and they had a complete neurological follow-up examination between 18 and 24 months. Only one subject was 1510 g of weight and of 30 weeks gestational age and all others were <1500 g; 21 (58%) were <1000 g. Seven infants were excluded because MRS acquisitions were incomplete. Fifteen remaining patients were excluded because of incomplete follow-up data. The final cohort was comprised of 36 patients. This study was approved with waiver of consent for retrospective review of already existing data by the Stanford University Administrative Panel on Human Subjects in Medical Research.
Magnetic resonance imaging
Magnetic resonance imaging studies were performed on a 1.5T Twin GE system. A standard (24-cm diameter, quadrature, birdcage) head coil was used. The examination included the following scans: a sagittal T1 spin-echo localizer with 500-ms repetition time (TR), 14-ms echo time (TE), 5-mm slice thickness, 1-mm gap between slices, 256 × 128 resolution, 22-cm field of view (FOV), 1 average (number of excitation, NEX) and 90° flip angle; an axial T2 fast spin-echo (FSE) with 5.6-s TR, 110-ms TE, 5-mm slice, 1-mm gap, 256 × 192, 22-cm FOV, 1 NEX, 17 echoes (echo train length, ETL) and 90° flip; a fluid-attenuated inversion recovery with 9-s TR, 125-ms TE, 2.2-s inversion time (TI), 5-mm slice, 1-mm gap, 256 × 192, 22-cm FOV, 1 NEX and 90° flip; a gradient-recalled echo with 650-ms TR, 15-ms TE, 5-mm slice, 1-mm gap, 256 × 192, 22-cm FOV, 1 NEX and 15° flip; and a diffusion-weighted imaging sequence with 9-s TR, 87-ms TE, 5-mm slice, 5-mm gap, 128 × 128, 32-cm FOV, 1 NEX, 90° flip and b-value of 750.
Review of magnetic resonance images
Magnetic resonance imaging examinations were reviewed by an experienced pediatric neuroradiologist (P.D.B.). The MRI findings were categorized by the degree of brain injury into the following four categories (labeled C1 to C4): C1, no abnormality; C2, minimal subependymal hemorrhage or mineralization, for example, grade 1 and 2 germinal matrix/intraventricular hemorrhage; C3, moderate or severe ventriculomegaly, that is, >25 to 50% ventriculocephalic ratio (for example, grade 2 intraventricular hemorrhage, post-hemorrhagic hydrocephalus or atrophy); C4, parenchymal abnormality, including evidence of injury (for example, T1, T2, or gradient-recalled echo intensity abnormalities or structural alterations) as a result of hemorrhage or ischemia (for example, grade 4 intraventricular hemorrhage or periventricular leukomalacia). For the purposes of this study, C1 and C2 were considered normal, and C3 and C4 were considered abnormal.
Magnetic resonance spectroscopy
Two axial MRS slices, one at the level of the basal ganglia and one at a supraventricular level, were acquired using a point-resolved spectroscopy technique with 1-s TR, 144-ms TE, 24-cm FOV, 16 × 16 matrix, 1-cm slice, 2.25-ml voxel size and 4:30 min acquisition time. The raw MRS data and MRI images were transferred offline to a Unix workstation (Advantage Windows, GE Healthcare, Milwaukee, Wisconsin, USA). A total of two regions of interest were analyzed, one from each of the two MRS slices. The combined left and right thalamus and basal ganglia comprised the first region of interest (Figure 1a). The cortex within the point-resolved spectroscopy box, including tissue from the frontal, parietal and occipital lobes, comprised the second region of interest (Figure 1b). The multivoxel acquisitions allowed for more versatile spatial processing: if the signal-to-noise ratio (SNR) within a single voxel was found to be insufficient for accurate quantization, the signals from adjacent voxels could be averaged to improve the SNR at the cost of reduced spatial resolution. In this way, the effective SNR could be enhanced as regions of contiguous voxels were hierarchically merged up to the boundary of the point-resolved spectroscopy box. Peak areas were determined by simple integration of the manually identified metabolite peaks using a graphical user interface (Functool, GE Healthcare). Spectra with NAA, Cre and Ch had signal-to-noise ratios of approximately 20:1, 12:1 and 12:1, respectively. The observed SNR of the lactate signal was too low for accurate analysis using our acquisition and processing methods. Myo-inositol, which is suggested to be a glial marker, was also not detectable due to the long TE (144 ms). Representative spectra are shown in Figure 2. The metabolite ratios of Ch/Cr, NAA/Cr and NAA/Ch were calculated from the relative peak areas.
Figure 1.
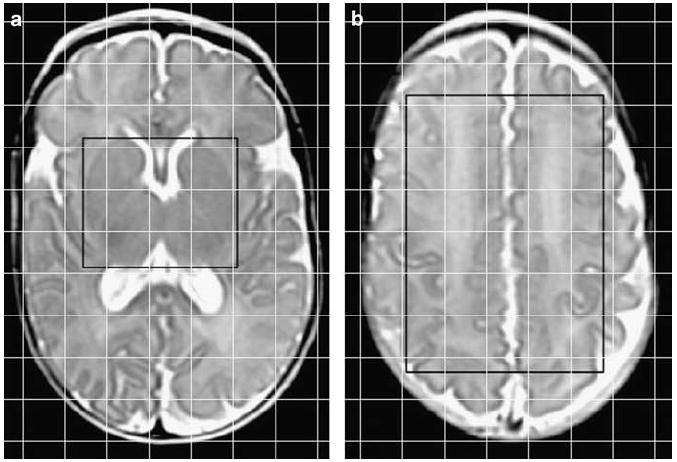
Location of proton MRS regions of interest demonstrated on axial T2 images of an infant (27-week gestation) scanned at a postmenstrual age of 40 weeks. The regions of interest are within the point-resolved spectroscopy box displayed as a black rectangle, and the 2D voxel locations are displayed as a superimposed grid of white lines. (a) The region of interest at the level of the basal ganglia was comprised of the combined left and right thalamus and basal ganglia. (b) The region of interest at a supraventricular level was comprised of the cortex including tissue from the frontal, parietal and occipital lobes.
Figure 2.
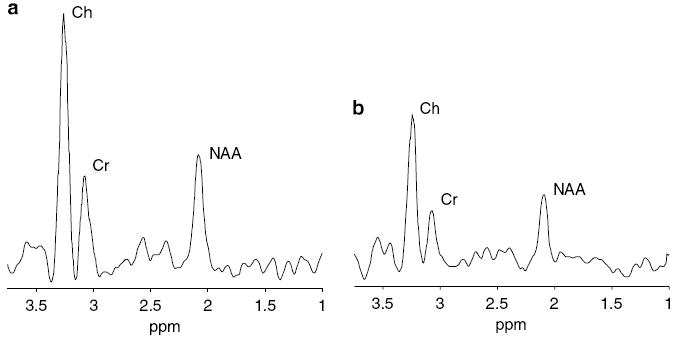
Representative proton MRS spectra of the (a) combined thalamus and basal ganglia and (b) cortex in an infant (26-week gestation) scanned at a postmenstrual age of 37 weeks. Choline (Ch), creatine (Cr) and N-acetylaspartate (NAA) peaks are labeled.
Developmental follow-up examinations
All patients were evaluated at Lucile Packard Children’s Hospital by the Development and Behavior Clinic between 18 and 24 months of age adjusted for prematurity (mean, 19.1. months). The Amiel–Tison standardized neurological examination was done to identify abnormal muscle tone, movement or reflex in at least one extremity in addition to abnormal control of movement and posture26 to diagnose CP. The Bayley Scales of Infant Development27 for the Mental Developmental Index (MDI) and Psychomotor Developmental Index (PDI) were also performed. The MDI assesses speech, language and cognitive skills, whereas the PDI evaluates gross and fine motor skills. Both developmental indices have a mean of 100 and a standard deviation of 15 points. On the basis of MDI and PDI scores for adjusted age due to prematurity, patients were categorized as normal (≥85) or abnormal (<85). The follow-up team is qualified by a NIH-supported Neonatal Research Network.
Statistical analysis
Regression analyses were used to compare metabolite ratios with (1) PMA at the time of the MRS scan, (2) gestational age and (3) birth weight. Unpaired t-tests were performed to compare metabolite ratios to developmental outcome and MRI classification. A value of P<0.05 was considered statistically significant.
Results
None of the 36 patients in our study were diagnosed with CP at 18 to 24 months. On the basis of MDI scores, 25 (69%) infants were classified as normal and 11 (31%) as abnormal. On the basis of PDI scores, 26 (76%) infants were classified as normal and 8 (24%) as abnormal. Owing to lack of participation, two infants did not receive a PDI score. Clinical data are summarized in Table 1.
Table 1.
Clinical data by MRI classification and developmental outcome
| All Patients | MRI |
MDI |
PDI |
||||
|---|---|---|---|---|---|---|---|
| Normal (C1 or C2) | Abnormal (C3 or C4) | Normal (≥85) | Abnormal (<85) | Normal (≥85) | Abnormal (<85) | ||
| Total number | 36 | 24 | 12 | 25 | 11 | 26a | 8a |
| Gender (% males) | 47% | 58% | 25% | 42% | 58% | 46% | 38% |
| mean±s.d. (range) | mean±s.d. (range) | mean±s.d. (range) | mean±s.d (range) | ||||
| Gestational age (weeks) | 28.4±2.2 (25.0–32.0) | 28.5±2.2 (25.0–32.0) | 28.1±2.2 (25.6–32.0) | 28.6±2.5 (25.0–32.0) | 28.0±1.5 (26.3–30.6) | 28.7±2.2 (25.0–32.0) | 27.2±2.0 (25.6±32.0) |
| PMA at MRS Scan (weeks) | 37.9±1.8 (35.4–43.0) | 38.0±1.9 (35.4–43.0) | 37.6±1.6 (35.6–40.3) | 37.9±1.9 (35.4–43.0) | 37.7±1.4 (36.3–40.3) | 37.7±1.7 (35.4–43.0) | 38.6±2.0 (35.6–40.9) |
| Birth weight (g) | 966±269 (463–1510) | 997±263 (665–1510) | 903±282 (463–1458) | 974±290 (463–1465) | 949±234 (582–1510) | 1015±246 (624–1465) | 741±193 (463–955) |
| Head Circ (cm)b | 25.2±2.3 | 25.3±2.2 | 25.0±2.6 | 25.2±2.4 | 25.1±2.3 | 25.7±2.0 | 23.4±2.0 |
| Length (cm)b | 35.3±3.1 | 35.9±2.8 | 34.1±3.5 | 35.3±3.3 | 35.3±3.0 | 36.2±2.6 | 32.6±3.1 |
| Apgar (1 min)b | 5.6±2.5 | 5.4±2.4 | 6.1±2.9 | 6.1±2.4 | 4.8±2.6 | 5.6±2.5 | 6.0±2.9 |
| Apgar (5 min)b | 7.7±1.9 | 7.8±1.9 | 7.6±2.0 | 8.3±1.2 | 6.7±2.5 | 7.9±1.6 | 7.3±2.9 |
Abbreviations: C, category; Circ, circumference; MDI, mental developmental index; MRI, magnetic resonance imaging; MRS, magnetic resonance spectroscopy; PDI, psychomotor developmental index; PMA, postmenstrual age.
Two patients did not receive a PDI score.
Complete data were not available for all 36 infants.
Magnetic resonance spectroscopy data were analyzed in two regions of interest. Analysis was not performed on incomplete data: that is, data acquired with the slices incorrectly positioned. Metabolite ratios could be calculated for 19 infants in the thalamus and basal ganglia and 33 infants in the cortex.
Regression analyses revealed a positive correlation (r2 = 0.31, P = 0.01) between PMA at MRS scan and NAA/Ch in the thalamus and basal ganglia. This same correlation was found when analyses were limited to children with a normal MDI (r2 = 0.35, P = 0.04) and PDI (r2 = 0.36, P = 0.02) (Figure 3). A significant correlation was not found when analyses were limited to children with an abnormal MDI or PDI. No other significant relationships were found.
Figure 3.
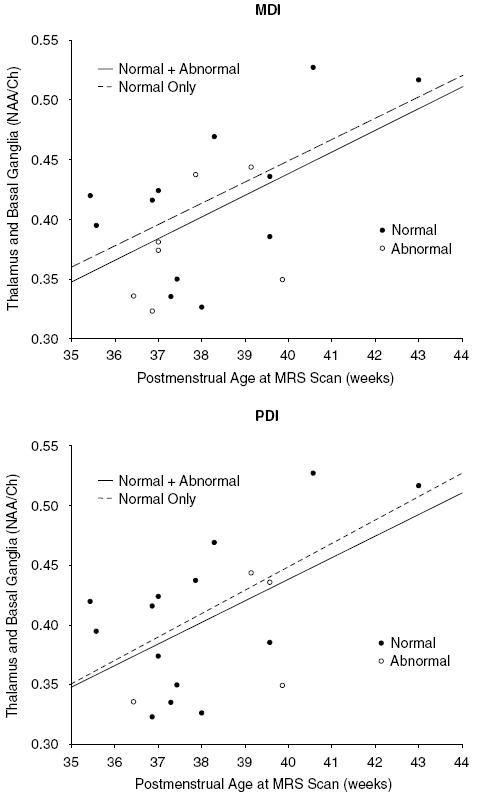
Regression plots demonstrating a positive correlation between postmenstrual age and NAA/Ch in the thalamus and basal ganglia for infants with a normal (●) and abnormal (○) MDI and PDI. Analyses were performed for: infants with either a normal or abnormal MDI (y = −0.288 + 0.018 x, r2 = 0.31, P = 0.01); infants with only a normal MDI (y = −0.259 + 0.018 x, r2 = 0.35, P = 0.04); infants with either a normal or abnormal PDI (y = −0.288 + 0.018 x, r2 = 0.31, P = 0.01); and infants with only a normal PDI (y = −0.330 + 0.019 x, r2 = 0.36, P = 0.02).
Unpaired t-tests did not demonstrate a statistically significant difference in metabolite ratios between infants with normal and abnormal developmental outcome. The ratios for both outcome groups according to the MDI and PDI are summarized in Table 2.
Table 2.
Metabolite ratios by MRI classification and developmental outcome
| Metabolite ratios in thalamus and basal ganglia |
|||||
|---|---|---|---|---|---|
| na | Ch/Cr (mean±s.d.) | NAA/Cr (mean±s.d.) | NAA/Ch (mean±s.d.) | ||
| MRI | Normal | 14 | 2.46±0.36 | 0.99±0.16 | 0.41±0.06 |
| Abnormal | 5 | 2.67±0.43 | 1.04±0.14 | 0.40±0.06 | |
| MDI | Normal | 12 | 2.47±0.30 | 1.02±0.14 | 0.42±0.06 |
| Abnormal | 7 | 2.60±0.49 | 0.98±0.19 | 0.38±0.05 | |
| PDI | Normal | 14 | 2.49±0.33 | 1.00±0.13 | 0.41±0.07 |
| Abnormal | 4 | 2.54±0.58 | 1.00±0.27 | 0.39±0.06 | |
| Metabolite ratios in cortex | |||||
| MRI | Normal | 23 | 2.50±0.42 | 1.11±0.25 | 0.45±0.11 |
| Abnormal | 10 | 2.44±0.24 | 1.09±0.16 | 0.46±0.09 | |
| MDI | Normal | 22 | 2.47±0.40 | 1.12±0.24 | 0.46±0.09 |
| Abnormal | 11 | 2.49±0.31 | 1.08±0.20 | 0.44±0.12 | |
| PDI | Normal | 24 | 2.49±0.39 | 1.15±0.23 | 0.47±0.11 |
| Abnormal | 7 | 2.50±0.22 | 1.02±0.12 | 0.41±0.05 | |
Abbreviations: Ch, choline; Cr, creatine; MDI, mental developmental index; MRI, magnetic resonance imaging; NAA, N-acetylaspartate; PDI, psychomotor developmental index.
Satisfactory metabolite spectra were not available for all infants in each region of interest.
On the basis of MR images, 24 (67%) infants were classified as normal and 12 (33%) as abnormal (Table 3). Unpaired t-tests did not demonstrate a statistically significant difference in metabolite ratios between infants with normal and abnormal MRI. The sensitivity, specificity, positive predictive value and negative predictive value of MRI in predicting MDI and PDI scores at 18 to 24 months of age adjusted for prematurity are also shown in Table 3.
Table 3.
MRI: sensitivity, specificity, positive and negative predictive value
| MRI |
||||||
|---|---|---|---|---|---|---|
| Normal | Abnormal | Sensitivity | Specificity | PPV | NPV | |
| MDI | ||||||
| Normal | 17 | 7 | 42% | 71% | 42% | 71% |
| Abnormal | 7 | 5 | ||||
| PDI | ||||||
| Normal | 19 | 7 | 63% | 73% | 42% | 86% |
| Abnormal | 3 | 5 | ||||
Abbreviations: MRI, magnetic resonance imaging; MDI, mental developmental index; PDI, psychomotor developmental index; PPV, positive predictive value; NPV, negative predictive value.
Discussion
The three metabolites measured in our study were Ch, Cr and NAA. The concentration of Ch, a component of cell membrane, reflects myelination and cell membrane turnover.28 The normal decrease in Ch during early brain development likely reflects the incorporation of Ch into macromolecules associated with myelin.15,29 The metabolite NAA, which is primarily stored and synthesized in neurons,30 increases as the brain develops. Relative decreases in NAA likely reflect decreased neuronal viability, neuronal function or neuronal loss.15 The single Cr resonance represents both Cr and phosphocreatine. Cr is converted to phosphocreatine, a high energy compound critical for maintaining cellular energy-dependent systems.15 In adults, Cr is often used as a reference metabolite based on the assumption that Cr is relatively unaffected by age or various pathologies.31
In our study, NAA/Ch in the thalamus and basal ganglia increased significantly between 35 and 43 postmenstrual weeks (Figure 3). This finding is consistent with the literature and indicates an increase in NAA and/or a decrease in Ch as a function of age. Roelants-van Rijn et al.17 reported increased NAA/Ch in both periventricular white matter and the basal ganglia in preterm infants at 32 and 41 postmenstrual weeks. Similarly, Kreis et al.16 reported a significant increase in the amount of NAA measured in the cortical gray matter, thalamus and white matter between 32 and 43 postmenstrual weeks. This same study did not find significant changes in Ch during the same developmental period. In an antenatal MRS study, Heerschap et al.18 found increased NAA between 30 and 41 weeks of gestation. Our study found the positive correlation between postmenstrual age and NAA/Ch when all infants were included, and when the analyses were limited to infants with a normal MDI and a normal PDI. A significant increase in NAA/Ch was not found when analyses were limited to infants with an abnormal MDI or an abnormal PDI. Although this result may be influenced by the limited number of children with developmental delay, it may well reflect abnormal changes in metabolite concentrations near term age in these infants. Neurodevelopmental outcome was not reported in the studies by Kreis et al.16 or Heerschap et al.,18 but Roelants-van Rijn et al.17 reported an abnormal outcome in 2 of the 40 infants in the study.
Numerous studies have found decreased NAA/Cr and/or NAA/Ch to be predictive of abnormal neuromotor outcome in term newborns with hypoxic–ischemic encephalopathy scanned at term age.19-24 These decreased ratios were measured in the basal ganglia,19,21,22,24 occipital cortex20,23 and intervascular boundary zone.21,22 However, in one study of preterm infants with a mean gestational age of 27.9±3.1, no difference in NAA/Cr or Ch/Cr was found at a postnatal age of 9.8±4.1 weeks between infants with and without white matter damage on MRI scan.32 Another study compared children younger than 2 years with developmental delay (1.5, 0.5 to 2 years; mean, range) with age-matched control subjects (1, 0.5 to 2 years), and compared children older than 2 years with developmental delay (5, 3 to 10 years) with age-matched control subjects (5.7, 3 to 10 years).29 Significant differences in NAA/Cr and Ch/Cr between children with developmental delay and age-matched control subjects were only found in the older group. In children older than 2 years with developmental delay, the NAA/ Cr ratio was decreased in frontal and parieto-occipital subcortical white matter, and the Ch/Cr ratio was increased in the parieto-occipital subcortical white matter. A future longitudinal study could allow a more accurate assessment as to whether the developmental changes in metabolites over time are abnormal in the poor outcome group. Our study is consistent with the limited data available and suggests that although NAA/Cr and/or NAA/Ch are useful in predicting outcome of asphyxiated neonates scanned at term, the ratios are not predictive of future development in VLBW preterm infants scanned near the same PMA.
Lactate accumulates during acute cerebral hypoxic–ischemic insults due to anaerobic metabolism. Numerous studies of term neonates with encephalopathy have found an association between increased lactate and poor developmental outcome.19-24 In the majority of these infants, MRS was performed within 1 week after delivery. As would be expected in these studies, lactate levels were found to be increased because injury was still acute or subacute at the time of MRS. Given the SNR of the data acquisition and the postnatal age of the infants (9.5±3.0 weeks) in our study, lactate peaks were not distinguished from the noise. In one study, however, comparing metabolite ratios of preterm infants scanned at a postnatal age of 9.8±4.1 weeks, Robertson et al.32 reported a significant difference in lactate/Cr in the periventricular area between infants with and without white matter damage observed on MRI. In our study, 12 infants had MRI abnormalities, but no infants had classic periventricular leukomalacia. Lactate peaks were not significantly above the noise in any of the infants in our study, perhaps because compared with the study by Robertson et al., the white matter damage was not as severe, the SNR was lower, fewer MRS spectra were acquired at the level of the thalamus and basal ganglia or selected regions of interest were different.
Magnetic resonance imaging did not correlate with metabolite ratios measured by MRS in our study. MRI has been shown to be more sensitive than ultrasonography in predicting white matter abnormalities.8-10 In our previous study using the same MRI classification criteria, the sensitivity, specificity, positive predictive value and negative predictive value of MRI in predicting CP at 20 months were reported as 71, 91, 50 and 96%, respectively.8 The values reported in our study at predicting normal and abnormal outcomes based on the MDI and PDI at 18–24 months adjusted age were 42, 71, 42 and 71%, and 63, 73, 42 and 86%, respectively. These differences likely reflect the differing outcome measure, CP versus Bayley developmental indices. No infants in our study were diagnosed with CP, and only 4 and 2 infants had abnormal MDI and PDI scores at least two standard deviations below the mean (score <70), respectively. Earlier studies by Cook et al.33 have shown that after removing children with CP from a population of preterm infants, MRI even at 8 years of age was not predictive of school performance.
There were several notable limitations to this retrospective study. The sample size was relatively small and few infants exhibited abnormal developmental outcome. Only one MRI/MRS scan was performed on each subject; data at multiple developmental time points would strengthen the conclusions. Because MRS scans were performed after a series of MRI scans, it was not always possible to obtain spectra from all subjects who were not compliant for the full duration of the exam. Ratios, rather than absolute concentrations,34,35 were computed from the spectra. Owing to certain factors, such as magnetic field inhomogeneity over a large volume and patient motion, it was necessary to average signals over a large region of interest to obtain sufficiently high SNR for quantitative analysis. Volumetric MRS imaging acquisition techniques36-38 for increased spatial coverage, as well as shorter echo times39 and phased-array head coils40 for an increased SNR, would improve the metabolite data. MRI data was not included in determining the accuracy of predicting long-term outcome; this study was intended to focus solely on the potential of MRS to make clinical diagnoses, whereas in the clinical setting, MRS is typically used as supplemental data to aid in clarifying MRI findings.
A normal Bayley exam at 2 years of age may not necessarily reflect normal cognitive and motor function at an older age.1 Another study of term neonates with encephalopathy found that 15% of the children considered normal at 2 years had minor neurological dysfunction and/or perceptual motor difficulties at 5 to 6 years of age.41 As a result, follow-up at a later date is necessary.
Conclusion
The ratio of NAA/Ch in the thalamus and basal ganglia increased between 35 and 43 postmenstrual weeks, possibly reflecting neuronal maturation.11 Near-term proton MRS did not predict neurodevelopmental outcome in VLBW preterm infants. Specifically, the ratios of Ch/Cr, NAA/Cr and NAA/Ch were not predictive of Bayley MDI and PDI scores at 18 to 24 months adjusted age in our patient cohort.
Acknowledgments
EMA was an American Pediatric Society/Society for Pediatric Research Program Awardee and received NIH grant HD007446. EMA also received travel award and presented this work in Poster Session at the 2006 Pediatric Academic Society Meeting. We gratefully acknowledge support from NIH Grant RR 09784 and thank Roger B Baldwin for assistance with data analysis.
References
- 1.Hack M, Taylor HG, Drotar D, Schluchter M, Cartar L, Andreias L, et al. Chronic conditions, functional limitations, and special health care needs of school-aged children born with extremely low-birth-weight in the 1990s. JAMA. 2005;294:318–325. doi: 10.1001/jama.294.3.318. [DOI] [PubMed] [Google Scholar]
- 2.Wilson-Costello D, Friedman H, Minich N, Fanaroff AA, Hack M. Improved survival rates with increased neurodevelopmental disability for extremely low birth weight infants in the 1990s. Pediatrics. 2005;115:997–1003. doi: 10.1542/peds.2004-0221. [DOI] [PubMed] [Google Scholar]
- 3.Vohr BR, Wright LL, Dusick AM, Perritt R, Poole WK, Tyson JE, et al. Center differences and outcomes of extremely low birth weight infants. Pediatrics. 2004;113:781–789. doi: 10.1542/peds.113.4.781. [DOI] [PubMed] [Google Scholar]
- 4.Hack M, Flannery DJ, Schluchter M, Cartar L, Borawski E, Klein N. Outcomes in young adulthood for very-low-birth-weight infants. N Engl J Med. 2002;346:149–157. doi: 10.1056/NEJMoa010856. [DOI] [PubMed] [Google Scholar]
- 5.Lemons JA, Bauer CR, Oh W, Korones SB, Papile LA, Stoll BJ, et al. Very low birth weight outcomes of the National Institute of Child health and human development neonatal research network, January 1995 through December 1996. Pediatrics. 2001;107:E1. doi: 10.1542/peds.107.1.e1. [DOI] [PubMed] [Google Scholar]
- 6.Vohr BR, Wright LL, Dusick AM, Mele L, Verter J, Steichen JJ, et al. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993 to 1994. Pediatrics. 2000;105:1216–1226. doi: 10.1542/peds.105.6.1216. [DOI] [PubMed] [Google Scholar]
- 7.Ment LR, Bada HS, Barnes P, Grant PE, Hirtz D, Papile LA, et al. Practice parameter: neuroimaging of the neonate: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2002;58:1726–1738. doi: 10.1212/wnl.58.12.1726. [DOI] [PubMed] [Google Scholar]
- 8.Mirmiran M, Barnes PD, Keller K, Constantinou JC, Fleisher BE, Hintz SR, et al. Neonatal brain magnetic resonance imaging before discharge is better than serial cranial ultrasound in predicting cerebral palsy in very low birth weight preterm infants. Pediatrics. 2004;114:992–998. doi: 10.1542/peds.2003-0772-L. [DOI] [PubMed] [Google Scholar]
- 9.Inder TE, Anderson NJ, Spencer C, Wells S, Volpe JJ. White matter injury in the premature infant: a comparison between serial cranial sonographic and MR findings at term. AJNR Am J Neuroradiol. 2003;24:805–809. [PMC free article] [PubMed] [Google Scholar]
- 10.Miller SP, Cozzio CC, Goldstein RB, Ferriero DM, Patridge JC, Vigneron DB, et al. Comparing the diagnosis of white matter injury in premature newborns with serial MR imaging and transfontanel ultrasonography findings. AJNR Am J Neuroradiol. 2003;24:1661–1669. [PMC free article] [PubMed] [Google Scholar]
- 11.Cecil KM, Jones BV. Magnetic resonance spectroscopy of the pediatric brain. Topics Magn Reson Imaging. 2001;12(6):435–452. doi: 10.1097/00002142-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Scarabino T, Popolizio T, Bertolino A, Salvolini U. Proton magnetic resonance spectroscopy of the brain in pediatric patients. European J Radiol. 1999;30:142–153. doi: 10.1016/s0720-048x(99)00055-8. [DOI] [PubMed] [Google Scholar]
- 13.Babikian T, Freier MC, Ashwal S, Riggs ML, Burley T, Holshouser BA. MR spectroscopy: predicting long-term neuropsychological outcome following pediatric TBI. J Magn Reson Imaging. 2006;24:801–811. doi: 10.1002/jmri.20696. [DOI] [PubMed] [Google Scholar]
- 14.Filippi CG, Ulug AM, Deck MDF, Zimmerman RD, Heier LA. Developmental delay in children: assessment with proton MR spectroscopy. Am J Neuroradiol. 2002;23:882–888. [PMC free article] [PubMed] [Google Scholar]
- 15.Moore GJ. Proton magnetic resonance spectroscopy in pediatric neuroradiology. Pediatr Radiol. 1998;28:805–814. doi: 10.1007/s002470050470. [DOI] [PubMed] [Google Scholar]
- 16.Kreis R, Hofmann L, Kuhlmann B, Boesch C, Bossi E, Hüppi PS. Brain metabolite composition during early human brain development as measured by quantitative in vivo 1H magnetic resonance spectroscopy. Magn Reson Med. 2002;48:949–958. doi: 10.1002/mrm.10304. [DOI] [PubMed] [Google Scholar]
- 17.Roelants-van Rijn AM, van der Grond J, Stigter RH, de Vries LS, Groenendaal F. Cerebral structure and metabolism and long-term outcome in small-for-gestational-age preterm neonates. Pediatr Res. 2004;56:285–290. doi: 10.1203/01.PDR.0000132751.09067.3F. [DOI] [PubMed] [Google Scholar]
- 18.Heerschap A, Kok RD, van den Berg PP. Antenatal proton MR spectroscopy of the human brain in vivo. Childs Nerv Syst. 2003;19:418–421. doi: 10.1007/s00381-003-0774-5. [DOI] [PubMed] [Google Scholar]
- 19.Khong PL, Tse C, Wong IY, Lam BC, Cheung PT, Goh WH, et al. Diffusion-weighted imaging and proton magnetic resonance spectroscopy in perinatal hypoxic-ischemic encephalopathy: association with neuromotor outcome at 18 months of age. J Child Neurol. 2004;19:872–881. doi: 10.1177/08830738040190110501. [DOI] [PubMed] [Google Scholar]
- 20.Kadri M, Shu S, Holshouser B, Deming D, Hopper A, Peverini R, et al. Proton magnetic resonance spectroscopy improves outcome prediction in perinatal CNS insults. J Perinatol. 2003;23:181–185. doi: 10.1038/sj.jp.7210913. [DOI] [PubMed] [Google Scholar]
- 21.Miller SP, Newton N, Ferriero DM, Partridge JC, Glidden DV, Barnwell A, et al. Predictors of 30-month outcome after perinatal depression: role of proton MRS and socioeconomic factors. Pediatr Res. 2002;52:71–77. doi: 10.1203/00006450-200207000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Barkovich AJ, Baranski K, Vigneron D, Partridge JC, Hallam DK, Hajnal BL, et al. Proton MR spectroscopy for the evaluation of brain injury in asphyxiated, term neonates. Am J Neuroradiol. 1999;20:1399–1405. [PMC free article] [PubMed] [Google Scholar]
- 23.Shu SK, Ashwal S, Holshouser BA, Nystrom G, Hinshaw DBJ. Prognostic value of 1H-MRS in perinatal CNS insults. Pediatr Neurol. 1997;17:309–318. doi: 10.1016/s0887-8994(97)00140-9. [DOI] [PubMed] [Google Scholar]
- 24.Groenendaal F, Veenhoven RH, van der Grond J, Jansen GH, Witkamp TD, de Vries LS. Cerebral lactate and N-acetyl-aspartate/choline ratios in asphyxiated full-term neonates demonstrated in vivo using proton magnetic resonance spectroscopy. Pediatr Res. 1994;35:148–151. doi: 10.1203/00006450-199402000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Boichot C, Walker PM, Durand C, Grimaldi M, Chapuis S, Gouyon JB, et al. Term neonate prognoses after perinatal asphyxia: contributions of MR imaging, MR spectroscopy, relaxation times, and apparent diffusion coefficients. Radiology. 2006;239:839–848. doi: 10.1148/radiol.2393050027. [DOI] [PubMed] [Google Scholar]
- 26.Amiel-Tison C. Neuromotor status. In: Taeusch H, Yogman M, editors. Follow-up management of the high-risk infant Little. Brown and Company; Boston: 1987. pp. 115–126. [Google Scholar]
- 27.Bayley N. The Bayley Scales of Infant Development II. New York Psychological Corporation; New York: 1993. [Google Scholar]
- 28.Zimmerman RA, Wang ZJ. The value of proton MR spectroscopy in pediatric metabolic brain disease. AJNR Am J Neuroradiol. 1997;18:1872–1879. [PMC free article] [PubMed] [Google Scholar]
- 29.Filippi CG, Ulug AM, Deck MD, Zimmerman RD, Heier LA. Developmental delay in children: assessment with proton MR spectroscopy. AJNR Am J Neuroradiol. 2002;23:882–888. [PMC free article] [PubMed] [Google Scholar]
- 30.Baslow MH. N-acetylaspartate in the vertebrate brain: metabolism and function. Neurochem Res. 2003;28:941–953. doi: 10.1023/a:1023250721185. [DOI] [PubMed] [Google Scholar]
- 31.Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13:129–153. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 32.Robertson NJ, Kuint J, Counsell TJ, Rutherford TA, Coutts A, Cox IJ, et al. Characterization of cerebral white matter damage in preterm infants using 1H and 31P magnetic resonance spectroscopy. J Cereb Blood Flow Metab. 2000;20:1446–1456. doi: 10.1097/00004647-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Cooke RW, Abernethy LJ. Cranial magnetic resonance imaging and school performance in very low birth weight infants in adolescence. Arch Dis Child Fetal Neonatal Ed. 1999;81:F116–F121. doi: 10.1136/fn.81.2.f116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kugel H, Roth B, Pillekamp F, Kruger K, Schulte O, von Gontard A, et al. Proton spectroscopic metabolite signal relaxation times in preterm infants: a prerequisite for quantitative spectroscopy in infant brain. J Magn Reson Imaging. 2003;17:634–640. doi: 10.1002/jmri.10315. [DOI] [PubMed] [Google Scholar]
- 35.Cheong JL, Cady EB, Penrice J, Wyatt JS, Cox IJ, Robertson NJ. Proton MR spectroscopy in neonates with perinatal cerebral hypoxic-ischemic injury: metabolite peak-area ratios, relaxation times, and absolute concentrations. Radiology. 2006;239(3):839–848. [PMC free article] [PubMed] [Google Scholar]
- 36.Posse S, Tedeschi G, Risinger R, Ogg R, Le Bihan D. High speed 1H spectroscopic imaging in human brain by echo planar spatial-spectral encoding. Magn Reson Med. 1995;33:34–40. doi: 10.1002/mrm.1910330106. [DOI] [PubMed] [Google Scholar]
- 37.Adalsteinsson E, Irarrazabal P, Topp S, Meyer C, Macovski A, Spielman DM. Volumetric spectroscopic imaging with spiral-based k-space trajectories. Magn Reson Med. 1998;39:889–898. doi: 10.1002/mrm.1910390606. [DOI] [PubMed] [Google Scholar]
- 38.Vigneron DB, Barkovich J, Noworolski SM, von dem Bussche M, Henry RG, Lu Y, et al. Three-dimensional proton MR spectroscopic imaging of premature and term neonates. Am J Neuroradiol. 2001;22:1422–1424. [PMC free article] [PubMed] [Google Scholar]
- 39.Kim DH, Barkovich AJ, Vigneron DB. Short echo time MR spectroscopic imaging for neonatal pediatric imaging. Am J Neuroradiol. 2006;27:1370–1372. [PMC free article] [PubMed] [Google Scholar]
- 40.Wald LL, Moyher SE, Day MR, Nelson SJ, Vigneron DB. Proton spectroscopic imaging of the human brain using phased array detectors. Magn Reson Med. 1995;34:440–445. doi: 10.1002/mrm.1910340322. [DOI] [PubMed] [Google Scholar]
- 41.Barnett A, Mercuri E, Rutherford M, Haataja L, Frisone MF, Henderson S, et al. Neurological and perceptual-motor outcome at 5 to 6 years of age in children with neonatal encephalopathy: relationship with neonatal brain MRI. Neuropediatrics. 2002;33:242–248. doi: 10.1055/s-2002-36737. [DOI] [PubMed] [Google Scholar]


