Abstract
Background
The course of mild to moderate persistent asthma in children is not clearly established.
Objective
To determine the rate and predictors for remitting, periodic, and persistent asthma in adolescence.
Methods
The Childhood Asthma Management Program (CAMP) was a 4.3-year randomized, double-masked, multicenter trial in children with mild to moderate persistent asthma that compared continuous therapy with either budesonide or nedocromil, each to placebo, followed by 4 years observational follow-up period. Asthma activity during the observation period included remitting (no asthma activity in the last year), persistent (asthma activity in every quarter), and periodic asthma (neither remitting nor persistent).
Results
Asthma was identified as remitting in 6%, periodic in 39%, and persistent in 55% of the 909 participants, with no effect noted from earlier anti-inflammatory treatment during the CAMP trial. Within all three asthma activity categories, improvements in airway hyperresponsiveness, eosinophilia, and asthma morbidity were observed over time. Features at entry into CAMP associated with remitting vs. persistent asthma were lack of allergen sensitization and exposure to indoor allergens [OR=3.23, p<0.001], milder asthma [OR=2.01, p=0.03], older age [OR=1.23, p=0.01], less airway hyperresponsiveness (higher log methacholine FEV1 PC20 [OR=1.39, p=0.03]), higher pre-bronchodilator FEV1 % predicted [OR=1.05, p=0.02], and lower FVC % predicted [OR=0.96, p=0.04].
Conclusion
Remission of asthma in adolescence is infrequent and not impacted by 4 years of anti-inflammatory controller therapy. Factors such as sensitization and exposure, low lung function, and airway greater hyperresponsiveness decrease the likelihood of remitting asthma.
Keywords: Remission, Natural history, Persistent asthma
INTRODUCTION
Current asthma prevalence is higher for children than for adults,1 suggesting that children with asthma may “outgrow” their disease. Variable remission rates of 14 to 75% have been reported in unselected population-based or pre-selected cohorts.2–17 This variability could be largely due to a lack of a standard definition for asthma remission and the heterogeneity of the populations from which the estimates were derived. To these questions parents commonly ask, “Will this child with persistent asthma outgrow his/her disease?” and “if so, what can be done to help this?”, the response is not as straightforward and the answer may even depend on the severity or nature of the asthma. In fact, there seems to be no consensus as to how long asthma controller medications should be used. In addition, the impact of long-term controller therapy on remission is also poorly understood.
The Childhood Asthma Management Program (CAMP) is the largest and longest asthma clinical trial in children with mild to moderate asthma. CAMP was a 4.3 year, randomized, double-masked trial that compared budesonide or nedocromil, each versus placebo, followed by 4 additional years of observation. The results of the clinical trial and follow-up phase have been published.18–19 Briefly, improvements in various measures of asthma control were found with budesonide or nedocromil over placebo during the intervention phase, but the trends in asthma morbidity and other physiologic parameters were no longer different between the initial active treatment vs. placebo groups when the study medications were discontinued and the children were followed for an additional 4 years of observation while their physicians managed care.19 Although CAMP was initially intended to be a clinical trial, it has now evolved into an observational “natural history” study of mild to moderate persistent asthma. From this well characterized cohort, we determined the rates of remitting, periodic (episodic), and persistent asthma and identified features associated with these asthma outcomes.
METHODS
CAMP randomized 1041 children 5–12 years of age from 1993 to 1995, after a 2–4 month screening phase, to a 4–6 year treatment phase consisting of budesonide (200 mcg twice daily), nedocromil (8 mg twice daily), or placebo. The CAMP trial was followed by a 4-month washout after discontinuation of treatment which was completed in 1999 and a 4-year observational phase completed in 2003 in which asthma treatment was delegated to a participant’s personal physician. CAMP eligibility included a history of asthma for at least 6 months; presence of symptoms or low peak flow readings on at least 8 of the 28 day run in period, pre-bronchodilator FEV1 ≥65% predicted, airway hyperresponsiveness (AHR) using methacholine PC20 ≤ 12.5 mg/ml, and an overall assessment by clinic staff at baseline of mild or moderate asthma (i.e. without a pre-specified set of criteria).18–20
Questionnaire information (three times a year during trial and four times a year during the observational phase including 2 phone interviews), spirometric lung function measurement (three times a year during the clinical trial and twice a year in the observational phase) and methacholine challenge (yearly in both phases) were obtained. Percutaneous skin testing using a standard battery of aeroallergens20 and locally important pollen and fungal spores, and measurements of serum IgE and circulating eosinophil count were obtained on average every 4 years. At the start of CAMP, indoor allergen exposures were ascertained from the environmental survey and household dust sampling for major indoor allergens (cat, dog, mite, cockroach, and mold).21 Significant exposure was considered if either environmental survey was positive or house dust analysis levels exceeded specified values for the following: Fel d 1 >8 μg per gram of dust collected; Can f 1 >10 μg/g; Der p 1 + Der f 1 >2 μg/g; and Bla g 1 >2 U/g; and mold plate from house dust >25,000 colonies.
Definitions of the course of asthma are based on reported asthma activity during the 4-year observational phase. Asthma activity was defined to occur if any of the following were present during the observational phase: contact with local medical provider, school absence, emergency department (ED) visit, and hospitalization due to asthma; presence of wheezing or any other exercise-related symptoms; or use of rescue or controller medications. Remitting asthma is defined as absence of any asthma activity at the last 4 encounters, approximately within the last year of observation. Persistent asthma includes participants with asthma activity reported at every encounter in the observational phase. Periodic asthma includes participants who had neither remitting nor persistent asthma. Participants included had at least 8 clinic or telephone encounters in the observational phase.
CAMP was approved by the Institutional Review Board of all study sites and monitored by a Data and Safety Monitoring Board appointed by the National Heart Lung and Blood Institute. Informed consent and assent were obtained from all participants and their guardians.
Statistical analysis
The statistical significance of comparisons of characteristics at enrollment (baseline) across the three patterns of asthma groups (remitting, periodic, and persistent) as determined from p-values derived from either chi-square tests (for categorical variables), Fisher’s exact test (for categorical variables with small expected numbers: race, maternal and paternal asthma, and food allergy), analysis of variance (for normally-distributed continuous variables), or Kruskal-Wallis tests (for variables that were not normally distributed: duration of asthma, age at diagnosis, number of positive skin tests, bronchodilator reversibility, serum IgE, and eosinophil count). P-values were also calculated to compare enrollment characteristics for the following pairs: remitting vs. periodic; remitting vs. persistent; and periodic vs. persistent using the same methods as above except that the Wilcoxon ranksum test was used for variables that were not normally distributed. For subsequent analyses, methacholine PC20 and IgE were log transformed to enhance symmetry of their distributions.
The trends with time across the three patterns of asthma groups are presented as the adjusted mean values of annual prednisone course rate, annual urgent care visit rate, pre-bronchodilator percent of predicted FEV1, pre-bronchodilator FEV1/FVC, bronchodilator reversibility (%), log FEV1 PC20, total eosinophil count, and log IgE. The adjusted means22 were least-squares means derived from multiple linear regression models for each continuous outcome regressed on asthma group, follow-up time, asthma group by follow-up time interaction terms, and age at randomization, race or ethnic group (African American, Hispanic, or other; 2 indicator variables), sex, clinic (7 indicator variables), duration of asthma at randomization (at least 7 years, less than 3 years, or other; 2 indicator variables), and skin test reactivity at randomization (any reactivity vs none). The number of time points available varied: 24 time points for prednisone course and urgent care visit rates, 15 time points for pre-bronchodilator percent of predicted FEV1, pre-bronchodilator FEV1/FVC, and bronchodilator reversibility (%), 10 time points for FEV1 PC20, and 4 time points for total eosinophil count and log IgE. The statistical significance of whether the differences in the time course of each outcome measure differed among pairwise comparisons of asthma groups (remitting vs. periodic, remitting vs. persistent, and periodic vs. persistent) was determined from F-tests with from 4 to 25 degrees of freedom (depending on the number of available time points) of the asthma group indicator plus the interaction terms between the asthma group indicator variable and follow-up time indicator variables, using generalized estimating equations with robust variance estimation to allow for within-individual repeated measures. The test of the null hypothesis of identical asthma patterns over time between groups required both main effects and the pattern group by time interaction to be zero. To increase the robustness of the p-values, ranks of the outcome measures were used in the linear regression models.23
Backwards stepwise multinomial logistic regression analysis was used to identify significant predictors of the three patterns of asthma groups (remitting vs. periodic, remitting vs. persistent, and periodic vs. persistent) from among candidate predictors at enrollment: age at randomization (years), race or ethnic group (African American, Hispanic, or other; 2 indicator variables), sex, treatment group (2 indicator variables), BMI (kg/m2), age at symptom onset (years), age at diagnosis (years), smoke exposure, hayfever, eczema, food allergies, indoor allergen sensitivity/exposure, paternal asthma, maternal asthma, asthma severity, pre-bronchodilator percent of predicted FEV1, pre-bronchodilator percent of predicted FVC, pre-bronchodilator FEV1/FVC, bronchodilator reversibility (%), log FEV1 PC20, eosinophil count (at or above median vs. below), and IgE (at or above median vs. below).24 The p-value for elimination from the model was p<0.05 and racial/ethnic group, sex, and treatment group were forced to be included in the model. The multinomial model estimated conditional odds of each asthma pattern category relative to the persistent asthma reference category for each candidate predictor at enrollment.
All analyses were performed using SAS statistical software (version 9, SAS Institute, Cary, NC) 25, except for the multinomial logistic regression analysis which was performed using Stata (Release 10.0, Stata Corporation, College Station, TX).24 All p-values were 2-sided and were considered to be statistically significant if P <0.05.
RESULTS
909 of the 1041 children initially enrolled in the CAMP trial (86%) had sufficient data for this analysis (i.e. at least 8 clinic or telephone contacts in the 4-year observational phase). The mean duration of the CAMP trial and observational phase was 9.3 (range 7.0 to 10.0) years.
In at least one encounter in the 4-year observational phase, 95% of participants reported wheezing and beta-agonist rescue use; 85% had exercise-induced symptoms; 68% used at least one controller medication; 37% required at least one oral corticosteroid course; 52% incurred school absences due to asthma; 22% had ED or urgent care visits; and 4% were hospitalized due to asthma.
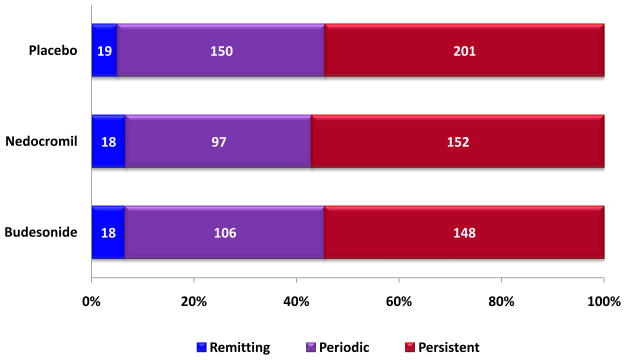
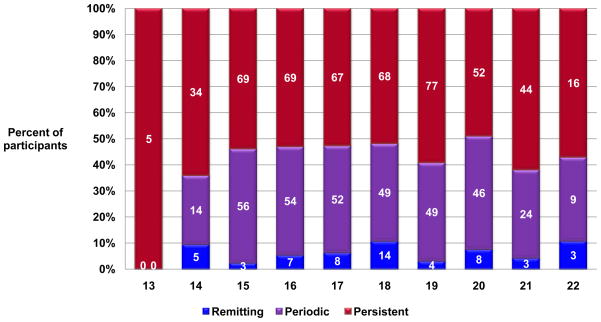
Based on our definitions of the natural course of asthma, 6% had remitting asthma, 39% had periodic asthma, and 55% had persistent asthma, with no difference by original CAMP treatments of budesonide, nedocromil, or placebo (Figure 1). Only 3 participants had no asthma activity throughout the observational phase. The mean ages of the remitting (18.3±2.1 years), periodic (18.1±2.1), and persistent groups (18.1±2.2) at the end of the study were comparable (p=0.83). There was no overrepresentation of participants with remitting asthma among the older adolescents, although the numbers of subjects in early and late adolescent years were low (Figure 2).
Figure 1.
Percentages of remitting, periodic, and persistent asthma evaluated in the follow up phase in CAMP were similar for the budesonide, nedocromil, and placebo treated groups.
Figure 2.
Distribution of CAMP participants with remitting [blue], periodic [purple], and persistent [red]) course by age at the end of the observational phase (N = the number within the bars). The percentage of participants having remitting asthma did not increase with age, although the numbers of older adolescents were low.
Differential features of the asthma activity groups before or at randomization
The cohort is 60% male, mostly having a diagnosis of asthma at preschool age, largely atopic based on presence of any positive skin test at entry, and on average having no significant evidence of airflow limitation based on spirometric measures of lung function, yet with moderate to severe AHR. Features at baseline that significantly differed across the three asthma course groups were number of positive skin tests, being both sensitized and exposed to indoor allergens, eczema status, paternal history of asthma, asthma severity, spirometric lung function measures, bronchodilator reversibility, methacholine responsiveness, serum IgE level, and circulating eosinophil count (Table 1).
Table 1.
Comparisons of baseline characteristics of the remitting, periodic, and persistent asthma groups
| P value* |
|||||||
|---|---|---|---|---|---|---|---|
| Characteristics | Remitting (N=55) | Periodic (N=353) | Persistent (N=501) | P-value* | Remitting vs. Periodic | Remitting vs. Persistent | Periodic vs. Persistent |
| Age (years) | 9.0 ± 2.1 | 8.9 ± 2.0 | 8.9 ± 2.2 | 0.84 | 0.68 | 0.59 | 0.76 |
| Gender (% male) | 61.8% | 61.2% | 59.9% | 0.91 | 0.93 | 0.78 | 0.70 |
| Ethnicity | 0.40 | 0.11 | 0.15 | 0.97 | |||
| White | 72.7% | 68.3% | 69.5% | ||||
| Black | 7.3% | 15.0% | 14.0% | ||||
| Hispanic | 5.5% | 9.6% | 9.2% | ||||
| Other | 14.6% | 7.1% | 7.4% | ||||
| Duration of asthma (years) | 4.6 (2.8–7.8) | 4.5 (2.7–6.4) | 5.0 (3.2–6.9) | 0.12 | 0.50 | 0.84 | 0.04 |
| Age at diagnosis (years) | 4.0 (1.6–5.1) | 4.0 (2.0–6.0) | 3.0 (2.0–5.0) | 0.19 | 0.65 | 0.68 | 0.07 |
| At least one positive skin test (%) | 78.2% | 83.0% | 92.0% | <0.0001 | 0.38 | 0.001 | <0.0001 |
| Number of positive skin tests | 4 (1–6) | 4 (1–7) | 5 (3–9) | <0.0001 | 0.78 | 0.01 | <0.0001 |
| Sensitive and exposed† to indoor allergens | 47.3% | 64.6% | 77.1% | <0.0001 | 0.01 | <0.0001 | <0.0001 |
| Eczema (%) | 20.0% | 24.1% | 31.5% | 0.02 | 0.51 | 0.08 | 0.02 |
| Hayfever (%) | 52.7% | 51.6% | 53.7% | 0.82 | 0.87 | 0.89 | 0.53 |
| Food allergy (%) | 16.4% | 15.9% | 20.6% | 0.21 | 1.0 | 0.60 | 0.08 |
| Asthma in mother (%) | 21.8% | 26.3% | 26.8% | 0.77 | 0.62 | 0.52 | 0.87 |
| Asthma in father (%) | 13.5% | 16.6% | 25.3% | 0.005 | 0.69 | 0.06 | 0.004 |
| Asthma severity rating | <0.001 | 0.17 | 0.002 | <0.001 | |||
| Mild | 35 (63.6%) | 190 (53.8%) | 210 (41.9%) | ||||
| Moderate | 20 (36.4%) | 163 (46.2%) | 291 (58.1%) | ||||
| Lung function | |||||||
| Pre-bronchodilator FEV1 (% predicted) | 95.9 ± 12.5 | 96.3 ± 13.5 | 91.3 ± 14.4 | <0.0001 | 0.82 | 0.03 | <0.0001 |
| Post- bronchodilator FEV1 | 102.3 ± 11.8 | 103.8 ± 12.5 | 102.0 ± 12.9 | 0.11 | 0.41 | 0.84 | 0.04 |
| Pre-bronchodilator FVC (% predicted) | 103.0 ± 11.7 | 104.0 ± 12.7 | 103.7 ± 13.3 | 0.86 | 0.59 | 0.71 | 0.76 |
| Post-bronchodilator FVC (% predicted) | 104.4 ± 12.8 | 105.3 ± 12.3 | 106.8 ± 12.7 | 0.12 | 0.63 | 0.18 | 0.07 |
| Pre-bronchodilator FEV1/FVC | 82.6 ± 8.1 | 81.8 ± 7.4 | 77.9 ± 8.6 | <0.0001 | 0.49 | <0.001 | <0.0001 |
| Post-bronchodilator FEV1/FVC | 86.9 ± 6.5 | 87.1± 5.8 | 84.4 ± 6.7 | <0.0001 | 0.83 | 0.01 | <0.0001 |
| Bronchodilator reversibility (%) | 5.4 (3.0–9.7) | 6.8 (3.5–11.6) | 10.4 (5.5– 17.5) | <0.0001 | 0.14 | <0.0001 | <0.0001 |
| Methacholine FEV1 PC20 (geometric mean mg/ml) | 1.8 ± 3.0 | 1.5 ± 3.1 | 0.8 ± 3.1 | <0.0001 | 0.29 | <0.0001 | <0.0001 |
| Biomarkers | |||||||
| Serum IgE (ng/ml) | 261 (86–883) | 349 (111–970) | 671 (267–1610) | <0.0001 | 0.43 | <0.001 | <0.0001 |
| % at or above median (433 ng/ml) | 35.9% | 39.0% | 61.1% | <0.0001 | 0.66 | <0.001 | <0.0001 |
| Eosinophil count (cells/μl) | 271 (194–546) | 306 (176–539) | 471 (261–739) | <0.0001 | 0.92 | 0.002 | <0.0001 |
| % at or above median (400 cells/μl) | 37.0% | 41.9% | 59.8% | <0.0001 | 0.50 | 0.001 | <0.0001 |
Definitions: Remitting asthma: no asthma activity during at least the last 4 consecutive visits; Persistent asthma: At least one asthma symptom at every visit in the observational phase; Periodic asthma: Patients not included in either the remitting or persistent category. Participants must have at least 8 visits in the observational phase
Values are means ± SD for age at randomization and lung function measures (except bronchodilator reversibility), medians (IQR) for duration of asthma, age at diagnosis, number of positive skin tests, bronchodilator reversibility, serum IgE, and eosinophil count, or percentages.
P-values derived from chi-square tests (Fisher’s Exact test for small expected numbers), ANOVA for normally distributed continuous variables, or Wilcoxon rank sum test for not normally distributed continuous variables (Kruskal-Wallis test for comparison across three asthma groups).
The criteria for significant allergen exposure are: cat: >8,000 ng/g (of dust sample) or cat allowed inside the home; dog: >10,000 ng/g (of dust sample) or dog allowed inside the home; mite: >2,000 ng/g (of dust sample); cockroach: >2 U/g (of dust sample) or cockroaches observed in the home; mold: >25,000 colonies or mold observed on any surface of the home.
Pairwise comparison
The remitting and periodic groups shared comparable features, except that fewer participants in the remitting group were sensitized and exposed to indoor allergens (p=0.01) (Table 1).
Compared to the persistent group, participants in the remitting group had significantly lower rates of having any sensitization, being sensitized and exposed, having moderate asthma, and having serum IgE levels or circulating eosinophil counts above the corresponding median levels. In addition, the remitting group had significantly fewer number of positive skin tests, better spirometric lung function measures, and lower bronchodilator reversibility, methacholine responsiveness, serum IgE, and circulating eosinophil count compared to the persistent group (Table 1).
Similar features that significantly differentiated between the remitting and persistent groups also differentiated between the periodic and the persistent group. Additional factors which were significantly different between the periodic and persistent groups included: duration of asthma, lower percentage of participants with eczema or fathers with asthma, and higher postbronchodilator FEV1 % predicted (Table 1).
Differential features of the asthma activity groups over the course of the treatment and follow up phase
Clinical course
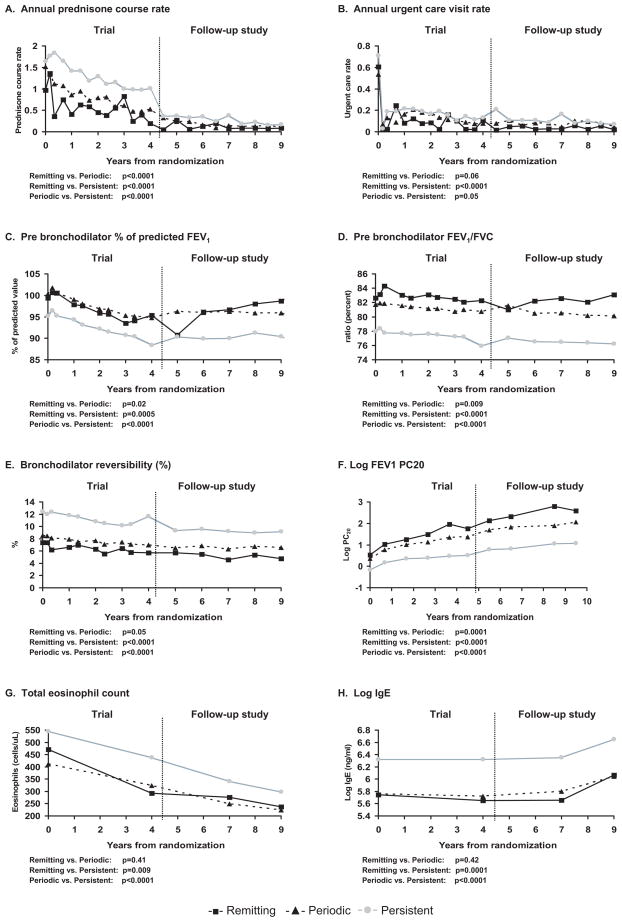
During the clinical trial phase, the remitting and periodic groups compared to the persistent group had lower rate of prednisone courses (33.8±51.5 vs. 58.5±76.3 vs. 88.8±112 prednisone courses/100 person years, respectively, p<0.001), and rates of ED visits (25.5% vs. 28.6%. vs. 35.7%, respectively, p=0.01) and hospital admissions for asthma (7.3% vs. 8.5% vs. 18.2%, respectively, p<0.001). In addition, lower rates of these events were found during the follow up phase compared to the clinical trial phase for the 3 groups (Figure 3a,b).
Figure 3.
The figures present the adjusted mean values for each continuous outcome measure: a: annual prednisone course rate; b: annual urgent care rate; c: prebronchodilator FEV1 % predicted; d: prebronchodilator FEV1/FVC; e: bronchodilator reversibility, f: log methacholine FEV1 PC20; g: total eosinophil count; and h: serum IgE in the remitting ( ), periodic (
), periodic ( ), persistent (
), persistent ( ) asthma groups during 9 years of follow-up after randomization in the CAMP trial.
) asthma groups during 9 years of follow-up after randomization in the CAMP trial.
Similarly there was a lower requirement for controller medication use in the follow up phase. Data at the last follow up visit noted that 0, 11% and 64% of remitting, periodic, and persistent groups, respectively, required daily asthma controller therapy, largely from inhaled corticosteroid use with the majority on combination therapy with a long-acting agonist, leukotriene receptor antagonist, or theophylline (eTable 1).
Lung function measurements
At randomization, the remitting and periodic groups already showed significantly higher FEV1 % predicted, FEV1/FVC ratio, methacholine FEV1 PC20, and lower BD response (Table 1 and Figure 3c–f) compared to the persistent group. The persistent group during both the trial and the follow up phases had the poorest lung function.
During the trial phase a reduction in pre-bronchodilator FEV1 % predicted was seen in all 3 activity groups, but then a plateau for the 3 groups was evident during the observational phase (Figure 3c). There was a difference in the trend in FEV1 % predicted between the remitting vs. periodic (p=0.02), remitting vs. persistent (p=0.0005), and periodic vs. persistent (p<0.0001) groups.
With respect to the FEV1/FVC ratio, there was an apparent steady drop in the 3 groups even through the observational phase (Figure 3d) with significant differences with pair-wise comparisons between the remitting vs. periodic (p=0.009), remitting vs. persistent (p<0.0001), and periodic vs. persistent (p<0.0001).
A similar gradual drop in trend for bronchodilator response was demonstrated by the 3 groups over time, with differences found between remitting vs. persistent (p<0.0001) and periodic vs. persistent (p<0.0001), but not remitting vs. periodic (p=0.05) (Figure 3e).
There were improvements in methacholine FEV1 PC20 (Figure 3f) over time, both during the trial and follow up phase, for all 3 groups. The remitting group had the highest methacholine FEV1 PC20 while the persistent group had the lowest methacholine FEV1 PC20 across all time periods (p<0.001 for differences). By the end of the study, 50% of the remitters and only 35% and 15% of the periodic and persistent groups, respectively, had a methacholine FEV1 PC20 considered within the normal range (≥ 25 mg/ml) (p<0.001).
Biologic markers
A reduction in circulating eosinophil counts from the beginning of the trial to the follow up phase was similar for all 3 activity groups. Log serum IgE levels remained steady for all activity groups during the trial and initial part of the follow up phase, but increased by the end of observation. The remitting and periodic groups had similar circulating eosinophil counts (p=0.41) and total serum IgE (p=0.42) across all time periods, but each lower than those for the persistent group (p<0.01) for both pair-wise comparisons (Fig 3g–h).
Predictors of remitting asthma using pre-treatment characteristics
A backwards multinomial logistic regression analysis was used to identify significant baseline predictors of the three asthma activity groups. Children with mild asthma were more likely to have a remitting vs. persistent asthma [OR (95% confidence interval): 2.01 (1.08, 3.74)] or periodic vs. persistent asthma [OR (95% confidence interval): 1.37, (1.02, 1.85)]. Those who were not sensitized and exposed to relevant indoor allergens at enrollment had 3 times the odds of having remitting vs. persistent asthma [OR (95% confidence interval): 3.23 (1.69, 6.25)]; and more likely to have periodic vs. persistent asthma [OR (95% confidence interval): 1.37, (1.02, 1.85)]. In addition, those with serum IgE values below the median of 502 ng/mL, compared to those at or above the median value, had 2 fold greater odds of periodic vs. persistent asthma [OR (95% confidence interval): 2.08 (1.49, 2.86)], but not remitting vs. persistent asthma (p=0.10). Older age at randomization was also associated with increased odds of remitting vs. persistent [OR (95% confidence interval): 1.23 (1.05, 1.43)] and periodic vs. persistent asthma [OR (95% confidence interval): 1.11 (1.03 1.19)]. Higher baseline pre-bronchodilator FEV1 % predicted was associated with increased odds of remitting vs. persistent [OR (95% confidence interval): 1.05 (1.01, 1.09)], or periodic vs. persistent [OR (95% confidence interval): 1.05 (1.03, 1.07)] while lower pre-bronchodilator FVC % predicted was associated with increased odds of remitting vs. persistent asthma [OR (95% confidence interval): 0.96 (0.92, 1.00)] or periodic vs. persistent asthma [OR (95% confidence interval): 0.96 (0.95, 0.98)]. Lastly, higher methacholine FEV1 log PC20 was associated with increased odds of remitting vs. persistent asthma [OR (95% confidence interval): 1.39 (1.03, 1.87)] and periodic vs. persistent asthma [OR (95% confidence interval): 1.24 (1.07, 1.43)].
DISCUSSION
This extended analysis of the clinical course of children with mild to moderate persistent asthma in the CAMP cohort revealed important observations on remitting asthma during adolescence. While the course of asthma in this cohort is one of an overall decrease in morbidity over 7 to 10 years of follow up19, only 1 out of 16 children is likely to remit for at least one year. Further, remitting asthma is not influenced by long-term anti-inflammatory treatment, but is associated with not being sensitized and exposed to indoor allergens, milder AHR, less airflow limitation, and older age (Tables 1 and 2).
Table 2.
Baseline predictors of remitting, periodic, or persistent asthma in childhood (N=884)
| Remitting vs. Persistent | Periodic vs. Persistent | |||
|---|---|---|---|---|
| Baseline measures | Odds Ratio (95% Confidence Interval) | P-value | Odds Ratio (95% Confidence Interval) | P-value |
| Mild vs. moderate asthma | 2.01 (1.08, 3.74) | 0.03 | 1.37 (1.02, 1.85) | 0.04 |
| Not sensitive or exposed to any indoor allergen* | 3.23 (1.69, 6.25) | <0.001 | 1.49 (1.05, 2.08) | 0.02 |
| IgE<502 ng/mL (median) vs. ≥ 502 ng/mL | 1.75 (0.90, 3.45) | 0.10 | 2.08 (1.49, 2.86) | <0.001 |
| Age at randomization (years) | 1.23 (1.05, 1.43) | 0.01 | 1.11 (1.03 1.19) | 0.01 |
| Pre-BD FEV1 (% predicted) | 1.05 (1.01, 1.09) | 0.02 | 1.05 (1.03, 1.07) | <0.001 |
| Pre-BD FVC (% predicted) | 0.96 (0.92, 1.00) | 0.04 | 0.96 (0.95, 0.98) | <0.001 |
| log methacholine FEV1 PC20 (mg/ml) | 1.39 (1.03, 1.87) | 0.03 | 1.24 (1.07, 1.43) | 0.003 |
Backwards multinomial logistic regression model included candidate baseline predictors: height, weight, BMI, age at randomization, age at symptom onset, age at diagnosis, household smoking exposure, history of hayfever, eczema, and food allergies, mother or father has asthma, mild vs. moderate asthma, pre-bronchodilator FEV1 and FVC in % predicted, log methacholine PC20, serum IgE, being sensitized and exposed to indoor allergens, and peripheral blood eosinophils. The model was forced to include terms for gender, race, and treatment group (p>0.05 results not shown in the table).
Perennial allergens include cat, dog, dust mite, cockroach, and mold.
The CAMP long-term follow-up noted less frequent remissions than other reports.2–17 Reasons for this difference may be attributable to differences in the definition of remission (using clinical with or without physiologic measurements), age and duration of follow-up, retention, and inherent differences in cohort characteristics (i.e. other studies had unselected cohorts largely composed of mild intermittent asthma). The strength and reliability of the present study derive from its multi-center composition, large sample size of children with documented mild to moderate persistent asthma at enrollment, high retention rate of 90% over 7 to 10 years, and comprehensiveness of data collection. Interviews were conducted 4 times per year in contrast to other studies conducted years apart. Frequent and stringent data collection reduced the potential for recall bias in reporting of information.
Controller therapy for persistent asthma has an immediate impact at alleviating symptoms, improving lung function, markers of inflammation, and quality of life, and reducing morbidity during the period of treatment18–19, 26, but probably not progression of airflow limitation over a longer period27,28 or the development of persistent asthma in very young children.29 Our observation that equal percentages of participants in the inhaled corticosteroid, nedocromil, and placebo groups had remitting, periodic, and persistent asthma, and that treatment was not identified as a predictor of remitting asthma, supports the conclusion that long-term treatment with an inhaled corticosteroid does not influence this aspect of the natural history of asthma.
Children with mild to moderate persistent asthma, regardless of their outcome activity categories, generally exhibited improvement over time. While only 3 of over 900 subjects had complete remission during the 4 year-follow up phase, 55% reported regular asthma activity (i.e. persistent asthma) at every visit. Notwithstanding the reduced use of inhaled corticosteroid therapy in our persistent activity group during the observational phase, there was still an overall reduction in asthma morbidity (prednisone requirement, ED visits, and hospitalization) during the clinical trial period and in the observational phase.19
The clinical course of asthma with respect to morbidity was associated with improvement in AHR and reduction in peripheral eosinophil count over time, but not necessarily with improvements in spirometry. Fifty percent, 35%, and 15% in the remitting, periodic, and persistent asthma activity groups, respectively, had a methacholine FEV1 PC20 ≥ 25 mg/ml during the observational phase, despite having AHR at enrollment. Sex differences in the natural history of AHR have been reported in this CAMP cohort, with post-pubertal males demonstrating better improvement in AHR over time compared to post-pubertal females.30 However, we did not see a higher proportion of males in the remitting or periodic groups, consistent with other studies demonstrating lack of differences in asthma prognosis related to sex.17,31–34
Not surprisingly, these independent factors were associated with remitting asthma in our study: milder asthma, higher pre-bronchodilator FEV1 % predicted and methacholine FEV1 PC20, lower FVC % predicted (perhaps suggestive of less airtrapping), and older age. The association of sensitization and exposure to indoor allergens with the development 34 and severity 35 of asthma and impaired lung function 36 in childhood has been recognized in previous studies, but now we report an association with a decreased likelihood of remitting asthma. A child who is not sensitized and exposed to relevant indoor allergen is 3 times more likely to have remitting asthma (Table 2). Therefore, avoiding or limiting exposure to relevant indoor allergens in children who are allergic may in fact alter the course of asthma in childhood.
Supportive of our findings, a study that enrolled mainly allergic children with relatively moderate to severe asthma noted that lower serum IgE levels, fewer positive skin tests, and less medication requirement were predictive of remission.17 In contrast to our findings and possibly due to their smaller cohort, no difference in FEV1 % predicted was found between the 13 remitting, 19 intermittent, and 53 persistent young adult asthmatics.17 A separate study of 119 allergic asthmatic children (age 5 to 14 years) seen at a specialty university clinic, however, also found that either a high FEV1 % predicted in childhood or a larger increase in FEV1 with age was significantly associated with both complete and clinical remission at age 32 to 42 years.32
Important insights into the natural history of childhood asthma are generated from this CAMP analysis. First, our findings provide physicians with information to guide parents about the prognosis of their child’s asthma. A school-aged child with mild to moderate persistent asthma is likely to improve but has only a small chance of complete remission in adolescence, and in as many as two-thirds of the patient population, controller therapy may still be indicated. Second, inhaled corticosteroid therapy should be utilized to improve disease control and not expected to alter the course of the disease. Third, perhaps modifying the effect of sensitization and exposure to relevant indoor allergens may prove to be an important intervention to increase the likelihood of remitting disease. Finally, adolescents in CAMP with remitting asthma activity may represent a distinct phenotype with milder, less atopic and less hyperresponsive asthma during school years.
Acknowledgments
The Childhood Asthma Management Program is supported by contracts NO1-HR-16044, 16045, 16046, 16047, 16048, 16049, 16050, 16051, and 16052 with the National Heart, Lung, and Blood Institute and General Clinical Research Center grants M01RR00051, M01RR0099718-24, M01RR02719-14, and RR00036 from the National Center for Research Resources.
We are indebted to the CAMP participants for their involvement in this study. We also acknowledge the CAMP study coordinators and investigators over the years for their valuable contribution. The CAMP roster is available as an online supplement.
Abbreviations
- AHR
Airway hyperresponsiveness
- CAMP
Childhood Asthma Management Program
- FEV1
Forced expiratory volume in one second
- FVC
Forced vital capacity
- PC20
Provocative concentration of methacholine causing a 20% decline in FEV1
Footnotes
Clinical implications
A general decrease in asthma morbidity in children with mild to moderate persistent asthma can be expected in adolescence, not altered by long-term controller therapy; yet complete remission is infrequent.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Center for Health Statistics Web Site. Health E-Stays Asthma. [Accessed October 2005];Asthma prevalence, health care use and mortality, 2000–2001. at http://www.cdc.gov/asthma/NHIS/2001_table4-1.htm.
- 2.Rackemann FM, Edwards MC. Asthma in Childhood: A follow-up study of 688 patients after an interval of twenty years. New Engl J Med. 1952;246:815–863. doi: 10.1056/NEJM195205222462104. [DOI] [PubMed] [Google Scholar]
- 3.Jenkins MA, Hopper JL, Bowes G, Carlin JB, Flander LB, Giles GG. Factors in childhood as predictors of asthma in adult life. BMJ. 1994;309:90–3. doi: 10.1136/bmj.309.6947.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strachan DP, Butland BK, Anderson HR. Incidence and prognosis of asthma and wheezing illness from early childhood to age 33 in a national British cohort. BMJ. 1996;312:1195–9. doi: 10.1136/bmj.312.7040.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelly WJ, Hudson I, Raven J, Phelan PD, Pain MC, Olinsky A. Childhood asthma and adult lung function. Am Rev Respir Dis. 1988;138:26–30. doi: 10.1164/ajrccm/138.1.26. [DOI] [PubMed] [Google Scholar]
- 6.Phelan PD, Robertson CF, Olinsky A. J The Melbourne Asthma Study: 1964–1999. J Allergy Clin Immunol. 2002;10:189–94. doi: 10.1067/mai.2002.120951. [DOI] [PubMed] [Google Scholar]
- 7.Godden DJ, Ross S, Abdalla M, et al. Outcome of wheeze in childhood. Symptoms and pulmonary function 25 years later. Am J Respir Crit Care Med. 1994;149:106–12. doi: 10.1164/ajrccm.149.1.8111567. [DOI] [PubMed] [Google Scholar]
- 8.Sears MR, Greene JM, Willan AR, et al. A longitudinal, population-based cohort of childhood asthma followed to adulthood. N Engl J Med. 2003;349:1414–1422. doi: 10.1056/NEJMoa022363. [DOI] [PubMed] [Google Scholar]
- 9.Ryssing E, Flensborg EW. Prognosis after puberty for 442 asthmatic children examined and treated on specific allergologic principles. Acta Paediatr. 1963;52:97–105. doi: 10.1111/j.1651-2227.1963.tb04083.x. [DOI] [PubMed] [Google Scholar]
- 10.Buffum WP, Settipane GA. Prognosis of asthma in childhood. Am J Dis Child. 1966;112:214–7. doi: 10.1001/archpedi.1966.02090120082007. [DOI] [PubMed] [Google Scholar]
- 11.Johnstone DE. A study of the natural history of bronchial asthma in children. Am J Dis Child. 1968;115:213–6. doi: 10.1001/archpedi.1968.02100010215010. [DOI] [PubMed] [Google Scholar]
- 12.Blair H. Natural history of childhood asthma. 20-year follow-up. Arch Dis Child. 1977;52:613–9. doi: 10.1136/adc.52.8.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerritsen J, Koeter GH, Postma DS, Schouten JP, Knol K. Prognosis of asthma from childhood to adulthood. Am Rev Respir Dis. 1989;140:1325–30. doi: 10.1164/ajrccm/140.5.1325. [DOI] [PubMed] [Google Scholar]
- 14.Kokkonen J, Linna O. The state of childhood asthma in young adulthood. Eur Resp J. 1993;6:657–61. [PubMed] [Google Scholar]
- 15.Roorda RJ, Gerritsen J, van Aalderen WMC, et al. Follow-up of asthma from childhood to adulthood: Influence of potential childhood risk factors on the outcome of pulmonary function and bronchial responsiveness in adulthood. J Allergy Clin Immunol. 1994;93:575–584. doi: 10.1016/s0091-6749(94)70069-9. [DOI] [PubMed] [Google Scholar]
- 16.Ulrik CS, Backer V, Dirksen A, Pedersen M, Koch C. Extrinsic and intrinsic asthma from childhood to adult age: a 10-yr follow-up. Respir Med. 1995;89:547. doi: 10.1016/0954-6111(95)90156-6. [DOI] [PubMed] [Google Scholar]
- 17.Limb SL, Brown KC, Wood RA, et al. Adult asthma severity in individuals with a history of childhood asthma. J Allergy Clin Immunol. 2005;115:61–6. doi: 10.1016/j.jaci.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 18.The Childhood Asthma Management Program Research Group. Long term effects of budesonide or nedocromil in children with asthma. N Engl J Med. 2000;343:1054–1063. doi: 10.1056/NEJM200010123431501. [DOI] [PubMed] [Google Scholar]
- 19.The Childhood Asthma Management Program Research Group. Long-term budesonide or nedocromil treatment, once discontinued, does not alter the course of mild to moderate asthma in children and adolescents. J Pediatr. 2009;154:682–7. doi: 10.1016/j.jpeds.2008.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Childhood Asthma Management Program Research Group. The Childhood Asthma Management Program (CAMP): design, rationale, and methods. Controlled Clin Trials. 1999;20:91–120. [PubMed] [Google Scholar]
- 21.Nelson HS, Szefler SJ, Jacobs J, Huss K, Shapiro G, Sternberg AL. The relationships among environmental allergen sensitization, allergen exposure, pulmonary function, and bronchial hyperresponsiveness in the Childhood Asthma Management Program. J Allergy Clin Immunol. 1999;104:775–85. doi: 10.1016/s0091-6749(99)70287-3. [DOI] [PubMed] [Google Scholar]
- 22.Searle S, Speed F, Miliken G. Population marginal means in the linear model: an alternative to least squares means. Am Statistician. 1980;34:216–21. [Google Scholar]
- 23.Conover WJ, Iman RL. Rank transformations as a bridge between parametric and nonparametric statistics. The American Statistician. 1981;35:124–129. [Google Scholar]
- 24.StataCorp. Stata Statistical Software: Release 10. College Station, TX: StataCorp LP; 2007. [Google Scholar]
- 25.SAS Institute, Inc. SAS software, Version 8 of the SAS System for Windows. Cary, NC: 1999. [Google Scholar]
- 26.Szefler SJ, Phillips BR, Martinez FD, et al. Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. J Allergy Clin Immunol. 2005;115:233–42. doi: 10.1016/j.jaci.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 27.Covar RA, Spahn JD, Murphy JR, Szefler SJ for the CAMP Research Group. Progression of asthma measured by lung function in the Childhood Asthma Management Program. Am J Respir Crit Care Med. 2004;170:234–41. doi: 10.1164/rccm.200308-1174OC. [DOI] [PubMed] [Google Scholar]
- 28.Pauwels RA, Pedersen S, Busse WW, Tan WC, Chen YZ, Ohlsson SV, Ullman A, Lamm CJ, O’Byrne PM START Investigators Group. Early intervention with budesonide in mild persistent asthma: a randomised, double-blind trial. Lancet. 2003;361:1071–6. doi: 10.1016/S0140-6736(03)12891-7. [DOI] [PubMed] [Google Scholar]
- 29.Guilbert TW, Morgan WJ, Zeiger RS, Mauger DT, Boehmer SJ, Szefler SJ, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006;354:1985–97. doi: 10.1056/NEJMoa051378. [DOI] [PubMed] [Google Scholar]
- 30.Tantisira KG, Colvin R, Tonascia J, Strunk RC, Weiss ST, Fuhlbrigge AL Childhood Asthma Management Program Research Group. Airway responsiveness in mild to moderate childhood asthma: sex influences on the natural history. Am J Respir Crit Care Med. 2008;178:325–31. doi: 10.1164/rccm.200708-1174OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guerra S, Wright AL, Morgan WJ, Sherrill DL, Holberg CJ, Martinez FD. Persistence of asthma symptoms during adolescence: role of obesity and age at the onset of puberty. Am J Respir Crit Care Med. 2004;170:78–85. doi: 10.1164/rccm.200309-1224OC. [DOI] [PubMed] [Google Scholar]
- 32.Vonk JM, Postma DS, Boezen HM, et al. Childhood factors associated with asthma remission after 30 year follow up. Thorax. 2004;59:925–9. doi: 10.1136/thx.2003.016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Marco R, Pattaro C, Locatelli F, Svanes C ECRHS Study Group. Influence of early life exposures on incidence and remission of asthma throughout life. J Allergy Clin Immunol. 2004;113:845–52. doi: 10.1016/j.jaci.2004.01.780. [DOI] [PubMed] [Google Scholar]
- 34.Sporik R, Holgate ST, Platts-Mills TA, Cogswell JJ. Exposure to house-dust mite allergen (Der p I) and the development of asthma in childhood. A prospective study. N Engl J Med. 1990;323:502–7. doi: 10.1056/NEJM199008233230802. [DOI] [PubMed] [Google Scholar]
- 35.Gruchalla RS, Pongracic J, Plaut M, et al. The Inner City Asthma Study: relationships among sensitivity, allergen exposure, and asthma morbidity. J Allergy Clin Immunol. 2005;115:478–85. doi: 10.1016/j.jaci.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 36.Illi S, von Mutius E, Lau S, Niggemann B, Gruber C, Wahn U Multicenter Allergy Study group. Perennial allergen sensitization early in life and chronic asthma in children: a birth cohort study. Lancet. 2006;368:763–70. doi: 10.1016/S0140-6736(06)69286-6. [DOI] [PubMed] [Google Scholar]