Abstract
Purpose
Ispinesib (SB-715992) is a potent inhibitor of kinesin spindle protein, a kinesin motor protein essential for the formation of a bipolar mitotic spindle and cell cycle progression through mitosis. Clinical studies of ispinesib have shown a 9% response rate in patients with locally advanced or metastatic breast cancer and a favorable safety profile without significant neurotoxicities, gastrointestinal toxicities, or hair loss. To better understand the potential of ispinesib in the treatment of breast cancer, we explored the activity of ispinesib alone and in combination with several therapies approved for the treatment of breast cancer.
Experimental Design
We measured the ispinesib sensitivity and pharmacodynamic response of breast cancer cell lines representative of various subtypes in vitro and as xenografts in vivo and tested the ability of ispinesib to enhance the antitumor activity of approved therapies.
Results
In vitro, ispinesib displayed broad antiproliferative activity against a panel of 53 breast cell lines. In vivo, ispinesib produced regressions in each of five breast cancer models and tumor-free survivors in three of these models. The effects of ispinesib treatment on pharmacodynamic markers of mitosis and apoptosis were examined in vitro and in vivo, revealing a greater increase in both mitotic and apoptotic markers in the MDA-MB-468 model than in the less sensitive BT-474 model. In vivo, ispinesib enhanced the antitumor activity of trastuzumab, lapatinib, doxorubicin, and capecitabine and exhibited activity comparable with paclitaxel and ixabepilone.
Conclusions
These findings support further clinical exploration of kinesin spindle protein inhibitors for the treatment of breast cancer.
Chemotherapy remains a cornerstone in the treatment of breast cancer. Microtubule-targeted antimitotic agents feature prominently in therapeutic regimens. These include paclitaxel and docetaxel, vinorelbine, vinblastine, and the recently approved ixabepilone, usually administered as part of a combination regimen with an anthracycline (doxorubicin), an antimetabolite (capecitabine), a platinum (carboplatin), or with HER2-targeted therapy such as trastuzumab.
Therapeutic regimens containing microtubule-targeted agents often produce clinically limiting toxicities, including myelosuppression, neuropathy, alopecia, and gastrointestinal toxicities (1, 2). Neuropathy is the only toxicity unrelated to antiproliferative activity and is likely due to the effects of these drugs on neuronal microtubules (3). One strategy to identify novel antimitotic cancer therapies with improved tolerability profiles is to target mitosis-specific enzymes, eliminating target-related neurotoxicities (4).
Kinesin spindle protein (KSP) is a motor protein with an exclusive and essential role in mitosis (5–7). It is required early in mitosis to separate the centrosomes of the emerging spindle poles, thus driving establishment of a bipolar mitotic spindle. Failure to establish a bipolar spindle results in a mitotic arrest, after which cells may experience a variety of fates, including abnormal exit from mitosis, resumption of the cell cycle, and apoptosis (8–12). The essential role of KSP in cell cycle progression through mitosis in normal and tumor cells alike suggests that antitumor activity of KSP inhibitors is most likely attributable to postmitotic response pathways that remain poorly understood.
Ispinesib (SB-715992), an allosteric small-molecule inhibitor of KSP kinesin motor ATPase (13), was the first small-molecule inhibitor of KSP that advanced to cancer clinical trials. Results from multiple clinical studies of ispinesib confirm the absence of significant neurotoxicities, alopecia, or gastrointestinal toxicities (14–22). The most common toxicity was reversible neutropenia. A preliminary phase II trial of ispinesib in women with locally advanced or metastatic breast cancer progressing despite anthracycline and taxane treatment produced a response rate of 9%, with reductions in tumor size of 46% to 69% (20).
In this first in-depth biological examination of KSP inhibition in preclinical models of breast cancer, we have evaluated the activity of ispinesib as a single agent and in combination with various standards of care in estrogen receptor (ER)–positive, HER2-positive, and triple-negative disease models.
Materials and Methods
Cell culture
Cell lines were obtained from the American Type Culture Collection and from collections developed by Drs. Steve Ethier and Adi Gazdar (23). KPL4 was kindly provided by Dr J. Kurebayashi (Kawasaki Medical School, Kurashiki, Okayama, Japan). Cell culture reagents were from Cellgro-Mediatech. Cells were plated in log phase of growth in 96-well plates and treated for 72 h with ispinesib at concentrations of 3.3 × 10−5 to 8.5 × 10−11 mol/L. Cell growth was measured using CellTiter-Glo (Promega), and luminescence was recorded using BioTek FLx800. Data were analyzed according to the method described previously by the National Cancer Institute/NIH Developmental Therapeutics Program Human Tumor Cell Line Screen Process (24). The GI50 value is the drug concentration that results in 50% growth inhibition after 72 h of drug exposure relative to control.
Western blot analyses
Cells were treated with 150 nmol/L ispinesib and lysed in radioimmunoprecipitation assay buffer. Primary antibodies for Bax, Bid, xIAP, Bcl2, phospho-Bcl2 (Ser70), and Bcl-XL (54H6) were from Cell Signaling. Other primary antibodies used were cyclin B and cyclin E (HE12; Upstate-Millipore). Secondary antibodies were IR 680/800CW LI-COR, and signal detection and analysis were done on a LI-COR Odyssey imaging system.
DNA cell cycle analysis by flow cytometry
Cells were treated with 150 nmol/L ispinesib, fixed in 85% ice-cold ethanol, resuspended in PBS containing 10 μg/mL propidium iodide DNA stain (Sigma-Aldrich) and 250 μg/mL RNase A (Sigma-Aldrich), and analyzed with a FACSCalibur flow cytometer (Becton Dickinson). Cell cycle analyses were done with FlowJo (TreeStar, Inc.).
Xenograft studies
Protocols for xenograft studies were approved by the Cytokinetics Institutional Animal Care and Use Committee. Female mice (7–8 wk) obtained from Charles River were implanted on their flank with 107 cells in 100 μL of 1:1 PBS/Matrigel (BD Biosciences). nu/nu mice were used for all tumor models, except BT-474 and MDA-MB-468, which were established in Fox Chase severe combined immunodeficient (SCID) mice. BT-474 tumors were generated by s.c. implanting 30 mm3 tumor fragments from previously established xenografts. For MCF7 xenografts, mice were implanted s.c. at the base of the neck with 90-d release 0.36 mg 17β-estradiol pellets (Innovative Research of America) 3 d before tumor cell implantation. Tumor volume (length × width2)/2 and body weight were measured twice weekly. For efficacy studies, drug treatment started when tumor volume was ~100 mm3 and mice were sacrificed at 60 d after treatment or when tumor volume reached 1,500 mm3. Drug-treated mice were categorized as a partial regression (PR) if three consecutive tumor measurements were less than half the starting tumor volume on day 0 of treatment, a complete regression (CR) if tumor volume was <12.5 mm3 for three consecutive measurements, and a tumor-free survivor (TFS) if it had no measurable tumor or remained a CR at the end of the study. Tumor growth inhibition (TGI) is the percentage difference in tumor volume between vehicle- and drug-treated groups, determined on the final day when all tumor volumes in the vehicle group are <1,000 mm3.
Statistical analyses of tumor volume differences between only two groups, such as single-agent ispinesib and vehicle control, were conducted using unpaired t tests of tumor volumes at the end of the study (day 60). Statistical analyses of multiple treatment groups were conducted using one-way ANOVA followed by Newman Keuls post hoc test to determine the significance of differences in tumor volumes on day 60 (unless otherwise noted) among treatment groups.
Drugs
All drugs were dosed at their maximum tolerated dose (MTD) unless otherwise stated, and drug volumes were 200 μL/25 g mouse. Ispinesib was formulated in 10% ethanol, 10% cremophor, and 80% D5W (dextrose 5%) and dosed i.p. on a q4d×3 schedule (three doses, every 4 d) at 10 mg/kg in nu/nu mice or 8 mg/kg in SCID mice, unless otherwise stated. Trastuzumab (Genentech) was dosed i.p. twice weekly for 4 wk at 10 mg/kg. Doxorubicin (LGM Pharmaceuticals) was formulated in 0.9% saline and dosed q4d×3 at 3 mg/kg in nu/nu mice or on days 1, 7, and 21 at 2.5 mg/kg in SCID mice. Lapatinib (GlaxoSmithKline) was formulated in 0.5% hydroxypropylmethylcellulose and 0.1% Tween 80 in water and dosed orally twice daily for 3 wk at 40 mg/kg. Capecitabine (Roche) was formulated in 40 mmol/L citrate buffer (pH 6) in 0.5% methylcellulose and orally dosed daily at 450 mg/kg for 14 d. Paclitaxel (Natural Pharmaceuticals) and ixabepilone (Bristol-Myers Squibb) were formulated in 10% ethanol, 10% cremophor, and 80% D5W and dosed i.v. q4d×3 at their respective MTDs of 30 and 5 mg/kg. Vehicle-treated control mice were injected i.p. q4d×3 with a formulation of 10% ethanol, 10% cremophor, and 80% D5W.
Immunohistochemistry
Mice with a tumor volume of ~250 mm3 received a single dose of ispinesib (10 mg/kg). Tumors were dissected, fixed in 10% buffered formalin, and embedded in paraffin, and 5-μm tissue sections were prepared. Antigen retrieval was done by boiling in 50 mmol/L citrate buffer (pH 5.5), and sections were then incubated in 3% hydrogen peroxide, washed in PBS–0.1% Tween, and blocked in 10% goat serum (The Jackson Laboratory). Phospho–histone H3 (PH3) antibody (Upstate) was detected using Alexa Fluor 488 secondary antibody (Molecular Probes-Invitrogen). Images were taken with a Nikon Eclipse TE-2000U microscope at ×10 magnification and captured using MetaMorph software to quantify PH3 expression by computing the area ratio of PH3-positive cells per total cells. Ki67/cleaved caspase-3 staining was done according to the manufacturer’s guidelines (Biocare Medical LLC). Nonfluorescent images were taken on an Olympus BX41 microscope at ×20 magnification.
Results
Sensitivity of human breast cancer cell lines to ispinesib in vitro
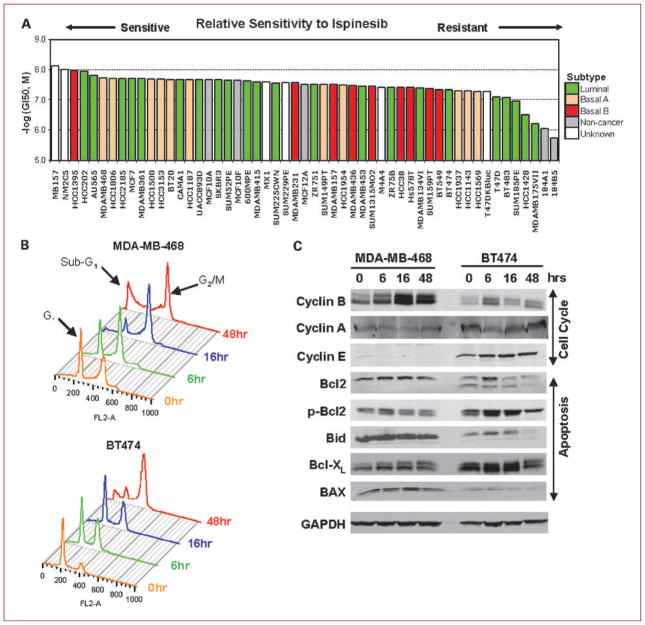
We investigated the possibility that specific breast cancer subtypes might exhibit particular sensitivity to ispinesib in a panel of 50 human breast tumor cell lines representative of diverse primary tumor histotypes and genetic backgrounds and in three normal mammary epithelial lines: MCF10A, MCF10F, and MCF12A (Fig. 1A; ref. 23). Cells were treated with increasing concentrations of ispinesib and ranked according to the concentration of drug required to reduce growth by 50% (GI50; Fig. 1A). All lines exhibited sensitivities between 7.4 and 600 nmol/L, with most falling within a 10-fold range, between 7.4 and 80 nmol/L. Three lines of luminal subtype exhibited sensitivities between 100 and 600 nmol/L. Across this relatively narrow range of sensitivity, we were unable to discern any obvious correlation with subtype, receptor expression, or mutational status.
Fig. 1.
Antitumor activity of ispinesib in vitro against models of breast cancer. A, growth inhibition at 50% (GI50) induced by ispinesib was determined for 53 breast cell lines of luminal, basal A, basal B, and noncancerous origin. B, differences in cell cycle profiles in MDA-MB-468, a cell line sensitive to ispinesib, compared with a less sensitive cell line, BT-474, after treatment with 150 nmol/L ispinesib (3- to 7-fold GI50). C, expression of cell cycle markers (cyclin A, cyclin B, and cyclin E) and apoptotic proteins [Bax, Bid, phospho-Bcl2 (p-Bcl2), Bcl2, and Bcl-XL] was analyzed by Western blotting in MDA-MB-468 and BT-474 cells following treatment with 150 nmol/L ispinesib.
We selected two cell lines, BT-474, a HER2-positive luminal cell line (GI50, 45 nmol/L), and MDA-MB-468, a basal A triple-negative cell line (GI50, 19 nmol/L), and characterized the kinetics of cell cycle and apoptotic responses in vitro following exposure to 150 nmol/L ispinesib, >3-fold the GI50 value for both cell lines (Fig. 1B), and exceeding the concentration required to produce a 90% loss in viability (IC90) in clonogenic survival assays of multiple cell lines (25). This dose (150 nmol/L) is also comparable with the estimated free fraction of ispinesib at Cmax at doses producing detectable antitumor activity in clinical studies (~120 nmol/L; refs. 16, 20). In the absence of drug, the proportion of cells within G2 or M phases of the cell cycle in MDA-MB-468 was twice that of BT-474. After exposure to 150 nmol/L ispinesib, this proportion increased transiently in both lines, consistent with KSP-induced mitotic arrest. Maximal accumulation of mitotic cells occurred after 16 hours of treatment in MDA-MB-468 cells and 48 hours in BT-474 cells. At 48 hours, MDA-MB-468 displayed a much higher proportion of apoptotic cells (sub-G1 DNA content; 35%) than BT-474 cells (13.6%; Supplementary Data 1). These findings are consistent with a more rapid and penetrant onset of cell death following mitotic arrest in MDA-MB-468 than in BT-474.
We also evaluated the effects of ispinesib on the abundance of cell cycle and apoptosis-related proteins (Fig. 1C). Expression of the proapoptotic proteins Bax and Bid was higher in MDA-MB-468 than in BT-474, whereas the antiapoptotic protein Bcl-XL was lower. Bcl2 levels were not different between the two lines, although phosphorylation on Ser70 was greater in BT-474. The significance of this modification is unclear but has been previously associated with potentiating and abrogating Bcl2 antiapoptotic activity (26).
The onset of apoptosis was preceded by accumulation of cyclin B, a marker of mitosis (27). In MDA-MB-468 cells, cyclin B expression was maximal at 16 hours and remained elevated for at least 48 hours, consistent with an abundance of mitotic cells. In contrast, in BT-474 cells, cyclin B levels were lower, and maximal accumulation was observed at 6 hours and diminished thereafter. Cyclin E, which normally accumulates to maximal levels in late G1 phase of the cell cycle (28), increased slightly in BT-474 after ispinesib treatment, but in MDA-MB-468 cells, it was almost undetectable. The abundance of cyclin A was minimally affected by drug exposure, and we observed no changes in the abundance of cyclin D (data not shown).
Efficacy of ispinesib as a single agent in preclinical breast cancer models
To determine the extent of ispinesib antitumor activity in breast cancer models in vivo, we chose cell lines that exhibited different in vitro sensitivity to ispinesib and represent different subtypes of human breast tumors. Their rank from most sensitive to less sensitive to ispinesib in vitro is as follows: MDA-MB-468 > HCC1954 = MCF7 > BT-474.
MCF7 is a well-characterized ER-positive luminal breast cancer cell line. MDA-MB-468 is a model for basal triple-negative breast cancer. To represent HER2-overexpressing breast cancer, we chose BT-474, HCC1954, and KPL4, a breast tumor line of metastatic origin (29). The transcriptomic, genomic, and functional characteristics of these cell lines, except KPL4, have been characterized previously (23).
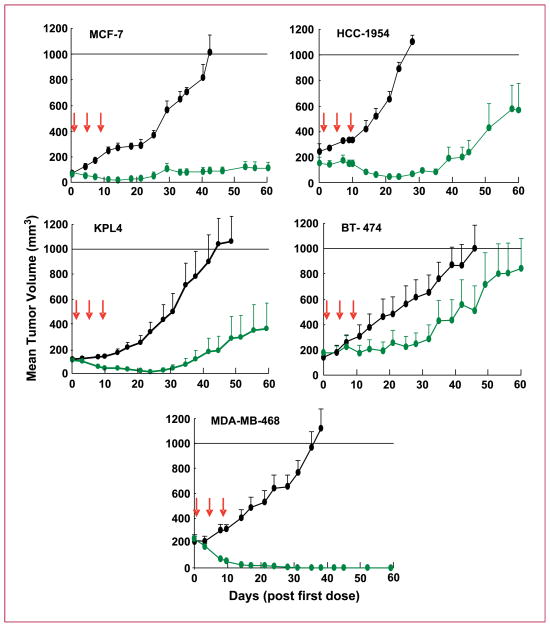
Mice bearing tumor xenografts of the lines listed were treated i.p. with ispinesib at its MTD (SCID, 8 mg/kg; nude, 10 mg/kg) on its optimal q4d×3 schedule (30). Ispinesib was active in all models tested (Fig. 2; Table 1), producing regressions in each. However, the respective tumors differed in sensitivity as judged by the extent of tumor shrinkage, the number of regressions, and extent of tumor regrowth.
Fig. 2.
Antitumor activity of ispinesib in vivo in preclinical models of breast cancer. Vehicle control (black) and ispinesib (green) at its MTD (10 mg/kg in nude mice, 8 mg/kg in SCID mice) were dosed i.p. q4d×3 in models of ER-positive (MCF7), HER2-positive (KPL4, HCC1954, and BT-474), and triple-negative (MDA-MB-468) breast cancer. All cell lines were grown in nude mice, except BT-474 and MDA-MB-468, which were grown in SCID mice. Arrows indicate the days on which ispinesib was administered.
Table 1.
Ispinesib single-agent activity in xenograft models of breast cancer
| Xenograft | n | PR | CR | TFS | Best response | TGI (%) | Mean tumor volume (mm3) day 60 | P (t test) | Type | Origin | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ER positive | MCF7* | 9 | 1 | 4 | 2 | TFS | 92 (d40) | 111 | 0.0164 | Luminal | IDC |
| Triple negative | MDA-MB-468* | 7 | 0 | 7 | 7 | TFS | 100 (d35) | 0 | 0.0023 | Basal | AC |
| HER2 positive | KPL4* | 10 | 4 | 6 | 4 | TFS | 94 (d35) | 379 | 0.0012 | Luminal | IDC |
| BT-474 | 8 | 0 | 2 | 0 | CR | 61 (d25) | 875 | 0.125 | Luminal | IDC | |
| HCC1954* | 5 | 3 | 1 | 0 | CR | 95 (d24) | 563 | 0.0068 | Basal | DC |
Abbreviations: n, mice per group; AC, adenocarcinoma; IDC, invasive ductal carcinoma; DC, ductal carcinoma.
Tumor size at study end was significantly different from vehicle control (P < 0.05).
The triple-negative xenograft model MDA-MB-468, among the most sensitive lines in vitro (Fig. 1A), exhibited the greatest ispinesib sensitivity in vivo. On ispinesib treatment, MDA-MB-468 tumors regressed completely in all mice, each scoring as TFS at the end of the study and 30 days beyond (data not shown).
In the ER-positive model MCF7, ispinesib caused tumor regressions in five of nine mice (one PR and four CR, two of which were TFS at study end) and a TGI of 92%.
Of the HER2-positive models, KPL4 showed the best response to ispinesib treatment. All 10 treated mice exhibited regressions (four PR, six CR, and four TFS). In the HCC1954 model, ispinesib caused regressions in four of the five treated mice. However, in both of these models, tumor regrowth began 35 days after treatment in the less responsive tumors. In the third HER2-positive model BT-474, ispinesib caused a CR in 2 of 8 mice, a lower TGI (61%) than that observed in the other models, and tumors had regrown in all mice by the end of the study (mean tumor volume, 875 mm3).
MDA-MB-468 xenografts are hypersensitive to ispinesib
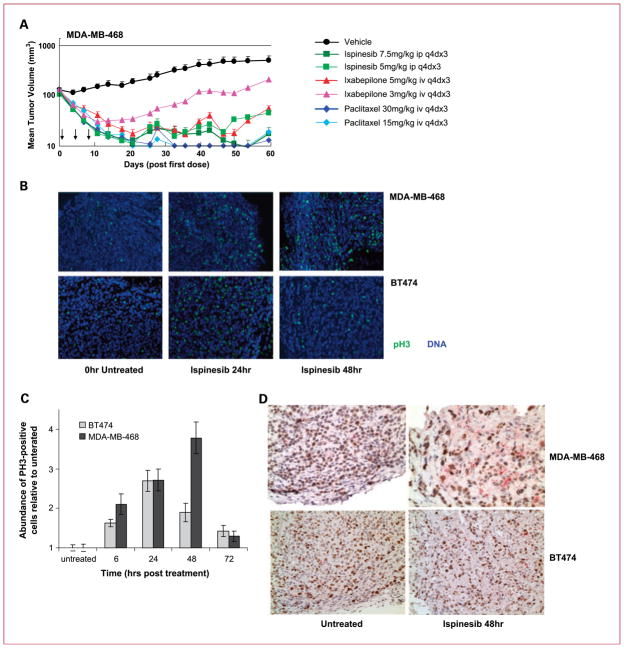
To investigate further the hypersensitivity of the MDA-MB-468 tumors to ispinesib, we compared the antitumor activity of ispinesib with that of ixabepilone or paclitaxel, two antimitotic therapies approved for the treatment of breast cancer. We administered each agent to two cohorts of tumor-bearing animals, receiving either the MTD or a lower dose. Ispinesib antitumor activity was comparable with that of paclitaxel and ixabepilone in terms of TGI and regressions (Fig. 3A; Supplementary Data 2). One of nine mice treated with the higher dose of ixabepilone (5 mg/kg) developed limb paralysis and was sacrificed early. No such toxicity was observed with paclitaxel or ispinesib.
Fig. 3.
Mitotic arrest and apoptosis induced in the MDA-MB-468 ispinesib-hypersensitive xenograft model. A, antitumor activity of ispinesib compared with paclitaxel and ixabepilone in MDA-MB-468 xenografts in SCID mice. Arrows indicate the days on which the respective drugs were administered. B, mice with MDA-MB-468 and BT-474 xenografts were treated with a single 10 mg/kg i.p. dose of ispinesib, and tumor sections were stained for the mitotic antigen PH3 (green) and nuclear 4′,6-diamidino-2-phenylindole (blue). PH3 images were taken at ×10 magnification. C, quantification of ispinesib-induced PH3 staining in MDA-MB-468 and BT-474 xenografts (calculated as the area of PH3-positive signal relative to the area of DNA-positive signal). D, Ki67 (brown) was used as a marker of cellular proliferation and cleaved caspase-3 (pink) as a marker of apoptosis; images were taken at ×20 magnification.
We compared primary and secondary pharmacodynamic responses to ispinesib in MDA-MB-468 and the less sensitive BT-474 tumors. For primary pharmacodynamic response (mitotic arrest), we stained tumor sections for the mitotic antigen PH3 (Fig. 3B; ref. 31). Quantification of PH3 immunofluorescence (Fig. 3C) showed that its expression increased in both tumor lines by 6 hours after treatment. At 48 hours, PH3 levels declined sharply in BT-474 tumors but continued increasing in MDA-MB-468 to levels representing more than twice those in BT-474. At 72 hours, PH3 expression returned to near untreated levels in both lines. For secondary pharmacodynamic responses, we stained tumor sections for markers of proliferation (Ki67) and apoptosis (cleaved caspase-3; Fig. 3D). Forty-eight hours after ispinesib administration to mice with MDA-MB-468 tumors, we observed a sharp reduction in Ki67 expression (brown), a simultaneous marked induction of cleaved caspase-3 (pink), and decreased cellularity consistent with cell death and tumor shrinkage. In BT-474, however, we observed a more modest decrease in Ki67 expression, no noticeable induction of cleaved caspase-3, and little change in tumor cellularity. These responses to ispinesib in vivo were similar to those observed in vitro, with cell cycle arrest in mitosis and cell death occurring more efficiently and rapidly in MDA-MB-468 than in BT-474.
Activity of ispinesib in combination with standards of care in breast cancer
We sought to identify potentially beneficial combination regimens of ispinesib with agents commonly used to treat breast cancer: the HER2-targeted therapies, trastuzumab and lapatinib, doxorubicin (anthracycline), and capecitabine (antimetabolite). In all combination studies, we dosed the approved agent at MTD and optimal dosing schedule and adjusted the dose of ispinesib as necessary to achieve a tolerated combination regimen.
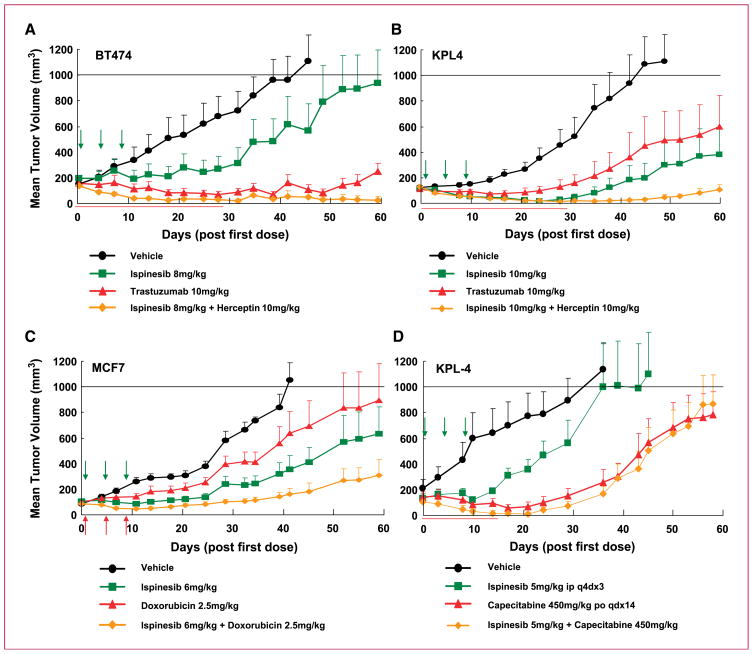
We combined ispinesib with trastuzumab in two different tumor models overexpressing HER2: the luminal model BT-474 (Fig. 4A) and the metastasis-derived model KPL4 (Fig. 4B). In both models, the absence of trastuzumab toxicity allowed combination with the single-agent MTD of ispinesib. The combination proved superior to treatment with either single agent. In BT-474, the combined agents caused a TGI of 99% compared with 61% and 88% with ispinesib and trastuzumab, respectively (Table 2), and cured seven of eight mice. In KPL4, all 10 mice receiving the combination experienced PR or CR, 4 remained tumor-free at the end of the study, and TGI was 97%.
Fig. 4.
Ispinesib enhances the antitumor activity of therapies approved for the treatment of breast cancer. Combination of ispinesib with trastuzumab in BT-474 (A) and KPL4 (B) xenografts. Ispinesib was dosed i.p. q4d×3 at its MTD (10 mg/kg) in nu/nu mice (KPL4 xenografts) and 8 mg/kg in Fox Chase SCID mice (BT-474 xenografts). Trastuzumab was dosed i.p. twice weekly for 4 wk at 10 mg/kg. C, combination of ispinesib with the anthracycline doxorubicin. nu/nu mice with MCF7 xenografts were treated i.p q4d×3 with ispinesib (6 mg/kg) and i.v. q4d×3 with doxorubicin (2.5 mg/kg). D, combination of ispinesib with capecitabine in KPL4 xenografts in nu/nu mice. Ispinesib was dosed q4d×3 i.p. at 5 mg/kg (0.5× MTD), and capecitabine was dosed at 450 mg/kg (MTD) orally daily for 14 d (qdx14). Arrows indicate the days on which ispinesib was administered.
Table 2.
Efficacy of standard-of-care breast cancer therapies when used in combination with ispinesib
| Xenograft | Drug | Dose/route | Schedule | n | PR | CR | TFS (day 60) | TGI (%) | Mean tumor volume (mm3) day 60 | P (ANOVA) | Max mean % weight loss |
|---|---|---|---|---|---|---|---|---|---|---|---|
| KPL4 | Ispinesib | 10 mg/kg i.p. | q4d×3 | 10 | 4 | 6 | 4 | 89% | 379 | 3 | |
| Trastuzumab | 10 mg/kg i.p. | (2×/wk)×4 | 10 | 1 | 4 | 3 | 73% | 569 | 1 | ||
| Combination | As above | 10 | 6 | 4 | 4 | 97% | 107 | 0.032 | 3 | ||
| (day 35) | |||||||||||
| BT-474 | Ispinesib | 8 mg/kg i.p. | q4d×3 | 8 | 0 | 2 | 0 | 61% | 875 | 4 | |
| Trastuzumab | 10 mg/kg i.p. | (2×/wk)×4 | 10 | 1 | 4 | 4 | 88% | 234 | 5 | ||
| Combination | As above | 8 | 0 | 7 | 7 | 99% | 23 | <0.0001 | 7 | ||
| 7 (day 90) | (day 32) | ||||||||||
| BT-474 | Ispinesib | 3 mg/kg i.p. | q4d×3 | 10 | 0 | 0 | 0 | 11% | 1091 | 5 | |
| Lapatinib | 40/k mg/kg p.o. | bid 11/6/17 | 10 | 1 | 0 | 0 | 57% | 668 | 15 | ||
| Combination | As above | 6 | 2 | 0 | 0 | 81% | 445 | 0.0015 | 11 | ||
| (day 41) | |||||||||||
| MCF7 | Ispinesib | 6 mg/kg i.p. | q4d×3 | 12 | 2 | 1 | 0 | 67% | 612 | 5 | |
| Doxorubicin | 2.5 mg/kg i.v. | q4d×3 | 7 | 0 | 0 | 0 | 40% | 872 | 8 | ||
| Combination | As above | 7 | 3 | 1 | 0 | 86% | 295 | 0.0355 | 8 | ||
| (day 40) | |||||||||||
| MDA-MB-468 | Ispinesib | 4 mg/kg i.p. | q4d×3 | 10 | 7 | 0 | 0 | 21% | 692 | 3 | |
| Doxorubicin | 3 mg/kg i.v. | d1,7,21 | 9 | 0 | 0 | 0 | 47% | 560 | 10 | ||
| Combination | As above | 8 | 5 | 0 | 0 | 65% | 377 | 0.0126 | 10 | ||
| (day 32) | |||||||||||
| KPL4 | Ispinesib | 5 mg/kg i.p. | q4d×3 | 7 | 0 | 0 | 0 | 56% | 1035 | 5 | |
| Capecitabine | 450 mg/kg p.o. | qd×14 | 9 | 2 | 1 | 0 | 81% | 780 | 7 | ||
| Combination | As above | 10 | 8 | 2 | 1 | 94% | 858 | 0.0431 | 5 | ||
| (day 21) | (day 14) |
NOTE: For each xenograft study, the combination treatment was superior to treatment with approved single-agent therapy (bold). Efficacy was judged by the number and extent of regressions and number of TFS produced by each treatment. P values (ANOVA followed by Newman Keuls post test) are for comparison of end-of-study tumor volumes produced by combination treatment and by approved single-agent therapy. For the study of combination with capecitabine, tumor volume was not statistically different after day 14 of study.
Abbreviations: n, number of mice per group; p.o., oral dosing; bid, twice daily.
The benefit of combining trastuzumab with ispinesib suggested that similar effects might be observed with lapatinib, a small-molecule HER2/HER1-targeting therapy (32). Although lapatinib proved less effective as a single agent than trastuzumab in the BT-474 model, the addition of a tolerable dose of ispinesib to the MTD of lapatinib improved the TGI from 57% to 81%. The combination did not increase the number of regressions (Table 2).
We also studied the combination of ispinesib with the anthracycline doxorubicin in two different models: MCF7 and MDA-MB-468 (Fig. 4C; Table 2). At the doxorubicin MTD, concomitant administration of ispinesib increased the TGI in both models compared with that obtained with single agents, but no change in the number of regressions was detected (Table 2).
Lastly, we assessed the antitumor activity of ispinesib in combination with capecitabine in the KPL4 model (Fig. 4D). We found that capecitabine dosed at its MTD (33) could only be coadministered with ispinesib at a dose half its single-agent MTD (5 mg/kg). In these conditions, although the mean tumor volume was similar to that in mice treated with capecitabine alone, we observed a clear increase in the number of tumor regressions, including one TFS and an increase in TGI (Table 2).
Discussion
In this study, ispinesib has shown significant antitumor activity in diverse preclinical models of breast cancer, supporting its potential for therapeutic intervention in breast cancer. In vitro, ispinesib inhibited proliferation of all 53 breast cell lines tested. GI50 values spanned a 100-fold range and fall between 10 and 100 nmol/L for most cell lines. Ispinesib exhibited no apparent specificity for histopathologic subtype (luminal A, luminal B, basal) or receptor status (HER2, ER/PR). Interestingly, its profile of activity differed from that of other antimitotic agents such as paclitaxel that inhibited cell growth over a larger concentration range and were more potent against models of basal breast cancer (34). Identification of genomic and transcriptomic differences correlating with relative sensitivity to ispinesib may reveal the basis for differential sensitivity of these cell lines in culture and as xenografts, providing biomarkers predictive of disease response to ispinesib.
Ispinesib was also active in vivo in various breast cancer subtypes, inducing CRs or cures in ER-positive (MCF7), HER2-positive (BT-474, HCC1954, and KPL4), and triple-negative (MDA-MB-468) models, suggesting that it might be useful in the treatment of a broad range of breast cancers.
Xenografts of the triple-negative MDA-MB-468 cell line were exquisitely sensitive to ispinesib. In vitro, this cell line scored among the most sensitive. In vivo, all mice were cured and remained tumor-free for at least 30 additional days. The basis for this strikingly efficacious response is unclear, but our data suggest that cell cycle abnormalities might present a favorable environment for ispinesib activity.
Before drug treatment in vitro and in vivo, MDA-MB-468 cells displayed a relatively high proportion of cells in mitosis (with a 4N DNA content or positive for the mitotic antigen PH3) compared with the less sensitive BT-474 cells. BT-474 cells seem to transiently arrest in mitosis and then escape from M phase, reentering interphase as suggested by accumulation of cyclin E. The loss of cyclin E expression and the increased and longer duration of cyclin B expression in MDA-MB-468 cells are consistent with ispinesib, inducing a penetrant and sustained mitotic arrest in these cells. This suggests a deregulation of the G1-S transition, and interestingly, deregulation of cyclin E expression is commonly observed in breast cancer (35). MDA-MB-468 cells also harbor mutations in the regulators of the G1 checkpoint Rb and p53. Additional experiments will be required to determine if these cell cycle alterations play a role in the increased sensitivity of MDA-MB-468 to ispinesib.
The elevated expression of the proapoptotic proteins Bax and Bid and the reduced expression of antiapoptotic proteins phospho-Bcl2 and Bcl-XL are consistent with increased induction of apoptosis following ispinesib treatment. Previous observations have linked elevated Bax expression to the induction of apoptosis by KSP inhibition (8, 9), and differences in apoptotic responses have been proposed to be predictive of sensitivity to antimitotic drugs such as KSP inhibitors (10). Importantly, our in vitro observations were confirmed by pharmacodynamic studies in vivo. In both MDA-MB-468 and BT-474 tumors, we observed ispinesib-induced increases in the mitotic antigen PH3. Ispinesib treatment was also associated with cleavage of caspase-3 (a marker of apoptosis), decreased staining for Ki67 (a marker of active proliferation), and decrease in tumor cellularity. These ispinesib-induced pharmacodynamic changes were greater in MDA-MB-468 tumors compared with BT-474 tumors, consistent with the greater rate of regressions observed in MDA-MB-468 xenografts compared with BT-474 xenografts.
Ispinesib compared favorably with approved antimitotic agents (paclitaxel and ixabepilone) in the MDA-MB-468 model of the basal subtype of breast cancer, with all three agents producing CR and PR. Consistent with neuropathy being a common side effect of ixabepilone therapy (3, 36, 37), we observed severe limb paralysis in some mice receiving ixabepilone. However, neurotoxicity is uncommon in patients receiving ispinesib, and this side effect was not recorded in preclinical studies, most likely due to the absence of the target KSP of ispinesib from postmitotic neurons (6).
Our findings also show that preclinically ispinesib is well tolerated when combined with doxorubicin, capecitabine, trastuzumab and lapatanib, therapies commonly used in treatment of breast cancer. Administration of ispinesib with these agents enhanced their antitumor activity as shown by higher TGI values and increased tumor regressions.
A particularly beneficial combination was that of ispinesib with trastuzumab. The HER2-positive models we tested were somewhat less sensitive to single-agent ispinesib than either the ER-positive or triple-negative models. However, in two models of HER2-positive breast cancer, ispinesib combined with trastuzumab enhanced the activity of either single agent. HER2 inhibition is known to potentiate the activity of antimitotic agents such as paclitaxel (38, 39), and this drug combination is currently the standard of care for patients with advanced/metastatic HER2-positive breast cancer (40, 41). The improved efficacy of combining KSP inhibition and HER2 inhibition in the preclinical setting, together with the favorable clinical toxicity profile of ispinesib (20, 42), suggests that a combination with trastuzumab may be of clinical benefit.
In patients with advanced or metastatic breast cancer, capecitabine is a standard of care (43). A phase I study showed that the commonly used dose of capecitabine, 2,000 mg/m2, is well tolerated with the full recommended phase II dose of ispinesib administered every 21 days (18 mg/m2; ref. 44). In preclinical studies, toxicity prevented us from combining capecitabine and ispinesib at the respective single-agent MTD. However, we found that doses of ispinesib below MTD potentiated the antitumor activity of capecitabine administered at its MTD. These findings suggest that ispinesib and capecitabine represent a potentially beneficial combination for the treatment of advanced breast cancer, a setting where ispinesib has already shown activity.
We have shown that in vitro and in vivo, ispinesib displays a broad spectrum of activity against breast cancer models representative of various breast tumor types and can enhance the antitumor activity of several therapies that are current standards of care for the treatment of breast cancer. This robust preclinical antitumor activity, coupled with evidence of clinical activity and favorable tolerability profile in patients with breast cancer, supports the continued investigation of ispinesib as a promising therapeutic agent in breast cancer.
Translational Relevance.
Microtubule-targeted therapies form an integral part of treatment regimens for breast cancers, most often in combination with other therapies. Of the clinically limiting toxicities of microtubule-targeted therapies, neuropathy is uniquely unrelated to antiproliferative drug action. Inhibitors of kinesin spindle protein (KSP) have emerged as candidate nonneurotoxic anti-mitotic cancer therapies. One of these KSP inhibitors, ispinesib, has been evaluated in a phase II clinical trial in women with locally advanced or metastatic breast cancer, producing several partial responses. In this report, we evaluate the activity of ispinesib as a single agent in models of several breast cancer subgroups and examined tolerability and efficacy of ispinesib combined with various standards of care. This is the first study to explore activity of a KSP inhibitor in models of breast cancer and to identify attractive KSP inhibitor drug combinations. Our results highlight clinical settings in which KSP inhibitors may be of clinical utility.
Supplementary Material
Acknowledgments
We thank Peter Lambert for advice on statistical analysis.
Grant Support
This work was supported by Cytokinetics, Inc. J.W. Gray received grant support from the Director, Office of Science, Office of Biological and Environmental Research, of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231; NIH, National Cancer Institute grants P50 CA 58207 and P50 CA 83639; and Cytokinetics, Inc.
Footnotes
Disclosure of Potential Conflicts of Interest
J.W. Purcell, J. Davis, M. Reddy, S. Martin, K. Samoya, H. Vo, K. Thomsen, P. Bean, K.W. Wood, and S. Cases are employees and consultants and/or have an ownership interest in Cytokinetics. J.W. Gray is a consultant for Agendia, Cepheid, Sirna/Merck, New Leaf Ventures, and Aveo Pharmaceuticals and received grant support from Cytokinetics, Inc. The other authors disclosed no potential conflicts of interest.
References
- 1.Bishop JF, Dewar J, Toner GC, et al. Initial paclitaxel improves outcome compared with CMFP combination chemotherapy as front-line therapy in untreated metastatic breast cancer. J Clin Oncol. 1999;17:2355–64. doi: 10.1200/JCO.1999.17.8.2355. [DOI] [PubMed] [Google Scholar]
- 2.Nabholtz JM, Riva A. Taxane/anthracycline combinations: setting a new standard in breast cancer? Oncologist. 2001;6 (Suppl 3):5–12. doi: 10.1634/theoncologist.6-suppl_3-5. [DOI] [PubMed] [Google Scholar]
- 3.Rowinsky EK, Eisenhauer EA, Chaudhry V, Arbuck SG, Donehower RC. Clinical toxicities encountered with paclitaxel (Taxol) Semin Oncol. 1993;20:1–15. [PubMed] [Google Scholar]
- 4.Jackson JR, Patrick DR, Dar MM, Huang PS. Targeted anti-mitotic therapies: can we improve on tubulin agents? Nat Rev Cancer. 2007;7:107–17. doi: 10.1038/nrc2049. [DOI] [PubMed] [Google Scholar]
- 5.Sawin KE, LeGuellec K, Philippe M, Mitchison TJ. Mitotic spindle organization by a plus-end-directed microtubule motor. Nature. 1992;359:540–3. doi: 10.1038/359540a0. [DOI] [PubMed] [Google Scholar]
- 6.Sakowicz R, Finer JT, Beraud C, et al. Anti-tumour activity of a kinesin inhibitor. Cancer Res. 2004;64:3276–80. doi: 10.1158/0008-5472.can-03-3839. [DOI] [PubMed] [Google Scholar]
- 7.Blangy A, Lane HA, d’Herin P, Harper M, Kress M, Nigg EA. Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell. 1995;83:1159–69. doi: 10.1016/0092-8674(95)90142-6. [DOI] [PubMed] [Google Scholar]
- 8.Tao W, South VJ, Diehl RE, et al. An inhibitor of the kinesin spindle protein activates the intrinsic apoptotic pathway independently of p53 and de novo protein synthesis. Mol Cell Biol. 2007;27:689–98. doi: 10.1128/MCB.01505-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tao W, South VJ, Zhang Y, et al. Induction of apoptosis by an inhibitor of the mitotic kinesin KSP requires both activation of the spindle assembly checkpoint and mitotic slippage. Cancer Cell. 2005;8:49–59. doi: 10.1016/j.ccr.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Shi J, Orth JD, Mitchison TJ. Cell type variation in responses to antimitotic drugs that target microtubules and kinesin-5. Cancer Res. 2008;68:3269–76. doi: 10.1158/0008-5472.CAN-07-6699. [DOI] [PubMed] [Google Scholar]
- 11.Gascoigne KE, Taylor SS. Cancer cells display profound intra- and interline variation following prolonged exposure to antimitotic drugs. Cancer Cell. 2008;14:111–22. doi: 10.1016/j.ccr.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Gascoigne KE, Taylor SS. How do anti-mitotic drugs kill cancer cells? J Cell Sci. 2009;122:2579–85. doi: 10.1242/jcs.039719. [DOI] [PubMed] [Google Scholar]
- 13.Lad L, Luo L, Carson JD, et al. Mechanism of inhibition of human KSP by ispinesib. Biochemistry. 2008;47:3576–85. doi: 10.1021/bi702061g. [DOI] [PubMed] [Google Scholar]
- 14.Burris HA, Lorusso P, Jones S, et al. Phase I trial of novel kinesin spindle protein (KSP) inhibitor SB-715992 IV days 1, 8, 15 q 28 days. J Clin Oncol 2004 ASCO Annual Meeting Proceedings. 2004;22:2004. [Google Scholar]
- 15.Beekman KW, Dunn R, Colevas D, et al. University of Chicago Consortium phase II study of ispinesib (SB-715992) in patients (pts) with advanced renal cell carcinoma (RCC) J Clin Oncol 2007 ASCO Annual Meeting Proceedings. 2007;25:15573. [Google Scholar]
- 16.Chu Q, Holen KD, Rowinsky EK, et al. A phase I study of novel kinesin spindle protein (KSP) inhibitor, SB-715992 administered intravenously once every 21 days. J Clin Oncol 2004 ASCO Annual Meeting Proceedings. 2004;22:2078. [Google Scholar]
- 17.Knox JJ, Gill S, Synold TW, et al. A phase II and pharmacokinetic study of SB-715992, in patients with metastatic hepatocellular carcinoma: a study of the National Cancer Institute of Canada Clinical Trials Group (NCIC CTG IND. 168) Invest New Drugs. 2008;26:265–72. doi: 10.1007/s10637-007-9103-2. [DOI] [PubMed] [Google Scholar]
- 18.Lee CW, Belanger K, Rao SC, et al. A phase II study of ispinesib (SB-715992) in patients with metastatic or recurrent malignant melanoma: a National Cancer Institute of Canada Clinical Trials Group trial. Invest New Drugs. 2008;26:249–55. doi: 10.1007/s10637-007-9097-9. [DOI] [PubMed] [Google Scholar]
- 19.LoRusso JB, Jones JB, Gadgeel S, et al. A phase I study to determine the safety and pharmacokinetics of intravenous administration of SB-715992, a novel kinesin spindle protein (KSP) inhibitor, on a once weekly for three consecutive weeks schedule in patients with refractory solid tumors. European Cancer Conference; 2003. [Google Scholar]
- 20.Miller K, Ng C, Ang P, et al. Phase II, open label study of SB-715992 (ispinesib) in subjects with advanced or metastatic breast cancer [abstract 1089]. San Antonio (TX). 28th Annual San Antonio Breast Cancer Symposium; 2005. [Google Scholar]
- 21.Shahin MS, Braly P, Rose P, et al. A phase II, open-label study of ispinesib (SB-715992) in patients with platinum/taxane refractory or resistant relapsed ovarian cancer. J Clin Oncol; ASCO Annual Meeting Proceedings; 2007; 2007. p. 5562. [Google Scholar]
- 22.Tang PA, Siu LL, Chen EX, et al. Phase II study of ispinesib in recurrent or metastatic squamous cell carcinoma of the head and neck. Invest New Drugs. 2008;26:257–64. doi: 10.1007/s10637-007-9098-8. [DOI] [PubMed] [Google Scholar]
- 23.Neve RM, Chin K, Fridlyand J, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–27. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monks A, Scudiero D, Skehan P, et al. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J Natl Cancer Inst. 1991;83:757–66. doi: 10.1093/jnci/83.11.757. [DOI] [PubMed] [Google Scholar]
- 25.Jackson JR, Gilmartin AG, Williams T, et al. A pharmacodynamic marker of mitosis demonstrates the anti-mitotic activity of SB-715992, an inhibitor of the mitotic kinesin KSP. Proc Am Assoc Cancer Res. 2002;43:1336. [Google Scholar]
- 26.Haldar S, Basu A, Croce CM. Serine-70 is one of the critical sites for drug-induced Bcl2 phosphorylation in cancer cells. Cancer Res. 1998;58:1609–15. [PubMed] [Google Scholar]
- 27.Dutta A, Chandra R, Leiter LM, Lester S. Cyclins as markers of tumor proliferation: immunocytochemical studies in breast cancer. Proc Natl Acad Sci U S A. 1995;92:5386–90. doi: 10.1073/pnas.92.12.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lew DJ, Dulic V, Reed SI. Isolation of three novel human cyclins by rescue of G1 cyclin (Cln) function in yeast. Cell. 1991;66:1197–206. doi: 10.1016/0092-8674(91)90042-w. [DOI] [PubMed] [Google Scholar]
- 29.Kurebayashi J, Otsuki T, Tang CK, et al. Isolation and characterization of a new human breast cancer cell line, KPL-4, expressing the Erb B family receptors and interleukin-6. Br J Cancer. 1999;79:707–17. doi: 10.1038/sj.bjc.6690114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson RK, McCabe FL, Cauder E, et al. SB-715992, a potent and selective inhibitor of the mitotic kinesin KSP, demonstrates broad-spectrum activity in advanced murine tumors and human tumor xenografts. Proc Am Assoc Cancer Res. 2002;43:1335. [Google Scholar]
- 31.Juan G, Traganos F, James WM, et al. Histone H3 phosphorylation and expression of cyclins A and B1 measured in individual cells during their progression through G2 and mitosis. Cytometry. 1998;32:71–7. doi: 10.1002/(sici)1097-0320(19980601)32:2<71::aid-cyto1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 32.Moy B, Goss PE. Lapatinib: current status and future directions in breast cancer. Oncologist. 2006;11:1047–57. doi: 10.1634/theoncologist.11-10-1047. [DOI] [PubMed] [Google Scholar]
- 33.Higgins B, Kolinsky K, Linn M, et al. Antitumor activity of capecitabine and bevacizumab combination in a human estrogen receptor-negative breast adenocarcinoma xenograft model. Anticancer Res. 2007;27:2279–87. [PubMed] [Google Scholar]
- 34.O’Brien C, Cavet G, Pandita A, et al. Functional genomics identifies ABCC3 as a mediator of taxane resistance in HER2-amplified breast cancer. Cancer Res. 2008;68:5380–9. doi: 10.1158/0008-5472.CAN-08-0234. [DOI] [PubMed] [Google Scholar]
- 35.Smith AP, Henze M, Lee JA, et al. Deregulated cyclin E promotes p53 loss of heterozygosity and tumorigenesis in the mouse mammary gland. Oncogene. 2006;25:7245–59. doi: 10.1038/sj.onc.1209713. [DOI] [PubMed] [Google Scholar]
- 36.Denduluri N, Low JA, Lee JJ, et al. Phase II trial of ixabepilone, an epothilone B analog, in patients with metastatic breast cancer previously untreated with taxanes. J Clin Oncol. 2007;25:3421–7. doi: 10.1200/JCO.2006.10.0784. [DOI] [PubMed] [Google Scholar]
- 37.Perez EA, Lerzo G, Pivot X, et al. Efficacy and safety of ixabepilone (BMS-247550) in a phase II study of patients with advanced breast cancer resistant to an anthracycline, a taxane, and capecitabine. J Clin Oncol. 2007;25:3407–14. doi: 10.1200/JCO.2006.09.3849. [DOI] [PubMed] [Google Scholar]
- 38.Baselga J, Norton L, Albanell J, Kim YM, Mendelsohn J. Recombinant humanized anti-HER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res. 1998;58:2825–31. [PubMed] [Google Scholar]
- 39.Lee S, Yang W, Lan KH, et al. Enhanced sensitization to taxol-induced apoptosis by herceptin pretreatment in ErbB2-overexpressing breast cancer cells. Cancer Res. 2002;62:5703–10. [PubMed] [Google Scholar]
- 40.Buzdar AU, Ibrahim NK, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676–85. doi: 10.1200/JCO.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 41.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 42.Gomez H, Castaneda C, Philco M, et al. A phase I-II trial of ispinesib, a kinesin spindle protein inhibitor, dosed every two weeks in patients with locally advanced or metastatic breast cancer previously untreated with chemotherapy for metastatic disease or recurrence [abstract 2148]. San Antonio (TX). 31st Annual San Antonio Breast Cancer Symposium; 2008. [Google Scholar]
- 43.Findlay M, von Minckwitz G, Wardley A. Effective oral chemotherapy for breast cancer: pillars of strength. Ann Oncol. 2008;19:212–22. doi: 10.1093/annonc/mdm285. [DOI] [PubMed] [Google Scholar]
- 44.Rodon J, Till E, Patnaik A, et al. Phase I study of ispinesib in combination with capecitabine in patients with advanced solid tumors. Prague (Czech Republic). 18th Annual EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics; 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.