Abstract
A critical period in establishing pregnancy occurs after the onset of implantation but before placental development. Evidence strongly suggests that abnormalities occurring during this period can result in pregnancy termination or in pre-eclampsia; the latter may lead to small-for-gestational-weight offspring that are likely to be unhealthy. Clearly, events occurring in the endometrium during the implantation process are crucial for proper fetal development and for optimal offspring health. In several mammalian species bi-directional communication between the conceptus and endometrium during implantation is required for successful pregnancy. Although different implantation and placentation modes occur in different mammalian species, common aspects of this bi-directional signaling may exist. The molecular signals from the trophoblast cells of the conceptus, which direct endometrial changes during implantation progression, are well known in some non-rodent species. Currently, we know little about such signaling in rodents during implantation progression, when the endometrium undergoes decidualization. This review focuses on data that support the hypothesis that paracrine signals from the rodent conceptus influence decidualization. Where possible, these findings are compared and contrasted to information currently known in other species that exhibit different implantation modes.
INTRODUCTION
In mammals implantation of the conceptus (embryonic and extra-embryonic cells arising from the fertilized oocyte) involves a series of processes that begins with the attachment of the blastocyst to the uterine wall and ends when the mature structure of the placenta is formed. In rodents and humans implantation begins at Day 4.5–5 and Days 5–7 of pregnancy, respectively (Finn and McLaren, '67; Psychoyos, '73; Enders and Schlafke, '86; Enders and Schlafke, '67). As reviewed elsewhere (Dey and others, '04; Psychoyos, '73; Yoshinaga, '88; Tabibzadeh, '02; Finn, '77), implantation only occurs during a specific period after (a) the endometrium has undergone a series of hormone-dependent changes to become receptive to the implanting blastocyst and (b) the embryo is at the proper developmental stage. Initially relating to rodents, Enders and Sclafke (Enders and Schlafke, '67; Enders and Schlafke, '69) described three stages of implantation that can be applied to mammals in general. Although implantation in all mammals involves the first two stages, apposition and adhesion, only those having invasive conceptuses exhibit the third stage, invasion and embedding to varying degrees (Carson and others, '00; Wooding, '02). Thus, implantation varies from relatively non-invasive to invasive resulting in the formation of epitheliochorial (e.g. pig, sheep, horse, cow) and hemochorial (e.g. mouse, rat, human, baboon) placental types, respectively (Wooding and Flint, '94; Wooding, '02; Johnson, '07). In laboratory rodents, implantation first involves apposition and adhesion of the blastocyst to the endometrium. This process is followed by the apoptotic displacement of epithelial cells, leading to blastocyst invasion (Weitlauf, '88).
Research efforts to date have focused on determining the molecular mechanisms and the embryo-endometrial dialogue involved in the apposition and adhesion stages in early mammalian implantation. In rodents, apposition and adhesion result in the fixation of the “hatched” blastocyst embryo to the epithelial cells lining the antimesometrial chamber of the uterine lumen. In recent years, a great deal has been learned about the molecules involved in blastocyst-endometrial signaling during apposition and adhesion (Carson and others, '00; Imakawa and others, '04; Paria and others, '01; Wang and Dey, '06; Kennedy and others, '07; Kimber and Spanswick, '02). The first result of the interaction between the rodent blastocyst and luminal epithelium is an immediate increase in endometrial vascular permeability (Psychoyos, '60) (see Fig. 1A and B). Increased endometrial vascular permeability occurs only in implantation segments; it is the first macroscopically observable sign that implantation has begun, and can be visualized using an intravenous injection of a macromolecular dye such as Evans blue. The change in permeability is followed by apoptosis of the adjacent luminal epithelial cells (Parr and others, '87; Schlafke and others, '85; Welsh and Enders, '91). Conceptus-derived paracrine signals do not appear to be required for initiating characteristic changes in the rodent endometrium. A review of recent literature highlights the fact that the physical stimulus, not the signaling molecules of the implanting conceptus, elicits these changes, as long as the endometrium is properly hormonally “sensitized” for the deciduogenic stimulus (Kennedy and others, '07). Similar changes in vascular permeability and epithelial cell displacement can also occur in response to an artificial implantation stimulus in a non-pregnant endometrium that has been adequately sensitized for a deciduogenic stimulus (Lundkvist, '78; Milligan, '87; Milligan and Mirembe, '85; Andrade and others, '96).
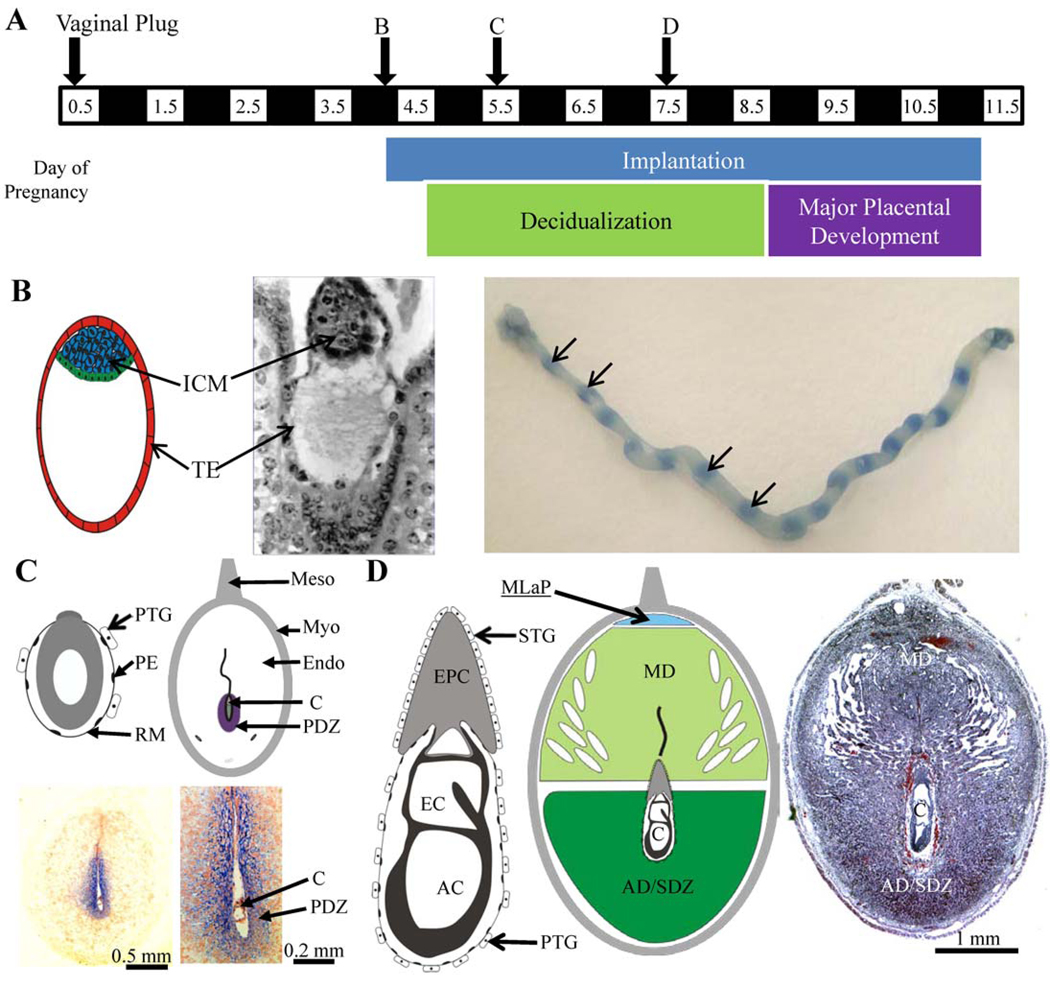
Figure 1.
Timing of events and histology of during the first half of mouse pregnancy. A. Timeline of Days 0.5 to 11.5 of pregnancy. Implantation begins in the dark phase between Days 3.5 and 4.5. B. With the onset of implantation of the blastocyst the first macroscopically observable sign it has begun is the increased vascular permeability or “bluing reaction” (arrows) at implantation segments of the uterus where the implantation has begun along the uterine horns. C. Conceptus (egg cylinder embryo) and uterine implantation site on Day 5.5. With the onset of decidualization, a primary decidual zone (PDZ) is fully formed in the antimesometrial endometrium by Day 5.5 as shown by Tjp1 protein immunohistochemical staining (blue). D. Conceptus and uterine implantation site on Day 7.5. As decidualization continues in the antimesometrial region and mesometrial region, an antimesometrial (AD) mesometrial (MD) decidua are formed by Day 7.5. Conceptus acronyms: amniotic cavity (AC), conceptus (C), ectoplacental cone (EPC), exocelomic cavity (EC), ICM, inner cell mass (ICM), parietal endoderm (PE), trophectoderm (TE), trophoblast giant cells (primary, PTG; secondary, STG), Reichert’s membrane (RM). For more detail on conceptus histology during decidualization please see books by Kaufman or Theiler (Kaufman, '02; Theiler, '89).
Paracrine factors from the conceptus likely play important roles in the endometrium during the progression of implantation. During early implantation in rodents, endometrial stromal differentiation begins before complete displacement of the luminal epithelial cells in contact with the blastocyst. This stromal differentiation transforms endometrial tissue into decidual tissue in a process commonly referred to as decidualization. As discussed below, the onset of decidualization can occur when an artificial implantation stimulus is applied; paracrine signals directly from the conceptus are not required. However, signaling from the conceptus remains essential. There is significant evidence that certain molecules play critical roles in facilitating the bi-directional dialog between the conceptus and endometrium during implantation in several non-rodent species. For example, there is evidence that a trophoblast-derived type I interferon, interferon tau (IFNτ), exerts paracrine and endocrine effects on ungulate uteri and ovaries, respectively (Roberts, '07; Spencer and others, '07; Thatcher and others, '01). In humans and some non-human primates there is strong evidence that trophoblast-derived chorionic gonadotropin provides paracrine signals to the endometrium during early pregnancy, before the hormone exerts its endocrine effects on the ovary (Sherwin and others, '08; Licht and others, '07). These observations indicate that trophoblast-derived paracrine factors play important roles to ensure that successful changes in the endometrium enable the normal progression of implantation in several non-rodent species. Rodents do not appear to possess genes orthologous to IFNτ or to chorionic gonadotropin in their genomes. Compared to certain other species, relatively little is known about the target of conceptus-derived paracrine signals in rodents and their influence on gene expression during implantation progression. Although signaling between the conceptus and endometrium at this stage is likely bi-directional, this review is confined to an assessment of some key aspects of potential paracrine effects of the conceptus on the endometrium during decidualization in rodent species. Some of the non-conserved and potentially conserved features between rodent and other mammalian species are described below.
FORMATION OF THE DECIDUA IN RODENTS
A broad description of decidualization includes the endometrial changes required to form decidual tissue. These changes include the re-differentiation of endometrial fibroblast-like cells into epithelial-like decidual cells (decidual cell differentiation), as well as the immune cell changes, vascular changes and extensive tissue remodeling activities that occur independently of embryo invasion. Readers are referred to several excellent reviews on this topic (Abrahamsohn and Zorn, '93; Abrahamsohn and others, '91; Abrahamsohn and others, '02; Kennedy and others, '07; Krehbiel, '37; Tachi and Tachi, '74). Unlike humans, in whom decidualization begins during later menstrual cycle phases under the influence of progesterone, rodent decidualization does not begin naturally during the estrous cycle (Gellersen and others, '07). Rather, rodent decidualization occurs after normal entrance into pregnancy following cervical stimulation and insemination. The conceptus must be at the proper developmental stage to provide an “implantation stimulus” to the responsive endometrium. After such a stimulus ensues in mice and rats, local increased endometrial vascular permeability is easily observed at 6–8 h; the onset of decidualization is observed at 24–36 h (see Fig. 1). Initially, decidualization occurs in several cell layers of the endometrial stroma immediately adjacent to the implanting conceptus. This area is known as the primary decidual zone (PDZ) and is located adjacent to the antimesometrial chamber of the uterine lumen that surrounds the conceptus (Fig. 1C). The PDZ is fully formed by Day 5.5 of pregnancy in mice. Decidualization then extends to the remainder of the antimesometrial region of the endometrium, forming the secondary decidual zone (SDZ; Fig. 1D). Decidualization in the mesometrial region of the endometrium ensues, forming the mesometrial decidua (Fig. 1D). Since the mesometrial decidua is the site of placental development, changes that occur in this region are more complex than the formation of the PDZ and SDZ in the antimesometrial region. In the antimesometrial region a thin layer of endometrial fibroblast-like cells adjacent to the myometrium do not differentiate; these cells may help repopulate the cells of the endometrium after parturition.
During implantation, the endometrial tissue undergoing decidualization must provide a supportive environment for the conceptus. Initially, the PDZ (Fig. 1C) is avascular and thus less permeable to macromolecules compared to regions of non-decidualized endometrium. This avascular barrier is believed to protect the developing embryo early in implantation (Parr and Parr, '86). However, the PDZ disappears shortly after it forms. Three days after the onset of implantation (Day 7.5) the conceptus is surrounded by decidual tissue of the SDZ and mesometrial decidua (Fig 1D). This decidual tissue is highly vascular; both primary and secondary trophoblast giant cells come into direct contact with the maternal blood and immune cells. Later in pregnancy, once the mature placenta structure is formed, only a few of the large and polyploid antimesometrial decidual cells remain; these cells form the decidua capsularis while the decidual cells in the mesometrial region form the maternal component of the placenta called the decidua basalis.
THE DECIDUA VERSUS DECIDUOMA
Since the early 20th century, several studies have established methods for artificially inducing decidual-like tissue formation in rodents (Krehbiel, '37; Loeb, '07; Loeb, '08; Parkes, '14). Artificial induction of decidual-like tissue is commonly achieved by injecting sesame oil into the uterine lumens of either pseudopregnant or ovariectomized hormone-treated rodents as a deciduogenic stimulus. The tissue that forms in response to an artificial stimulus is termed the ‘deciduoma’, which distinguishes it from the natural decidua formed during normal pregnancy. To date, the precise mechanism by which an artificial stimulus causes decidualization remains unknown, however it is believed to be initiated via physical trauma rather than through hormonal or chemical conceptus secretions, and to involve prostaglandins (Kennedy and others, '07). Although this proposed mechanism clearly demonstrates that paracrine signals from the conceptus are not absolutely required for decidualization, it is unclear whether the two tissue types are identical. Several studies have highlighted differences between decidua and deciduoma formation in rodents. For example, Krehbiel (Krehbiel, '37), Deanesly (Deanesly, '71) and Wang et al. (Wang and others, '04) described small morphohistological differences in the deciduoma, particularly in the equivalent of the PDZ and mesometrial decidua. These studies also indicated that the rate of decidualization itself may differ between the decidua and deciduoma. Thus, morphohistological differences between the decidua and deciduoma may exist. Most workers in the field believe that these differences depend on the type of artificial deciduogenic stimulus employed, the timing of when the stimulus is applied, and the strain of animal used. Comparative studies are needed to determine which of the currently used artificial models are more biologically relevant. Arguably, the best deciduoma model would be one in which the morphohistological and molecular changes in the endometrium during decidualization are spatially and temporally similar to those observed during deciduas formation. Periodically in the literature gene expression studies have utilized both natural and artificially-induced decidualization models to study potential effects of paracrine factors from the conceptus on the endometrium during decidualization in rodents. We provide descriptions of some of these studies in the following sections.
EFFECT OF THE CONCEPTUS ON ENDOMETRIAL IMMUNE CELL POPULATIONS
Uterine natural killer (uNK) cells are the most abundant immune cells present in the endometrium during decidualization in rodents. The biology of uNK cells in mammals has been reviewed extensively (Archer, '07; Ashkar and Croy, '01; Bilinski and others, '08; Bulmer and Lash, '05; Croy and others, '06; Croy and others, '00a; Croy and others, '00b; Croy and others, '03a; Croy and others, '03b; Dietl and others, '06; Dosiou and Giudice, '05; Engelhardt and King, '96; Head, '96; King and others, '96; Leonard and others, '06; Liu and Young, '01; Quenby and Farquharson, '06; Santoni and others, '08; Tayade and others, '07; van den Heuvel and others, '05; Zhang and others, '05; Zhang and Tian, '07), therefore discussion here is limited. Briefly, uNK cell numbers are low in the uteri of non-pregnant rodents; at the onset of decidualization during early pregnancy, however, uNK cells increase in number. Decidualization is progesterone-dependent, and progesterone is believed to play a key role in uNK cell biology in both rodents and humans (Croy and others, '06; Yoshinaga, '08). As rodent decidualization proceeds, uNK cells are activated, develop into granulated mature uNK cells, and become the major source of IFNγ. Because mice deficient in uNK/NK cells exhibit normal fertility competence (Croy and others, '06), uNK cells are not absolutely required for successful murine pregnancy. However, research with these mice clearly shows that uNK cells are required for maintaining a normal decidua and spiral arteriole modifications, as seen in the second half of pregnancy (Ashkar and Croy, '01; Croy and others, '00b; Ashkar and others, '03; Chantakru and others, '02; Guimond and others, '98; Guimond and others, '96). Finally, the importance of uNK cells in implantation is likely not only restricted to rodents since uNK cell recruitment during early pregnancy may be a conserved feature in other mammalian species, albeit with different modes of implantation (Croy and others, '06; Arrighi and others, '07; Engelhardt and others, '02).
Regardless of whether a conceptus is present, uNK cells emerge in increasing numbers in the rodent uterus at sites undergoing decidualization during decidua and deciduoma formation. This finding implies that the conceptus is not required for emergence and development of uNK cells in the rodent endometrium during decidualization (Bany and Cross, '06; Herington and Bany, '07a; Herington and Bany, '07b; Martel and others, '89; Peel, '89). However, compared to the decidua of pregnant mice, the total number of uNK cells in the mouse deciduoma is dramatically reduced, and their pattern of localization is abnormal (Bany and Cross, '06; Herington and Bany, '07b). Otherwise, the development and maturation of uNK cells in the deciduoma and in the decidua appear to be relatively similar (Herington and Bany, '07b). Therefore, subsequent observations of increased gene expression levels (Kashiwagi and others, '07), including secreted phosphoprotein 1 (Spp1) (Herington and Bany, '07a), in the decidua compared to their mRNA levels in the deciduoma may be due to larger uNK cell populations in the decidua. These results indicate that signals from the conceptus can influence total uNK cell numbers and their distribution patterns in the endometrium during decidualization. The precise mechanism by which the conceptus achieves this effect remains to be determined. Notably, evidence suggests that the effect of the conceptus on cell numbers does not likely involve the modulation of uNK cell proliferation (Herington and Bany, '07b). Therefore the conceptus may provide signals that either trigger an increased recruitment of circulating pre-uNK cells into the endometrium, or enhance uNK cell survival.
The prolactin family 4 subfamily a member 1 (Prl4a1) gene encodes a protein (PRL4A1) that is believed to regulate uNK cells. The highest expression level of Prl4a1 during pregnancy in mice and rats occurs in trophoblast cells from mid-pregnancy onwards (Campbell and others, '89; Deb and Soares, '90; Lin and others, '97; Ma and Linzer, '00; Muller and others, '98), after implantation is complete. Although Prl4a1 expression is greatest in the latter half of pregnancy, trophoblast cells also express Prl4a1 in the pregnant mouse and rat during the late phase of decidualization (Ain and others, '03b; Bany and Cross, '06). PRL4A1 has been shown to bind activated Fc receptor-like 6 (also called GP42)-positive uNK cells at mid-pregnancy (Muller and others, '99). Although its putative receptor remains unknown, PRL4A1 increases during intracellular calcium mobilization and suppresses IFNγ production in rodent uNK cells (Ain and others, '03b). Trophoblast-derived PRL4A1 might play an important role in uNK cell trafficking and may control the ability of the uNK cells to limit trophoblast invasion during the latter half of pregnancy (Ain and others, '03a; Ain and others, '03b). Recently, Prl4a1 knockout mice were shown to undergo normal pregnancies and exhibit normal uNK cell distributions, although significantly elevated IFNγ expression levels were observed in their mesometrial decidua (Ain and others, '04). It appears that neither maternal nor conceptus Prl4a1 expression is required for normal pregnancy. Notably, however, when these knockout mothers with knockout conceptuses were exposed to the abnormal environmental condition of hypobaric hypoxia, placental development was severely impacted, and pregnancy was terminated. Notably, wild-type mothers pregnant with wild-type embryos were not affected under these conditions (Ain and others, '04).
Many important questions remain concerning the effect of the conceptus on immune cell populations in the rodent uterus during decidualization. Little is known about how pre-uNK cell recruitment into the rodent uterus is controlled by decidualization and the conceptus. Perhaps mechanisms required for immune cell trafficking to other tissues are involved. Nonetheless, additional information is necessary to understand the potential effects of the conceptus on other immune cell types known to be present in the uterus during decidualization, including dendritic, eosinophil, neutrophil, macrophage, and regulatory T cells (De and Wood, '91; Tachi and Tachi, '86; Finn and Pope, '91; Rogers and others, '92; Brandon, '93; McMaster and others, '93; Saito and others, '07; Wang and others, '00).
PROGESTERONE AND DECIDUALIZATION
Progesterone action on the uterus is essential for conceptus implantation in rodents. This requirement is demonstrated by the fact that progesterone receptor (Pgr)-knockout female mice are infertile due to defects in ovulation, fertilization, and failure of implantation plus decidualization (Lydon and others, '95). The A and B isoforms of PGR appear to play roles in negatively and positively regulating endometrial epithelial cell proliferation, respectively (Conneely and others, '01; Mulac-Jericevic and others, '03); only the PGR A isoform is required to mediate the decidualization response in the endometrium (Conneely and others, '02; Conneely and others, '01; Mulac-Jericevic and others, '00; Mulac-Jericevic and others, '03). Expression of the PGR A isoform is essential for adequate uterine responses to progesterone because, unlike the B isoform, the A isoform can control the expression of a subset of genes in the endometrium required for implantation (Mulac-Jericevic and others, '03; Mulac-Jericevic and others, '00; Mulac-Jericevic and Conneely, '04).
No evidence exists to suggest that the expression of progesterone receptors themselves may be under the control of paracrine signals from the conceptus. Limited evidence suggests that paracrine signals from the conceptus might influence progesterone-regulated responses in the endometrium that are important for decidualization. The FK506-binding protein 52 gene (Fkbp4) encodes an immunophilin protein that acts as a chaperone for steroid receptor signaling (Smith and Toft, '08). FKBP4 was originally identified as a downstream target of HoxA10 function in the peri-implantation mouse uterus (Daikoku and others, '05), and to play a critical role in establishing uterine receptivity for embryo implantation (Tranguch and others, '05). Moreover, FKBP4 was found to regulate the PGR A isoform (Yang and others, '06), and FKBP4 deficiency confers uterine progesterone resistance and implantation failure (Tranguch and others, '07). However, this resistance is specific to genetic background and pregnancy-stage; implantation can be restored with progesterone supplementation. Artificially-induced decidualization cannot be restored by progesterone supplementation, suggesting that a role for cellular signals arising from the conceptus (Tranguch and others, '07).
Bone morphogenetic protein 2 (Bmp2) gene expression, along with stromal cell proliferation and decidual cell differentiation in the endometrium, is significantly lower in the decidua compared to that in the deciduoma (Kashiwagi and others, '07). BMP2 is a key regulator of progesterone-dependent endometrial stromal cell proliferation and differentiation into decidual cells (Lee and others, '07b). Several BMP genes are expressed in a spatiotemporally specific fashion in the rodent endometrium during decidualization (Ying and Zhao, '00). Expression of Bmp2 in the endometrial stroma is under the control of an epithelial-stromal signaling and progesterone receptor-regulated pathway; this pathway involves Indian hedgehog (IHH) and nuclear receptor subfamily 2 group F member 2 (NR2F2, also called COUP-TFII) proteins (Kurihara and others, '07). BMP2, in turn, regulates the expression levels of target genes in the endometrium during decidualization (Lee and others, '07b). An effect of paracrine signals from the conceptus on the BMP2 pathway is intriguing, and the differential expression of Bmp2 between the decidua and deciduoma (Kashiwagi and others, '07) warrants further investigation. Recently we have shown little difference in temporal changes in Bmp2 gene expression (or that of the several downstream genes it regulates) in the mouse uterus during the formation of the decidua compared to that in the deciduoma (Herington and Bany, submitted). Notably, our work involved the use of what seems to be a more “physiological” implantation stimulus in pseudopregnant mice that has only been reported in a single past publication (Sakoff and Murdoch, '94). In our hands, the use of this stimulus resulted in a similar decidualization response compared to time-matched pregnant uteri. There is a need for additional work in comparing and contrasting the strengths and weaknesses of some of the current models of deciduoma induction and their usage in research studying the decidualization process.
DISCOVERY-BASED APPROACHES FOR STUDYING CONCEPTUS EFFECTS ON THE ENDOMETRIUM DURING DECIDUALIZATION
Discovery-based approaches at the protein level have been used to identify the emergence of conceptus-dependent protein secretions by the rodent endometrium during decidualization. In a series of studies conducted 30 years ago, a comparison was made between the secreted proteins from mouse decidua and/or deciduoma tissue explants using 2-dimensional polyacrylamide gel electrophoresis (2-D PAGE) and fluorography techniques (Weitlauf, '87; Weitlauf, '89; Weitlauf and Suda-Hartman, '88). Differences were detected between pregnant uteri and uteri administered an artificially-induced deciduogenic stimulus. Embryo-conditioned medium caused detectable differences in specific secreted protein levels compared to explants not incubated with the conditioned medium. Due to technological limitations, none of these studies identified the protein identity or its cellular source. The advent of new high-throughput proteomics technologies enables scientists to re-evaluate the effect of the rodent conceptus on endometrial tissue proteome profiles. A myriad of powerful techniques are now available to evaluate proteome differences among different samples (Aebersold and Mann, '03; Gorg and others, '04; Minden, '07; Pandey and Mann, '00; Patterson and Aebersold, '03; Patton, '02). New approaches at the protein level are currently used to study other aspects of implantation biology in rodents and other species. Recently, Burnam and colleagues (Burnum and others, '08) reported on their use of matrix-assisted laser desorption/ionization mass spectrometry directly on uterine sections. This study highlighted differences in the proteome signatures of pregnant mouse implantations verses inter-implantation sites, and between the implantation sites of phospholipase A2 group 4a (Pla2g4a)-knockout verses wild-type mothers.
Little is known about rodent endometrial gene expression during decidualization that is under the control of conceptus-derived paracrine factors. However, the availability of cDNA membrane arrays (Bany and Cross, '06) and more powerful microarrays (Kashiwagi and others, '07) has enabled more comprehensive gene expression comparisons between the developing decidua and the deciduoma. In recent work, the differential expression of several gene groups sharing a common function has been verified using independent techniques. Furthermore, these gene groups have been identified as potential targets of molecular signals from the conceptus. For example, uNK cell-specific transcripts such as those encoding certain granzyme genes, perforin, and Spp1 are expressed at greater levels in the decidua compared to the deciduoma (see Table 1) (Herington and Bany, '07a; Kashiwagi and others, '07). That these genes may be targeted by conceptus signaling is a reasonable conclusion, since uNK cell numbers distribution areas are smaller in the deciduoma than in time-matched deciduas (Bany and Cross, '06; Herington and Bany, '07b). Other gene groups expressed differentially between the decidua and deciduoma include genes exhibiting a potential function in vascular changes (such as Angpt1, Angpt2, Fgfr1, and Procr), IFN-regulated processes (Irf8, Rtp4/Iarp, Ifi202b, Iigp1, Ifi27/Isg12), tissue growth and remodeling (Htra1, Htra2, Mmp9, Timp3, Fstl3, Bmp2, and Bmp7) and immune cell regulation or function (Nkg7, Prl4a1, Prl8a2)(see Table 1). These array studies, and additional work on smaller numbers of genes (e.g. (Bany and Cross, '06; Kashiwagi and others, '07)), provide valuable insight into the identity of possible conceptus-directed genes in the rodent endometrium during decidualization. Verification of much of these findings is required, and potentially should involve more suitable deciduoma models. Once verified, more work is required to determine the mechanism by which the conceptus exerts its effects and to determine the precise functions of these target genes in the endometrium during decidualization. Although a list of potential target endometrial genes has been suggested, little is known of the identity or source of the potential conceptus paracrine factors that may be responsible for controlling their expression (see Table 1). Undoubtedly, the use of genetic and in vitro models will assist in such studies. Overall, the use of decidua versus deciduoma models and array profiling methods appear valuable as an initial step toward identifying relevant paracrine factors.
Table 1.
The expression of target genes of various functions in regions/cells of the endometrium that may be regulated by paracrine factors from the conceptus.
| ENDOMETRIUM | CONCEPTUS | ||
|---|---|---|---|
| TARGET GENE | SOURCE | PARACRINE FACTOR | SOURCE |
| Barrier Function | |||
| Tjp1 | PDZ | ? | Trophoblast/Ectoplacental cone? |
| Interferon-regulated | |||
| Irf8 | ? | ? | ? |
| Ifi202b | ? | ? | ? |
| Iigp | ? | ? | ? |
| Ifi27/Isg12 | ? | ? | ? |
| Isg15 | Decidua | Type I INTERFERON? | Giant Cells |
| Rtp4/Iarp | ? | ? | ? |
| Tissue Growth/Remodeling | |||
| Cst3 | Decidua | ? | ? |
| Bmp2 | Decidua | ? | ? |
| Bmp7 | Decidua | ? | ? |
| Fstl3 | Decidua | ? | ? |
| Htra1 | Decidua | ? | ? |
| Htra2 | Decidua | ? | ? |
| Mmp9 | Decidua | ? | Trophoblast MMP9 |
| Timp3 | Decidua | ? | ? |
| Upa | Decidua | ? | ? |
| Immune Cell Regulation/Function | |||
| GzmE | uNK? | ? | ? |
| Gzmf | uNK? | ? | ? |
| Nkg7 | uNK? | ? | ? |
| Perforin | uNK | ? | ? |
| Prl4a1 | uNK? | ? | ? |
| Prl8a2 | Decidua | ? | ? |
| Spp1 | uNK | ? | ? |
| Vascular Changes | |||
| Angpt1 | ? | ? | ? |
| Angpt2 | Decidua | ? | ? |
| Fgfr1 | ? | ? | ? |
| Procr | Decidua | ? | ? |
| ? | endothelial? | Proliferins | Trophoblast |
| Unknown Function? | |||
| Pglyrp1 | ? | ? | ? |
| Prlpb | Decidua | ? | ? |
THE VALUE OF GENETIC MODELS FOR STUDYING CONCEPTUS EFFECTS ON DECIDUALIZATION
The trophectoderm cells (see Fig. 1B) of the blastocyst differentiate into the various trophoblast cell types of the mature placenta (Simmons and Cross, '05). The trophoblast cells that arise early during decidualization are the primary and secondary trophoblast giant cells that originate from trophectoderm and ectoplacental cone cells, respectively (Fig. 1C–D). When these giant cells initially appear, they are localized at the interface of the developing conceptus and the maternal endometrium. Therefore, trophectoderm cells are well-positioned to profoundly impact the endometrium during decidualization. Using genetic models, a great deal has been learned about specific gene functions toward successful implantation (Lee and others, '07a). Most of these genes appear to play roles in critical events either just prior to or at the onset of implantation in mice. However, genetic mouse models have provided little information on trophoblast giant cell-derived paracrine factors that affect the endometrium during decidualization after implantation onset. Examples of paracrine factors that are known are those encoded by proliferin, placental lactogen I (Prl3d1), and Prl4a genes (Carney and others, '93; Fang and others, '99; Campbell and others, '89; Deb and Soares, '90; Lin and others, '97; Ma and Linzer, '00; Muller and others, '98). Of these only Prl4a1 knockout mice have been generated and pregnancies with knockout conceptuses in knockout mothers appear normal unless the mothers are exposed to a hypobaric plus hypoxic environmental stress (Ain and others, '04).
Using genetic models, two major clues suggest that secreted paracrine factors of the trophoblast giant cells may play key roles in the endometrium during decidualization. Jun-B oncogene (Junb) and GATA binding proteins 2 (Gata2) and 3 (Gata3) genes encode transcriptional regulators of trophoblast gene expression (Ma and Linzer, '00; Ma and others, '97; Ng and others, '94). The Junb-encoded protein is a component of the AP-1 transcription factor complex that regulates trophoblast cell gene expression, including Mmp9, Prl3d1, and proliferin (Schorpp-Kistner and others, '99). The protein encoded by Gata2 (GATA2) is a major regulator of Prl4a1 gene expression in primary trophoblast giant cells (Ma and Linzer, '00), whereas GATA2 and GATA3 together are major expression regulators of several trophoblast cell genes. Notably, Junb- (Schorpp-Kistner and others, '99) and Gata2/Gata3-knockout (Ain and others, '04) mice trigger diminished expression of trophoblast proliferin and potentially other genes that, in turn, may cause poor decidual vascular development. Since proliferin has been shown to be a major angiogenic factor for endothelial cells (Groskopf and others, '97; Jackson and others, '94), it has been suggested that the abnormal proliferin expression in these knockout mice cause the poor development of the decidual vasculature observed at midpregnancy (Ma and others, '97; Schorpp-Kistner and others, '99). As major placental development begins when decidualization is almost complete, shortly after mid-pregnancy (see Fig. 1A), many of the trophoblast cell types express a variety of angiogenic and anti-angiogenic factors (Hemberger and others, '03; Cross and others, '02). The altered expression levels of these or other genes in the Gata2/3 and Junb knockout mice may also contribute to the altered decidual vascular phenotype. Many other prolactin-like protein genes that are expressed in the developing and mature placenta play potential roles in conceptus-decidual paracrine signaling (Soares and others, '07; Soares, '04; Ain and others, '03a; Ain and others, '06; Alam and others, '06; Wiemers and others, '03) later in pregnancy, after decidualization is complete. Although there is a significant amount of evidence suggesting that specific trophoblast-secreted paracrine factors participate in conceptus-endometrial-vascular signaling in the rodent, more definitive work is required to identify these factors and/or determine if their effect occurs during decidualization or later, when major placental development events are taking place.
IN VITRO APPROACHES TO STUDYING CONCPETUS EFFECTS ON DECIDUALIZATION
In vitro studies of trophoblast-endometrial signaling have verified some of the in vivo evidence that rodent conceptuses can regulate endometrial gene expression during decidualization. The placement of ectoplacental cones on mouse endometrial stromal cells undergoing decidualization dramatically up-regulates Cst3 expression in endometrial stromal cells (Afonso and others, '02). Incubation of similar endometrial cells with trophoblast giant-cell conditioned medium induces Isg15 expression (Bany and Cross, '06). Recent microarray analysis of the transcriptomic changes in human endometrial stromal cells undergoing decidualization in response to human trophoblast cell-conditioned medium has identified several target endometrium genes regulated by the human conceptus, including immune and angiogenic modulators (Hess and others, '07). This in vitro approach will be a valuable tool for future studies aiming to determine the effect of conceptus-derived paracrine factors on rodent endometrial stromal/decidual cell gene expression.
ASSESSING EXPRESSION OF GENES ONE-BY-ONE: WHAT HAVE WE LEARNED?
Much of the research examining conceptus-uterine signaling during decidualization in rodents comes from descriptive work on uterine gene expression in which single genes or small gene groups were examined. Over the years, the expression levels and patterns of numerous genes in the endometrium during decidualization has been reported. Many of these genes are thought to play key roles in the general processes of decidualization, including decidual cell differentiation, angiogenesis, cytokine signaling, immune cell regulation and tissue remodeling. However, little is known about what specific factors and pathways control their expression. On the conceptus side, factors expressed by trophoblast giant cells have been described that have a potential impact on the endometrium. No results prove unequivocally that these conceptus-produced paracrine factors have specific paracrine effects on the rodent endometrium when it is undergoing decidualization. Although they have received less attention, some gene expression data have been collected for both rodent deciduas and deciduomas together in the same study. Using this dual approach, researchers have found that the expression of some genes may be under paracrine signaling control, originating from the rodent conceptus during decidualization; some of these studies are summarized in Table 1. The following three topics provide preliminary evidence that trophoblast cell signals from the rodent conceptus may influence the rodent endometrium during decidualization.
Barrier Function of the PDZ
Specialized permeability junctions form between PDZ cells in the decidua and deciduoma with the initiation of decidualization of the rodent endometrium. This zone of the pregnant endometrium (Parr and Parr, '86; Rogers and others, '83; Tung and others, '86) and its equivalent in the deciduoma (Abrahamsohn and Zorn, '93; Kleinfeld and others, '76; O’Shea and others, '83) shows similar histological and ultrastructural features. It contains tightly packed polypoid cells that form a tissue that is avascular and contains abundant tight, gap- or desmosome-like junctions. The PDZ of the pregnant endometrium is more impermeable to macromolecules compared to adjacent regions, and forms a partial permeability barrier between the conceptus and endometrium (Parr and Parr, '86; Rogers and others, '83). Of the many genes encoding proteins that may be involved in forming various inter-decidual cell junctions, only a few have been studied in the endometrium during early decidualization. Expression of the tight junction protein 1 (Tjp1) (see Fig. 1C, lower panels), tight junction protein 2 (Tjp2), claudin 1 (Cldn1) and cadherin 1 (Cdh1) genes has been shown in the mouse PDZ on Day 5.5 of pregnancy, and the encoded proteins may form functional complexes (Paria and others, '99; Wang and others, '04). Recently, it was reported that the expression of Tjp1 and formation of a permeability barrier is not found in the deciduoma of pseudopregnant mice on Day 5.5 of pseudopregnancy using sesame oil as the deciduogenic stimulus (Wang and others, '04). In addition, trophoblast vesicles can induce decidualization onset in pseudopregnant mice, with the formation of a permeability barrier equivalent to the PDZ of the decidua (Wang and others, '04). Therefore, currently available evidence suggests that trophoblastic tissue directs the formation of the permeability barrier of the PDZ during rodent pregnancy. The potential paracrine effect of trophoblast cells early in decidualization requires further exploration.
Interferon-Stimulated Gene 15 (Isg15)
Trophoblast cell-derived paracrine factors that control Isg15 gene expression in the endometrium during implantation have been demonstrated in the cow (Austin and others, '96; Hansen and others, '97; Johnson and others, '98), sheep (Johnson and others, '00; Johnson and others, '99), mouse (Austin and others, '03; Bany and Cross, '06), baboon and human (Bebington and others, '99a; Bebington and others, '99b). Although little is known about its precise function in the endometrium during implantation in any of these species, many potential functions of the protein (ISG15) encoded by this gene have been reviewed recently (Andersen and Hassel, '06; Dao and Zhang, '05; Ritchie and Zhang, '04). Briefly, ISG15 is an ubiquitin-like protein that is covalently conjugated to intracellular proteins; it may also leave cells in a non-conjugated form to act as a cytokine. Conjugating and de-conjugating complexes or enzymes also exist for ISG15. Unlike ubiquitinylation, this “ISG15ylation” alone does not directly target proteins for proteosome degradation.
Expression of Isg15 occurs in different endometrium cell types during implantation, depending on the mode of implantation of the conceptus; its expression was initially described during implantation in the uteri of cows (Austin and others, '96; Hansen and others, '97; Johnson and others, '98) and sheep (Johnson and others, '00; Johnson and others, '99). In these species, with a more superficial implantation type, Isg15 expression is located mainly in the epithelial cells of the endometrium. A significant volume of research shows that Isg15 expression in epithelial cells is regulated by trophoblast-derived IFNτ (Roberts, '07; Spencer and others, '07; Thatcher and others, '01). Isg15 expression also occurs in the endometrial decidual cells of the baboon and human during early pregnancy (Bebington and others, '99a; Bebington and others, '99b). In these species, Isg15 expression is located in the epithelial cells of the endometrium during the menstrual cycle, with a change in expression pattern to the decidual cells during early pregnancy. Murine Isg15 gene expression is undetectable in the pregnant endometrium until after the onset of decidualization on Day 5.5 of pregnancy. As in primates, this expression level dramatically increases during the progression of decidualization over the following 3 days (Austin and others, '03; Bany and Cross, '06). Therefore, the endometrial expression of Isg15 during implantation appears to be somewhat conserved in certain mammals; however, more work is required to determine if its expression in primates and rodents is controlled by paracrine factors from the trophoblast cells in a similar fashion to that in the cow and sheep.
Isg15 gene expression has been localized to the antimesometrial decidual cells on Day 7.5 of mouse pregnancy (Austin and others, '03; Bany and Cross, '06). Four observations suggest that paracrine factors from the rodent conceptus control Isg15 expression during decidualization. First, Isg15 expression is only detected in the implantation regions whereby the endometrium is undergoing decidualization, and not in inter-implantation regions (Bany and Cross, '06). Second, its expression in the uteri of pseudopregnant mice is undetectable or barely detectable, compared to that in pregnant animals as decidualization progresses (Austin and others, '03). Third, at several time points the expression level of Isg15 in uteri undergoing artificially-induced decidualization is dramatically lower than that in the pregnant uteri (Bany and Cross, '06). Finally, type I IFNs (Austin and others, '03) or a presumptive type I IFN produced by trophoblast giant cells in vitro (Bany and Cross, '06) induces Isg15 expression in cultured mouse endometrial stromal and decidual cells. Therefore, current evidence suggests that the mouse trophoblast giant cells secrete a paracrine factor that controls endometrial Isg15 expression during decidualization. An ortholog to ruminant IFNτ does not exist in the mouse or human genomes. Since type I IFNs induce Isg15 expression in cultured mouse endometrial decidual cells (Austin and others, '03; Bany and Cross, '06), the trophoblast-derived molecule may be at least a type I IFN. However, little is known about type I IFN production and the expression of type I IFN receptor genes in the rodent trophoblast and endometrium during decidualization.
Although murine Isg15 expression during decidualization appears in the antimesometrial decidua (Austin and others, '03; Bany and Cross, '06), little is known about the role of ISG15 and other ubiquitin-like modifiers in the rodent endometrium during implantation. Furthermore, a recent study showed that Isg15 is expressed in both the antimesometrial plus mesometrial decidua and placenta on Days 12.5 and 17.5 of murine pregnancy, after decidualization is complete (Rempel and others, '07). Therefore, ISG15 likely also plays a role in the post-decidualization period. Although Isg15 knockout mice appear to have no problems during pregnancy (Osiak and others, '05), it has been suggested this observation may depend on their genetic background (Rempel and others, '07). Regardless, it seems that Isg15 is not absolutely required for successful pregnancy. This implication is complicated further by the finding that mice deficient in functional ubiquitin specific peptidase 18 (USP18), a protease that de-conjugates ISG15 from proteins, have abnormal placentas and suffer from significant fetal loss (Rempel and others, '07).
Notably, other ubiquitin-like protein modifications may also occur in the endometrium during decidualization in rodents. Neural precursor cell expressed, developmentally down-regulated gene 8 (Nedd8) and SMT3 suppressor of mif two 3 homologs 1 to 3 (Sumo1, Sumo2 and Sumo3) transcripts are also found in the mouse uterus during decidualization (Bany, unpublished data). Although these genes encode proteins that can also be conjugated to other proteins (Herrmann and others, '07; Kerscher and others, '06; Yeh and others, '00), even less is known about their function during decidualization. There is some evidence to suggest that such “neddylation” and “sumoylation” pathways may be important modulators of steroid hormone responsiveness in the uterus (Fan and others, '02; Jones and others, '06). Since most of what happens in the endometrium during decidualization is dependent upon progesterone, Nedd8 and Sumo1–3 may indeed play an important role in decidualization processes. More work is required to determine whether the “neddylation” or “sumoylation” pathways play roles in the endometrium during decidualization and whether they are influenced by the conceptus.
Countering Trophoblast Invasion
A delicate balance exists between proteinases and their inhibitors in the endometrium during mammalian implantation. This balance determines where and when tissue remodeling occurs in the endometrium in species with not only invasive (Salamonsen, '99) but also relatively non-invasive (Hashizume, '07; Roberts, '07) modes of implantation. For rodents and humans, the endometrium must allow trophoblast cell subtypes to invade and enable placental development as well as the tissue remodeling associated with decidualization. In species that exhibit a more superficial-type implantation, extensive tissue remodeling also occurs in the endometrial tissue during implantation. In ungulates, the caruncular endometrial tissue is the site of implantation and undergoes a rapid enlargement due to proliferation and differentiation (Hafez, '87). Although not well studied, it has been suggested that a decidual cell-like differentiation occurs in these species (MacIntyre and others, '02; Joyce and others, '05; King and others, '80; Johnson and others, '03; Kellas, '66; Mossman, '37). Thus, regardless of the depth of invasion of trophoblast cells, endometrial tissue remodeling and differentiation may be a common implantation feature in mammals that are not commonly believed to exhibit endometrial decidualization (Johnson, '08).
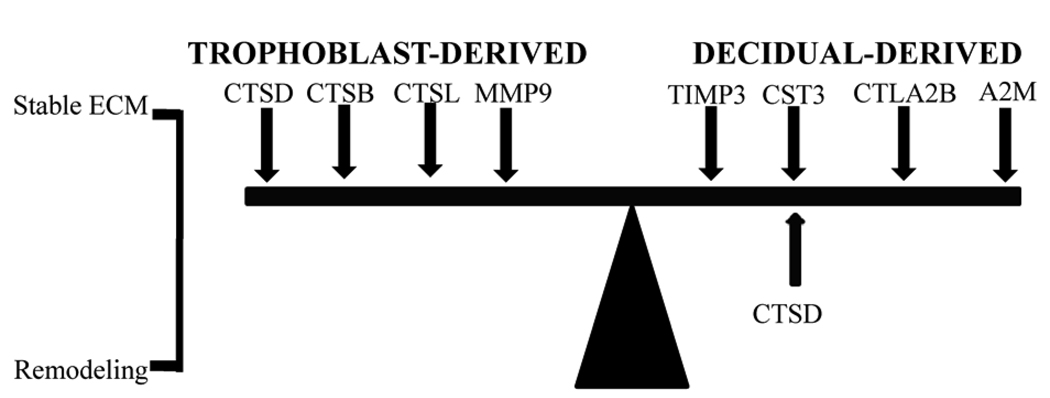
The levels and localization patterns of proteinase and proteinase inhibitor gene expression determine when and where protein degradation occurs in the extracellular space of the endometrium during mammalian implantation. In some cases, specific proteinases from trophoblast cells enter the endometrial tissue to promote embryo invasion, while the endometrium produces inhibitors to prevent over-invasion (see Fig. 2). An example of this scenario in the rodent uterus during decidualization involves the matrix metalloproteinase-9 (MMP9) produced by giant cells. Its inhibitor, tissue inhibitor of metalloproteinase-3 (TIMP3), is produced in adjacent decidua (Alexander and others, '96; Salamonsen, '99; Curry and Osteen, '03; Leco and others, '96). Other examples are cathepsin enzymes produced by the trophoblast giant cells; at least one of their inhibitors (cystatin 3, CST3) is produced in adjacent decidua.
Figure 2.
Examples of balancing of proteinase and anti-proteinases produced by the conceptus and endometrium during decidualization. The balance of these in the extracellular space determines if the extracellular matrix (ECM) is stable or undergoing remodeling.
Several cathepsin proteinases are expressed in the rodent uterus or trophoblast cells during decidualization. As recently reviewed, cathepsins B (CTSB) and L (CTSL) are C1A clan cysteine cathepsins that play diverse roles in several intracellular compartments; they may also be secreted into the extracellular space (Brix and others, '08). During rodent decidualization, a major source of CTSB and CTSL are the trophoblast giant cells (Afonso and others, '97; Afonso and others, '99; Cheon and others, '04; Afonso and others, '02). Thus, CTSB and CTSL may be involved in trophoblast invasion if, when they are secreted, they reach the extracellular space of the endometrium and are present in an active state. Treatment of mice with E-64 on Day 4.5–5.5 of pregnancy, after the onset of implantation, severely impairs implantation and decidualization (Afonso and others, '97). Since this compound is an inhibitor of cysteine proteinases (Barrett and others, '82; Barrett and others, '81), it was speculated that the effects of E-64 were due to the inhibition of trophoblast-derived CTSB and CTSL. However, knockout mice deficient in either or both CTSB and CTSL are viable and fertile (Halangk and others, '00; Stypmann and others, '02; Felbor and others, '02).
The expression of several C1A clan cathepsin inhibitors is observable on the maternal side in the rodent endometrium during decidualization (Fig. 2). The cystatin 3 gene (Cst3) encodes low molecular weight protein (CST3) that is secreted by many cell types; it inhibits extracellular C1 cysteine proteinases, including CTSB and CTSL (Barrett and others, '84; Abrahamson and others, '03; Abrahamson and others, '86; Abrahamson and others, '90; Hall and others, '95; Tavera and others, '90). In the mouse and rat, Cst3 expression increases in the endometrium following decidualization onset (Afonso and others, '97; Quinn and others, '06). However, to the best of our knowledge, there is currently no published experimental evidence clearly demonstrating a difference in expression between the decidua and deciduoma in these species. The cytotoxic T lymphocyte-associated protein 2 beta (Ctla2b) gene encodes a second inhibitor of cysteine proteinases, including CTSB and CTSL, which are found in the mouse endometrium during decidualization (Cheon and others, '04). Expression of this gene is induced in response to an artificial-deciduogenic stimulus (Cheon and others, '04); therefore paracrine factors from the conceptus are not required. During decidualization, other modulators in the uterus also directly or indirectly influence cathepsin activity secreted from the trophoblast cells. For example, the cathepsin D gene (Ctsd) encodes an aspartyl proteinase that is also produced by trophoblast giant cells (Afonso and others, '99) as well as in the endometrium (Elangovan and Moulton, '80; Moulton, '74). Although levels of this protein are dramatically down-regulated during decidualization (Elangovan and Moulton, '80; Moulton, '74), it reportedly inactivates the anti-proteolytic activity of CST3 (Lenarcic and others, '91). Finally, alpha-2-macroglobulin (A2m) is also expressed in the rodent endometrium (He and others, '05; Gu and others, '92). The protein encoded by this gene inhibits CTSB and CTSL activities in other tissues (Buttle and others, '91; Peloille and others, '97). Therefore, the control of trophoblast-secreted cathepsin activity in the uterus during decidualization might be quite complex.
Several observations suggest that during decidualization Cst3 expression in the rodent endometrium is induced by paracrine signals from the conceptus (Table 1). First, as the mouse endometrium undergoes decidualization, mRNA and protein levels increase continuously (Afonso and others, '97). Second, Cst3 expression is not detectable in mouse trophoblast giant and ectoplacental cone cells. The highest level of Cst3 expression has been detected in the adjacent decidual cells (Afonso and others, '97). Third, mouse endometrial decidual cells isolated from Day 7.5 pregnant uteri express Cst3 in vitro; this expression pattern is significantly enhanced upon co-culture with ectoplacental cone cells (Afonso and others, '02), suggesting that these cone cells might secrete a paracrine factor that induces decidual cell Cst3 expression. Although transforming growth factor beta (TGFβ) and epidermal growth factor (EGF) signaling can control Cst3 expression by decidual cells (Afonso and others, '02), the exact identity of the paracrine factor(s) involved remains undetermined. Notably, there is some evidence suggesting that the conceptus of non-rodent species may also control Cst3 expression in the uterus during implantation. For example, IFNτ is secreted by sheep trophoblast cells and appears to stimulate endometrial Cst3 expression in the sheep uterus during implantation (Song and others, '06). Currently it is unknown whether rodent giant or ectoplacental cone cell-derived type I IFNs play a role in stimulating Cst3 expression in rodent decidual cells. However, Cst3 knockout mice have normal fertility capacity, indicating that Cst3 is not absolutely required for successful pregnancy (Huh and others, '99).
CONSERVATION AMONG MAMMALIAN SPECIES?
Throughout this review, we have discussed certain potentially common features regarding the conceptus effects on the endometrium in non-rodent species. It is surprising, however, that common features exist between some of these species, because they exhibit different modes of implantation and placentation. Trophoblast IFNτ is a major regulator of endometrial function during implantation in ruminants, while chorionic gonadotrophin is a major regulator in humans and some other primates. Although mice, humans and baboons apparently do not encode IFNτ in their genomes, another type I IFN from the trophoblast giant cells in these species may control Isg15 expression in the endometrium during decidualization. Since several other type I IFN-regulated genes are also controlled by IFNτ in ungulates, it would be interesting to identify the potential type I IFN(s) responsible for this effect in rodents. The effect of the rodent trophoblast giant cells on the expression of the type I IFN-regulated genes in the endometrium needs further assessment. Type I IFN-induced genes may play an important role in implantation in mammals in general.
SUMMARY AND PERSPECTIVE
Mammalian species have developed a spectrum of strategies to undergo implantation. In all mammalian species it is likely that bi-directional paracrine signaling between the conceptus and endometrium plays an important role throughout pregnancy. The focus of this review was to cover what is currently known on the effects of conceptus-induced paracrine signals on the endometrium while it is undergoing decidualization in rodents. Current evidence indicates that such signals exist in rodents and exert their effects on PDZ formation, endometrial stromal cell gene expression, endometrial proteinase-antiproteinase balance, vascular changes, and immune cell populations in the endometrium during decidualization. It appears that trophoblast cells, especially the giant cells, may be the major source of paracrine factors that exert at least some of these effects. An alternative explanation is that paracrine signals from the conceptus play a negligible role in decidualization and merely the mechanical stimulus provided by the conceptus is all that is required to set into motion all of the requisite changes in endometrial gene expression for normal decidualization. At this time we feel that more work is required to understand fully whether these paracrine effects exist and if they play a role in decidualization; such work will require genetic models to identify and test the paracrine factors and their targets in the endometrium during decidualization.
Coordination of embryo development, decidualization and placenta formation is important for successful pregnancy. It is believed that abnormalities in the paracrine interactions between trophoblast cells and the endometrium can lead to complications later in pregnancy, abnormal conceptus development, or even pregnancy loss in humans (Heazell and others, '08; Huppertz, '08; Goldman-Wohl and Yagel, '07). For example, during early human pregnancy, preeclampsia and intrauterine growth restriction are believed to be the results of improper decidual processes leading to improper trophoblast-maternal endothelial cell signaling and shallow placentation (Brosens and others, '02). Studying the biology of conceptus-endometrial communications during decidualization in other species may help us understand potential causes of and treatments for clinically relevant issues in human pregnancy. For obvious reasons, these processes are challenging to study in vivo during human implantation. Studies of other species may provide valuable insights into conceptus-endometrial interactions during decidualization. The rodent provides a good model of decidualization due to well-elaborated and numerous powerful genetic approaches available in these species. Such approaches include transgenesis and tissue-specific plus whole animal gene knockout techniques, which are particularly useful in the mouse.
Acknowledgments
We thank NIH for current funding in the area of research that falls under the topic of this review (RO1-HD049010 to BB).
REFERENCES
- Abrahamsohn PA, Zorn TM. Implantation and decidualization in rodents. J Exp Zool. 1993;266(6):603–628. doi: 10.1002/jez.1402660610. [DOI] [PubMed] [Google Scholar]
- Abrahamsohn PA, Zorn TM, Oliveira SF. Extracellular matrix of the mouse endometrium during decidualization. Mem Inst Oswaldo Cruz. 1991;86 Suppl 3:23–25. doi: 10.1590/s0074-02761991000700004. [DOI] [PubMed] [Google Scholar]
- Abrahamsohn PA, Zorn TMT, Oliveira SF. Decidua in rodents. In: Glasser SR, Aplin JD, Giudice LC, Tabibzadeh S, editors. The Endometrium. New York: Taylor & Francis; 2002. pp. 279–293. [Google Scholar]
- Abrahamson M, Alvarez-Fernandez M, Nathanson CM. Cystatins. Biochem Soc Symp. 2003;(70):179–199. doi: 10.1042/bss0700179. [DOI] [PubMed] [Google Scholar]
- Abrahamson M, Barrett AJ, Salvesen G, Grubb A. Isolation of six cysteine proteinase inhibitors from human urine. Their physicochemical and enzyme kinetic properties and concentrations in biological fluids. J Biol Chem. 1986;261(24):11282–11289. [PubMed] [Google Scholar]
- Abrahamson M, Olafsson I, Palsdottir A, Ulvsback M, Lundwall A, Jensson O, Grubb A. Structure and expression of the human cystatin C gene. Biochem J. 1990;268(2):287–294. doi: 10.1042/bj2680287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aebersold R, Mann M. Mass spectrometry-based proteomics. Nature. 2003;422(6928):198–207. doi: 10.1038/nature01511. [DOI] [PubMed] [Google Scholar]
- Afonso S, Romagnano L, Babiarz B. The expression and function of cystatin C and cathepsin B and cathepsin L during mouse embryo implantation and placentation. Development. 1997;124(17):3415–3425. doi: 10.1242/dev.124.17.3415. [DOI] [PubMed] [Google Scholar]
- Afonso S, Romagnano L, Babiarz B. Expression of cathepsin proteinases by mouse trophoblast in vivo and in vitro. Dev Dyn. 1999;216(4–5):374–384. doi: 10.1002/(SICI)1097-0177(199912)216:4/5<374::AID-DVDY6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Afonso S, Tovar C, Romagnano L, Babiarz B. Control and expression of cystatin C by mouse decidual cultures. Mol Reprod Dev. 2002;61(2):155–163. doi: 10.1002/mrd.1142. [DOI] [PubMed] [Google Scholar]
- Ain R, Canham LN, Soares MJ. Gestation stage-dependent intrauterine trophoblast cell invasion in the rat and mouse: novel endocrine phenotype and regulation. Dev Biol. 2003a;260(1):176–190. doi: 10.1016/s0012-1606(03)00210-0. [DOI] [PubMed] [Google Scholar]
- Ain R, Dai G, Dunmore JH, Godwin AR, Soares MJ. A prolactin family paralog regulates reproductive adaptations to a physiological stressor. Proc Natl Acad Sci U S A. 2004;101(47):16543–16548. doi: 10.1073/pnas.0406185101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ain R, Konno T, Canham LN, Soares MJ. Phenotypic analysis of the rat placenta. Methods Mol Med. 2006;121:295–313. doi: 10.1385/1-59259-983-4:293. [DOI] [PubMed] [Google Scholar]
- Ain R, Tash JS, Soares MJ. Prolactin-like protein-A is a functional modulator of natural killer cells at the maternal-fetal interface. Mol Cell Endocrinol. 2003b;204(1–2):65–74. doi: 10.1016/s0303-7207(03)00125-4. [DOI] [PubMed] [Google Scholar]
- Alam SM, Ain R, Konno T, Ho-Chen JK, Soares MJ. The rat prolactin gene family locus: species-specific gene family expansion. Mamm Genome. 2006;17(8):858–877. doi: 10.1007/s00335-006-0010-1. [DOI] [PubMed] [Google Scholar]
- Alexander CM, Hansell EJ, Behrendtsen O, Flannery ML, Kishnani NS, Hawkes SP, Werb Z. Expression and function of matrix metalloproteinases and their inhibitors at the maternal-embryonic boundary during mouse embryo implantation. Development. 1996;122(6):1723–1736. doi: 10.1242/dev.122.6.1723. [DOI] [PubMed] [Google Scholar]
- Andersen JB, Hassel BA. The interferon regulated ubiquitin-like protein, ISG15, in tumorigenesis: friend or foe? Cytokine Growth Factor Rev. 2006;17(6):411–421. doi: 10.1016/j.cytogfr.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Andrade CG, Abrahamsohn PA, Godinho F, Samuel E, Zorn TM. Death and replacement of uterine epithelial cells during oil-induced deciduoma development in the mouse. Anat Rec. 1996;244(3):316–326. doi: 10.1002/(SICI)1097-0185(199603)244:3<316::AID-AR4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Archer DF. Endometrial bleeding during hormone therapy: the effect of progestogens. Maturitas. 2007;57(1):71–76. doi: 10.1016/j.maturitas.2007.02.022. [DOI] [PubMed] [Google Scholar]
- Arrighi S, Cremonesi F, Bosi G, Groppetti D, Pecile A. Characterization of a population of unique granular lymphocytes in a bitch deciduoma, using a panel of histo- and immunohistochemical markers. Vet Pathol. 2007;44(4):521–524. doi: 10.1354/vp.44-4-521. [DOI] [PubMed] [Google Scholar]
- Ashkar AA, Black GP, Wei Q, He H, Liang L, Head JR, Croy BA. Assessment of requirements for IL-15 and IFN regulatory factors in uterine NK cell differentiation and function during pregnancy. J Immunol. 2003;171(6):2937–2944. doi: 10.4049/jimmunol.171.6.2937. [DOI] [PubMed] [Google Scholar]
- Ashkar AA, Croy BA. Functions of uterine natural killer cells are mediated by interferon gamma production during murine pregnancy. Semin Immunol. 2001;13(4):235–241. doi: 10.1006/smim.2000.0319. [DOI] [PubMed] [Google Scholar]
- Austin KJ, Bany BM, Belden EL, Rempel LA, Cross JC, Hansen TR. Interferon-stimulated gene-15 (Isg15) expression is up-regulated in the mouse uterus in response to the implanting conceptus. Endocrinology. 2003;144(7):3107–3113. doi: 10.1210/en.2002-0031. [DOI] [PubMed] [Google Scholar]
- Austin KJ, Ward SK, Teixeira MG, Dean VC, Moore DW, Hansen TR. Ubiquitin cross-reactive protein is released by the bovine uterus in response to interferon during early pregnancy. Biol Reprod. 1996;54(3):600–606. doi: 10.1095/biolreprod54.3.600. [DOI] [PubMed] [Google Scholar]
- Bany BM, Cross JC. Post-implantation mouse conceptuses produce paracrine signals that regulate the uterine endometrium undergoing decidualization. Dev Biol. 2006;294(2):445–456. doi: 10.1016/j.ydbio.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Barrett AJ, Davies ME, Grubb A. The place of human gamma-trace (cystatin C) amongst the cysteine proteinase inhibitors. Biochem Biophys Res Commun. 1984;120(2):631–636. doi: 10.1016/0006-291x(84)91302-0. [DOI] [PubMed] [Google Scholar]
- Barrett AJ, Kembhavi AA, Brown MA, Kirschke H, Knight CG, Tamai M, Hanada K. L-trans-Epoxysuccinyl-leucylamido(4-guanidino)butane (E-64) and its analogues as inhibitors of cysteine proteinases including cathepsins B, H and L. Biochem J. 1982;201(1):189–198. doi: 10.1042/bj2010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett AJ, Kembhavi AA, Hanada K. E-64 [L-trans-epoxysuccinyl-leucyl-amido(4-guanidino)butane] and related epoxides as inhibitors of cysteine proteinases. Acta Biol Med Ger. 1981;40(10–11):1513–1517. [PubMed] [Google Scholar]
- Bebington C, Bell SC, Doherty FJ, Fazleabas AT, Fleming SD. Localization of ubiquitin and ubiquitin cross-reactive protein in human and baboon endometrium and decidua during the menstrual cycle and early pregnancy. Biol Reprod. 1999a;60(4):920–928. doi: 10.1095/biolreprod60.4.920. [DOI] [PubMed] [Google Scholar]
- Bebington C, Doherty FJ, Fleming SD. Ubiquitin cross-reactive protein gene expression is increased in decidualized endometrial stromal cells at the initiation of pregnancy. Mol Hum Reprod. 1999b;5(10):966–972. doi: 10.1093/molehr/5.10.966. [DOI] [PubMed] [Google Scholar]
- Bilinski MJ, Thorne JG, Oh MJ, Leonard S, Murrant C, Tayade C, Croy BA. Uterine NK cells in murine pregnancy. Reprod Biomed Online. 2008;16(2):218–226. doi: 10.1016/s1472-6483(10)60577-9. [DOI] [PubMed] [Google Scholar]
- Brandon JM. Leucocyte distribution in the uterus during the preimplantation period of pregnancy and phagocyte recruitment to sites of blastocyst attachment in mice. J Reprod Fertil. 1993;98(2):567–576. doi: 10.1530/jrf.0.0980567. [DOI] [PubMed] [Google Scholar]
- Brix K, Dunkhorst A, Mayer K, Jordans S. Cysteine cathepsins: cellular roadmap to different functions. Biochimie. 2008;90(2):194–207. doi: 10.1016/j.biochi.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Brosens JJ, Pijnenborg R, Brosens IA. The myometrial junctional zone spiral arteries in normal and abnormal pregnancies: a review of the literature. Am J Obstet Gynecol. 2002;187(5):1416–1423. doi: 10.1067/mob.2002.127305. [DOI] [PubMed] [Google Scholar]
- Bulmer JN, Lash GE. Human uterine natural killer cells: a reappraisal. Mol Immunol. 2005;42(4):511–521. doi: 10.1016/j.molimm.2004.07.035. [DOI] [PubMed] [Google Scholar]
- Burnum KE, Tranguch S, Mi D, Daikoku T, Dey SK, Caprioli RM. Imaging Mass Spectrometry Reveals Unique Protein Profiles During Embryo Implantation. Endocrinology. 2008 doi: 10.1210/en.2008-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttle DJ, Abrahamson M, Burnett D, Mort JS, Barrett AJ, Dando PM, Hill SL. Human sputum cathepsin B degrades proteoglycan, is inhibited by alpha 2-macroglobulin and is modulated by neutrophil elastase cleavage of cathepsin B precursor and cystatin C. Biochem J. 1991;276(Pt 2):325–331. doi: 10.1042/bj2760325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell WJ, Deb S, Kwok SC, Joslin JA, Soares MJ. Differential expression of placental lactogen-II and prolactin-like protein-A in the rat chorioallantoic placenta. Endocrinology. 1989;125(3):1565–1574. doi: 10.1210/endo-125-3-1565. [DOI] [PubMed] [Google Scholar]
- Carney EW, Prideaux V, Lye SJ, Rossant J. Progressive expression of trophoblast-specific genes during formation of mouse trophoblast giant cells in vitro. Mol Reprod Dev. 1993;34(4):357–368. doi: 10.1002/mrd.1080340403. [DOI] [PubMed] [Google Scholar]
- Carson DD, Bagchi I, Dey SK, Enders AC, Fazleabas AT, Lessey BA, Yoshinaga K. Embryo implantation. Dev Biol. 2000;223(2):217–237. doi: 10.1006/dbio.2000.9767. [DOI] [PubMed] [Google Scholar]
- Chantakru S, Miller C, Roach LE, Kuziel WA, Maeda N, Wang WC, Evans SS, Croy BA. Contributions from self-renewal and trafficking to the uterine NK cell population of early pregnancy. J Immunol. 2002;168(1):22–28. doi: 10.4049/jimmunol.168.1.22. [DOI] [PubMed] [Google Scholar]
- Cheon YP, DeMayo FJ, Bagchi MK, Bagchi IC. Induction of cytotoxic T-lymphocyte antigen-2beta, a cysteine protease inhibitor in decidua: a potential regulator of embryo implantation. J Biol Chem. 2004;279(11):10357–10363. doi: 10.1074/jbc.M309434200. [DOI] [PubMed] [Google Scholar]
- Conneely OM, Mulac-Jericevic B, DeMayo F, Lydon JP, O'Malley BW. Reproductive functions of progesterone receptors. Recent Prog Horm Res. 2002;57:339–355. doi: 10.1210/rp.57.1.339. [DOI] [PubMed] [Google Scholar]
- Conneely OM, Mulac-Jericevic B, Lydon JP, De Mayo FJ. Reproductive functions of the progesterone receptor isoforms: lessons from knock-out mice. Mol Cell Endocrinol. 2001;179(1–2):97–103. doi: 10.1016/s0303-7207(01)00465-8. [DOI] [PubMed] [Google Scholar]
- Cross JC, Hemberger M, Lu Y, Nozaki T, Whiteley K, Masutani M, Adamson SL. Trophoblast functions, angiogenesis and remodeling of the maternal vasculature in the placenta. Mol Cell Endocrinol. 2002;187(1–2):207–212. doi: 10.1016/s0303-7207(01)00703-1. [DOI] [PubMed] [Google Scholar]
- Croy AB, van den Heuvel MJ, Borzychowski AM, Tayade C. Uterine natural killer cells: a specialized differentiation regulated by ovarian hormones. Immunol Rev. 2006;214:161–185. doi: 10.1111/j.1600-065X.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- Croy BA, Ashkar AA, Minhas K, Greenwood JD. Can murine uterine natural killer cells give insights into the pathogenesis of preeclampsia? J Soc Gynecol Investig. 2000a;7(1):12–20. doi: 10.1016/s1071-5576(99)00049-0. [DOI] [PubMed] [Google Scholar]
- Croy BA, Di Santo JP, Greenwood JD, Chantakru S, Ashkar AA. Transplantation into genetically alymphoid mice as an approach to dissect the roles of uterine natural killer cells during pregnancy--a review. Placenta. 2000b;21 Suppl A:S77–S80. doi: 10.1053/plac.1999.0518. [DOI] [PubMed] [Google Scholar]
- Croy BA, Esadeg S, Chantakru S, van den Heuvel M, Paffaro VA, He H, Black GP, Ashkar AA, Kiso Y, Zhang J. Update on pathways regulating the activation of uterine Natural Killer cells, their interactions with decidual spiral arteries and homing of their precursors to the uterus. J Reprod Immunol. 2003a;59(2):175–191. doi: 10.1016/s0165-0378(03)00046-9. [DOI] [PubMed] [Google Scholar]
- Croy BA, He H, Esadeg S, Wei Q, McCartney D, Zhang J, Borzychowski A, Ashkar AA, Black GP, Evans SS, Chantakru S, van den Heuvel M, Paffaro VA, Jr, Yamada AT. Uterine natural killer cells: insights into their cellular and molecular biology from mouse modelling. Reproduction. 2003b;126(2):149–160. doi: 10.1530/rep.0.1260149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry TE, Jr, Osteen KG. The matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr Rev. 2003;24(4):428–465. doi: 10.1210/er.2002-0005. [DOI] [PubMed] [Google Scholar]
- Daikoku T, Tranguch S, Friedman DB, Das SK, Smith DF, Dey SK. Proteomic analysis identifies immunophilin FK506 binding protein 4 (FKBP52) as a downstream target of Hoxa10 in the periimplantation mouse uterus. Mol Endocrinol. 2005;19(3):683–697. doi: 10.1210/me.2004-0332. [DOI] [PubMed] [Google Scholar]
- Dao CT, Zhang DE. ISG15: a ubiquitin-like enigma. Front Biosci. 2005;10:2701–2722. doi: 10.2741/1730. [DOI] [PubMed] [Google Scholar]
- De M, Wood GW. Analysis of the number and distribution of macrophages, lymphocytes, and granulocytes in the mouse uterus from implantation through parturition. J Leukoc Biol. 1991;50(4):381–392. doi: 10.1002/jlb.50.4.381. [DOI] [PubMed] [Google Scholar]
- Deanesly R. The differentiation of the decidua at ovo-implantation in the guinea-pig contrasted with that of the traumatic deciduoma. J Reprod Fertil. 1971;26(1):91–97. doi: 10.1530/jrf.0.0260091. [DOI] [PubMed] [Google Scholar]
- Deb S, Soares MJ. Characterization of placental prolactin-like protein-A in intracellular and extracellular compartments. Mol Cell Endocrinol. 1990;74(2):163–172. doi: 10.1016/0303-7207(90)90118-r. [DOI] [PubMed] [Google Scholar]
- Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T, Wang H. Molecular cues to implantation. Endocr Rev. 2004;25(3):341–373. doi: 10.1210/er.2003-0020. [DOI] [PubMed] [Google Scholar]
- Dietl J, Honig A, Kammerer U, Rieger L. Natural killer cells and dendritic cells at the human feto-maternal interface: an effective cooperation? Placenta. 2006;27(4–5):341–347. doi: 10.1016/j.placenta.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Dosiou C, Giudice LC. Natural killer cells in pregnancy and recurrent pregnancy loss: endocrine and immunologic perspectives. Endocr Rev. 2005;26(1):44–62. doi: 10.1210/er.2003-0021. [DOI] [PubMed] [Google Scholar]
- Elangovan S, Moulton BC. Blastocyst implantation in the rat and the immunohistochemical distribution and rate of synthesis of uterine lysosomal cathepsin D. Biol Reprod. 1980;23(3):663–668. doi: 10.1095/biolreprod23.3.663. [DOI] [PubMed] [Google Scholar]
- Enders AC, Schlafke S. A morphological analysis of the early implantation stages in the rat. Am J Anat. 1967;120(2):185–225. [Google Scholar]
- Enders AC, Schlafke S. Cytological aspects of trophoblast-uterine interaction in early implantation. Am J Anat. 1969;125(1):1–29. doi: 10.1002/aja.1001250102. [DOI] [PubMed] [Google Scholar]
- Enders AC, Schlafke S. Implantation in nonhuman primates and in human. In: Dukelow WR, Erwin J, editors. Reproduction and Development. New York: Alan R. Liss; 1986. pp. 291–310. [Google Scholar]
- Engelhardt H, Croy BA, King GJ. Evaluation of natural killer cell recruitment to embryonic attachment sites during early porcine pregnancy. Biol Reprod. 2002;66(4):1185–1192. doi: 10.1095/biolreprod66.4.1185. [DOI] [PubMed] [Google Scholar]
- Engelhardt H, King GJ. Uterine natural killer cells in species with epitheliochorial placentation. Nat Immun. 1996;15(1):53–69. [PubMed] [Google Scholar]
- Fan M, Long X, Bailey JA, Reed CA, Osborne E, Gize EA, Kirk EA, Bigsby RM, Nephew KP. The activating enzyme of NEDD8 inhibits steroid receptor function. Mol Endocrinol. 2002;16(2):315–330. doi: 10.1210/mend.16.2.0778. [DOI] [PubMed] [Google Scholar]
- Fang Y, Lepont P, Fassett JT, Ford SP, Mubaidin A, Hamilton RT, Nilsen-Hamilton M. Signaling between the placenta and the uterus involving the mitogen-regulated protein/proliferins. Endocrinology. 1999;140(11):5239–5249. doi: 10.1210/endo.140.11.7142. [DOI] [PubMed] [Google Scholar]
- Felbor U, Kessler B, Mothes W, Goebel HH, Ploegh HL, Bronson RT, Olsen BR. Neuronal loss and brain atrophy in mice lacking cathepsins B and L. Proc Natl Acad Sci U S A. 2002;99(12):7883–7888. doi: 10.1073/pnas.112632299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn CA. The implantation reaction. In: Wynn RM, editor. Biology of the Uterus. New York: Plenum Press; 1977. pp. 245–308. [Google Scholar]
- Finn CA, McLaren A. A study of the early stages of implantation in mice. J Reprod Fertil. 1967;13(2):259–267. doi: 10.1530/jrf.0.0130259. [DOI] [PubMed] [Google Scholar]
- Finn CA, Pope MD. Infiltration of neutrophil polymorphonuclear leucocytes into the endometrial stroma at the time of implantation of ova and the initiation of the oil decidual cell reaction in mice. J Reprod Fertil. 1991;91(1):365–369. doi: 10.1530/jrf.0.0910365. [DOI] [PubMed] [Google Scholar]
- Gellersen B, Brosens IA, Brosens JJ. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med. 2007;25(6):445–453. doi: 10.1055/s-2007-991042. [DOI] [PubMed] [Google Scholar]
- Goldman-Wohl DS, Yagel S. Examination of distinct fetal and maternal molecular pathways suggests a mechanism for the development of preeclampsia. J Reprod Immunol. 2007;76(1–2):54–60. doi: 10.1016/j.jri.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Gorg A, Weiss W, Dunn MJ. Current two-dimensional electrophoresis technology for proteomics. Proteomics. 2004;4(12):3665–3685. doi: 10.1002/pmic.200401031. [DOI] [PubMed] [Google Scholar]
- Groskopf JC, Syu LJ, Saltiel AR, Linzer DI. Proliferin induces endothelial cell chemotaxis through a G protein-coupled, mitogen-activated protein kinase-dependent pathway. Endocrinology. 1997;138(7):2835–2840. doi: 10.1210/endo.138.7.5276. [DOI] [PubMed] [Google Scholar]
- Gu Y, Jayatilak PG, Parmer TG, Gauldie J, Fey GH, Gibori G. Alpha 2-macroglobulin expression in the mesometrial decidua and its regulation by decidual luteotropin and prolactin. Endocrinology. 1992;131(3):1321–1328. doi: 10.1210/endo.131.3.1380439. [DOI] [PubMed] [Google Scholar]
- Guimond MJ, Wang B, Croy BA. Engraftment of bone marrow from severe combined immunodeficient (SCID) mice reverses the reproductive deficits in natural killer cell-deficient tg epsilon 26 mice. J Exp Med. 1998;187(2):217–223. doi: 10.1084/jem.187.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimond MJ, Wang B, Fujita J, Terhorst C, Croy BA. Pregnancy-associated uterine granulated metrial gland cells in mutant and transgenic mice. Am J Reprod Immunol. 1996;35(6):501–509. doi: 10.1111/j.1600-0897.1996.tb00049.x. [DOI] [PubMed] [Google Scholar]
- Hafez ESE. Functional histology in reproduction. In: Hafez ESE, editor. Reproduction in Farm Animals. 5th ed. Philadelphia: Lea & Febiger; 1987. pp. 65–82. [Google Scholar]
- Halangk W, Lerch MM, Brandt-Nedelev B, Roth W, Ruthenbuerger M, Reinheckel T, Domschke W, Lippert H, Peters C, Deussing J. Role of cathepsin B in intracellular trypsinogen activation and the onset of acute pancreatitis. J Clin Invest. 2000;106(6):773–781. doi: 10.1172/JCI9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A, Hakansson K, Mason RW, Grubb A, Abrahamson M. Structural basis for the biological specificity of cystatin C. Identification of leucine 9 in the N-terminal binding region as a selectivity-conferring residue in the inhibition of mammalian cysteine peptidases. J Biol Chem. 1995;270(10):5115–5121. doi: 10.1074/jbc.270.10.5115. [DOI] [PubMed] [Google Scholar]
- Hansen TR, Austin KJ, Johnson GA. Transient ubiquitin cross-reactive protein gene expression in the bovine endometrium. Endocrinology. 1997;138(11):5079–5082. doi: 10.1210/endo.138.11.5655. [DOI] [PubMed] [Google Scholar]
- Hashizume K. Analysis of uteroplacental-specific molecules and their functions during implantation and placentation in the bovine. J Reprod Dev. 2007;53(1):1–11. doi: 10.1262/jrd.18123. [DOI] [PubMed] [Google Scholar]
- He H, McCartney DJ, Wei Q, Esadeg S, Zhang J, Foster RA, Hayes MA, Tayade C, Van Leuven F, Croy BA. Characterization of a murine alpha 2 macroglobulin gene expressed in reproductive and cardiovascular tissue. Biol Reprod. 2005;72(2):266–275. doi: 10.1095/biolreprod.104.029835. [DOI] [PubMed] [Google Scholar]
- Head JR. Uterine natural killer cells during pregnancy in rodents. Nat Immun. 1996;15(1):7–21. [PubMed] [Google Scholar]
- Heazell AEP, Nugent J, Jones RL, Harris LK, Baker PN. Preeclampsia and intrauterine growth restriction. In: Aplin JD, Fazleabas AT, Glasser SR, Giudice LC, editors. The Endometrium: Molecular, Cellular and Clinical Perspectives. second ed. London: Informa UK Ltd; 2008. pp. 745–760. [Google Scholar]
- Hemberger M, Nozaki T, Masutani M, Cross JC. Differential expression of angiogenic and vasodilatory factors by invasive trophoblast giant cells depending on depth of invasion. Dev Dyn. 2003;227(2):185–191. doi: 10.1002/dvdy.10291. [DOI] [PubMed] [Google Scholar]
- Herington JL, Bany BM. The conceptus increases secreted phosphoprotein 1 gene expression in the mouse uterus during the progression of decidualization mainly due to its effects on uterine natural killer cells. Reproduction. 2007a;133(6):1213–1221. doi: 10.1530/REP-07-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herington JL, Bany BM. Effect of the conceptus on uterine natural killer cell numbers and function in the mouse uterus during decidualization. Biol Reprod. 2007b;76(4):579–588. doi: 10.1095/biolreprod.106.056630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann J, Lerman LO, Lerman A. Ubiquitin and ubiquitin-like proteins in protein regulation. Circ Res. 2007;100(9):1276–1291. doi: 10.1161/01.RES.0000264500.11888.f0. [DOI] [PubMed] [Google Scholar]
- Hess AP, Hamilton AE, Talbi S, Dosiou C, Nyegaard M, Nayak N, Genbecev-Krtolica O, Mavrogianis P, Ferrer K, Kruessel J, Fazleabas AT, Fisher SJ, Giudice LC. Decidual stromal cell response to paracrine signals from the trophoblast: amplification of immune and angiogenic modulators. Biol Reprod. 2007;76(1):102–117. doi: 10.1095/biolreprod.106.054791. [DOI] [PubMed] [Google Scholar]
- Huh CG, Hakansson K, Nathanson CM, Thorgeirsson UP, Jonsson N, Grubb A, Abrahamson M, Karlsson S. Decreased metastatic spread in mice homozygous for a null allele of the cystatin C protease inhibitor gene. Mol Pathol. 1999;52(6):332–340. doi: 10.1136/mp.52.6.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppertz B. Placental origins of preeclampsia: challenging the current hypothesis. Hypertension. 2008;51(4):970–975. doi: 10.1161/HYPERTENSIONAHA.107.107607. [DOI] [PubMed] [Google Scholar]
- Imakawa K, Chang KT, Christenson RK. Pre-implantation conceptus and maternal uterine communications: molecular events leading to successful implantation. J Reprod Dev. 2004;50(2):155–169. doi: 10.1262/jrd.50.155. [DOI] [PubMed] [Google Scholar]
- Jackson D, Volpert OV, Bouck N, Linzer DI. Stimulation and inhibition of angiogenesis by placental proliferin and proliferin-related protein. Science. 1994;266(5190):1581–1584. doi: 10.1126/science.7527157. [DOI] [PubMed] [Google Scholar]
- Johnson GA. Uterine stromal cell differentiation in non-decidualizaing species. In: Aplin JD, Fazleabas AT, Glasser SR, Giudice LC, editors. The Endometrium: Molecular, Cellular and Clinical Perspectives. Second ed. London: Informa UK Ltd; 2008. pp. 409–421. [Google Scholar]
- Johnson GA, Austin KJ, Van Kirk EA, Hansen TR. Pregnancy and interferon-tau induce conjugation of bovine ubiquitin cross-reactive protein to cytosolic uterine proteins. Biol Reprod. 1998;58(4):898–904. doi: 10.1095/biolreprod58.4.898. [DOI] [PubMed] [Google Scholar]
- Johnson GA, Burghardt RC, Joyce MM, Spencer TE, Bazer FW, Pfarrer C, Gray CA. Osteopontin expression in uterine stroma indicates a decidualization-like differentiation during ovine pregnancy. Biol Reprod. 2003;68(6):1951–1958. doi: 10.1095/biolreprod.102.012948. [DOI] [PubMed] [Google Scholar]
- Johnson GA, Spencer TE, Burghardt RC, Joyce MM, Bazer FW. Interferon-tau and progesterone regulate ubiquitin cross-reactive protein expression in the ovine uterus. Biol Reprod. 2000;62(3):622–627. doi: 10.1095/biolreprod62.3.622. [DOI] [PubMed] [Google Scholar]
- Johnson GA, Spencer TE, Hansen TR, Austin KJ, Burghardt RC, Bazer FW. Expression of the interferon tau inducible ubiquitin cross-reactive protein in the ovine uterus. Biol Reprod. 1999;61(1):312–318. doi: 10.1095/biolreprod61.1.312. [DOI] [PubMed] [Google Scholar]
- Johnson MH. Implantation and establishment of the placenta. In: Johnson MH, editor. Essential Reproduction. Sixth ed. Malden: Blackwell Publishing; 2007. pp. 189–211. [Google Scholar]
- Jones MC, Fusi L, Higham JH, Abdel-Hafiz H, Horwitz KB, Lam EW, Brosens JJ. Regulation of the SUMO pathway sensitizes differentiating human endometrial stromal cells to progesterone. Proc Natl Acad Sci U S A. 2006;103(44):16272–16277. doi: 10.1073/pnas.0603002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce MM, Gonzalez JF, Lewis S, Woldesenbet S, Burghardt RC, Newton GR, Johnson GA. Caprine uterine and placental osteopontin expression is distinct among epitheliochorial implanting species. Placenta. 2005;26(2–3):160–170. doi: 10.1016/j.placenta.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Kashiwagi A, DiGirolamo CM, Kanda Y, Niikura Y, Esmon CT, Hansen TR, Shioda T, Pru JK. The postimplantation embryo differentially regulates endometrial gene expression and decidualization. Endocrinology. 2007;148(9):4173–4184. doi: 10.1210/en.2007-0268. [DOI] [PubMed] [Google Scholar]
- Kaufman MH. The Atlas of Mouse Development. Amsterdam: Academic Press; 2002. p. 525. [Google Scholar]
- Kellas LM. The placenta and foetal membranes of the antelope Ourebia ourebi (Zimmermann) Acta Anat. 1966;64:390–445. [Google Scholar]
- Kennedy TG, Gillio-Meina C, Phang SH. Prostaglandins and the initiation of blastocyst implantation and decidualization. Reproduction. 2007;134(5):635–643. doi: 10.1530/REP-07-0328. [DOI] [PubMed] [Google Scholar]
- Kerscher O, Felberbaum R, Hochstrasser M. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu Rev Cell Dev Biol. 2006;22:159–180. doi: 10.1146/annurev.cellbio.22.010605.093503. [DOI] [PubMed] [Google Scholar]
- Kimber SJ, Spanswick C. The adhesion cascade. In: Glasser SR, Aplin JD, Giudice LC, Tabibzadeh S, editors. The Endometrium. New York: Taylor & Francis; 2002. pp. 229–248. [Google Scholar]
- King A, Burrows T, Loke YW. Human uterine natural killer cells. Nat Immun. 1996;15(1):41–52. [PubMed] [Google Scholar]
- King GJ, Atkinson BA, Robertson HA. Development of the bovine placentome from days 20 to 29 of gestation. J Reprod Fertil. 1980;59(1):95–100. doi: 10.1530/jrf.0.0590095. [DOI] [PubMed] [Google Scholar]
- Kleinfeld RG, Morrow H, DeFeo VJ. Intercellular junctions between decidual cells in the growing deciduoma of the pseudopregnant rat uterus. Biol Reprod. 1976;15(5):593–603. doi: 10.1095/biolreprod15.5.593. [DOI] [PubMed] [Google Scholar]
- Krehbiel RH. Cytological studies of the decidual reaction in the rat during early pregnancy and in the production of deciduomata. Physiological Zoölogy. 1937;10(2):212–234. [Google Scholar]
- Kurihara I, Lee DK, Petit FG, Jeong J, Lee K, Lydon JP, DeMayo FJ, Tsai MJ, Tsai SY. COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS Genet. 2007;3(6):e102. doi: 10.1371/journal.pgen.0030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leco KJ, Edwards DR, Schultz GA. Tissue inhibitor of metalloproteinases-3 is the major metalloproteinase inhibitor in the decidualizing murine uterus. Mol Reprod Dev. 1996;45(4):458–465. doi: 10.1002/(SICI)1098-2795(199612)45:4<458::AID-MRD8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Lee KY, Jeong JW, Tsai SY, Lydon JP, DeMayo FJ. Mouse models of implantation. Trends Endocrinol Metab. 2007a;18(6):234–239. doi: 10.1016/j.tem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Lee KY, Jeong JW, Wang J, Ma L, Martin JF, Tsai SY, Lydon JP, DeMayo FJ. Bmp2 is critical for the murine uterine decidual response. Mol Cell Biol. 2007b;27(15):5468–5478. doi: 10.1128/MCB.00342-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenarcic B, Krasovec M, Ritonja A, Olafsson I, Turk V. Inactivation of human cystatin C and kininogen by human cathepsin D. FEBS Lett. 1991;280(2):211–215. doi: 10.1016/0014-5793(91)80295-e. [DOI] [PubMed] [Google Scholar]
- Leonard S, Murrant C, Tayade C, van den Heuvel M, Watering R, Croy BA. Mechanisms regulating immune cell contributions to spiral artery modification -- facts and hypotheses -- a review. Placenta. 2006;27 Suppl A:S40–S46. doi: 10.1016/j.placenta.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Licht P, Fluhr H, Neuwinger J, Wallwiener D, Wildt L. Is human chorionic gonadotropin directly involved in the regulation of human implantation? Mol Cell Endocrinol. 2007;269(1–2):85–92. doi: 10.1016/j.mce.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Lin J, Poole J, Linzer DI. Three new members of the mouse prolactin/growth hormone family are homologous to proteins expressed in the rat. Endocrinology. 1997;138(12):5541–5549. doi: 10.1210/endo.138.12.5626. [DOI] [PubMed] [Google Scholar]
- Liu CC, Young JD. Uterine natural killer cells in the pregnant uterus. Adv Immunol. 2001;79:297–329. doi: 10.1016/s0065-2776(01)79007-4. [DOI] [PubMed] [Google Scholar]
- Loeb L. Uber die experimentelle erzeugung von knoten von deciduagewebe in dem uterus des meerschweinchens nach statgefundener copulation. Centralbl F Allg Path u Anat. 1907;18:563. [Google Scholar]
- Loeb L. The production of deciduomata and the relation between the ovaries and the formation of the decidua. J Am Med Assoc. 1908;50:1897–1901. [Google Scholar]
- Lundkvist O. Morphological studies of the stromal changes after artificial induction of the decidual reaction in rats. Cell Tissue Res. 1978;194(2):287–296. doi: 10.1007/BF00220395. [DOI] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O'Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9(18):2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- Ma GT, Linzer DI. GATA-2 restricts prolactin-like protein A expression to secondary trophoblast giant cells in the mouse. Biol Reprod. 2000;63(2):570–574. doi: 10.1095/biolreprod63.2.570. [DOI] [PubMed] [Google Scholar]
- Ma GT, Roth ME, Groskopf JC, Tsai FY, Orkin SH, Grosveld F, Engel JD, Linzer DI. GATA-2 and GATA-3 regulate trophoblast-specific gene expression in vivo. Development. 1997;124(4):907–914. doi: 10.1242/dev.124.4.907. [DOI] [PubMed] [Google Scholar]
- MacIntyre DM, Lim HC, Ryan K, Kimmins S, Small JA, MacLaren LA. Implantation-associated changes in bovine uterine expression of integrins and extracellular matrix. Biol Reprod. 2002;66(5):1430–1436. doi: 10.1095/biolreprod66.5.1430. [DOI] [PubMed] [Google Scholar]
- Martel D, Monier MN, Roche D, De Feo VJ, Psychoyos A. Hormonal dependence of the metrial gland: further studies on oestradiol and progesterone receptor levels in the rat. J Endocrinol. 1989;120(3):465–472. doi: 10.1677/joe.0.1200465. [DOI] [PubMed] [Google Scholar]
- McMaster MT, Dey SK, Andrews GK. Association of monocytes and neutrophils with early events of blastocyst implantation in mice. J Reprod Fertil. 1993;99(2):561–569. doi: 10.1530/jrf.0.0990561. [DOI] [PubMed] [Google Scholar]
- Milligan SR. The sensitivity of the uterus of the mouse and rat to intraluminal instillation. J Reprod Fertil. 1987;79(1):251–259. doi: 10.1530/jrf.0.0790251. [DOI] [PubMed] [Google Scholar]
- Milligan SR, Mirembe FM. Intraluminally injected oil induces changes in vascular permeability in the 'sensitized' and 'non-sensitized' uterus of the mouse. J Reprod Fertil. 1985;74(1):95–104. doi: 10.1530/jrf.0.0740095. [DOI] [PubMed] [Google Scholar]
- Minden J. Comparative proteomics and difference gel electrophoresis. Biotechniques. 2007;43(6):739–741. doi: 10.2144/000112653. 743 passim. [DOI] [PubMed] [Google Scholar]
- Mossman HW. Comparative morphogenesis of the foetal membranes and accessory uterine structures. Contr Embryol Carnegie Inst. 1937;26:129–246. doi: 10.1016/0143-4004(91)90504-9. [DOI] [PubMed] [Google Scholar]
- Moulton BC. Ovum implantation and uterine lysosomal enzyme activity. Biol Reprod. 1974;10(5):543–548. doi: 10.1095/biolreprod10.5.543. [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Conneely OM. Reproductive tissue selective actions of progesterone receptors. Reproduction. 2004;128(2):139–146. doi: 10.1530/rep.1.00189. [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci U S A. 2003;100(17):9744–9749. doi: 10.1073/pnas.1732707100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science. 2000;289(5485):1751–1754. doi: 10.1126/science.289.5485.1751. [DOI] [PubMed] [Google Scholar]
- Muller H, Ishimura R, Orwig KE, Liu B, Soares MJ. Homologues for prolactin-like proteins A and B are present in the mouse. Biol Reprod. 1998;58(1):45–51. doi: 10.1095/biolreprod58.1.45. [DOI] [PubMed] [Google Scholar]
- Muller H, Liu B, Croy BA, Head JR, Hunt JS, Dai G, Soares MJ. Uterine natural killer cells are targets for a trophoblast cell-specific cytokine, prolactin-like protein A. Endocrinology. 1999;140(6):2711–2720. doi: 10.1210/endo.140.6.6828. [DOI] [PubMed] [Google Scholar]
- Ng YK, George KM, Engel JD, Linzer DI. GATA factor activity is required for the trophoblast-specific transcriptional regulation of the mouse placental lactogen I gene. Development. 1994;120(11):3257–3266. doi: 10.1242/dev.120.11.3257. [DOI] [PubMed] [Google Scholar]
- O'Shea JD, Kleinfeld RG, Morrow HA. Ultrastructure of decidualization in the pseudopregnant rat. Am J Anat. 1983;166(3):271–298. doi: 10.1002/aja.1001660304. [DOI] [PubMed] [Google Scholar]
- Osiak A, Utermohlen O, Niendorf S, Horak I, Knobeloch KP. ISG15, an interferon-stimulated ubiquitin-like protein, is not essential for STAT1 signaling and responses against vesicular stomatitis and lymphocytic choriomeningitis virus. Mol Cell Biol. 2005;25(15):6338–6345. doi: 10.1128/MCB.25.15.6338-6345.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey A, Mann M. Proteomics to study genes and genomes. Nature. 2000;405(6788):837–846. doi: 10.1038/35015709. [DOI] [PubMed] [Google Scholar]
- Paria BC, Song H, Dey SK. Implantation: molecular basis of embryo-uterine dialogue. Int J Dev Biol. 2001;45(3):597–605. [PubMed] [Google Scholar]
- Paria BC, Zhao X, Das SK, Dey SK, Yoshinaga K. Zonula occludens-1 and E-cadherin are coordinately expressed in the mouse uterus with the initiation of implantation and decidualization. Dev Biol. 1999;208(2):488–501. doi: 10.1006/dbio.1999.9206. [DOI] [PubMed] [Google Scholar]
- Parkes AS. The functions of the corpus luteum. II.--The experimental production of placentomata in the mouse. Proc Royal Soc London. 1914;104:183–188. [Google Scholar]
- Parr EL, Tung HN, Parr MB. Apoptosis as the mode of uterine epithelial cell death during embryo implantation in mice and rats. Biol Reprod. 1987;36(1):211–225. doi: 10.1095/biolreprod36.1.211. [DOI] [PubMed] [Google Scholar]
- Parr MB, Parr EL. Permeability of the primary decidual zone in the rat uterus: studies using fluorescein-labeled proteins and dextrans. Biol Reprod. 1986;34(2):393–403. doi: 10.1095/biolreprod34.2.393. [DOI] [PubMed] [Google Scholar]
- Patterson SD, Aebersold RH. Proteomics: the first decade and beyond. Nat Genet. 2003;33 Suppl:311–323. doi: 10.1038/ng1106. [DOI] [PubMed] [Google Scholar]
- Patton WF. Detection technologies in proteome analysis. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;771(1–2):3–31. doi: 10.1016/s1570-0232(02)00043-0. [DOI] [PubMed] [Google Scholar]
- Peel S. Granulated metrial gland cells. Adv Anat Embryol Cell Biol. 1989;115:1–112. doi: 10.1007/978-3-642-74170-8. [DOI] [PubMed] [Google Scholar]
- Peloille S, Esnard A, Dacheux JL, Guillou F, Gauthier F, Esnard F. Interactions between ovine cathepsin L, cystatin C and alpha 2-macroglobulin. Potential role in the genital tract. Eur J Biochem. 1997;244(1):140–146. doi: 10.1111/j.1432-1033.1997.00140.x. [DOI] [PubMed] [Google Scholar]
- Psychoyos A. La réaction déciduale est précédée de modifications précoses de la perméabilité capillaire de l'utérus. C R Seances Soc Biol Fil. 1960;154:1384–1387. [PubMed] [Google Scholar]
- Psychoyos A. Endocrine control of egg implantation. In: Greep ROAEB, Geiger SR, editors. Handbook of Physiology. Bethesda: American Physiological Society; 1973. pp. 187–215. [Google Scholar]
- Quenby S, Farquharson R. Uterine natural killer cells, implantation failure and recurrent miscarriage. Reprod Biomed Online. 2006;13(1):24–28. doi: 10.1016/s1472-6483(10)62012-3. [DOI] [PubMed] [Google Scholar]
- Quinn CE, Simmons DG, Kennedy TG. Expression of Cystatin C in the rat endometrium during the peri-implantation period. Biochem Biophys Res Commun. 2006;349(1):236–244. doi: 10.1016/j.bbrc.2006.08.054. [DOI] [PubMed] [Google Scholar]
- Rempel LA, Austin KJ, Ritchie KJ, Yan M, Shen M, Zhang DE, Henkes LE, Hansen TR. Ubp43 gene expression is required for normal Isg15 expression and fetal development. Reprod Biol Endocrinol. 2007;5:13. doi: 10.1186/1477-7827-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie KJ, Zhang DE. ISG15: the immunological kin of ubiquitin. Semin Cell Dev Biol. 2004;15(2):237–246. doi: 10.1016/j.semcdb.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Roberts RM. Interferon-tau, a Type 1 interferon involved in maternal recognition of pregnancy. Cytokine Growth Factor Rev. 2007;18(5–6):403–408. doi: 10.1016/j.cytogfr.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers PA, Macpherson AM, Beaton L. Reduction in endometrial neutrophils in proximity to implanting rat blastocysts. J Reprod Fertil. 1992;96(1):283–288. doi: 10.1530/jrf.0.0960283. [DOI] [PubMed] [Google Scholar]
- Rogers PA, Murphy CR, Rogers AW, Gannon BJ. Capillary patency and permeability in the endometrium surrounding the implanting rat blastocyst. Int J Microcirc Clin Exp. 1983;2(3):241–249. [PubMed] [Google Scholar]
- Saito S, Shima T, Nakashima A, Shiozaki A, Ito M, Sasaki Y. What is the role of regulatory T cells in the success of implantation and early pregnancy? J Assist Reprod Genet. 2007;24(9):379–386. doi: 10.1007/s10815-007-9140-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakoff JA, Murdoch RN. Alterations in uterine calcium ions during induction of the decidual cell reaction in pseudopregnant mice. J Reprod Fertil. 1994;101(1):97–102. doi: 10.1530/jrf.0.1010097. [DOI] [PubMed] [Google Scholar]
- Salamonsen LA. Role of proteases in implantation. Rev Reprod. 1999;4(1):11–22. doi: 10.1530/ror.0.0040011. [DOI] [PubMed] [Google Scholar]
- Santoni A, Carlino C, Gismondi A. Uterine NK cell development, migration and function. Reprod Biomed Online. 2008;16(2):202–210. doi: 10.1016/s1472-6483(10)60575-5. [DOI] [PubMed] [Google Scholar]
- Schlafke S, Welsh AO, Enders AC. Penetration of the basal lamina of the uterine luminal epithelium during implantation in the rat. Anat Rec. 1985;212(1):47–56. doi: 10.1002/ar.1092120107. [DOI] [PubMed] [Google Scholar]
- Schorpp-Kistner M, Wang ZQ, Angel P, Wagner EF. JunB is essential for mammalian placentation. Embo J. 1999;18(4):934–948. doi: 10.1093/emboj/18.4.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin JRA, Sharkey AM, Fazleabas AT. Chorionic gonadotropin signalling at the maternal-fetal interface. In: Aplin JD, Fazleabas AT, Glasser SR, Giudice LC, editors. The Endometrium: Molecular, Cellular and Clinical Perspectives. Second ed. London: Informa UK Ltd; 2008. pp. 286–295. [Google Scholar]
- Simmons DG, Cross JC. Determinants of trophoblast lineage and cell subtype specification in the mouse placenta. Dev Biol. 2005;284(1):12–24. doi: 10.1016/j.ydbio.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Smith DF, Toft DO. Minireview: the intersection of steroid receptors with molecular chaperones: observations and questions. Mol Endocrinol. 2008;22(10):2229–2240. doi: 10.1210/me.2008-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares MJ. The prolactin and growth hormone families: pregnancy-specific hormones/cytokines at the maternal-fetal interface. Reprod Biol Endocrinol. 2004;2:51. doi: 10.1186/1477-7827-2-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares MJ, Konno T, Alam SM. The prolactin family: effectors of pregnancy-dependent adaptations. Trends Endocrinol Metab. 2007;18(3):114–121. doi: 10.1016/j.tem.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Song G, Spencer TE, Bazer FW. Progesterone and interferon-tau regulate cystatin C in the endometrium. Endocrinology. 2006;147(7):3478–3483. doi: 10.1210/en.2006-0122. [DOI] [PubMed] [Google Scholar]
- Spencer TE, Johnson GA, Bazer FW, Burghardt RC, Palmarini M. Pregnancy recognition and conceptus implantation in domestic ruminants: roles of progesterone, interferons and endogenous retroviruses. Reprod Fertil Dev. 2007;19(1):65–78. doi: 10.1071/rd06102. [DOI] [PubMed] [Google Scholar]
- Stypmann J, Glaser K, Roth W, Tobin DJ, Petermann I, Matthias R, Monnig G, Haverkamp W, Breithardt G, Schmahl W, Peters C, Reinheckel T. Dilated cardiomyopathy in mice deficient for the lysosomal cysteine peptidase cathepsin L. Proc Natl Acad Sci U S A. 2002;99(9):6234–6239. doi: 10.1073/pnas.092637699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabibzadeh S. Current concepts in endometrial receptivity and implantation. In: Glasser SR, Aplin JD, Giudice LC, Tabibzadeh S, editors. The Endometrium. New York: Taylor & Francis; 2002. pp. 249–257. [Google Scholar]
- Tachi C, Tachi S. Cellular aspects of ovum implantation and decidualization in the rat. Basic Life Sci. 1974;4(PT B):263–286. doi: 10.1007/978-1-4684-2892-6_19. [DOI] [PubMed] [Google Scholar]
- Tachi C, Tachi S. Macrophages and implantation. Ann N Y Acad Sci. 1986;476:158–182. doi: 10.1111/j.1749-6632.1986.tb20929.x. [DOI] [PubMed] [Google Scholar]
- Tavera C, Prevot D, Girolami JP, Leung-Tack J, Colle A. Tissue and biological fluid distribution of cysteine proteinases inhibitor: rat cystatin C. Biol Chem Hoppe Seyler. 1990;371 Suppl:187–192. [PubMed] [Google Scholar]
- Tayade C, Fang Y, Croy BA. A review of gene expression in porcine endometrial lymphocytes, endothelium and trophoblast during pregnancy success and failure. J Reprod Dev. 2007;53(3):455–463. doi: 10.1262/jrd.18170. [DOI] [PubMed] [Google Scholar]
- Thatcher WW, Guzeloglu A, Mattos R, Binelli M, Hansen TR, Pru JK. Uterine-conceptus interactions and reproductive failure in cattle. Theriogenology. 2001;56(9):1435–1450. doi: 10.1016/s0093-691x(01)00645-8. [DOI] [PubMed] [Google Scholar]
- Theiler K. The House Mouse: Atlas of Embryonic Development. New York: Springer-Verlag; 1989. [Google Scholar]
- Tranguch S, Cheung-Flynn J, Daikoku T, Prapapanich V, Cox MB, Xie H, Wang H, Das SK, Smith DF, Dey SK. Cochaperone immunophilin FKBP52 is critical to uterine receptivity for embryo implantation. Proc Natl Acad Sci U S A. 2005;102(40):14326–14331. doi: 10.1073/pnas.0505775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranguch S, Wang H, Daikoku T, Xie H, Smith DF, Dey SK. FKBP52 deficiency-conferred uterine progesterone resistance is genetic background and pregnancy stage specific. J Clin Invest. 2007;117(7):1824–1834. doi: 10.1172/JCI31622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung HN, Parr MB, Parr EL. The permeability of the primary decidual zone in the rat uterus: an ultrastructural tracer and freeze-fracture study. Biol Reprod. 1986;35(4):1045–1058. doi: 10.1095/biolreprod35.4.1045. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MJ, Xie X, Tayade C, Peralta C, Fang Y, Leonard S, Paffaro VA, Jr, Sheikhi AK, Murrant C, Croy BA. A review of trafficking and activation of uterine natural killer cells. Am J Reprod Immunol. 2005;54(6):322–331. doi: 10.1111/j.1600-0897.2005.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Ishimura R, Walia DS, Muller H, Dai G, Hunt JS, Lee NA, Lee JJ, Soares MJ. Eosinophils are cellular targets of the novel uteroplacental heparin-binding cytokine decidual/trophoblast prolactin-related protein. J Endocrinol. 2000;167(1):15–28. doi: 10.1677/joe.0.1670015. [DOI] [PubMed] [Google Scholar]
- Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet. 2006;7(3):185–199. doi: 10.1038/nrg1808. [DOI] [PubMed] [Google Scholar]
- Wang X, Matsumoto H, Zhao X, Das SK, Paria BC. Embryonic signals direct the formation of tight junctional permeability barrier in the decidualizing stroma during embryo implantation. J Cell Sci. 2004;117(Pt 1):53–62. doi: 10.1242/jcs.00826. [DOI] [PubMed] [Google Scholar]
- Weitlauf HM. Implantation associated changes in uterine secreted proteins. Adv Exp Med Biol. 1987;230:207–220. doi: 10.1007/978-1-4684-1297-0_12. [DOI] [PubMed] [Google Scholar]
- Weitlauf HM. Biology of implantation. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. New York: Raven Press; 1988. pp. 231–262. [Google Scholar]
- Weitlauf HM. Embryonic signaling at implantation in the mouse. Prog Clin Biol Res. 1989;294:359–376. [PubMed] [Google Scholar]
- Weitlauf HM, Suda-Hartman M. Changes in secreted uterine proteins associated with embryo implantation in the mouse. J Reprod Fertil. 1988;84(2):539–549. doi: 10.1530/jrf.0.0840539. [DOI] [PubMed] [Google Scholar]
- Welsh AO, Enders AC. Chorioallantoic placenta formation in the rat: I. Luminal epithelial cell death and extracellular matrix modifications in the mesometrial region of implantation chambers. Am J Anat. 1991;192(3):215–231. doi: 10.1002/aja.1001920302. [DOI] [PubMed] [Google Scholar]
- Wiemers DO, Shao LJ, Ain R, Dai G, Soares MJ. The mouse prolactin gene family locus. Endocrinology. 2003;144(1):313–325. doi: 10.1210/en.2002-220724. [DOI] [PubMed] [Google Scholar]
- Wooding FBP. Implantation of the blastocyst: I. Comparative studies. In: Glasser SR, Aplin JD, Giudice LC, Tabibzadeh S, editors. The Endometrium. New York: Taylor & Francis; 2002. pp. 335–340. [Google Scholar]
- Wooding FBP, Flint APF. Placentation. In: Lamming GE, editor. Marshall’s Physiology of Reproduction. London: Chapman & Hall; 1994. pp. 230–466. [Google Scholar]
- Yang Z, Wolf IM, Chen H, Periyasamy S, Chen Z, Yong W, Shi S, Zhao W, Xu J, Srivastava A, Sanchez ER, Shou W. FK506-binding protein 52 is essential to uterine reproductive physiology controlled by the progesterone receptor A isoform. Mol Endocrinol. 2006;20(11):2682–2694. doi: 10.1210/me.2006-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh ET, Gong L, Kamitani T. Ubiquitin-like proteins: new wines in new bottles. Gene. 2000;248(1–2):1–14. doi: 10.1016/s0378-1119(00)00139-6. [DOI] [PubMed] [Google Scholar]
- Ying Y, Zhao GQ. Detection of multiple bone morphogenetic protein messenger ribonucleic acids and their signal transducer, Smad1, during mouse decidualization. Biol Reprod. 2000;63(6):1781–1786. doi: 10.1095/biolreprod63.6.1781. [DOI] [PubMed] [Google Scholar]
- Yoshinaga K. Uterine receptivity for blastocyst implantation. Ann N Y Acad Sci. 1988;541:424–431. doi: 10.1111/j.1749-6632.1988.tb22279.x. [DOI] [PubMed] [Google Scholar]
- Yoshinaga K. Review of factors essential for blastocyst implantation for their modulating effects on the maternal immune system. Semin Cell Dev Biol. 2008;19(2):161–169. doi: 10.1016/j.semcdb.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Zhang J, Croy BA, Tian Z. Uterine natural killer cells: their choices, their missions. Cell Mol Immunol. 2005;2(2):123–129. [PubMed] [Google Scholar]
- Zhang J, Tian Z. UNK cells: their role in tissue re-modelling and preeclampsia. Semin Immunopathol. 2007;29(2):123–133. doi: 10.1007/s00281-007-0068-1. [DOI] [PubMed] [Google Scholar]