Abstract
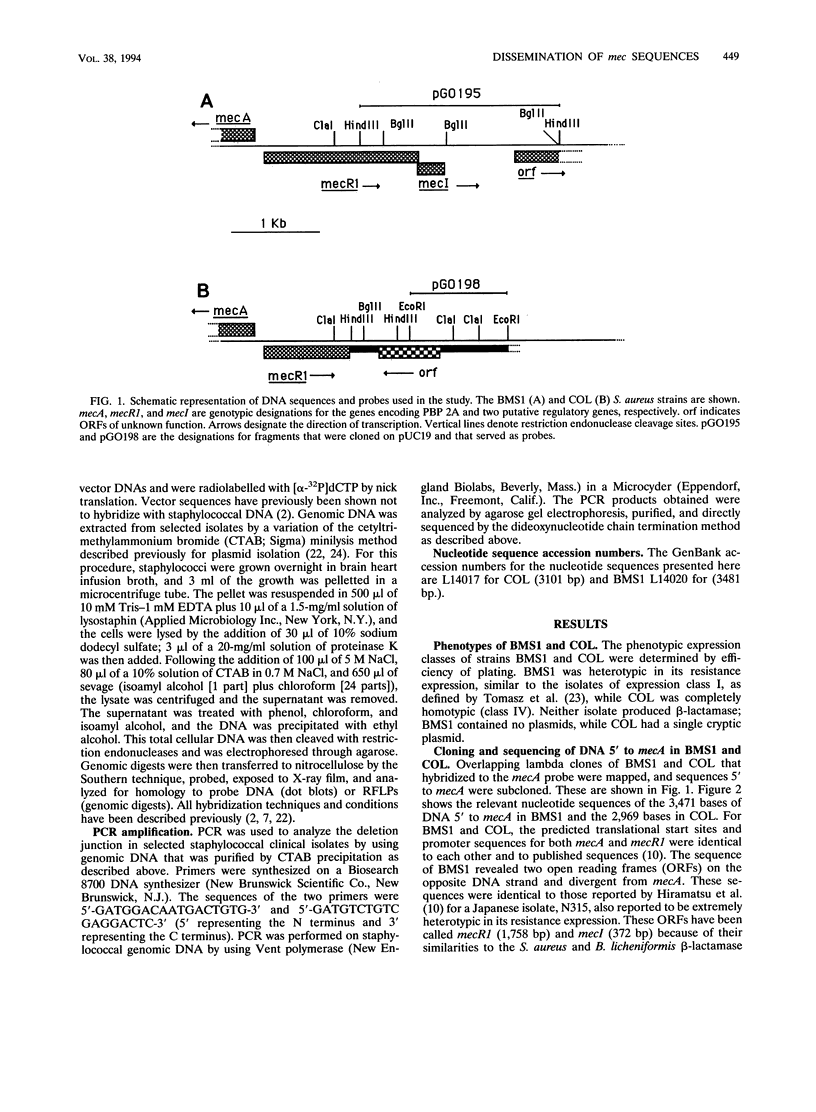
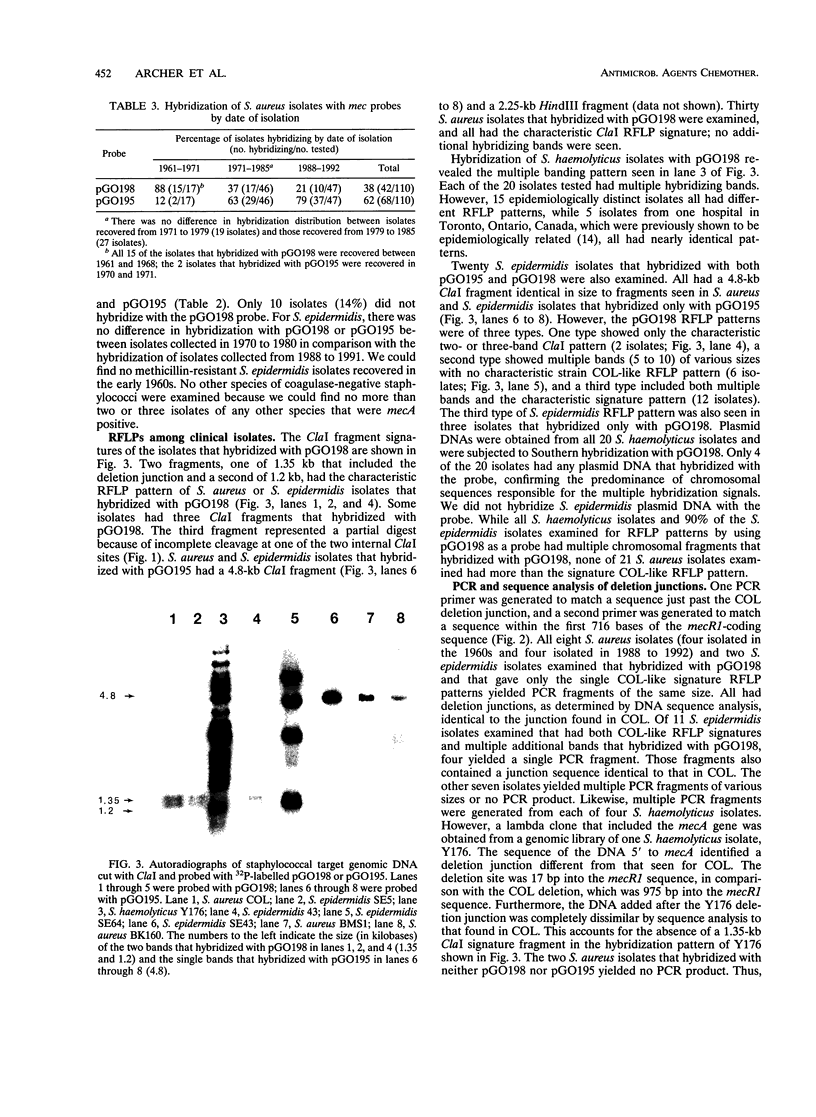
DNA probes consisting of pUC19 containing cloned Staphylococcus aureus chromosomal fragments were constructed from two methicillin-resistant S. aureus strains with different DNA sequences 5' to mecA, the gene that mediates methicillin resistance. The probe from one strain, BMS1, contained a portion of the regulatory sequences (the terminal 641 bp of mecR1 and all of mecI) associated with the induction and repression of mecA transcription (pGO195). The second probe, from strain COL (pGO198), contained DNA not found in strain BMS1. This DNA was within the sequences added at the site of a mecR1 deletion. Genomic digests of 14 S. aureus isolates recovered between 1961 and 1969 all hybridized with pGO198. In contrast, 78% (36 of 46) of the S. aureus organisms isolated since 1988 hybridized with pGO195 but not with pGO198; the remainder hybridized with pGO198. No S. aureus isolates hybridized with both probes. Staphylococcus epidermidis digests hybridized with pGO198 (46%), pGO195 (14%), or both probes (35%); all 20 Staphylococcus haemolyticus isolates hybridized with pGO198. The restriction fragment length polymorphism patterns of all pGO198-hybridizing regions in S. aureus were identical to those in strain COL. In addition, the mecR1 deletion junction nucleotide sequences of eight S. aureus and six S. epidermidis isolates were identical. However, 21 of 23 S. epidermidis and all 20 S. haemolyticus isolates had from 5 to more than 20 additional chromosomal bands that hybridized with pGO198; none of 21 S. aureus isolates had additional hybridizing bands. These data suggest that the additional DNA responsible for the mecR1 deletion was part of a repetitive, and possibly mobile, element resident in coagulase-negative staphylococci but not in S. aureus. These data also support a hypothesis that the deletion event occurred in a coagulase-negative staphylococcus with subsequent acquisition of the interrupted sequences by S. aureus.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer G. L., Pennell E. Detection of methicillin resistance in staphylococci by using a DNA probe. Antimicrob Agents Chemother. 1990 Sep;34(9):1720–1724. doi: 10.1128/aac.34.9.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck W. D., Berger-Bächi B., Kayser F. H. Additional DNA in methicillin-resistant Staphylococcus aureus and molecular cloning of mec-specific DNA. J Bacteriol. 1986 Feb;165(2):373–378. doi: 10.1128/jb.165.2.373-378.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumfitt W., Hamilton-Miller J. Methicillin-resistant Staphylococcus aureus. N Engl J Med. 1989 May 4;320(18):1188–1196. doi: 10.1056/NEJM198905043201806. [DOI] [PubMed] [Google Scholar]
- Dubin D. T., Matthews P. R., Chikramane S. G., Stewart P. R. Physical mapping of the mec region of an American methicillin-resistant Staphylococcus aureus strain. Antimicrob Agents Chemother. 1991 Aug;35(8):1661–1665. doi: 10.1128/aac.35.8.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froggatt J. W., Johnston J. L., Galetto D. W., Archer G. L. Antimicrobial resistance in nosocomial isolates of Staphylococcus haemolyticus. Antimicrob Agents Chemother. 1989 Apr;33(4):460–466. doi: 10.1128/aac.33.4.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman B. J., Tomasz A. Low-affinity penicillin-binding protein associated with beta-lactam resistance in Staphylococcus aureus. J Bacteriol. 1984 May;158(2):513–516. doi: 10.1128/jb.158.2.513-516.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himeno T., Imanaka T., Aiba S. Nucleotide sequence of the penicillinase repressor gene penI of Bacillus licheniformis and regulation of penP and penI by the repressor. J Bacteriol. 1986 Dec;168(3):1128–1132. doi: 10.1128/jb.168.3.1128-1132.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramatsu K., Asada K., Suzuki E., Okonogi K., Yokota T. Molecular cloning and nucleotide sequence determination of the regulator region of mecA gene in methicillin-resistant Staphylococcus aureus (MRSA). FEBS Lett. 1992 Feb 24;298(2-3):133–136. doi: 10.1016/0014-5793(92)80039-j. [DOI] [PubMed] [Google Scholar]
- Hürlimann-Dalel R. L., Ryffel C., Kayser F. H., Berger-Bächi B. Survey of the methicillin resistance-associated genes mecA, mecR1-mecI, and femA-femB in clinical isolates of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1992 Dec;36(12):2617–2621. doi: 10.1128/aac.36.12.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T., Zhu Y. F., Nicholls N. J., Lampen J. O. A second regulatory gene, blaR1, encoding a potential penicillin-binding protein required for induction of beta-lactamase in Bacillus licheniformis. J Bacteriol. 1987 Sep;169(9):3873–3878. doi: 10.1128/jb.169.9.3873-3878.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiswirth B., Kornblum J., Arbeit R. D., Eisner W., Maslow J. N., McGeer A., Low D. E., Novick R. P. Evidence for a clonal origin of methicillin resistance in Staphylococcus aureus. Science. 1993 Jan 8;259(5092):227–230. doi: 10.1126/science.8093647. [DOI] [PubMed] [Google Scholar]
- Low D. E., Schmidt B. K., Kirpalani H. M., Moodie R., Kreiswirth B., Matlow A., Ford-Jones E. L. An endemic strain of Staphylococcus haemolyticus colonizing and causing bacteremia in neonatal intensive care unit patients. Pediatrics. 1992 Apr;89(4 Pt 2):696–700. [PubMed] [Google Scholar]
- Morton T. M., Eaton D. M., Johnston J. L., Archer G. L. DNA sequence and units of transcription of the conjugative transfer gene complex (trs) of Staphylococcus aureus plasmid pGO1. J Bacteriol. 1993 Jul;175(14):4436–4447. doi: 10.1128/jb.175.14.4436-4447.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser J. M., Kapur V. Clonal analysis of methicillin-resistant Staphylococcus aureus strains from intercontinental sources: association of the mec gene with divergent phylogenetic lineages implies dissemination by horizontal transfer and recombination. J Clin Microbiol. 1992 Aug;30(8):2058–2063. doi: 10.1128/jcm.30.8.2058-2063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland S. J., Dyke K. G. Tn552, a novel transposable element from Staphylococcus aureus. Mol Microbiol. 1990 Jun;4(6):961–975. doi: 10.1111/j.1365-2958.1990.tb00669.x. [DOI] [PubMed] [Google Scholar]
- Suzuki E., Kuwahara-Arai K., Richardson J. F., Hiramatsu K. Distribution of mec regulator genes in methicillin-resistant Staphylococcus clinical strains. Antimicrob Agents Chemother. 1993 Jun;37(6):1219–1226. doi: 10.1128/aac.37.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesch W., Ryffel C., Strässle A., Kayser F. H., Berger-Bächi B. Evidence of a novel staphylococcal mec-encoded element (mecR) controlling expression of penicillin-binding protein 2'. Antimicrob Agents Chemother. 1990 Sep;34(9):1703–1706. doi: 10.1128/aac.34.9.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas W. D., Jr, Archer G. L. Identification and cloning of the conjugative transfer region of Staphylococcus aureus plasmid pGO1. J Bacteriol. 1989 Feb;171(2):684–691. doi: 10.1128/jb.171.2.684-691.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Nachman S., Leaf H. Stable classes of phenotypic expression in methicillin-resistant clinical isolates of staphylococci. Antimicrob Agents Chemother. 1991 Jan;35(1):124–129. doi: 10.1128/aac.35.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubukata K., Nonoguchi R., Matsuhashi M., Konno M. Expression and inducibility in Staphylococcus aureus of the mecA gene, which encodes a methicillin-resistant S. aureus-specific penicillin-binding protein. J Bacteriol. 1989 May;171(5):2882–2885. doi: 10.1128/jb.171.5.2882-2885.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]