Abstract
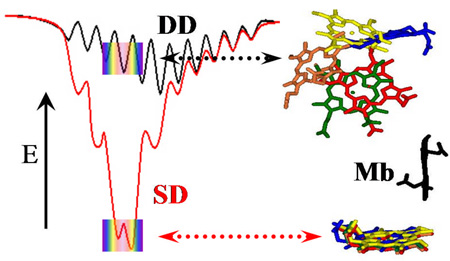
Cyt b5 is the electron-carrier ‘repair’ protein that reduces met-Mb and met-Hb to their O2-carrying ferroheme forms. Studies of electron transfer (ET) between Mb and cyt b5 revealed that they react on a “Dynamic Docking” (DD) energy landscape on which binding and reactivity are uncoupled: binding is weak and involves an ensemble of nearly isoenergetic configurations, only a few of which are reactive; those few contribute negligibly to binding. We set the task of redesigning the surface of Mb so that its reaction with cyt b5 instead would occur on a conventional ‘simple docking’ (SD) energy landscape, on which a complex exhibits a well-defined (set of) reactive binding configuration(s), with binding and reactivity thus no longer being decoupled. We prepared a myoglobin (Mb) triple mutant (D44K/D60K/E85K; Mb(+6)) substituted with Zn-deuteroporphyrin, and monitored cytochrome b5 (cyt b5) binding and electron transfer (ET) quenching of the 3ZnMb(+6) triplet state. In contrast, to Mb(WT), the three charge-reversals around the ‘front-face’ heme edge of Mb(+6) have directed cyt b5 to a surface area of Mb adjacent to its heme, created a well-defined, most-stable structure that supports good ET pathways, and apparently coupled binding and ET: both Ka and ket are increased by the same factor of ~ 2×102, creating a complex that exhibits a large ET rate constant, ket = 106 1s−1, and is in slow exchange (koff ≪ ket). In short, these mutations indeed appear to have created the sought-for conversion from DD to simple docking (SD) energy landscapes.
Interactions between macromolecules are fundamental to most of biology. The goal of understanding and controlling protein-protein interactions has inspired numerous efforts to modulate the binding within high-affinity complexes of known structure,1–6 with most alterations serving to weaken binding. To begin such efforts at the other extreme, with a pair of proteins that bind weakly, and by altering them to create a well-defined, highly functional complex, offers a different level of challenge. We here report dramatic progress in creating such a complex between the weakly interacting physiological electron-transfer (ET) partners, myoglobin (Mb) and cytochrome b5 (cyt b5).
Cyt b5 is the electron-carrier ‘repair’ protein that reduces met-Mb7 and met-Hb8,9 to their O2-carrying ferroheme forms. Studies of ET between Mb and cyt b5 revealed that they react on a “Dynamic Docking” (DD) energy landscape (Fig 1, upper),10–12 on which binding and reactivity are uncoupled: binding is weak and involves an ensemble of nearly isoenergetic configurations, only a few of which are reactive; those few contribute negligibly to binding. The sharp contrast of such behavior with that for the conventional ‘simple docking’ (SD) energy landscape (Fig 1, lower), on which a complex exhibits a well-defined (set of) reactive binding configuration(s), led us to ask whether it was possible to redesign the surface of Mb so that its reaction with cyt b5 instead would occur on a SD landscape, with binding and reactivity thus no longer being decoupled.
Fig 1.
Conversion of ‘Dynamic Docking’ (DD) to ‘Simple Docking’ (SD) Energy landscapes; rainbows represent ET-active configurations.
The low affinity of these partners is the consequence of poor shape complementarity, weak overall electrostatic attractions between the highly charged cyt b5 and the nearly neutral Mb (qb5 = − 5.72 C; qMb ~ − 0.29 C), and the absence of optimally placed complementary charge pairs on the surfaces of the partners. Our initial attempt to enhance their affinity electrostatically13 by neutralizing the Mb heme propionates through esterification increased the ET rate by ~100-fold but surprisingly, did not significantly alter the affinity; this observation in fact led us to formulate the DD paradigm.10
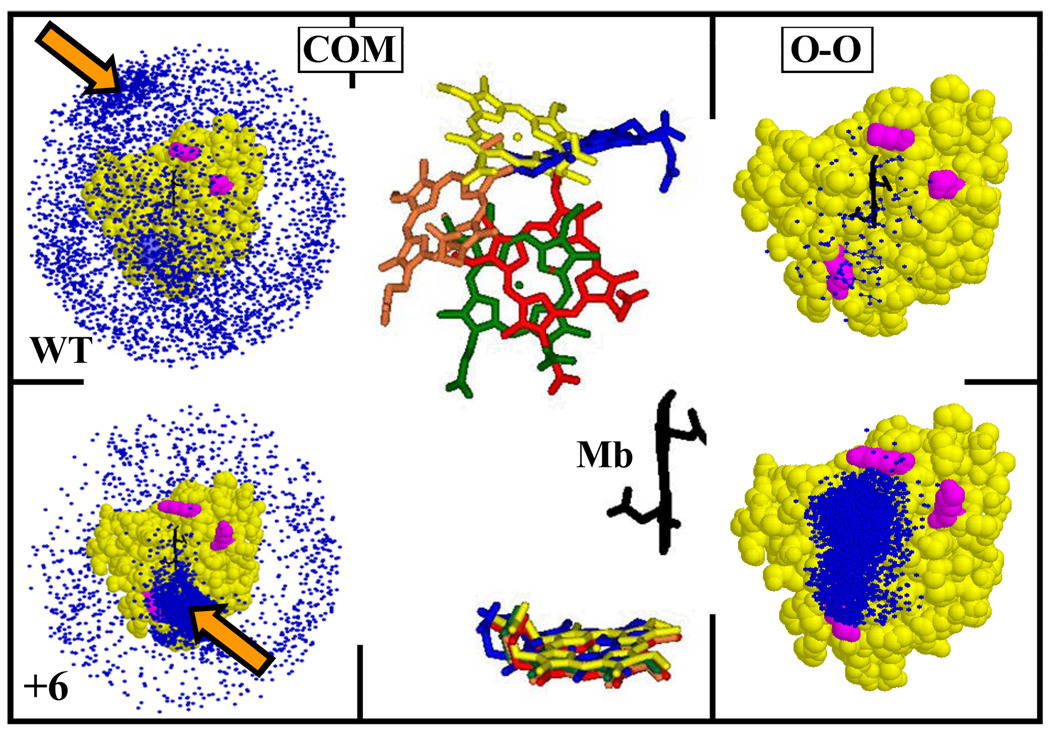
To identify surface residues of Mb where mutation would best enhance reactive binding, we performed Brownian Dynamics (BD) simulations14 of the docking between cyt b5 and all Mb surface charge reversal mutants (D/E → K). To probe net binding we used a center-of-mass (COM) criterion to define a BD trajectory ‘hit’; to probe reactive binding we defined a hit to have a short distance between one O of a cyt b5 heme carboxylate and one O of a Mb heme carboxylate (O-O criterion).15,16 The BD profile for Mb(WT) (Fig 2, top left) shows that cyt b5 ‘binding hits’ (COM criterion) are distributed over the entire Mb surface, with some clustering in patches away from the heme edge, and few ‘reactive hits’ (OO criterion) near the Mb(WT) heme edge (Fig 2, top, right). The twenty most stable binding hits (COM criterion) fall at a Mb surface patch ‘above and to the left’ of the heme edge. Among these, there is no correlation of the cyt b5 orientations (backbone RMSD, 11.2 Å) as illustrated by their differing cyt b5 heme orientations (Fig 2, top center).
Fig 2.
BD simulations for docking Mb(WT) (upper) and Mb(+6) (lower) to cyt b5 (104 trajectories; pH 7; : = 18mM); mutation sites, magenta. ‘Hit’ profiles using COM (Left) and O-O (Right) reaction criteria; blue dots, cyt b5 COM at a hit (see refs 12,14). Center: Mb heme (black) plus superpositions of the cyt b5 hemes for the five most-stable configurations from the COM simulations with Mb(WT) (upper), Mb(+6) (lower); hemes properly oriented relative to the Mb heme. Arrows on left indicate corresponding locations of the cyt b5 COM relative to Mb face.
Not surprisingly, computations with the mutant Mbs showed that binding of the negatively charged cyt b5 to Mb is opposed by the three acidic residues surrounding the exposed heme on the Mb front face (D44, D60, and E85). We then tested whether the cumulative effects of reversing the charge at these three sites would stabilize the complex in a reactive configuration with the hemes proximate to one another, thereby reshaping the energy landscape to more closely resemble one of simple docking, Fig 1.
Simulations for the triple mutant (Fig 2, lower) indeed show a high density of hits near the Mb heme edge with both criteria. Moreover, the twenty most-stable cyt b5 configurations now correspond to a single well-defined structure (RMSD, 0.6 Å),17 as illustrated by the close overlap of their hemes, (Fig 2, center, lower) with their heme edges facing that of Mb. Thus, the simulations suggest that cyt b5 might bind strongly to the Mb(+6) triple mutant (D44K/D60K/E85K) as a reactive ‘simple-docking’ complex in which binding and reactivity are coupled.
To test these predictions, we prepared ZnMb(+6), the (D44K/D60K/E85K) triple mutant substituted with Zn deuteroporphyrin, and monitored binding and ET quenching of the 3ZnMb triplet state formed by rapid intersystem crossing from the laser-flash generated singlet state of ZnMb.18,19,20
Throughout a titration of Mb(WT) with Fe3+b5 at pH 6 (µ = 5.3 mM), the ZnMb triplet decay traces remain exponential, with a decay constant (kobs) that increases linearly with [Fe3+b5] from the intrinsic decay constant, kD, without evidence of saturation: kobs = kD + k2 [Fe3+b5]. The slope of this line is the bimolecular quenching rate constant, k2 = 4.7×106 M−1s−1, similar to the value reported previously.10,12 The measurements indicate this complex is in fast exchange, koff ≫ ket, where ket represents the apparent intracomplex ET rate constant, and as a result, k2(WT) = ket(WT)Ka(WT), where Ka is the overall binding constant. Combination of the measured k2(WT) and reported value, Ka(WT) ≈ 103 M−1,21,22 gives, ket(WT) ≈ 4–5 ×103 s−1.
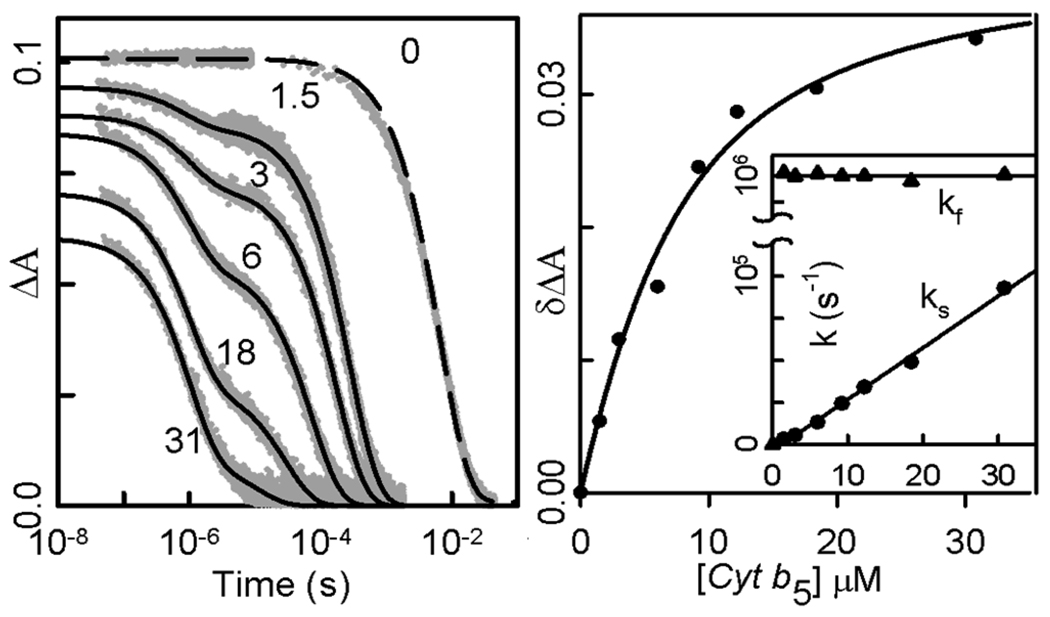
In contrast, the triplet decay traces for ZnMb(+6) are biexponential throughout a titration with Fe3+b5, and the larger rate constant is invariant with [Fe3+b5], kf = 106 s−1 (Fig 3). These observations indicate that the complex is in slow exchange with its unbound partners (koff ≪ ket), and that this phase represents rapid ET quenching within the bound [ZnMb, Fe3+b5] complex: kf. = ket(+6) = 106 s−1.23 The rate constant for the slower process increases linearly with addition of Fe3+b5, ks = k2 [Fe3+b5] + kD, and thus is associated with the bimolecular reaction between free ZnMb(+6) and Fe3+b5; the second-order rate constant is, k2 = 3.1×109 M−1s−1, corresponding to diffusion-limited ET.
Fig 3.
Titration of ZnMb(+6) with Fe3+ cyt b5. Left. Triplet decay traces as a function of [Fe3+ cyt b5] (:M). Conditions: 5 µM ZnMb; 5 mM KPi, pH 6; 20°C; 475 nm. Right. Zero-time absorbance decrease for cyt b5 binding to Mb(+6) and fit to eq 1: Ka = 2.2 × 105 M−1; *)A0max = 0.042. Inset: Rate constants from fitting triplet decays to bi-exponential.
Typically, one would fit the cyt b5-dependent fraction of the intra-complex phase, f(Fe3+b5), with a standard binding isotherm to obtain the binding constant, Ka. However, as seen in Fig 3, the total amplitude of the triplet signal, measured as the extrapolated zero-time amplitude, )A0(Fe3+b5), decreases during a titration, an indication that the bound complex not only exhibits rapid ET from the ZnMb(+6) triplet state to Fe3+b5, but also extremely rapid intra-complex quenching of the singlet state. We instead determined Ka by obtaining f(Fe3+b5) from )A0(Fe3+b5) during a titration by use of the relationship,
| (1) |
and fitting *)A0 to a binding isotherm. The resulting binding constant, Ka(+6) = 2.2×105 M−1, is more than two orders of magnitude greater than that for ZnMb(WT).21,22
Thus, while cyt b5 reacts with Mb(WT) on a DD energy landscape, with weak binding in multiple configurations - most of which are non-reactive, a low second-order ET rate constant, and rapid exchange (koff ≫ kf) between the complex and its unbound components, the three charge-reversals around the ‘front-face’ heme edge of Mb(+6) have directed cyt b5 to a surface area of Mb adjacent to its heme, and created a well-defined, most-stable structure (Fig 2) that supports good ET pathways. The mutations indeed appear to have coupled binding and ET, increasing both Ka and ket by a factor of ~ 2×102, and creating a complex with a large ET rate constant, ket(+6) = 106 s−1, that is in slow exchange (koff ≪ ket). In short, it appears that these mutations have created the sought-for conversion from DD to SD energy landscapes.
ACKNOWLEDGMENT
We gratefully acknowledge NIH funding for this work (HL063203; GM008382) and Prof. Ishwar Radhakrishnan for assistance with the structural comparisons.
REFERENCES
- 1.Tetreault M, Cusanovich M, Meyer T, Axelrod H, Okamura MY. Biochemistry. 2002;41:5807–5815. doi: 10.1021/bi012053e. [DOI] [PubMed] [Google Scholar]
- 2.Selzer T, Albeck S, Schreiber G. Nat. Struct. Biol. 2000;7:537–541. doi: 10.1038/76744. [DOI] [PubMed] [Google Scholar]
- 3.Ivkovic-Jensen MM, Ullmann GM, Young S, Hansson O, Crnogorac MM, Ejdebaeck M, Kostic NM. Biochemistry. 1998;37:9557–9569. doi: 10.1021/bi9802871. [DOI] [PubMed] [Google Scholar]
- 4.Schreiber G, Haran G, Zhou H-X. Chem. Rev. (Washington, DC, U.S.) 2009;109:839–860. doi: 10.1021/cr800373w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pearl NM, Jacobson T, Meyen C, Clementz AG, Ok EY, Choi E, Wilson K, Vitello LB, Erman JE. Biochemistry. 2008;47:2766–2775. doi: 10.1021/bi702271r. [DOI] [PubMed] [Google Scholar]
- 6.Davidson VL. Acc. Chem. Res. 2008;41:730–738. doi: 10.1021/ar700252c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagler L, Coppes RI, Jr, Herman RH. J. Biol. Chem. 1979;254:6505–6514. [PubMed] [Google Scholar]
- 8.Hultquist DE, Passon PG. Nature New Biology. 1971;229:252–254. doi: 10.1038/newbio229252a0. [DOI] [PubMed] [Google Scholar]
- 9.Kuma F. J. Biol. Chem. 1981;256:5518–5523. [PubMed] [Google Scholar]
- 10.Liang Z-X, Nocek J, Huang K, Hayes RT, Kurnikov IV, Beratan DN, Hoffman BM. J. Am. Chem. Soc. 2002;124:6849–6859. doi: 10.1021/ja0127032. [DOI] [PubMed] [Google Scholar]
- 11.Liang Z-X, Kurnikov IV, Nocek JM, Mauk AG, Beratan DN, Hoffman BM. J. Am. Chem. Soc. 2004;126:2785–2798. doi: 10.1021/ja038163l. [DOI] [PubMed] [Google Scholar]
- 12.Wheeler KE, Nocek J, Cull DA, Yatsunyk LA, Rosenzweig AC, Hoffman BM. J. Am. Chem. Soc. 2007;129:3906–3917. doi: 10.1021/ja067598g. [DOI] [PubMed] [Google Scholar]
- 13.Shurki A, Strajbl M, Schutz CN, Warshel A. Methods Enzymol. 2004;380:52–84. doi: 10.1016/S0076-6879(04)80003-X. [DOI] [PubMed] [Google Scholar]
- 14.BD computations were done with MacroDox (see, Northrup SH, Thomasson KA, Miller CM, Barker PD, Eltis LD, Guillemette JG, Inglis SC, Mauk AG. Biochemistry. 1993;32:6613–6623. doi: 10.1021/bi00077a014. using procedures described in ref. 12.
- 15.Lin J, Balabin IA, Beratan DN. Science (Washington, DC, U. S.) 2005;310:1311–1313. doi: 10.1126/science.1118316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moser CC, Chobot SE, Page CC, Dutton PL. Biochim. Biophys. Acta, Bioenerg. 2008;1777:1032–1037. doi: 10.1016/j.bbabio.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Configurations were compared using Suppose, available through the Structural Biology Facility at Vanderbilt University
- 18.Nocek JM, Sishta BP, Cameron JC, Mauk AG, Hoffman BM. J. Am. Chem. Soc. 1997;119:2146–2155. [Google Scholar]
- 19.Trypsin-solubilized bovine cytb5 is used.(10, 11) The plasmid for the equine Mb(D44K/D60K) mutant (20) was used as template for Mb(+6), which was overexpressed in E. coli, purified and reconstituted with Zn-deuteroporphyrin IX as in refs 10, 11; complete characterization will be presented elsewhere. Flash photolysis employed LKS.60 (Applied Photophysics) equipped with a Xe-arc and a frequency-doubled Spectra- Physics INDI 40-10-HG Nd:YAG pulsed laser.
- 20.Hoffman BM, Celis LM, Cull DA, Patel AD, Seifert JL, Wheeler KE, Wang J, Yao J, Kurnikov IV, Nocek J. PNAS. 2005;102:3564–3569. doi: 10.1073/pnas.0408767102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang Z-X, Jiang M, Ning Q, Hoffman BM. JBIC. 2002;7:580–588. doi: 10.1007/s00775-001-0332-0. [DOI] [PubMed] [Google Scholar]
- 22.Worrall JAR, Liu Y, Crowley PB, Nocek J, Hoffman BM, Ubbink M. Biochemistry. 2002;41:11721–11730. doi: 10.1021/bi026296y. [DOI] [PubMed] [Google Scholar]
- 23.Preliminary kinetic measurements in viscous solutions suggest that this ET rate constant involves conformational gating, and not just the ET process itself.