Abstract
Objective
Age and excessive energy intake/obesity are risk factors for cerebrovascular disease, but it is not known if and how these factors affect the extent of brain damage and outcome in ischemic stroke. We therefore determined the interactions of age and energy intake on the outcome of ischemic brain injury, and elucidated the underlying mechanisms.
Methods
We utilized a novel microchip-based immunoaffinity capillary electrophoresis technology to measure a panel of neurotrophic factors, cytokines and cellular stress resistance proteins in brain tissue samples from young, middle age and old mice that had been maintained on control or energy restricted diets prior to middle cerebral artery occlusion and reperfusion (I/R).
Results
Mortality from focal ischemic stroke was increased with advancing age and reduced by an intermittent fasting (IF) diet. Brain damage and functional impairment were reduced by IF in young and middle age mice, but not in old mice. The basal and post-stroke levels of neurotrophic factors (BDNF and bFGF), protein chaperones (HSP70 and GRP78) and the antioxidant enzyme HO-1 were decreased, while levels of inflammatory cytokines were increased in the cerebral cortex and striatum of old mice compared to younger mice. IF coordinately increased levels of protective proteins and decreases inflammatory cytokines in young, but not in old mice.
Interpretation
Reduction in dietary energy intake differentially modulates neurotrophic and inflammatory pathways to protect neurons against ischemic injury, and these beneficial effects of IF are compromised during aging resulting in increased brain damage and poorer functional outcome.
Introduction
Stroke, a major cause of disability and mortality in the elderly, occurs when a cerebral blood vessel is occluded and/or ruptured resulting in ischemic damage and death of neurons 1. The neurodegenerative mechanism involves metabolic and oxidative stress, excitotoxicity, apoptosis, and inflammatory processes including activation of glial cells and infiltrating leukocytes 2–4. Studies using cell culture and animal models have identified several different proteins and signaling pathways that can protect neurons against ischemic injury including: neurotrophic factors such as brain-derived neurotrophic factor (BDNF) and basic fibroblast growth factor (bFGF); protein chaperones including heat shock protein 70 (HSP70) and glucose regulated protein 78 (GRP78); and antioxidant enzymes such as superoxide dismutases and heme oxygenase-1 (HO-1) 5–9. When young rodents are subjected to brief mild cerebral ischemia prior to a stroke, the extent of brain damage is reduced and functional outcome is improved; this “preconditioning” effect of mild ischemia involves increased expression of neurotrophic factors and protein chaperones 10–12. The existence of the ischemic preconditioning mechanism suggests that it may be possible to activate a similar adaptive neuroprotective response with a non-invasive mild energetic stress.
Dietary energy restriction, by daily calorie reduction (CR) or intermittent fasting (IF), extends lifespan and decreases the development of age-related diseases including diabetes, cardiovascular disease and cancers 13. In humans CR and IF can reduce circulating markers of oxidative stress and inflammation, and can improve cardiovascular disease risk 14 and symptoms in asthma patients 15. Dietary energy restriction may also benefit neurons as suggested by data showing that CR and IF protect neurons against dysfunction and degeneration in animal models of epileptic seizures 16, and Alzheimer’s 17, Parkinson’s 18 and Huntington’s 19 diseases. Recent findings suggest the possibility that IF may promote neuronal survival and plasticity, in part by inducing the expression of brain-derived neurotrophic factor (BDNF) 19 and HSP70 20. However, energy restriction did not increase BDNF levels in the brains of old rats 21. Because aging is a major risk factor for stroke, and stroke outcome is poorer in the elderly 22, we tested the hypothesis that aging impairs ability of brain cells to respond adaptively to IF and so to survive a stroke. A novel microchip-based immunoaffinity capillary electrophoresis-based analytical technology was used to quantify levels of a battery of neurotrophic factors, protein chaperones and cytokines in several brain regions after experimental stroke. We show that multiple neuroprotective pathways are activated and inflammatory pathways are suppressed by IF in young animals, and that aging impairs the ability of IF to modulate these pathways adaptively.
Materials and Methods
Animals and Blood Collection and Measurements of Glucose and Insulin Concentrations
Male C57BL6 mice of three initial different ages (3, 9 and 16 months) were obtained from the Aging Colony in National Institute on Aging. Upon arrival all mice were maintained with a standard NIH-07 diet (Harlan–Teklad, Indianapolis) and free access to water, and a 12 h light/12 h dark cycle (lights on at 0600). Two weeks after arrival, mice were randomly assigned to either ad libitum (AL) or intermittent dietary energy restriction (IF; alternate day fasting) diets. Body weights were recorded weekly; for mice in the IF group the body weight was recorded on two consecutive days (a feeding day and a fasting day). Mice were maintained on the diets for at least three months prior to the experimental manipulations. Blood samples were taken after an overnight fast (for both diet groups). Glucose concentrations (mg/dL) were determined from whole blood using a glucose meter (FreeStyle, TheraSense, Alameda, CA). All mice were euthanized at 72 h after cerebral ischemia or sham operation, at which time plasma and brain tissue samples were collected and stored at −80 °C. The levels of plasma insulin was measured using an ultrasensitive insulin ELISA kit (ALPCO Diagnostics, NH). All animal procedures were approved by the National Institute on Aging Animal Care and Use Committee.
Focal Ischemic Stroke Model and Evaluations of Functional Outcome and Brain Damage
Mice were subjected to transient middle cerebral artery occlusion/reperfusion, as reported previously 23 and described in the Supplementary Methods. The functional consequences of focal cerebral I/R injury were evaluated using a five-point neurological deficit score (0, no deficit; 1, failure to extend right paw; 2, circling to the right; 3, falling to the right; and 4, unable to walk spontaneously) and were assessed in a blinded fashion. Two millimeter-thick coronal brain sections taken after 72 h of reperfusion were stained with 2% 2,3,5-triphenyltetrazolium chloride and evaluated for infarct size using standard methodology 23.
Cerebral Blood Flow Measurement
Animal’s head was placed in a fixed frame after it had been anesthetized and prepared for MCAO. Craniotomy performed to access the left MCA was extended to allow positioning of a 0.5-mm Doppler probe (PeriFlux System 5000, Jarfalla, Sweden) over the underlying parietal cortex approximately 1 mm posterior to bregma and 1 mm lateral to the midline. Laser-Doppler recordings are expressed as percentages of the pre-ischemic baseline and averaged over 30-min periods during 1 h of ischemia, and 30, 90, and 180 min of post-ischemic reperfusion.
Measurements of Neurotrophic Factor, Stress Protein and Cytokine Concentrations
Upon sacrifice, cerebral cortical and striatal tissue samples were rapidly removed from both the ipsilateral and contralateral hemispheres and were flash frozen and stored at -80°C. Levels of BDNF, bFGF, HSP-70, GRP-78, HO-1, TNFα, IL-1β, IL-6, IL-10 and IL-17 were quantified by immunoaffinity capillary electrophoresis using methods described previously 13, 24, 25 and detailed in Supplementary Methods. Briefly, the frozen samples were hand homogenized in 100 mM phosphate buffer and clarified by centrifugation at 10,000 g before being injected into the immunoaffinity port of a glass microchip (Micralyne, Edmonton, Alberta), which contained immobilized antibodies (R & D Systems, Mineapolis, MN; Bachem Biochemicals, King of Prussia, PA) thus capturing the analytes of interest, and removing them from the rest of the tissue extract. The bound analytes were labeled in-situ, eluted in acid buffer (pH 1.0), separated by electrophoresis, and measured by on-line laser-induced fluorescence detection.
Statistical Analysis
Several general linear model procedures including multivariate repeated measures, one-way or two-way ANOVA were applied to the data analyses in which the appropriate models were applicable. The neurological data were analyzed with the Generalized Estimating Equations (GEE) approach fit a marginal model that accounts for the repeated measures in the data set. The model analyzed ordinal multinomial data using a cumulative logit. Details of the analyses performed are available in Supplementary Methods.
Results
Dietary energy restriction protects against cerebral ischemia-induced mortality, brain cell death and functional deficits in a mouse stroke model
Groups of C57BL/6 mice of three different ages (3, 9 and 16 months) were maintained on ad libitum (AL) or alternate day fasting (IF) diets for 4–5 months prior to the experimental stroke. As expected from previous studies 26, young and middle age mice on the IF diet maintained significantly lower body weights (on both feeding and fasting days) compared to mice on the AL diet (Supplementary Fig. 1). The body weights of old AL fed mice were not different from those of old IF mice on feeding days, but were significantly greater than the body weights of IF mice on fasting days. Blood glucose concentrations were significantly lower in mice on IF (75–80 mg/dL) compared to mice on the AL diet (90–100 mg/dL) regardless of age (Supplementary Fig. 2). Plasma insulin concentrations were significantly lower in young mice on the IF diet compared to those on the AL diet, and there was a trend towards lower plasma insulin concentrations in middle and old age mice on the IF diet (Supplementary Fig. 2). The effects of IF on glucose and insulin concentrations are consistent with improved insulin sensitivity26, with a stronger effect of the diet in young compared to older mice.
To assess the effect of IF in experimental stroke, we used a mouse model that mimics the most common type of human stroke, transient ischemia and reperfusion (I/R). The experimental group consisted of three different age groups of C57BL/6 mice maintained on either an AL or an IF diet for 4–5 months prior to experimental stroke (n=30–40 mice in each age group). After 1 hour of ischemia we evaluated mortality rate during a 72 hour reperfusion period. Mortality was affected by both age and diet. Mortality was greater in older mice (35%), compared to young (13%) and middle age (23%) mice, and in mice on the AL diet compared to mice on the IF diet for all three age groups (Supplementary Table 1). The extent of anatomical and functional brain damage was also greatly reduced by IF in young and middle aged animals. Neurological impairment during a 3 day post-stroke period was significantly less in young and middle age IF mice compared with AL mice of the same ages (Table 1). However, in old mice IF had no significant effect on neurological deficit. Measurements of brain infarct volume showed a highly significant decrease in infarct volume in IF young and middle aged mice compared with mice on the AL diet (Fig. 1). In contrast, IF had no significant effect on infarct volume in old mice (Fig. 1). Stroke induced mortality, neurological deficits and brain infarct volume were increased with advancing age in both the AL and IF diet groups (Supplementary Table 1; Table 1; Fig. 1). Laser-Doppler measurements of cerebral blood flow prior to, during and for 180 min after I/R showed that middle cerebral artery occlusion was effective in reducing cerebral blood flow in mice of all ages and diet groups, and that there were no significant effects of age or diet on post-occlusion cerebral blood flow (Supplementary Figure 2 ).
Table 1.
Neurological scores (%) for each age and diet group during a 3 day post-stroke time period.
| Young | Middle | Old | |||||
|---|---|---|---|---|---|---|---|
| Time | Score | AL | IF | AL | IF | AL | IF |
| 0 | 0 | 23.1 | 0 | 15.4 | 0 | 0 | |
| 1 | 26.7 | 69.2 | 14.3 | 46.2 | 6.7 | 30.8 | |
| 24 hr | 2 | 33.3 | 7.7 | 7.1 | 7.7 | 0 | 30.8 |
| 3 | 20.0 | 0 | 50.0 | 30.8 | 33.3 | 23.1 | |
| 4 | 20.0 | 0 | 28.6 | 0 | 60.0 | 15.4 | |
| 0 | 0 | 33.3 | 0 | 15.4 | 0 | 0 | |
| 1 | 14.3 | 58.3 | 21.4 | 53.8 | 7.1 | 23.1 | |
| 48 hr | 2 | 57.1 | 8.3 | 28.6 | 15.4 | 7.1 | 46.2 |
| 3 | 0 | 0 | 35.7 | 15.4 | 35.7 | 23.1 | |
| 4 | 28.6 | 0 | 14.3 | 0 | 50.0 | 7.7 | |
| 0 | 0 | 38.4 | 7.1 | 15.4 | 0 | 0 | |
| 1 | 28.6 | 53.8 | 21.4 | 53.8 | 14.3 | 23.1 | |
| 72 hr | 2 | 42.9 | 7.7 | 28.6 | 23.1 | 14.3 | 38.5 |
| 3 | 7.1 | 0 | 21.4 | 7.7 | 35.7 | 23.1 | |
| 4 | 21.4 | 0 | 21.4 | 0 | 35.7 | 15.4 | |
| Source | DF | Chi-Square | P value |
| Age | 2 | 20.43 | <0.0001 |
| Time | 2 | 6.7 | <0.05 |
| Diet | 1 | 30.46 | <0.0001 |
Fig 1. Intermittent dietary energy restriction reduces brain damage in a mouse stroke model.
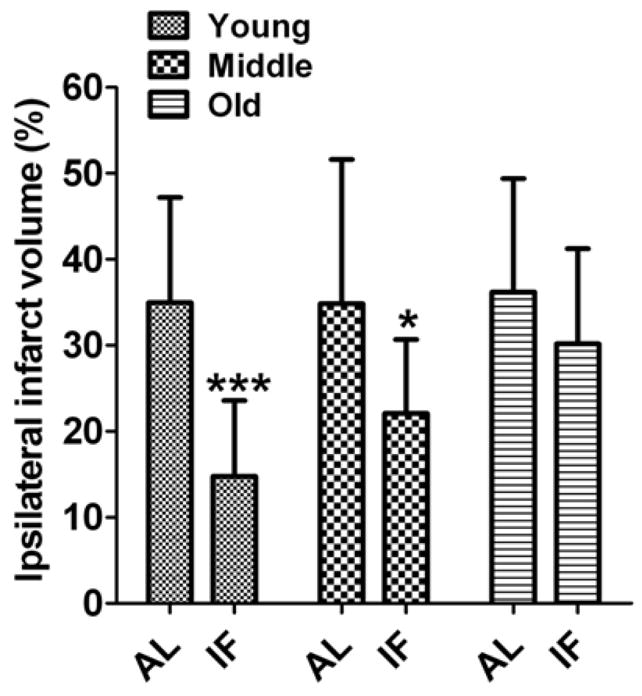
Ischemic infarct sizes 72 h after I/R in IF-fed (n=10–12) and AL-fed I/R (n=12–20) mice; ***p<0.001 and *p<0.05 compared with AL-fed mice value in each age group.
Levels of cortical and striatal neurotrophic factors are reduced during aging, and increased by IF and ischemic stroke in an age-related manner
Previous findings have demonstrated that bFGF 27 and BDNF 28 can reduce brain damage and improve functional outcome in stroke models. Using a novel microchip-based immunoaffinity capillary electrophoresis technology 29, we quantified levels of BDNF and bFGF in tissue samples from both the ipsilateral (IL; stroke side) and contralateral (CL) striatum and cortex of mice in all age and diet groups (Fig. 2; Supplementary Table 3). Concentrations of BDNF and bFGF were significantly lower in both the cortex and striatum of old mice compared to young and middle age mice; BDNF levels declining progressively whereas bFGF levels dropped from young to middle age with no further decline in old mice (Fig. 2; Supplementary Table 3). IF resulted in increased levels of cortical and striatal BDNF in mice of all three ages, though the absolute levels of BDNF were four- and two-fold greater in young and middle age mice, respectively, compared to old mice (Supplementary Table 3). Mice of all three ages exhibited increased levels of bFGF in the cortex and striatum when maintained on IF compared to AL diets, though this effect of IF was greater in young compared to older mice. In AL mice ischemia did not significantly affect BDNF levels in the cortex and striatum of young or old mice, but did cause a significant though small increase in BDNF levels in both the IL and CL cortex and striatum of middle age mice (Supplementary Table 3). In IF mice BDNF levels were significantly increased by ischemia in both the IL and CL cortex and striatum of middle age mice, but not in young or old mice. In young mice, but not in middle age or old mice, ischemia induced an increase in bFGF levels in both the cortex and striatum. The major statistical results using MANOVA repeated measures for BDNF and bFGF, as well as stress response proteins and cytokines in the following sections, are summarized in Supplementary Table 7).
Fig 2. Cortical and striatal levels of BDNF and bFGF decrease during aging and are increased by IF.
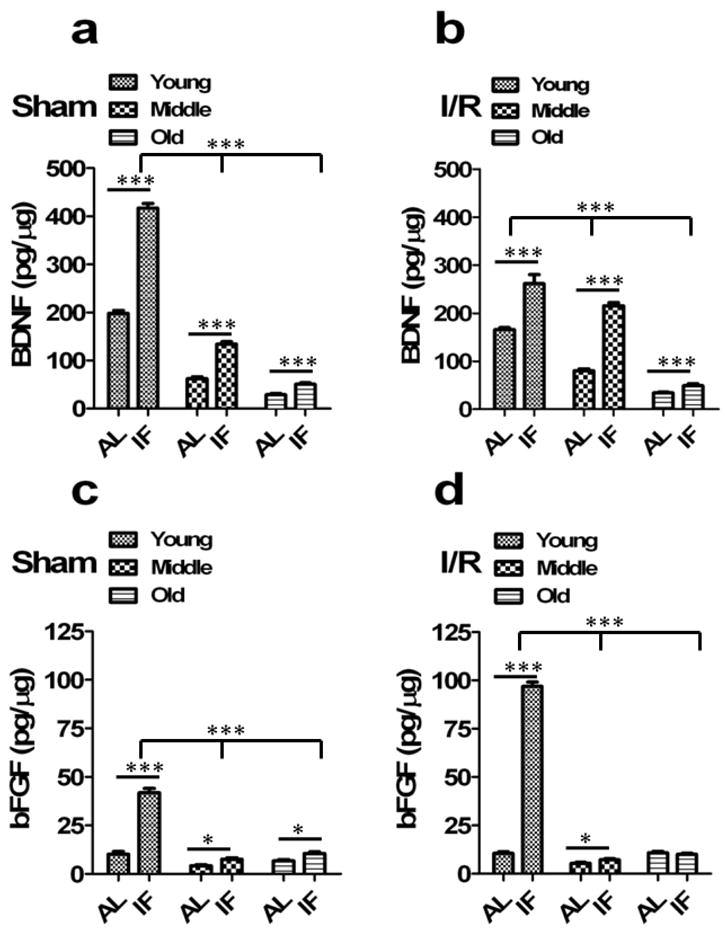
(a) BDNF levels were significantly decreased in middle aged and old sham animals brain compared to young sham animals (n=10 in each group). ***p<0.0001 compared with young animals. IF sham animals had significantly increased BDNF compared to AL-fed sham controls in all age group animals. ***p<0.0001 compared with AL animals in each group. (b) IF animals had significantly increased brain BDNF levels compared to AL-fed controls in all age group animals following cerebral I/R. ***p<0.0001 compared with AL-fed I/R controls in each group. (c) bFGF levels were significantly decreased in middle aged and old sham animals compared to young sham animals (n=10 in each group). ***p<0.0001 compared with young sham animals. IF sham animals had significantly increased bFGF compared to AL-fed sham controls in all age group animals. ***p<0.0001 compared with AL sham animals in each group. (d) Following cerebral I/R, IF animals had significantly increased bFGF compared to AL-fed I/R controls in all age group. ***p<0.0001 compared with AL I/R animals in each group. Values are the mean and SEM.
Levels of cortical and striatal stress response proteins are reduced during aging, and increased by IF and ischemic stroke in an age-dependent manner
Previous studies have shown that the expression of the protein chaperones HSP-70 and GRP-78, and the antioxidant enzyme HO-1 are induced by cerebral ischemia and can increase the resistance of neurons to ischemia 11, 12, 30, 31. We therefore measured levels of HSP-70, GRP-78 and HO-1 in samples of cerebral cortex and striatum in mice from all age and diet groups (Fig. 3; Supplementary Table 4). There were large reductions in levels of all three stress proteins in both the cortex and striatum with advancing age; in the cases of HSP-70 and GRP-78 the reductions were progressive, whereas HO-1 levels decreased from young to middle age with no further reduction in old mice. Levels of HSP-70 and GRP-78 were increased by IF in both brain regions and in all age groups HO-1 levels were increased in response to IF in young and middle age mice, but not in old mice. After a stroke in AL mice, levels of all three stress proteins were lowest in the cortex and striatum of old mice, compared to young and middle age mice (Fig. 3b, d and f; Supplementary Table 4). In IF mice, the post stroke levels of all three stress proteins were significantly greater in the cortex and striatum of young and middle age mice compared to the post stroke levels in AL mice. The ability of brain cells to respond to IF by increasing levels of HSP-70 and HO-1 was significantly attenuated in old mice compared to young and middle age mice. However, old mice subjected to a stroke did exhibit a significant increase in GRP-78 levels compared to AL mice subjected to a stroke.
Fig 3. Levels of HSP-70, GRP-78 and HO-1 are increased in the brains of mice on the IF diet and are decreased with aging.
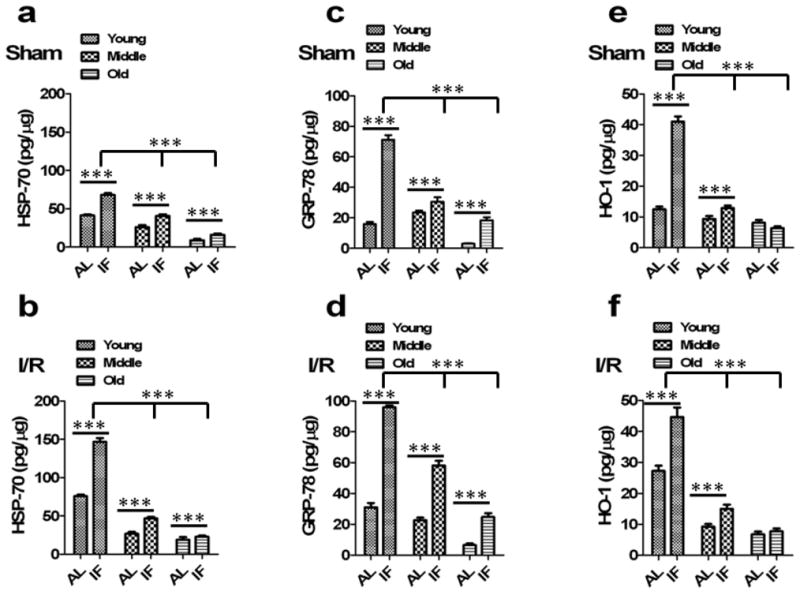
(a) HSP-70 quantified from brain lysates of sham animals. HSP-70 levels were significantly decreased in middle aged and old sham animals compared to young sham animals (n=10 in each group). ***p<0.0001 compared with young sham animals. IF sham animals had significantly increased HSP-70 compared to AL-fed sham controls in all age group animals. ***p<0.0001 compared with AL sham animals in each group. (b) IF I/R animals had significantly increased HSP-70 compared to AL-fed I/R controls in all age group following cerebral I/R. ***p<0.0001 compared with AL I/R animals in each group. (c) GRP-78 levels in the brain were significantly decreased in middle aged and old sham animals compared to young sham animals (n=10 in each group). ***p<0.0001 compared with young sham animals. IF sham animals had significantly increased GRP-78 compared to AL-fed sham controls in all age group animals. ***p<0.0001 compared with AL sham animals in each group. (d) Following cerebral I/R, IF animals had significantly increased GRP-78 compared to AL-fed I/R controls in all age group. ***p<0.0001 compared with AL I/R animals in each group. (e) HO-1 levels were significantly decreased in middle aged and old sham animals compared to young sham animals (n=10 in each group). ***p<0.0001 compared with young sham animals. IF sham animals had significantly increased HO-1 compared to AL-fed sham controls in young and middle age group. There was no significant increase observed in IF old animals compared to AL-fed old animals. ***p<0.0001 compared with AL sham animals. (f) IF young and middle aged I/R animals had significantly increased HO-1 compared to AL-fed I/R controls following cerebral I/R. ***p<0.0001 compared with AL I/R animals.
Age- and stroke-related alterations in pro- and anti-inflammatory cytokines are modulated by dietary energy restriction
Considerable evidence suggests that inflammation contributes to neuronal dysfunction and degeneration both chronically during aging and acutely after a stroke 32. Three pro-inflammatory cytokines (PIC) implicated in ischemic brain injury are tumor necrosis factor-α (TNF-α) 33, 34, interleukin-1β (IL-1β) 35 and interleukin-6 (IL-6) 36. To elucidate possible roles for changes in pro-inflammatory cytokines in the ameliorative effects of IF on stroke outcome in the context of advancing age, we therefore measured levels of TNF-α, IL-1β and IL-6 in the cortical and striatal tissue samples. In AL fed sham control mice levels of TNF-α and IL-6 in both the cortex and striatum, increased from young to middle age and remained elevated in old mice (Fig. 4; Supplementary Table 5). Levels of TNF-α and IL-6 were significantly lower in the cortex and striatum of mice on the IF diet compared to the AL diet. In response to ischemic stroke the levels of both TNF-α and IL-6 increased significantly in both the ipsilateral and contralateral cortex and striatum, with the magnitude of the elevations being greatest in young mice and least in old mice (Fig. 4a–d; Supplementary Table 5). IF suppressed the ischemia-induced increases of TNF-α and IL-6 levels, with this dietary effect being greatest in young mice and least in old mice.
Fig 4. Dietary energy restriction decreases pro-inflammatory cytokines TNF-α, IL-6 and IL-1β and increases anti-inflammatory cytokines IL-17A and IL-10 in the brain.

Pro-inflammatory cytokines TNF-α (a) and IL-6 (c) levels were significantly increased in middle aged and old sham animals compared young sham animals. +++p<0.0001 compared with young sham animals. IF significantly reduced both TNF-α (a) and IL-6 (c) levels compared to AL-fed animals in all three age groups. ***p<0.0001 compared with AL sham animals. TNF-α (b) and IL-6 (d) levels were significantly decreased in middle aged and old I/R animals compared young I/R animals. ***p<0.0001 compared with young sham animals. IF animals also had decreased levels of TNF-α (b) and IL-6 (d) compared to AL-fed animals in all three age groups. ***p<0.0001 compared with IL-fed animals. (e, f) IL-1β levels were significantly decreased in both middle aged and old sham animals compared to young sham animals. ***p<0.0001 compared with young animals. (e, f) IF young animals had significantly reduced IL-1β levels compared to AL-fed young animals in sham and I/R groups. ***p<0.0001 compared with AL-fed young animals. (g, h) Anti-inflammatory cytokine IL-17A levels were decreased with aging. Middle aged and old animals had significantly reduced IL-17A levels compared to young animals. ***p<0.0001 compared to young animals. IF animals had significantly increased levels of IL-17A compared to AL-fed animals in all aged group in sham animals and following cerebral I/R. ***p<0.0001 compared with AL-fed animals. (i, j) IL-10 levels were significantly increased with aging in middle aged and old animals compared to young animals. +++p<0.0001 compared to young animals. Young IF animals had significant increase in IL-10 in sham group *p<0.01 compared with AL-fed young sham animals) and young as well as old IF animals had significant increase in IL-10 following cerebral I/R compared to AL-fed I/R animals. ***p<0.0001 compared with AL-fed young and old I/R animals.
Surprisingly, changes in levels of the PIC IL-1β in response age, diet and stroke were strikingly different compared to TNF-α and IL-6. In both the cortex and striatum of AL-fed control mice the levels of IL-1β were thirty- to forty-fold greater in young mice compared to middle age and old mice (Fig. 4e; Supplementary Table 5). IF resulted in a significant reduction in cortical and striatal IL-1β levels in young mice, whereas IF increased IL-1β in middle age and old mice. In AL-fed mice ischemic stroke caused an increase of IL-1β levels in the cortex and striatum (both ipsilateral and contralateral) of young mice, while having little or no effect on IL-1β levels in middle age and old mice (Fig. 4f; Supplementary Table 5). IF suppressed the stroke-induced IL-1β increase in young mice, while increasing levels of IL-1β in middle age and old mice.
IL-10 most often serves an anti-inflammatory function 37, whereas IL-17A produced by lymphocytes is generally pro-inflammatory 38. Levels of IL-17A decreased significantly with advancing age and were increased by IF in both the cortex and striatum (Fig. 4g,h; Supplementary Table 6). Ischemia caused an approximately twofold increase in IL-17A levels in both the ipsilateral and contralateral cortex and striatum in mice of all three ages. The greatest levels of IL-17A were found in the ipsilateral ischemic cortex of mice on the IF diet (Supplementary Table 6). There was a striking progressive increase in IL-10 levels with advancing age in both the cortex and striatum of AL mice; IL-10 levels increased from approximately 25 pg/μg in young mice to 750 pg/μg in middle age mice and more than 1000 pg/μg in old mice (Fig. 4i, j; Supplementary Table 6). IF significantly increase IL-10 levels in the cortex and striatum of young mice, but did not affect IL-10 levels in middle age or old mice. In AL-fed mice ischemia induced a significant increase of IL-10 levels in both the ipsilateral and contralateral cortex and striatum of young and middle age mice, but not old mice (Supplementary Table 6). Young and old, but not middle age, mice on the IF diet exhibited elevated post-ischemia IL-10 levels in the cortex and striatum compared to the post-ischemia IL-10 levels in mice on the AL diet. Notably, the effects of age and diet on IL-10 levels and IL-1β levels were inversely related (Supplementary Tables 5 and 6).
Neuroprotective and inflammatory proteins are differentially regulated by dietary energy restriction
We next sought to establish relationships among and between neuroprotective factors (neurotrophic factors, protein chaperones and HO-1) and pro-inflammatory cytokines (TNF-α, IL-6 and IL-1β) in brain tissue samples from mice in all age and diet groups, under control and stroke conditions. To achieve this goal, we employed nonlinear mixed-effects regressions to model the data and to assess the product of the slopes for the concentrations of protein pairs in tissue samples from the ipsilateral cerebral cortex (Table 2). A significant negative relation (the product of the slopes was less than 0) indicated that an age-, diet- or ischemia-dependent elevation in one particular factor was associated with a reduction in another factor. In contrast, a significant positive relation indicated that both factors were altered in the same direction.
Table 2.
Summary of the tests of product of slopes = 0.
| Comparison | AL | IF | ||||||
|---|---|---|---|---|---|---|---|---|
| Sham | Ischemia | Sham | Ischemia | |||||
| Estimate | p-value | Estimate | p-value | Estimate | p-value | Estimate | p-value | |
| BDNF & bFGF | 3.5684 | 0.0363 | −0.3726 | 0.7477 | 69.5541 | <0.0001 | 107.31 | <0.0001 |
| BDNF & Hsp-70 | 32.6933 | <0.0001 | 43.1116 | <0.0001 | 114.11 | <0.0001 | 154.95 | <0.0001 |
| BDNF & HO1 | 4.4309 | 0.0009 | 15.5015 | <0.0001 | 76.4126 | <0.0001 | 46.1303 | <0.0001 |
| BDNF & Grp-78 | 12.5855 | 0.0012 | 19.03432 | <0.0001 | 116.53 | <0.0001 | 90.2596 | <0.0001 |
| bFGF & Hsp-70 | 0.6781 | 0.0361 | −0.1592 | 0.7497 | 9.8334 | <0.0001 | 60.6588 | <0.0001 |
| bFGF & HO1 | 0.0919 | 0.0654 | −0.0542 | 0.7479 | 6.5847 | <0.0001 | 18.0590 | <0.0001 |
| bFGF & Grp-78 | 0.2610 | 0.0677 | −0.07023 | 0.7479 | 10.0413 | <0.0001 | 35.3345 | <0.0001 |
| BDNF & TNFα | −241.99 | 0.0019 | 446.81 | <0.0001 | −786.69 | <0.0001 | −297.08 | 0.0007 |
| BDNF & IL6 | −189.22 | 0.0008 | 232.55 | 0.0028 | −691.34 | <0.0001 | −379.52 | <0.0001 |
| BDNF &IL1β | −568.02 | <0.0001 | 83.2698 | 0.4270 | −1925.34 | <0.0001 | −1701.99 | <0.0001 |
| bFGF & TNFα | −5.0174 | 0.0713 | −1.6495 | 0.7481 | −67.7901 | <0.0001 | −116.30 | 0.0015 |
| bFGF & IL6 | −3.9248 | 0.0650 | −0.8585 | 0.7490 | −59.5742 | <0.0001 | −148.57 | <0.0001 |
| bFGF &IL1β | −11.7815 | 0.0386 | −0.3074 | 0.7652 | −165.91 | <0.0001 | −666.29 | <0.0001 |
| Hsp-70 & TNFα | −45.9694 | 0.0018 | 190.86 | <0.0001 | −111.22 | <0.0001 | −167.93 | 0.0008 |
| Hsp-70 & IL6 | −35.9589 | 0.0008 | 99.3349 | 0.0040 | −97.7392 | <0.0001 | −214.54 | <0.0001 |
| Hsp-70 &IL1β | −107.94 | <0.0001 | 35.5696 | 0.4235 | −272.20 | <0.0001 | −962.12 | <0.0001 |
| HO1 & TNFα | −6.2302 | 0.0151 | 68.6432 | <0.0001 | −74.4753 | <0.0001 | −49.9964 | 0.0010 |
| HO1 & IL6 | −4.8735 | 0.0113 | 35.7259 | <0.0001 | −65.4486 | <0.0001 | −63.8718 | <0.0001 |
| HO1 &IL1β | −14.6293 | 0.0012 | 12.7927 | 0.4243 | −182.27 | <0.0001 | −286.44 | <0.0001 |
| Grp-78 & TNFα | −17.6963 | 0.0167 | 84.2675 | <0.0001 | −113.57 | <0.0001 | −97.8241 | 0.0005 |
| Grp-78 & IL6 | −13.8426 | 0.0126 | 43.8577 | 0.0042 | −99.8062 | <0.0001 | −124.97 | <0.0001 |
| Grp-78 &IL1β | −41.5529 | 0.0016 | 15.7045 | 0.4241 | −277.95 | <0.0001 | −560.45 | <0.0001 |
To accomplish this goal, the linear mixed-effects model was fit using a nonlinear mixed-effects regression model which allowed for the assessments on the product of the slopes. This revealed that the many of the changes in neurotrophic factors, protective protein chaperones and inflammatory products under the influences of dietary energy restriction, aging and ischemic lesion were significantly correlated (Table 2). Most notably, there were highly significant positive associations between different neuroprotective factors, and negative associations between neuroprotective factors and proinflammatory cytokines, in mice on the IF diet. This was the case in both non-ischemic and ischemic brain tissue samples. In control and ischemic tissue samples from mice maintained on IF there were significant positive associations between: BDNF and bFGF; BDNF and HSP70; BDNF and GRP78; BDNF and HO-1; bFGF and HSP70; bFGF and GRP78; and bFGF and HO-1 (Table 2). In mice fed AL there were also significant positive associations for most pairs of neuroprotective factors in control and ischemic cortical tissue samples for BDNF and HSP70, BDNF and GRP78, and BDNF and HO-1. However, in the case of bFGF, there were either weak or no significant associations with protein chaperones and HO-1 in mice fed AL.
In control and ischemic cortical tissue samples from mice maintained on IF there were highly significant negative associations between neurotrophic factors and pro-inflammatory cytokines in all cases (BDNF and TNF-α; BDNF and IL-6; BDNF and IL-1β; bFGF and TNF-α; bFGF and IL-6; bFGF and IL-1β). In contrast, associations between neurotrophic factors and pro-inflammatory cytokines were, in many cases, not significant in control and ischemic cortical tissue samples from mice fed AL (Table 2). Interestingly, and in contrast to the negative association between BDNF and pro-inflammatory cytokine levels in cortical tissue from mice on IF, BDNF and pro-inflammatory cytokines were positively correlated in ischemic cortical tissue from AL fed mice. Collectively, these findings suggest that IF enhances the ability of brain cells to protect neurons against ischemic injury by a mechanism involving the coordinate up-regulation of multiple neuroprotective proteins (neurotrophic factors, protein chaperones and antioxidant enzymes) and down-regulation of pro-inflammatory cytokines.
Discussion
Our findings suggest that aging compromises the ability of energy restriction to protect the brain against ischemic injury and improve functional outcome in a mouse model of stroke. The neuroprotective effect of IF was robust in young mice (infarct volume and neurological deficits were decreased by more than 50%), and was diminished in middle age mice and lacking in old mice. Aged mice that had been fed ad libitum did not show increased infarct volume compared to young and middle aged ad libitum animals. We believe the main reason for the latter result is that we occluded the middle cerebral artery for a relatively long time period (1 hour) that causes maximal or near-maximal damage to the striatum and cortex in young animals. The rationale for using this amount of ischemia was so that we would be more likely to detect robust protective effects of the intermittent fasting diet compared to the ad libitum diet in the young animals and, indeed, this was the case. However, our study shows that aged mice are more vulnerable to ischemic stroke-induced brain injury as their mortality was significantly greater than the young mice and the functional outcome of those that survived was worse than young or middle-age mice. However, we did not evaluate functional outcome beyond 72 hours of reperfusion as mortality was high in the aged mice on the ad libitum diet (~35% mortality). It will be of interest to evaluate long-term outcome, as well as the efficacy of post-stroke dietary energy restriction on recovery from stroke in future study.
Our analysis of neurotrophic factors, stress resistance proteins and cytokines suggests mechanisms by which aging impairs the ability of IF to protect brain cells against a stroke. Levels of BDNF and bFGF were diminished in the cortex and striatum of old mice compared to young mice. The amounts of BDNF and bFGF were increased by IF to much higher levels in young compared to middle age and old mice. Both BDNF 5, 39 and bFGF 6, 40 have previously been shown to protect neurons against ischemic injury. We found that at 72 hours after the stroke cortical and striatal bFGF levels were increased ten-fold in young IF mice, with little or no change in bFGF levels in AL young mice or IF middle age and old mice. Therefore, elevated bFGF levels were strongly associated with improved stroke outcome, suggesting an important role for bFGF in the protective effects of youth and IF against ischemic brain injury.
Levels of all three cellular stress proteins examined (HSP-70, GRP-78 and HO-1) were elevated in cortex and striatum in response to IF and a stroke in young mice, but with greatly diminished responses in middle age and old mice. Particularly striking was the robust post-stroke upregulation of HSP-70 and GRP-78 in mice on the IF diet compared to mice fed AL. These findings suggest that aging impairs the ability of brain cells to engage adaptive stress responses to both a beneficial environmental factor (IF) and an acute severe insult (stroke). HSP-70 and GRP-78 function as major protein chaperones in the cytosol and endoplasmic reticulum, respectively, whereas HO-1 is an antioxidant enzyme. All three of these stress proteins have previously been shown to protect neurons against stroke in vivo 9, 12, 30, 31 and can directly protect neurons against other insults relevant to stroke including excitotoxicity and oxidative stress 41, 42. HSP-70, GRP-78 and HO-1 may therefore contribute to the neuroprotective effect of IF, particularly in young animals. The specific signaling pathways and transcription factors that mediate IF- and stroke-induced increases in neurotrophic factors and cellular stress proteins, and those impaired in aging, remain to be established. Candidates include activation of glutamate receptors and calcium influx, mitogen-activated protein kinases, and the transcription factors CREB (BDNF), AP-1 (bFGF), heat shock factor-1 (HSP-70), activating transcription factor-6 (GRP78) and Nrf-2 (HO-1) 43–46.
Changes in cortical and striatal levels of TNFα and IL-6 in response to aging, IF and stroke were tightly correlated. TNFα and IL-6 levels increased during aging, and decreased in response to IF, particularly in young and middle age mice, in sham control mice. After a stroke the TNFα and IL-6 levels increased by two- to three-fold in AL mice, and these cytokine responses to stroke were attenuated by IF, particularly in young mice. Both TNFα and IL-6 are pro-inflammatory cytokines that are implicated in neuronal degenerative processes including ischemic brain injury 32–36. Our findings therefore suggest that suppression of TNFα and IL-6 production may contribute to the reduction in brain damage and improved functional outcome in mice on IF compared to AL-fed control mice. Interestingly, IF increased IL-17A levels in the cortex and striatum of sham mice, but suppressed stroke-induced IL-17A production. The IL-17 antibody used in our study was directed against a peptide sequence common to all IL-17 subtypes (IL-17A–IL-17F). Our findings therefore implicate one or more IL-17 family members in brain cell responses to IF and stroke.
There was a striking inverse relationship between levels of IL-10 and IL-1β across diet and age groups. IL-10 levels increased with advancing age whereas IL-1β levels decreased. IF increased IL-10 levels and decreased IL-1β levels in sham and stroke mice, but only in young animals. IL-10 is an anti-inflammatory molecule that can suppress microglial IL-1β production 47, and our data are therefore consistent with a role for IL-10 in protecting neurons against the damaging effects of IL-1β in ischemic stroke48, 49. Indeed, Vila and colleagues 50 reported lower levels of IL-10 in stroke patients with clinical deterioration, relative to those who remained stable or improved during the first two days after the stroke.
Previous studies have shown that a stroke induces changes in cellular energy metabolism and the expression of stress-responsive genes that are not limited to the region of tissue damage but, instead also occur at distant sites including the contralateral cerebral hemisphere 51–53. We found that the effects of age, IF and stroke on levels all neurotrophic factors, stress proteins and cytokines were nearly identical in both hemispheres. While there is no reason to believe that age or IF would differentially affect the expression of these proteins in right and left hemispheres, it is intriguing that stroke induced similar changes in their expression in both the damaged hemisphere and the undamaged contralateral hemisphere. Several mechanisms may underlie the upregulation of expression of neurotrophic factors, stress proteins and cytokines in the contralateral hemisphere. One possibility is that stroke induces oxidative and metabolic stress in cells in the contralateral hemisphere and, indeed, there is evidence to support this possibility 54, 55. Another possibility is that pro-inflammatory cytokines in the ischemic tissue are transferred to the contralateral hemisphere via the vasculature and cerebrospinal fluid as the result of blood-brain barrier disruption, a possibility suggested by previous findings 56. Yet another mechanism involves increased transhemispheric excitability 57, which is known to increase the production of neurotrophic factors, stress proteins and cytokines 58, 59.
IF increased levels of growth factors that promote neuronal survival and plasticity, as well as protein chaperones and an antioxidant enzyme, in the cortex and striatum revealing a mechanism by which IF protects the brain against injury. The ability of IF to increase levels of neurotrophic factors and stress proteins was diminished with advancing age and, accordingly, IF was less effective in reducing ischemic brain injury in old compared to young animals. Aging also increased stroke mortality and compromised IF-mediated suppression of pro-inflammatory cytokines and induction of the anti-inflammatory cytokine IL-10. Our findings suggest that individuals with relatively low energy intake are more likely to survive and recover function after a focal ischemic stroke compared to those with a higher energy intake. This possibility is supported by a recent study showing that caloric restriction improves recovery from transient global cerebral ischemia in rats 60. While stroke is relatively rare in children and young adults, traumatic injury to the central nervous system is common, and it was recently reported that IF initiated after spinal cord injury can improve recovery of function in rats 61. The present findings suggest that IF protects neurons against acute tissue injury and enhances brain function by simultaneously stimulating neurotrophic and neuroprotective pathways and suppressing inflammation.
Supplementary Material
Acknowledgments
This research was supported by the National Institute on Aging Intramural Research Program and the National Institute of Biomedical Imaging and Bioengineering Intramural Research Program of the National Institutes of Health (NIH).
References
- 1.Shuaib A, Boyle C. Stroke in the elderly. Curr Opin Neurol. 1994;7:41–47. doi: 10.1097/00019052-199402000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Zheng Z, Lee JE, Yenari M. Stroke: molecular mechanisms and potential targets for treatment. Curr Mol Med. 2003;3:361–372. doi: 10.2174/1566524033479717. [DOI] [PubMed] [Google Scholar]
- 3.Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nat Rev Neurosci. 2006;7:278–294. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol. 2006;147:S232–240. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Endres M, Fan G, Hirt L, et al. Ischemic brain damage in mice after selectively modifying BDNF or NT4 gene expression. J Cereb Blood Flow Metab. 2000;20:139–144. doi: 10.1097/00004647-200001000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Bethel A, Kirsch JR, Koehler RC, et al. Intravenous basic fibroblast growth factor decreases brain injury resulting from focal ischemia in cats. Stroke. 1997;28:609–615. doi: 10.1161/01.str.28.3.609. [DOI] [PubMed] [Google Scholar]
- 7.Kudo T, Kanemoto S, Hara H, et al. A molecular chaperone inducer protects neurons from ER stress. Cell Death Differ. 2008;15:364–375. doi: 10.1038/sj.cdd.4402276. [DOI] [PubMed] [Google Scholar]
- 8.Satoh T, Okamoto SI, Cui J, et al. Activation of the Keap1/Nrf2 pathway for neuroprotection by electrophilic phase II inducers. Proc Natl Acad Sci USA. 2006;103:768–773. doi: 10.1073/pnas.0505723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rajdev S, Hara K, Kokubo Y, et al. Mice overexpressing rat heat shock protein 70 are protected against cerebral infarction. Ann Neurol. 2000;47:782–791. [PubMed] [Google Scholar]
- 10.Jiang X, Tian F, Mearow K, Okagaki P, et al. The excitoprotective effect of N-methyl-D-aspartate receptors is mediated by a brain-derived neurotrophic factor autocrine loop in cultured hippocampal neurons. J Neurochem. 2005;94:713–722. doi: 10.1111/j.1471-4159.2005.03200.x. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Graham SH, Zhu RL, et al. Stress proteins and tolerance to focal cerebral ischemia. J Cereb Blood Flow Metab. 1996;16:566–577. doi: 10.1097/00004647-199607000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi T, Saito A, Okuno S, et al. Induction of GRP78 by ischemic preconditioning reduces endoplasmic reticulum stress and prevents delayed neuronal cell death. J Cereb Blood Flow Metab. 2003;23:949–961. doi: 10.1097/01.WCB.0000077641.41248.EA. [DOI] [PubMed] [Google Scholar]
- 13.Hursting SD, Lavigne JA, Berrigan D, et al. Calorie restriction, aging, and cancer prevention: mechanisms of action and applicability to humans. Annu Rev Med. 2003;54:131–152. doi: 10.1146/annurev.med.54.101601.152156. [DOI] [PubMed] [Google Scholar]
- 14.Lefevre M, Redman LM, Heilbronn LK, et al. Caloric restriction alone and with exercise improves CVD risk in healthy non-obese individuals. Atherosclerosis. 2008;203:206–213. doi: 10.1016/j.atherosclerosis.2008.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson JB, Summer W, Cutler RG, et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med. 2007;42:665–674. doi: 10.1016/j.freeradbiomed.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruce-Keller AJ, Umberger G, McFall R, et al. Food restriction reduces brain damage and improves behavioral outcome following excitotoxic and metabolic insults. Ann Neurol. 1999;45:8–15. [PubMed] [Google Scholar]
- 17.Halagappa VK, Guo Z, Pearson M, et al. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer’s disease. Neurobiol Dis. 2007;26:212–220. doi: 10.1016/j.nbd.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 18.Maswood N, Young J, Tilmont E, et al. Caloric restriction increases neurotrophic factor levels and attenuates neurochemical and behavioral deficits in a primate model of Parkinson’s disease. Proc Natl Acad Sci USA. 2004;101:18171–18176. doi: 10.1073/pnas.0405831102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duan W, Guo Z, Jiang H, et al. Dietary restriction normalizes glucose metabolism and BDNF levels, slows disease progression, and increases survival in huntingtin mutant mice. Proc Natl Acad Sci USA. 2003;100:2911–2916. doi: 10.1073/pnas.0536856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu ZF, Mattson MP. Dietary restriction and 2-deoxyglucose administration reduce focal ischemic brain damage and improve behavioral outcome: evidence for a preconditioning mechanism. J Neurosci Res. 1999;57:830–839. [PubMed] [Google Scholar]
- 21.Newton IG, Forbes ME, Legault C, et al. Caloric restriction does not reverse aging-related changes in hippocampal BDNF. Neurobiol Aging. 2005;26:683–688. doi: 10.1016/j.neurobiolaging.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Ay H, Koroshetz WJ, Vangel M, et al. Conversion of ischemic brain tissue into infarction increases with age. Stroke. 2005;36:2632–2636. doi: 10.1161/01.STR.0000189991.23918.01. [DOI] [PubMed] [Google Scholar]
- 23.Arumugam TV, Chan SL, Jo DG, et al. Gamma secretase-mediated Notch signaling worsens brain damage and functional outcome in ischemic stroke. Nat Med. 2006;12:621–623. doi: 10.1038/nm1403. [DOI] [PubMed] [Google Scholar]
- 24.Phillips TM. Rapid analysis of inflammatory cytokines in cerebrospinal fluid using chip-based immunoaffinity electrophoresis. Electrophoresis. 2004;10–11:1652–1659. doi: 10.1002/elps.200305873. [DOI] [PubMed] [Google Scholar]
- 25.Phillips TM, Wellner EF. Analysis of inflammatory biomarkers from tissue biopsies by chip-based immunoaffinity CE. Electrophoresis. 2007;28:3041–3048. doi: 10.1002/elps.200700193. [DOI] [PubMed] [Google Scholar]
- 26.Duan W, Guo Z, Jiang H, et al. Reversal of behavioral and metabolic abnormalities, and insulin resistance syndrome, by dietary restriction in mice deficient in brain-derived neurotrophic factor. Endocrinology. 2003;144:2446–2453. doi: 10.1210/en.2002-0113. [DOI] [PubMed] [Google Scholar]
- 27.Schäbitz WR, Steigleder T, Cooper-Kuhn CM, et al. Intravenous brain-derived neurotrophic factor enhances poststroke sensorimotor recovery and stimulates neurogenesis. Stroke. 2007;38:2165–2172. doi: 10.1161/STROKEAHA.106.477331. [DOI] [PubMed] [Google Scholar]
- 28.Ay H, Ay I, Koroshetz WJ, et al. Potential usefulness of basic fibroblast growth factor as a treatment for stroke. Cerebrovasc Dis. 1999;9:131–135. doi: 10.1159/000015941. [DOI] [PubMed] [Google Scholar]
- 29.Guzman NA, Blanc T, Phillips TM. Immunoaffinity capillary electrophoresis as a powerful strategy for the quantification of low-abundance biomarkers, drugs, and metabolites in biological matrices. Electrophoresis. 2008;29:3259–3278. doi: 10.1002/elps.200800058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee H. Targeted hsp70.1 disruption increases infarction volume after focal cerebral ischemia in mice. Stroke. 2001;32:2905–2912. doi: 10.1161/hs1201.099604. [DOI] [PubMed] [Google Scholar]
- 31.Panahian N, Yoshiura M, Maines MD. Overexpression of heme oxygenase-1 is neuroprotective in a model of permanent middle cerebral artery occlusion in transgenic mice. J Neurochem. 1999;72:1187–1203. doi: 10.1111/j.1471-4159.1999.721187.x. [DOI] [PubMed] [Google Scholar]
- 32.Barone FC, Feuerstein GZ. Inflammatory mediators and stroke: new opportunities for novel therapeutics. J Cereb Blood Flow Metab. 1999;19:819–834. doi: 10.1097/00004647-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Bruce AJ, Boling W, Kindy MS, et al. Altered neuronal and microglial responses to excitotoxic and ischemic brain injury in mice lacking TNF receptors. Nat Med. 1996;2:788–794. doi: 10.1038/nm0796-788. [DOI] [PubMed] [Google Scholar]
- 34.Barone FC, Arvin B, White RF, et al. Tumor necrosis factor-alpha. A mediator of focal ischemic brain injury. Stroke. 1997;28:1233–1244. doi: 10.1161/01.str.28.6.1233. [DOI] [PubMed] [Google Scholar]
- 35.Yamasaki Y, Matsuura N, Shozuhara H, et al. Interleukin-1 as a pathogenetic mediator of ischemic brain damage in rats. Stroke. 1995;26:676–680. doi: 10.1161/01.str.26.4.676. [DOI] [PubMed] [Google Scholar]
- 36.Waje-Andreassen U, Kråkenes J, Ulvestad E, et al. IL-6: an early marker for outcome in acute ischemic stroke. Acta Neurol Scand. 2005;111:360–365. doi: 10.1111/j.1600-0404.2005.00416.x. [DOI] [PubMed] [Google Scholar]
- 37.O’Garra A, Barrat FJ, Castro AG, et al. Strategies for use of IL-10 or its antagonists in human disease. Immunol Rev. 2008;223:114–131. doi: 10.1111/j.1600-065X.2008.00635.x. [DOI] [PubMed] [Google Scholar]
- 38.Matsuzaki G, Umemura M. Interleukin-17 as an effector molecule of innate and acquired immunity against infections. Microbiol Immunol. 2007;51:1139–1147. doi: 10.1111/j.1348-0421.2007.tb04008.x. [DOI] [PubMed] [Google Scholar]
- 39.Cheng Y, Gidday JM, Yan Q, et al. Marked age-dependent neuroprotection by brain-derived neurotrophic factor against neonatal hypoxic-ischemic brain injury. Ann Neurol. 1997;41:521–529. doi: 10.1002/ana.410410416. [DOI] [PubMed] [Google Scholar]
- 40.Fujiwara K, Date I, Shingo T, et al. Reduction of infarct volume and apoptosis by grafting of encapsulated basic fibroblast growth factor-secreting cells in a model of middle cerebral artery occlusion in rats. J Neurosurg. 2003;99:1053–1062. doi: 10.3171/jns.2003.99.6.1053. [DOI] [PubMed] [Google Scholar]
- 41.Yu Z, Luo H, Fu W, et al. The endoplasmic reticulum stress-responsive protein GRP78 protects neurons against excitotoxicity and apoptosis: suppression of oxidative stress and stabilization of calcium homeostasis. Exp Neurol. 1999;155:302–314. doi: 10.1006/exnr.1998.7002. [DOI] [PubMed] [Google Scholar]
- 42.Ahmad AS, Zhuang H, Doré S. Heme oxygenase-1 protects brain from acute excitotoxicity. Neuroscience. 2006;141:1703–1708. doi: 10.1016/j.neuroscience.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 43.Ding XZ, Tsokos GC, Kiang JG. Heat shock factor-1 protein in heat shock factor-1 gene-transfected human epIFmoid A431 cells requires phosphorylation before inducing heat shock protein-70 production. J Clin Invest. 1997;99:136–143. doi: 10.1172/JCI119124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.West AE, Chen WG, Dalva MB, et al. Calcium regulation of neuronal gene expression. Proc Natl Acad Sci USA. 2001;98:11024–11031. doi: 10.1073/pnas.191352298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Srisook K, Kim C, Cha YN. Molecular mechanisms involved in enhancing HO-1 expression: de-repression by heme and activation by Nrf2, the “one-two” punch. Antioxid Redox Signal. 2005;7:1674–1687. doi: 10.1089/ars.2005.7.1674. [DOI] [PubMed] [Google Scholar]
- 46.Galoforo SS, Berns CM, Erdos G, et al. Hypoglycemia-induced AP-1 transcription factor and basic fibroblast growth factor gene expression in multidrug resistant human breast carcinoma MCF-7/ADR cells. Mol Cell Biochem. 1996;155:163–171. doi: 10.1007/BF00229313. [DOI] [PubMed] [Google Scholar]
- 47.Sawada M, Suzumura A, Hosoya H, et al. Interleukin-10 inhibits both production of cytokines and expression of cytokine receptors in microglia. J Neurochem. 1999;72:1466–1471. doi: 10.1046/j.1471-4159.1999.721466.x. [DOI] [PubMed] [Google Scholar]
- 48.Grilli M, Barbieri I, Basudev H, et al. Interleukin-10 modulates neuronal threshold of vulnerability to ischaemic damage. Eur J Neurosci. 2000;12:2265–2272. doi: 10.1046/j.1460-9568.2000.00090.x. [DOI] [PubMed] [Google Scholar]
- 49.Frenkel D, Huang Z, Maron R, et al. Nasal vaccination with myelin oligodendrocyte glycoprotein reduces stroke size by inducing IL-10-producing CD4+ T cells. J Immunol. 2003;171:6549–6555. doi: 10.4049/jimmunol.171.12.6549. [DOI] [PubMed] [Google Scholar]
- 50.Vila N, Millán M, Ferrer X, et al. Levels of anti-inflammatory cytokines and neurological worsening in acute ischemic stroke. Stroke. 2003;34:671–675. doi: 10.1161/01.STR.0000057976.53301.69. [DOI] [PubMed] [Google Scholar]
- 51.Liu HM, Chen HH. Correlation between fibroblast growth factor expression and cell proliferation in experimental brain infarct: studied with proliferating cell nuclear antigen immunohistochemistry. J Neuropathol Exp Neurol. 1994;53:118–126. doi: 10.1097/00005072-199403000-00002. [DOI] [PubMed] [Google Scholar]
- 52.Pan W, Ding Y, Yu Y, et al. Stroke upregulates TNFalpha transport across the blood-brain barrier. Exp Neurol. 2006;198:222–233. doi: 10.1016/j.expneurol.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 53.Suzuki S. Temporal profile and cellular localization of interleukin-6 protein after focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 1999;19:1256–1262. doi: 10.1097/00004647-199911000-00010. [DOI] [PubMed] [Google Scholar]
- 54.Rissanen A, Sivenius J, Jolkkonen J. Prolonged bihemispheric alterations in unfolded protein response related gene expression after experimental stroke. Brain Res. 2006;1087:60–66. doi: 10.1016/j.brainres.2006.02.095. [DOI] [PubMed] [Google Scholar]
- 55.Lenzi GL, Frackowiak RS, Jones T. Cerebral oxygen metabolism and blood flow in human cerebral ischemic infarction. J Cereb Blood Flow Metab. 1982;2:321–335. doi: 10.1038/jcbfm.1982.33. [DOI] [PubMed] [Google Scholar]
- 56.Li HL, Kostulas N, Huang YM, et al. IL-17 and IFN-gamma mRNA expression is increased in the brain and systemically after permanent middle cerebral artery occlusion in the rat. J Neuroimmunol. 2001;116:5–14. doi: 10.1016/s0165-5728(01)00264-8. [DOI] [PubMed] [Google Scholar]
- 57.Reinecke S, Lutzenburg M, Hagemann G, et al. Electrophysiological transcortical diaschisis after middle cerebral artery occlusion (MCAO) in rats. Neurosci Lett. 1999;261:85–88. doi: 10.1016/s0304-3940(99)00014-2. [DOI] [PubMed] [Google Scholar]
- 58.Cohen S, Greenberg ME. Communication between the synapse and the nucleus in neuronal development, plasticity, and disease. Annu Rev Cell Dev Biol. 2008;24:183–209. doi: 10.1146/annurev.cellbio.24.110707.175235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Planas AM, Soriano MA, Ferrer I, et al. Kainic acid-induced heat shock protein-70, mRNA and protein expression is inhibited by MK-801 in certain rat brain regions. Eur J Neurosci. 1995;7:293–304. doi: 10.1111/j.1460-9568.1995.tb01065.x. [DOI] [PubMed] [Google Scholar]
- 60.Roberge MC, Messier C, Staines WA, et al. Food restriction induces long-lasting recovery of spatial memory deficits following global ischemia in delayed matching and non-matching-to-sample radial arm maze tasks. Neuroscience. 2008;156:11–29. doi: 10.1016/j.neuroscience.2008.05.062. [DOI] [PubMed] [Google Scholar]
- 61.Plunet WT, Streijger F, Lam CK, et al. Dietary restriction started after spinal cord injury improves functional recovery. Exp Neurol. 2008;213:28–35. doi: 10.1016/j.expneurol.2008.04.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


