Abstract
In naïve animals, γδ T cells are innate sources of IL-17, a potent proinflammatory cytokine mediating bacterial clearance as well as autoimmunity. However, mechanisms underlying the generation of these cells in vivo remain unclear. Here we show that TGFβ1 plays a key role in the generation of IL-17+ γδ T cells, and that it mainly occurs in the thymus particularly during the postnatal period. Interestingly, IL-17+ γδ TCR+ thymocytes were mainly CD44highCD25low cells, which seem to derive from DN4 γδ TCR+ cells that acquired CD44 and IL-17 expression. Our findings identify a novel developmental pathway during which IL-17-competent γδ T cells arise in the thymus by a TGFβ1-dependent mechanism.
Keywords: γδ T cells, IL-17, TGFβ
Introduction
γδ T cells constitute a small proportion (<5%) of total peripheral T lymphocytes, although they are widely distributed throughout the epithelial cell-rich tissues (1), and they are known to be an important source of IL-17 in response to a number of pathogens, recruiting neutrophils to the site of inflammation (2, 3). γδ T cells are also the major IL-17-producing cells in naïve animals (4–6). It was reported that Ag-naive CD122- γδ T cells preferentially produce IL-17 while Ag-experienced CD122+ γδ T cells produce IFNγ (5). It was recently shown that the expression of the tumor necrosis factor (TNF) family member CD27 better defines these cytokine producing γδ T cell subsets; IL-17+ γδ T cells are mostly CD27- and IFNγ+ γδ T cells are mostly CD27+ (7). However, how γδ T cells acquire IL-17 expression in vivo remains unclear.
Here, we report that developing γδ T cells acquire IL-17-producing capacity within the thymus via a TGFβ1-dependent mechanism. Interestingly, peripheral IL-17+ γδ T cells primarily accumulate in the peripheral but not in the mesenteric LN. An ontogeny study revealed that the highest frequency of IL-17+ γδ T cells was found in the postnatal thymus. IL-17+ γδ TCR+ thymocytes were CD44highCD25low (DN1) phenotype cells, which are in fact the DN4 cells that upregulated CD44 and acquired IL-17 expression. The generation of IL-17+ γδ T cells was dramatically abolished by the absence of TGFβ1 but not of other Th17-inducing cytokines. Consistent with this, γδ T cells in the thymus expressed the highest levels of TGFβ receptors. Taken together, the current study highlights a unique pathway of thymic γδ T cell development during which the differentiation of natural IL-17+ γδ T cells takes place, revealing an irreplaceable role for TGFβ1 to promote this process.
Materials and Methods
Mice
C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Lymphoid cells from IL-6−/− and IL-23 p19−/− mice were provided by Drs. Robert Fairchild (Cleveland Clinic Foundation) and Steve Stohlman (Cleveland Clinic Foundation). IL-21−/− mice were purchased from the MMRRC (Mutant Mouse Regional Resource Centers). Rag2p-GFP and Tgfβ1−/− mice were previously described (8, 9). All experimental procedures were conducted according to the guidelines of the Institutional Animal Care and Use Committee of the Cleveland Clinic Foundation, Case Western Reserve University, and the University of Washington.
Ex vivo stimulation
Spleen, pLN (axillary and cervical LN) and mesenteric LN (mLN) cells were separately harvested and ex vivo stimulated with PMA (10ng/ml) and Ionomycin (1 µM) for 4 hrs in the presence of 2 µM monensin (Calbiochem, San Diego, CA) during the last 2 hrs. Cells were immediately fixed with 4% paraformaldehyde, permeabilized, and stained with fluorescence conjugated antibodies (see below).
Flow cytometry
The following antibodies were used: biotinylated anti-γδ TCR (GL3), PE-anti-γδ TCR (GL3), PE-anti-CD25 (PC61), PE-anti-CD44 (IM7), PE-anti-CD62L (MEL-14), PE-anti-IFNγ (XMG1.2), streptavidin-PE, PE-Cy5-anti-CD44 (IM7), APC-anti-CD4 (RM4-5), APC-anti-CD8 (53-6.7), APC-anti-IL-17 (ebio17B7), FITC-anti-CD4 (RM4-5), FITC-anti-CD8(53-6.7), FITC-anti-NK1.1(PK136), FITC-anti-B220(RA3-6B2), and FITC-anti-IFNγ (XMG1.2) Abs. All antibodies were purchased from eBioscience (San Diego, CA) or PharMingen. Cells were acquired using a FACSCalibur (Becton Dickinson, San Diego, CA), and the data were analyzed using FlowJo software (Treestar, Ashland, OR).
Real time PCR
DN γδ TCR-, DN γδ TCR+, DP, and CD4 SP thymocytes were isolated by FACS sorting using a FACSAria cell sorter (Becton Dickinson). RNA was extracted using RNeasy reagent (Qiagen, Valencia, CA), and cDNA was subsequently generated by Superscript III RTase (Invitrogen). Taqman primers/probes specific for Tgfbr1 (Mm03024015_m1) and Tgfbr2 (Mm03024091_m1) were purchased from ABI (Applied Biosystem Inc., Foster city, CA) and their expression was determined using an ABI 7500 PCR system. Expression level was normalized based on the 18S rRNA (VIC-TAMRA, purchased from ABI) expression.
Data analysis
Statistical significance was determined by the Student’s t-test using the SigmaPlot 9.0 (SPSS Inc., Chicago, IL). p<0.05 was considered to indicate a significant difference.
Results and Discussion
γδ T cells are the major IL-17-producing cells in naïve animals
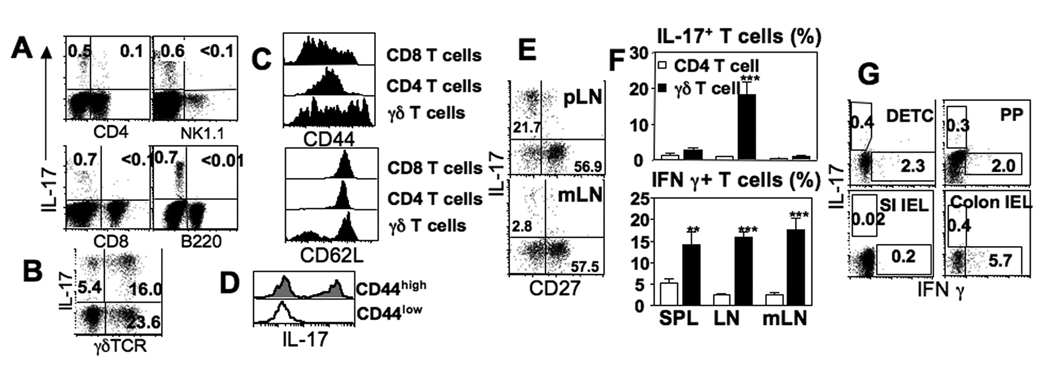
In naïve animals, very few (~0.1%) LN CD4 T cells expressed intracellular IL-17 following PMA/ionomycin stimulation but ~0.5% non-CD4 T cells expressed IL-17 under the same conditions (Fig. 1A). IL-17+ non-CD4 T cells were not CD8, B, or NK1.1+ cells (Fig. 1A); instead, ~75% of the IL-17+ cells expressed γδ TCR (Fig. 1B). A significant portion of γδ T cells expressed an activated phenotype compared to αβ T cells: CD44high and CD62Llow (Fig. 1C). IL-17 production was noticed only from CD44high γδ T cells (Fig. 1D), which differs from a previous study showing that IL-17 is preferentially produced from ‘naïve’ CD122low γδ T cells after TCR cross-linking (5). Our finding agrees with a recent report in that CD27low γδ T cells that mainly produce IL-17 are CD44highCD62Llow cells (7). Indeed, IL-17+ γδ T cells in the pLN displayed the same CD27low phenotype (Fig. 1E, top panel).
Figure 1. pLN γδ T cells preferentially express IL-17 following activation.
(A) LN cells were ex vivo stimulated with PMA/ionomycin and subsequently stained for CD4, NK1.1, CD8, B220, and IL-17. (B) LN cells stimulated as above were stained for B220, CD4, CD8, IL-17, and γδ TCR. Shown is γδ T cell IL-17 expression by non-B/non-T cells. (C) γ δ, CD4, and CD8 T cells from the indicated tissues were examined for the surface expression of CD44 and CD62L. (D) IL-17 expression of CD44low and CD44high γδ T cells (spleen and pLN) was examined by intracellular staining. (E) γδ T cells from the indicated tissues were stimulated and stained for IL-17 and CD27. (F) Proportions of IFNγ- and of IL-17-producing γδ T cells (and CD4 T cells) in the indicated tissues were examined. Shown is the mean ± SD (n=4). (G) Cells isolated from the indicated tissues were subsequently stimulated with PMA/Ionomycin, and cytokine production was determined by flow cytometric analysis. Shown are cytokine profiles of γδ TCR+ gated cells. All the experiments were repeated more than twice and similar results were observed. **, p<0.01, ***, p<0.001.
Interestingly, the proportion of IL-17+ γδ T cells in regional lymphoid tissues displayed a substantial heterogeneity; the highest frequency of IL-17+ γδ cells was found in pLN, while γδ T cells from mLN failed to express IL-17 (Fig. 1F). IL-17+ γδ T cells in the spleen were also present at a low frequency (~3%). Notably, less than 3% of CD27low mLN γδ T cells expressed IL-17, suggesting that CD27low phenotype does not necessarily define IL-17-producing γδ T cells (Fig. 1E). By contrast, IFNγ+ γδ T cells were found in all lymphoid tissues (Fig. 1F). Consistent with previous reports (5, 7), γδ T cells producing IL-17 and IFNγ did not overlap (data not shown). Unlike lymphoid γδ T cells, skin resident γδ T cells (DETC), γδ T cells from Peyer’s patches (PP), or intraepithelial γδ lymphocytes (IEL) from the small intestine (SI) and the colon expressed very little IL-17 (Fig. 1G). Why peripheral IL-17+ γδ T cells display a lymphoid tissue-specific accumulation is unclear. IL-17+ γδ T cells may express a chemokine receptor(s) that allows them to preferentially migrate to and accumulate in the pLN. Indeed, Martin et al. recently reported that IL-17+ γδ T cells uniformly express CCR6 (10). Whether CCR6 is necessary for IL-17+ γδ T cell accumulation in the pLN remains to be determined. Alternatively, a microenvironment within the mLN may suppress IL-17 expression by γδ T cells. These results demonstrate that CD44high γδ T cells display IL-17-producing capacity and that these IL-17+ γδ T cells are primarily enriched in the pLN but not in the mLN or in other epithelial cell-rich tissues.
Age-dependent generation of IL-17+ γδ T cells in the thymus
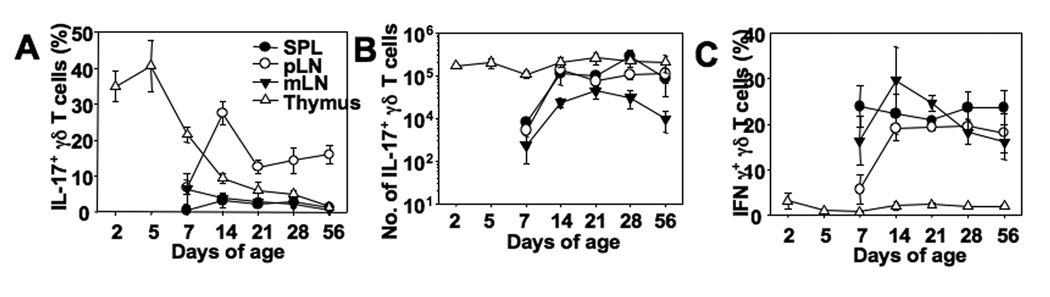
It was recently demonstrated that IL-17+ phenotype of γδ T cells is established during thymic development (5, 7). Analysis of γδ TCR+ thymocytes from mice of different ages revealed a striking pattern in IL-17 production. Thymus from newborn mice contained γδ TCR+ thymocytes, 30~40% of which expressed IL-17 following stimulation (Fig. 2A). The proportions of IL-17+ γδ TCR+ thymocytes reached a peak around 5 days of age and declined thereafter (Fig. 2A). Interestingly, IL-17+ γδ T cells in the pLN increased as thymic IL-17+ γδ TCR+ thymocytes declined (between 7–14 days of age), suggesting that the IL-17+ cells differentiated within the thymus appear to populate the periphery. Particularly striking is that the total numbers of IL-17+ γδ TCR+ thymocytes were constant regardless of age (Fig. 2B), strongly suggesting a tight homeostatic mechanism that controls the generation of IL-17+ γδ T cells in the thymus. Notably, thymic γδ TCR+ thymocytes expressed little IFNγ, while peripheral γδ T cells uniformly expressed IFNγ in all tested lymphoid tissues (Fig. 2C), suggesting that IFNγ production, unlike IL-17, may be acquired in the periphery at least at the protein level.
Figure 2. IL-17 expression of γδ T cells in mice of different ages.
(A–C) Spleen, pLN, mLN, and thymic cells from mice at the indicated ages were stimulated as described in Figure 1 were stained for IL-17, IFNγ, and γδ TCR. Proportions of IL-17- (A) and of IFNγ- (C) expressing γδ T cells were examined. (B) Total numbers of IL-17-expressing γδ T cells were determined by flow analysis. Shown are the mean ± SD (n = 3–4).
DN4 stage γδ T cells become CD44+ and express IL-17
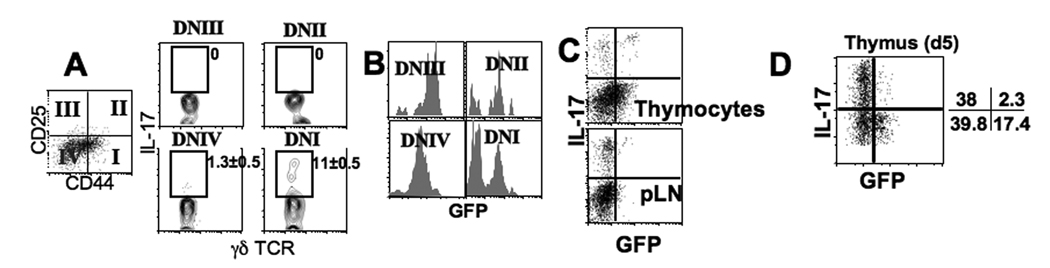
To characterize a developmental pathway leading to the generation of IL-17+ γδ thymocytes, adult thymocytes were stimulated ex vivo and the phenotypes of IL-17 producing γδ TCR+ cells were examined. IL-17+ γδ TCR+ thymocytes were mainly found within CD4negCD8negCD44highCD25low cells, the phenotype of DN1 thymocytes (Fig. 3A). Notably, developing DN thymocytes initiate γδ TCR rearrangement following the DN1 stage and start to express surface γδ TCR after the DN2/3 stage (11). Therefore, IL-17+ γδ T cells found in the DN1 stage might be peripheral γδ T cells that have recirculated from the periphery (12). Alternatively, it is possible that the DN1-stage γδ T cells are activated DN4 cells that upregulated CD44 and acquired IL-17 expression. To test this, we used Rag2p-GFP Tg mice (13). It was previously shown that Rag2 promoter-driven GFP expression is induced during the late DN2 stage, reaches peak expression in DP thymocytes, and gradually diminishes during the transition from the DP to the SP thymocytes (14). In the periphery, GFP expression is primarily found in recent thymic emigrants, yet the expression is lower than that in DP thymocytes (14). The distribution of γδ TCR+ thymocytes in Rag2p-GFP Tg adult mice among the DN subsets was comparable to that of non-transgenic mice (data not shown). Similarly, most IL-17+ γδ T cells were found within the DN1 subsets (data not shown). DN1 γδ TCR+ thymocytes showed two populations based on GFP expression (Fig. 3B), and IL-17+ γδ TCR+ thymocytes were mostly GFPneg cells (Fig. 3C). As expected, only GFPneg γδ T cells were found in the pLN (Fig. 3C).
Figure 3. IL-17-expressing thymic γδ T cells.
(A) Adult thymic cells stimulated as described in Figure 1 were stained for CD44, CD25, γδ TCR, and IL-17. Quadrant plot represents γδ TCR+ gated thymocytes. IL-17 expression of each subset (based on CD44 and CD25 expression) was indicated. (B) Thymic cells from adult Rag2p-GFP Tg mice were stained for γδ TCR, CD44, and CD25. GFP expression of each DN subset is shown. The results are representative of 4 individually tested mice. (C) Thymocytes and pLN cells from adult Rag2p-GFP Tg mice were stimulated and IL-17/GFP expression of γδ T cells was examined. (D) Thymic cells from 5-day old Rag2p-GFP mice were stimulated and stained for γδ TCR, CD44, CD25, and IL-17. Shown is GFP expression profiles of CD44highCD25low γδ T cells. The results are representative of 4 individually tested mice.
To further examine if GFPneg IL-17+ γδ T cells are mature cells recirculated from the periphery, we examined the thymus from 5-day old Rag2-GFP Tg mice, an age before IL-17+ γδ T cells are seen in the periphery (Fig. 2A). Even at this age, the majority of CD44high γδ TCR+ thymocytes did not express GFP (data not shown). Moreover, IL-17+ γδ TCR+ thymocytes from these mice were mostly GFPneg (Fig. 3D), while all DN4 γδ TCR- thymocytes expressed GFP (data not shown). Therefore, it is likely that the GFPneg IL-17+ γδ TCR+ thymocytes in 5-day old mice have undergone extensive proliferation and have lost GFP expression (15). A similar pattern was observed in 14-day old mice (data not shown). Therefore, our data strongly suggest that CD44high IL-17+ γδ TCR+ cells in the thymus are DN4 cells that acquire CD44 expression. To test this possibility sorted DN4 (CD44lowCD25low) γδ TCR+ thymocytes were cocultured with the stromal cell line OP9-DL4 (16). OP9-DL4 cells transduced with the Notch ligands (Delta-like 4) efficiently promoted T lineage development (17). Indeed, ~10% of cocultured DN4 γδ T cells upregulated CD44 (Fig. S1). DN4 γδ T cells cocultured with the OP9 control cell line failed to survive. These results further support the transition from DN4 to DN1-like cells.
TGFβ is required for the generation of IL-17+ γδ T cells
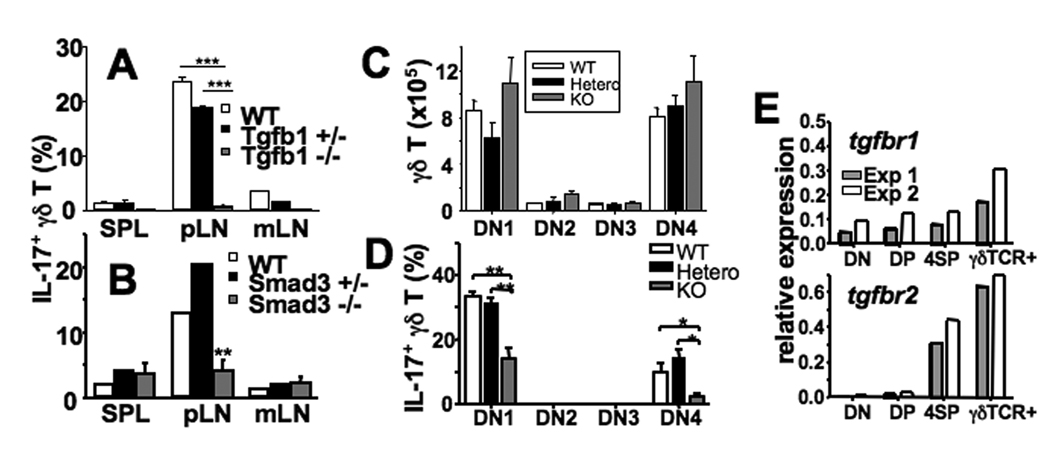
In case of CD4 T cells, multiple factors including IL-23, IL-21, IL-6, and TGFβ play roles in the differentiation of naïve CD4 T cells into Th17 cells (18–20). We thus explored whether these cytokines are required for the endogenous generation of IL-17+ γδ T cells. Lymphoid cells from the indicated gene deficient mice were stimulated and cytokine production was examined. As shown in Fig. S2, the lack of IL-23 p19 subunit and IL-6 did not alter IL-17 production by γδ T cells. IL-17 production of γδ T cells in the spleen and mLN of IL-21-deficient mice was slightly reduced; yet, pLN γδ T cell IL-17 production in these mice was not altered (Fig. S2). Notably, the total numbers of γδ T cells in the lymphoid tissues of these mice were equivalent, indicating that the generation of γδ T cells is independent of these cytokines (data not shown). Endogenous CD4 T cell IL-17 expression was partially reduced by IL-23 p19 deficiency, and completely abolished by the lack of IL-6 (data not shown). Therefore, these cytokines, while playing an important role in the generation of endogenous Th17 cells (21), play little or no role in γδ T cell acquisition of IL-17 production. Similarly, Lochner et al. also reported that IL-17+ RORγt+ γδ T cells were unaffected by the absence of IL-6 (6). Whether TGFβ1 plays a role in the generation of IL-17-producing γδ T cells was next examined. As all Tgfβ1−/− mice develop severe lymphoproliferative disease early in life, LN and spleen cells from 2~3 week-old mice were used. TGFβ1 deficiency completely abolished IL-17 expression by γδ T cells (Fig. 4A). Of note, γδ T cell generation was not impaired in Tgfβ1−/− mice (Fig. S3). Likewise, γδ T cells deficient in Smad3, a TGFβ-signaling adaptor molecule (22), expressed significantly lower levels of IL-17 compared to littermate controls (Fig. 4B). In contrast, IFNγ production of γδ T cells was not different in Tgfβ1−/− and in Smad3−/− mice (Fig. S4).
Figure 4. TGFβ1 controls γδ T cell IL-17 expression.
(A and B) γδ T cell cytokine production from Tgfβ1−/− (A), Smad3−/− (B), and littermate control mice was determined. Shown are the mean ± SD of 4~6 individually tested mice. (C and D) Thymic cells from 11-day old Tgfβ1−/− and littermate control mice were examined for DN subset distribution based on CD44 and CD25 expression (C), or stimulated and IL-17 expression of γδ TCR+ thymocytes in each DN subset was examined (D). Shown is the mean ± SD of 4 individually tested mice. (E) FACS sorted DN γδ TCR- (DN), DP, CD4 SP (4SP), and DN γδ TCR+ thymocytes were examined for Tgfβr1 and Tgfβr2 expression by real time PCR. *, p<0.05; **, p<0.01; ***, p<0.001.
We then examined if TGFβ1 is needed for the development of IL-17+ γδ T cells in the thymus. γδ TCR+ thymocytes from Tgfβ1−/− and littermate control mice were analyzed for IL-17 expression. The DN distribution of developing γδ TCR+ thymocytes was not different (Fig. 4C). However, IL-17-producing capacity of the developing γδ TCR+ thymocytes was greatly impaired in Tgfβ1−/− mice (Fig. 4D). In support of this finding, γδ TCR+ thymocytes expressed the highest levels of type I and type II TGFβ receptors (Fig. 4E). Notably, some thymic γδ T cells still acquire IL-17 expression in Tgfβ1−/− mice (Fig. 4D), and this finding might be supported by the expression of either TGFβ2 or TGFβ3 by thymic epithelia. However, these cells disappeared in the periphery (Fig. 4A), suggesting that TGFβ1 may play an important role in maintaining IL-17+ γδ T cells in the periphery. It was previously reported that thymic TGFβ is expressed on subcapsular and cortical thymic epithelium, which interacts with developing thymocytes (23). Identifying the source of TGFβ in the thymus as well as in the periphery will be an important subject for future study. Taken together, these results strongly suggest that TGFβ1, although dispensable for the phenotypic maturation (i.e., CD44 upregulation during DN4 to DN1-like transition), plays an irreplaceble role in the acquisition of IL-17-producing capacity in the thymus.
What are the immunologic roles of IL-17-producing γδ T cells in vivo? Following E. coli infection, γδ T cell-derived IL-17 was shown to play critical roles in recruiting neutrophils and in neutrophil-mediated bacterial clearance (2). IL-17 production by γδ T cells is also associated with lethal pulmonary aspergillosis in mice with chronic granulomatous disease (24). Moreover, γδ T cell IL-17 production is undoubtedly involved in exacerbating collagen-induced arthritis or autoimmunity (25, 26). The current study provides an important basis to define the mechanism(s) of how γδ T cells acquire IL-17 expression and of how endogenous γδ T cell-derived IL-17 influences immunity in vivo.
Acknowledgement
We thank Drs. Rob Fairchild and Steve Stohlman (Cleveland Clinic) for providing IL-6−/− and IL-23 p19−/− cells, respectively, Dr. Lan Zhou (Case Western Reserve Univ) for providing OP9 and OP9-DL4 cell lines, and Ms. Jennifer Powers for cell sorting.
Footnotes
Supported by NIH grant R01-AI074932 (B.M) and R01-AI064318 (P.J.F), and by the Intramural Research Program, National Heart, Lung, and Blood Institute, NIH (R.S. and W.J.L).
References
- 1.Carding SR, Egan PJ. Gammadelta T cells: functional plasticity and heterogeneity. Nat Rev Immunol. 2002;2:336–345. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- 2.Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. Resident Vdelta1+ gammadelta T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J Immunol. 2007;178:4466–4472. doi: 10.4049/jimmunol.178.7.4466. [DOI] [PubMed] [Google Scholar]
- 3.Roark CL, Simonian PL, Fontenot AP, Born WK, O'Brien RL. gammadelta T cells: an important source of IL-17. Curr Opin Immunol. 2008;20:353–357. doi: 10.1016/j.coi.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Jensen KD, Su X, Shin S, Li L, Youssef S, Yamasaki S, Steinman L, Saito T, Locksley RM, Davis MM, Baumgarth N, Chien YH. Thymic selection determines gammadelta T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity. 2008;29:90–100. doi: 10.1016/j.immuni.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lochner M, Peduto L, Cherrier M, Sawa S, Langa F, Varona R, Riethmacher D, Si-Tahar M, Di Santo JP, Eberl G. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORgamma t+ T cells. J Exp Med. 2008;205:1381–1393. doi: 10.1084/jem.20080034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ, Girardi M, Borst J, Hayday AC, Pennington DJ, Silva-Santos B. CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets. Nat Immunol. 2009;10:427–436. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hale JS, Boursalian TE, Turk GL, Fink PJ. Thymic output in aged mice. Proc Natl Acad Sci U S A. 2006;103:8447–8452. doi: 10.1073/pnas.0601040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diebold RJ, Eis MJ, Yin M, Ormsby I, Boivin GP, Darrow BJ, Saffitz JE, Doetschman T. Early-onset multifocal inflammation in the transforming growth factor beta 1-null mouse is lymphocyte mediated. Proc Natl Acad Sci U S A. 1995;92:12215–12219. doi: 10.1073/pnas.92.26.12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 11.Wilson A, Capone M, MacDonald HR. Unexpectedly late expression of intracellular CD3epsilon and TCR gammadelta proteins during adult thymus development. Int Immunol. 1999;11:1641–1650. doi: 10.1093/intimm/11.10.1641. [DOI] [PubMed] [Google Scholar]
- 12.Agus DB, Surh CD, Sprent J. Reentry of T cells to the adult thymus is restricted to activated T cells. J Exp Med. 1991;173:1039–1046. doi: 10.1084/jem.173.5.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu W, Nagaoka H, Jankovic M, Misulovin Z, Suh H, Rolink A, Melchers F, Meffre E, Nussenzweig MC. Continued RAG expression in late stages of B cell development and no apparent re-induction after immunization. Nature. 1999;400:682–687. doi: 10.1038/23287. [DOI] [PubMed] [Google Scholar]
- 14.Boursalian TE, Golob J, Soper DM, Cooper CJ, Fink PJ. Continued maturation of thymic emigrants in the periphery. Nat Immunol. 2004;5:418–425. doi: 10.1038/ni1049. [DOI] [PubMed] [Google Scholar]
- 15.Porritt HE, Rumfelt LL, Tabrizifard S, Schmitt TM, Zuniga-Pflucker JC, Petrie HT. Heterogeneity among DN1 prothymocytes reveals multiple progenitors with different capacities to generate T cell and non-T cell lineages. Immunity. 2004;20:735–745. doi: 10.1016/j.immuni.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Zhou L, Li LW, Yan Q, Petryniak B, Man Y, Su C, Shim J, Chervin S, Lowe JB. Notch-dependent control of myelopoiesis is regulated by fucosylation. Blood. 2008;112:308–319. doi: 10.1182/blood-2007-11-115204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhandoola A, von Boehmer H, Petrie HT, Zuniga-Pflucker JC. Commitment and developmental potential of extrathymic and intrathymic T cell precursors: plenty to choose from. Immunity. 2007;26:678–689. doi: 10.1016/j.immuni.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Zhou L, Ivanov, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 20.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 21.Marks BR, Nowyhed HN, Choi JY, Poholek AC, Odegard JM, Flavell RA, Craft J. Thymic self-reactivity selects natural interleukin 17-producing T cells that can regulate peripheral inflammation. Nat Immunol. 2009;10:1125–1132. doi: 10.1038/ni.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong Y, Tang L, Letterio JJ, Benveniste EN. The Smad3 protein is involved in TGF-beta inhibition of class II transactivator and class II MHC expression. J Immunol. 2001;167:311–319. doi: 10.4049/jimmunol.167.1.311. [DOI] [PubMed] [Google Scholar]
- 23.Takahama Y, Letterio JJ, Suzuki H, Farr AG, Singer A. Early progression of thymocytes along the CD4/CD8 developmental pathway is regulated by a subset of thymic epithelial cells expressing transforming growth factor beta. J Exp Med. 1994;179:1495–1506. doi: 10.1084/jem.179.5.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romani L, Fallarino F, De Luca A, Montagnoli C, D'Angelo C, Zelante T, Vacca C, Bistoni F, Fioretti MC, Grohmann U, Segal BH, Puccetti P. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature. 2008;451:211–215. doi: 10.1038/nature06471. [DOI] [PubMed] [Google Scholar]
- 25.Roark CL, French JD, Taylor MA, Bendele AM, Born WK, O'Brien RL. Exacerbation of collagen-induced arthritis by oligoclonal, IL-17-producing gamma delta T cells. J Immunol. 2007;179:5576–5583. doi: 10.4049/jimmunol.179.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]