Despite significant advancements in vascular medicine, including percutaneous interventional strategies, surgical revascularization remains the best available treatment option for many patients with coronary artery disease. Autologous saphenous vein grafts are still commonly used for coronary artery bypass grafts, especially for multi-vessel disease, when available arterial grafts are insufficient. However, it has been described that saphenous vein graft patency at 10 years is 61% and only 50% after 15 years and more – significantly lower than for internal mammary artery grafts.1,2 Thus, in clinical medicine, saphenous vein graft failure remains a major clinical problem.
The underlying pathophysiology of venous graft disease and late bypass failure includes intimal hyperplasia and subsequent accelerated atherosclerosis.3 Over 100 years ago, Alexis Carrel, who won the Nobel prize in recognition of his work on vascular suture and the transplantation of blood vessels and organs, made the first observations of intimal hyperplasia after vein grafting, which were later linked to vein graft stenosis and failure.4,5 Furthermore, it was shown that vein grafts implanted in the arterial circulation developed intimal hyperplasia, whereas those implanted in the venous circulation did not.6 Although these observations were made decades ago, the molecular mechanisms of vein graft intimal hyperplasia remain subject to intense research given the clinical importance of graft failure. Since vascular smooth muscle cell (VSMC) proliferation plays a key role in the pathophysiology of intimal hyperplasia,3,7 it is of particular interest to note that pulsatile stretch leads to VSCM proliferation in saphenous veins but not in internal mammary arteries.8
The process of converting mechanical forces into biochemical signals and the subsequent effect on cellular function has been termed ‘mechanotransduction’.9 Fluid shear stress, the frictional force per unit area from flowing blood, acts on the endothelial cells that line the vessels. Blood pressure, which drives fluid flow, causes circumferential stress on both endothelial cells and the VSMCs that surround the endothelium in arteries.9 Both shear stress and circumferential stress are markedly increased in the arterial system compared to the venous system and contribute to the development of intimal hyperplasia in vein grafts.
In this issue of Arteriosclerosis, Thrombosis, and Vascular Biology, new insight into the biology of vascular mechanotransduction is presented. In their study, Wu and coworkers investigated the transcriptional regulation of Insulin-like growth factor 1 receptor (IGF-1R) by the Early growth response-1 (Egr-1) transcription factor. IGF-1 is a growth factor that participates in multiple physiological and pathophysiological pathways including cellular growth and atherosclerosis.10–12 IGF-1 promotes VSMC migration as well as proliferation.13,14 Furthermore, both IGF-1 secretion and IGF-1R expression are increased by mechanical stretch in VSMC.15,16 Egr-1 is a mechano-sensitive transcription factor known to participate in multiple cardiovascular pathological processes such as atherosclerosis and intimal thickening.17 Egr-1 expression, like IGF-1 and IGF-1R expression, is increased by mechanical stretch in many cell types.18 Furthermore, it has been shown that stretch-inducible expression of the angiogenic factor CCN1 in VSMC is regulated by Egr-1.19
In their study, the authors confirmed that mechanical stretch increases both mRNA and protein expression of Egr-1 in VSMC. Using an Egr-1 knockout mouse, the authors then showed that Egr-1 deficiency attenuates the increase in IGF-1R expression and the proliferation of VSMC subjected to mechanical stretch. Furthermore, intimal hyperplasia was suppressed in vein grafts obtained from Egr-1 knockout mice, and mechanical stretch led to nuclear translocation of Egr-1 in VSMCs of vein grafts. Both electrophoretic mobility shift assays as well as chromosome immunoprecipitation confirmed binding of Egr-1 to the IGF-1R promoter. To explore the mechanical stretch-responsive regulation of IGF-1R, deletion analysis of the IGF-1R promoter was performed, and the mechanical stretch-responsive region is located between nucleotides −270 and −130 of the IGF-1R promoter. Further site-specific mutagenesis analysis showed that two regions (−170/−150 and −210/−190) of the IGF-1R promoter are necessary for mechanical stretch-induced transcriptional activity, and both of these regions contain an Egr-1 binding site.
Although Alexis Carrel made the first observations regarding intimal hyperplasia more than a century ago, the underlying pathophysiological processes remain incompletely defined and clinically important for hundreds of thousands of patients. The Egr-1 / IGF-1R interaction described in this issue represents a novel mechanotransduction pathway, and the results of this study lead us further down the road to understanding why vein grafts develop accelerated atherosclerosis in the arterial system. This study also raises new questions. Are there other regulators of IGF-1R transcription? Mechanical stretch-induced expression of IGF-1R was not completely normalized by genetic deletion of Egr-1. Therefore, it is likely that there are other mechanical stretch-induced transcription factors that control the IGF-1R promoter. How do upstream regulators of Egr-1 such as FGF-2 and the MEK/ERK pathway17 or c-Jun20 affect regulation of the IGF-1R promoter? An important, yet, unanswered question concerns the receptors that transduce mechanical forces into biochemical signals. What are those ‘mechanosensors’ and how are they linked to these pathways? And finally, how can the results of this study in mice be translated into meaningful therapeutic approaches to vein graft failure in patients undergoing bypass surgery? Hopefully, future research will point us in new directions that will allow vein grafts to function more like internal mammary artery grafts.
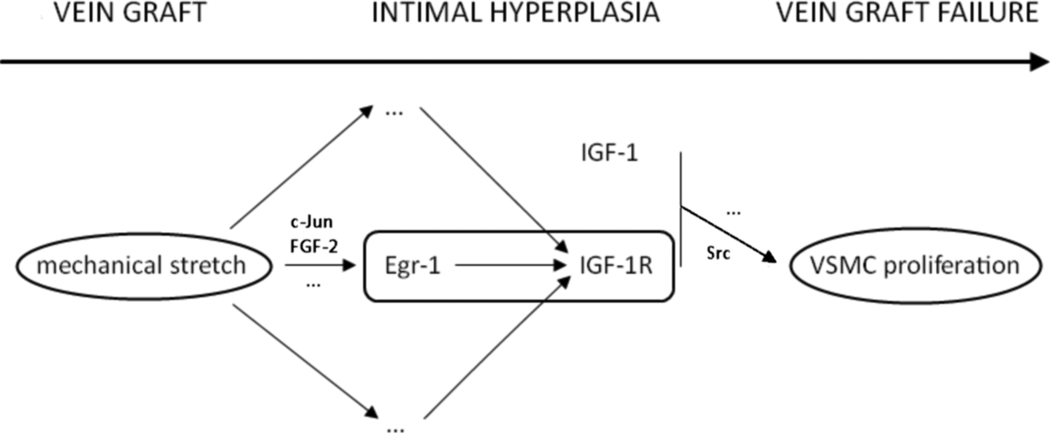
Figure 1.
Implantation of a vein graft exposes vascular smooth muscle cells (VSMC) to mechanical stretch. Through activation of the Early growth response-1 (Egr-1) transcription factor, Insulin-like growth factor-1 receptor (IGF-1R) transcription is upregulated. In combination with IGF-1 this leads to increased VSMC proliferation, intimal hyperplasia, and eventually to vein graft failure.
Acknowledgments
Sources of Funding
This work was supported by grants from the National Institutes of Health. S.L. is supported by the Koeln Fortune Program / Faculty of Medicine, University of Cologne, Germany.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Goldman S, Zadina K, Moritz T, Ovitt T, Sethi G, Copeland JG, Thottapurathu L, Krasnicka B, Ellis N, Anderson RJ, Henderson W Group VACS. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: results from a Department of Veterans Affairs Cooperative Study. J Am Coll Cardiol. 2004;44:2149–2156. doi: 10.1016/j.jacc.2004.08.064. [DOI] [PubMed] [Google Scholar]
- 2.Fitzgibbon GM, Kafka HP, Leach AJ, Keon WJ, Hooper GD, Burton JR. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years.[see comment] J Am Coll Cardiol. 1996;28:616–626. doi: 10.1016/0735-1097(96)00206-9. [DOI] [PubMed] [Google Scholar]
- 3.Mitra AK, Gangahar DM, Agrawal DK. Cellular, molecular and immunological mechanisms in the pathophysiology of vein graft intimal hyperplasia. Immunol Cell Biol. 2006;84:115–124. doi: 10.1111/j.1440-1711.2005.01407.x. [DOI] [PubMed] [Google Scholar]
- 4.Carrel A, Guthrie CG. Uniterminal and biterminal venous transplantations. Surg Gynecol Obstet. 1906;2:266–286. [Google Scholar]
- 5.Grondin CM, Meere C, Castonguay Y, Lepage G, Grondin P. Progressive and late obstruction of an aorto-coronary venous bypass graft. Circulation. 1971;43:698–702. doi: 10.1161/01.cir.43.5.698. [DOI] [PubMed] [Google Scholar]
- 6.Brody WR, Angeli WW, Kosek JC. Histologic fate of the venous coronary artery bypass in dogs. Am J Pathol. 1972;66:111–130. [PMC free article] [PubMed] [Google Scholar]
- 7.Berk BC. Vascular smooth muscle growth: autocrine growth mechanisms. Physiol Rev. 2001;81:999–1030. doi: 10.1152/physrev.2001.81.3.999. [DOI] [PubMed] [Google Scholar]
- 8.Predel HG, Yang Z, von Segesser L, Turina M, Buhler FR, Luscher TF. Implications of pulsatile stretch on growth of saphenous vein and mammary artery smooth muscle. Lancet. 1992;340:878–879. doi: 10.1016/0140-6736(92)93287-w. [DOI] [PubMed] [Google Scholar]
- 9.Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol. 2009;10:53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stiles CD, Capone GT, Scher CD, Antoniades HN, Van Wyk JJ, Pledger WJ. Dual control of cell growth by somatomedins and platelet-derived growth factor. Proc Natl Acad Sci U S A. 1979;76:1279–1283. doi: 10.1073/pnas.76.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stewart CE, Rotwein P. Growth, differentiation, and survival: multiple physiological functions for insulin-like growth factors. Physiol Rev. 1996;76:1005–1026. doi: 10.1152/physrev.1996.76.4.1005. [DOI] [PubMed] [Google Scholar]
- 12.Bayes-Genis A, Conover CA, Schwartz RS. The insulin-like growth factor axis: A review of atherosclerosis and restenosis. Circ Res. 2000;86:125–130. doi: 10.1161/01.res.86.2.125. [DOI] [PubMed] [Google Scholar]
- 13.Bornfeldt KE, Raines EW, Nakano T, Graves LM, Krebs EG, Ross R. Insulin-like growth factor-I and platelet-derived growth factor-BB induce directed migration of human arterial smooth muscle cells via signaling pathways that are distinct from those of proliferation. J Clin Invest. 1994;93:1266–1274. doi: 10.1172/JCI117081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du J, Delafontaine P. Inhibition of vascular smooth muscle cell growth through antisense transcription of a rat insulin-like growth factor I receptor cDNA. Circ Res. 1995;76:963–972. doi: 10.1161/01.res.76.6.963. [DOI] [PubMed] [Google Scholar]
- 15.Standley PR, Obards TJ, Martina CL. Cyclic stretch regulates autocrine IGF-I in vascular smooth muscle cells: implications in vascular hyperplasia. Am J Physiol. 1999;276:E697–E705. doi: 10.1152/ajpendo.1999.276.4.E697. [DOI] [PubMed] [Google Scholar]
- 16.Cheng J, Du J. Mechanical stretch simulates proliferation of venous smooth muscle cells through activation of the insulin-like growth factor-1 receptor.[see comment] Arterioscler Thromb Vasc Biol. 2007;27:1744–1751. doi: 10.1161/ATVBAHA.107.147371. [DOI] [PubMed] [Google Scholar]
- 17.Khachigian LM. Early growth response-1 in cardiovascular pathobiology. Circ Res. 2006;98:186–191. doi: 10.1161/01.RES.0000200177.53882.c3. [DOI] [PubMed] [Google Scholar]
- 18.Morawietz H, Ma YH, Vives F, Wilson E, Sukhatme VP, Holtz J, Ives HE. Rapid induction and translocation of Egr-1 in response to mechanical strain in vascular smooth muscle cells. Circ Res. 1999;84:678–687. doi: 10.1161/01.res.84.6.678. [DOI] [PubMed] [Google Scholar]
- 19.Grote K, Bavendiek U, Grothusen C, Flach I, Hilfiker-Kleiner D, Drexler H, Schieffer B. Stretch-inducible expression of the angiogenic factor CCN1 in vascular smooth muscle cells is mediated by Egr-1. J Biol Chem. 2004;279:55675–55681. doi: 10.1074/jbc.M406532200. [DOI] [PubMed] [Google Scholar]
- 20.Ni J, Waldman A, Khachigian LM. c-Jun regulates shear- and injury-inducible Egr-1 expression, vein graft stenosis after autologous end-to-side transplantation in rabbits and intimal hyperplasia in human saphenous veins. J Biol Chem. 2009 Nov 23; doi: 10.1074/jbc.M109.078345. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]