Abstract
OBJECTIVE
Sirtuins (SIRTs) are NAD+-dependent deacetylases that regulate metabolism and life span. We used peripheral blood mononuclear cells (PBMCs) to determine ex vivo whether insulin resistance/metabolic syndrome influences SIRTs. We also assessed the potential mechanisms linking metabolic alterations to SIRTs in human monocytes (THP-1) in vitro.
RESEARCH DESIGN AND METHODS
SIRT1-SIRT7 gene and protein expression was determined in PBMCs of 54 subjects (41 with normal glucose tolerance and 13 with metabolic syndrome). Insulin sensitivity was assessed by the minimal model analysis. Subclinical atherosclerosis was assessed by carotid intima-media thickness (IMT). In THP-1 cells exposed to high glucose or fatty acids in vitro, we explored SIRT1 expression, p53 acetylation, Jun NH2-terminal kinase (JNK) activation, NAD+ levels, and nicotinamide phosphoribosyltransferase (NAMPT) expression. The effects of SIRT1 induction by resveratrol and of SIRT1 gene silencing were also assessed.
RESULTS
In vivo, insulin resistance and metabolic syndrome were associated with low PBMC SIRT1 gene and protein expression. SIRT1 gene expression was negatively correlated with carotid IMT. In THP-1 cells, high glucose and palmitate reduced SIRT1 and NAMPT expression and reduced the levels of intracellular NAD+ through oxidative stress. No effect was observed in cells exposed to linoleate or insulin. High glucose and palmitate increased p53 acetylation and JNK phosphorylation; these effects were abolished in siRNA SIRT1–treated cells. Glucose- and palmitate-mediated effects on NAMPT and SIRT1 were prevented by resveratrol in vitro.
CONCLUSIONS
Insulin resistance and subclinical atherosclerosis are associated with SIRT1 downregulation in monocytes. Glucotoxicity and lypotoxicity play a relevant role in quenching SIRT1 expression.
Metabolic syndrome is increasingly prevalent in the general population. Excess caloric intake and nutrient availability are the obvious culprits that lead to obesity and insulin resistance. In turn, metabolic syndrome predisposes to early atherosclerosis and cardiovascular morbidity (1). The evolutionary conserved silent information regulator 2 (SIR2) is a NAD+-dependent deacetylase that regulates life span in response to caloric restriction in many organisms. Mammalian homologues of SIR2 comprise a family of seven proteins termed Sirtuins (SIRT1-SIRT7), which are implicated in metabolic processes and stress resistance (2,3). Caloric restriction extends life span in a variety of organisms through induction of SIRT (4). In mammals, SIRT1 deacetylates many key transcription factors and cofactors, such as the tumor suppressor p53, forkhead box class O (FOXO) proteins (5), peroxisome proliferator–activated receptor-γ coactivator-1α (PGC-1α) (6), and nuclear factor-κB (7). These specific actions may affect cellular pathways involved in glucose homeostasis. The effects of SIRT appear to be beneficial, as they trigger metabolic changes similar to those observed in caloric restriction. Indeed, calorie restriction increases the levels of SIRT1 in the liver and muscle, which are key insulin-sensitive organs (8). Moreover, SIRT1−/− mice are insensitive to the metabolic effects of caloric restriction (9).
In light of these observations, SIRTs have been proposed as a possible target for the treatment of metabolic syndrome (3,4,10). In white adipose tissue, SIRT1 was shown to inhibit adipogenesis and to reduce fat storage in differentiated cells (11). In parallel, pancreatic β-cells were found to be highly enriched in SIRT4: knocking out this SIRT in insulinoma cells and in mice triggers insulin hypersecretion (12,13).
Despite this considerable amount of data, no information is available on the relationships between insulin sensitivity and SIRTs in humans, and on the mechanisms that might potentially interfere with their expression. Specifically, there is no demonstration that SIRTs are altered in the setting of metabolic syndrome, a well-known condition of insulin resistance.
Thus, we sought to determine whether insulin resistance and metabolic syndrome and its components are associated with altered SIRT gene and protein expression in circulating peripheral blood mononuclear cells (PBMCs). Monocytes play a major role in pathogenetic processes linked to metabolic syndrome, such as inflammation of the adipose tissue and development of the atherosclerotic plaque (14,15). The use of these cells can also circumvent ethical concerns inherent to the invasive procedures needed to obtain adipose and muscle tissue samples. In addition, we aimed to extend the observations from in vivo studies by investigating the potential mechanisms linking excess nutrient and SIRT in a human monocyte cell line (THP-1).
RESEARCH DESIGN AND METHODS
Subjects.
We recruited by advertisement 54 consecutive volunteers who were employees of the Padova Province Offices. Their carbohydrate metabolism status was determined by a standard 75-g oral glucose tolerance test (OGTT) performed within the previous 6 months. Patients filled out a complete lifestyle questionnaire regarding medical history, parental history of cardiovascular disease, smoking habits, and physical activity. A physical examination ruled out peripheral vascular disease by clinical criteria (absence of peripheral pulses of the lower extremity) and ankle-brachial pressure indexes <0.9. The extent of subclinical atherosclerosis was measured by quantitative high-resolution B-mode ultrasound of the far wall of the right and left common carotid arteries. The measurements were carried out as previously reported according to a validated procedure with an HDI-5000 SONO CT ultrasound machine (Philips Medical System/ATL Spa) equipped with a phased-array 4- to 7-MHz transducer. Intima-media thickness (IMT) readings were performed independently by two trained operators as described (16). A resting 12-lead electrocardiogram was performed, and angina was excluded in each patient according to the World Health Organization Rose questionnaire. Four subjects were moderately hypertensive and were on antihypertensive drugs (two subjects on ACE inhibitors and two on angiotensin receptor blockers). This therapy was discontinued 3 days before the study. Smoking and alcohol intake were prohibited at least 24 h before the study. All subjects were following their standard Italian diet containing at least 50% of carbohydrates as energy.
Human experimental protocol.
Each subject was evaluated after overnight fasting at the Division of Metabolic Diseases, University School of Medicine, Padova, Italy. The protocol was approved by the Ethics Committee of the University Hospital of Padova and all subjects provided informed consent. Anthropometric parameters and multiple blood pressure measurements were recorded. A cannula was inserted in a superficial vein of the arm for blood sample collection. Then, a mixed meal containing 10 kcal/kg (55% carbohydrate, 15% protein, and 30% fat) was administered in 10–15 min. Plasma samples were drawn at −5, 0, 10, 20, 30, 60, 90, 120, 150, and 180 min, and plasma glucose, insulin, C-peptide, and free fatty acid (FFA) concentrations were determined to evaluate insulin sensitivity index (Si) (17). At baseline, lipid concentrations were measured as well. Metabolic syndrome was diagnosed according to the revised Adult Treatment Panel-III (ATP-III) criteria (18). At time 0 and 180 min, blood samples were obtained for the isolation of PBMCs to determine SIRT gene and protein expression.
The analytic methods, preparation of PBMCs, cell culture conditions, assessment of cell viability, quantitative Real Time RT-PCR (Q-PCR) (supplementary Table 1), Western blot analysis, p53-acetylation procedure, SIRT1 silencing, measurement of intracellular reactive oxygen species (ROSs), and NAD+ determination are reported in supplementary methods, available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db09-1187/DC1.
Insulin sensitivity.
Insulin sensitivity (defined as Si and expressed as 10−4 dl · kg−1 · min−1 per μU/ml) was estimated from plasma glucose and insulin concentrations measured during the meal test using the oral glucose minimal model (19). Si measures the overall effect of insulin to stimulate glucose disposal and to inhibit glucose production. The minimal model has been validated against the hyperinsulinemic-euglycemic clamp, which is considered the gold standard for assessment of insulin sensitivity. Subjects were divided into insulin sensitive and insulin resistant according to the median value of Si (9.5 × 10−4 dl · kg−1 · min−1 per μU/ml).
Statistical analysis.
Statistical analyses were performed with SPSS Version 13.0. Data are presented as mean ± SE. Normal distribution was verified with the Kolmogorov-Smirnov test. Differences between two groups were assessed using Student t test for unpaired data or Mann-Whitney test for nonnormally distributed variables. Pearson correlation was used to evaluate univariate correlations. To assess independent association between SIRT expression and clinical data, a stepwise multiple regression model was used. A P value ≤ 0.05 was accepted as significant.
RESULTS
Subjects' characteristics and metabolic responses.
A total of 13 subjects fulfilled ATP-III criteria for diagnosis of metabolic syndrome. Clinical characteristics of subjects divided into non–metabolic syndrome and metabolic syndrome are presented in Table 1. Upon OGTT, 41 subjects had normal glucose tolerance (NGT) and 13 had pre-diabetes. Of these, four had impaired fasting glucose (IFG, defined as preload plasma glucose ≥100 mg/dl); nine had impaired glucose tolerance (IGT, defined as postload plasma glucose ≥140 and <200 mg/dl); three had both IFG and IGT; and none had diabetes.
TABLE 1.
Demographic and metabolic characteristics of study subjects
| Characteristics | All (n = 54) | Non–metabolic syndrome (n = 41) | Metabolic syndrome (n = 13) |
|---|---|---|---|
| Sex (male/female) | 37/17 | 26/15 | 11/2 |
| Age (years) | 46.0 ± 1 | 45.3 ± 1.2 | 48.0 ± 1.5 |
| BMI (kg/m2) | 26.7 ± 0.8 | 24.5 ± 0.6 | 33.7 ± 1.1* |
| Waist (cm) | 93.5 ± 2.0 | 88.1 ± 1.8 | 110.8 ± 3.1* |
| Systolic blood pressure (mmHg) | 121.7 ± 2 | 118.5 ± 2.1 | 131.6 ± 3.7* |
| Diastolic blood pressure (mmHg) | 80.1 ± 1.0 | 78.1 ± 1.2 | 86.2 ± 2.8* |
| Fasting plasma glucose (mg/dl) | 85.7 ± 2.0 | 82.1 ± 1.6 | 97.3 ± 5.0* |
| Fasting plasma insulin (μU/ml) | 10.5 ± 1.1 | 7.3 ± 0.8 | 20.8 ± 2.4* |
| Fasting plasma C-peptide (ng/ml) | 1.6 ± 0.1 | 1.4 ± 0.1 | 2.3 ± 0.2* |
| Plasma triglyceride (mg/dl) | 120.2 ± 12.8 | 98.2 ± 7.7 | 189.6 ± 40.9* |
| Plasma total cholesterol (mg/dl) | 195.7 ± 5.0 | 191.2 ± 4.5 | 209.9 ± 10.9 |
| Plasma HDL cholesterol (mg/dl) | 49.4 ± 2.2 | 50.8 ± 1.8 | 40.6 ± 2.7* |
| Plasma LDL cholesterol (mg/dl) | 126.1 ± 4.0 | 120.6 ± 3.9 | 143.3 ± 9.4* |
| Plasma FFAs (μmol/l) | 592 ± 59 | 593 ± 42 | 588 ± 73 |
Data are expressed as mean ± SE.
*P < 0.05 versus non–metabolic syndrome.
Glucose and FFA areas under the curve (AUC) were calculated and stratified according to insulin sensitivity and glucose tolerance. Glucose AUC was significantly higher in insulin resistant versus insulin sensitive (10674 ± 751 vs. 16687 ± 782 mg · dl−1 · min; P < 0.001), in metabolic syndrome versus non–metabolic syndrome (21389 ± 1,276 vs. 17822 ± 634; P < 0.01) and in IFG/IGT versus NGT (22756 ± 1,233 vs. 17388 ± 562; P < 0.001). The FFA AUC was significantly greater in insulin resistant versus insulin sensitive (50.16 ± 2.98 vs. 33.76 ± 2.79 μmol · l−1 · min; P < 0.001), marginally significant in IFG/IGT versus NGT (49.69 ± 4.59 vs. 39.50 ± 2.59; P = 0.059), and similar in metabolic syndrome versus non–metabolic syndrome (47.74 ± 3.66 vs. 40.12 ± 2.78). The characteristics of subjects divided by insulin resistant and insulin sensitive and by glucose tolerance are shown in supplementary Tables 2 and 3.
SIRT gene and protein expression in relation to metabolic syndrome and insulin sensitivity.
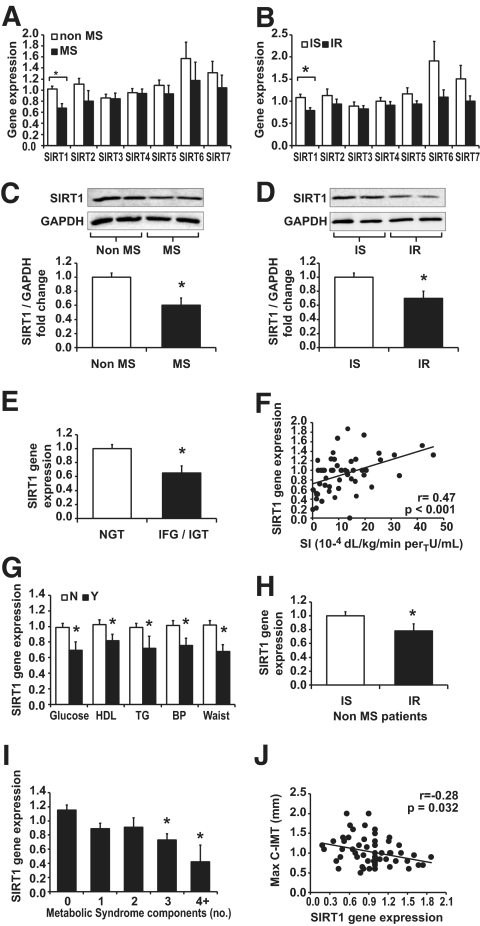
When the subjects were divided into two groups according to the presence (n = 13) or absence (n = 41) of metabolic syndrome, we found significantly lower levels of SIRT1 gene expression in metabolic syndrome versus non–metabolic syndrome subjects (0.67 ± 0.08 vs. 1.02 ± 0.06 comparative cycle threshold [ΔΔCt]; P = 0.003), whereas expression of other SIRTs (SIRT2-SIRT7) were not significantly altered (Fig. 1A). Similarly, when subjects were divided into insulin sensitive (n = 28) and insulin resistant (n = 26) on the basis of median value of their Si, we found significantly lower SIRT1 gene expression in insulin resistant compared with insulin sensitive (0.77 ± 0.06 vs. 1.09 ± 0.06 ΔΔCt; P = 0.001), whereas other SIRTs were unaffected (Fig. 1B). To confirm this result, we analyzed the protein level of SIRT1 in PBMCs from these subjects according to metabolic syndrome and insulin sensitivity. In PBMCs from metabolic syndrome and from insulin-resistant subjects, SIRT1 protein expression was significantly reduced in comparison with non–metabolic syndrome subjects and with insulin-sensitive subjects (metabolic syndrome versus non–metabolic syndrome: 0.37 ± 0.06 vs. 0.60 ± 0.06 arbitrary units; P = 0.010; insulin resistant versus insulin sensitive: 0.42 ± 0.04 vs. 0.67 ± 0.09; P = 0.021; representative blots are reported in Fig. 1C and D).
FIG. 1.
SIRT1-SIRT7 gene and protein expression in PBMCs. Subjects were first divided by the presence/absence of metabolic syndrome and by insulin-resistant or insulin-sensitive status. A: Q-PCR analysis of SIRT1-SIRT7 gene expression in PBMCs from metabolic syndrome and from non–metabolic syndrome subjects. B: Q-PCR analysis of SIRT1-SIRT7 gene expression in PBMCs from insulin-resistant and from insulin-sensitive subjects. The relative quantification was achieved by the expression of each gene of interest by housekeeping genes according to ΔΔCt formula. C and D: Western blot analysis of SIRT1 from PBMCs of two representative patients with and without metabolic syndrome or insulin resistance. Densitometric analysis of SIRT1 after normalization for glyceraldehyde-3-phosphate dehydrogenase in the whole cohort of subjects is described in the results section. E: Subjects were also divided according to OGTT results into subjects with NGT and pre-diabetic subjects (either IFG or IGT): SIRT1 expression in subjects with NGT was significantly reduced compared with pre-diabetic subjects (*P < 0.05). Protein expression data are described in the results section. F: SIRT1 gene expression was significantly directly correlated with Si. G: Subjects were also divided according to the presence (black columns) or absence (white columns) of each metabolic syndrome component, as defined by revised ATP-III criteria (*P < 0.05). I: When subjects were divided according to the number of metabolic syndrome components, SIRT1 expression was progressively downregulated by increasing number of components (*P < 0.05 vs. 0 components after α-correction; ANOVA, P < 0.05). H: Subjects divided according to metabolic syndrome and insulin sensitivity: in nine subjects without metabolic syndrome classified as insulin resistant, there was a significant SIRT1 downregulation compared with insulin-sensitive subjects. *P < 0.05. J: Negative significant correlation between SIRT1 gene expression and maximal carotid IMT. Data are expressed as mean ± SE.
Because sex might influence SIRT1, we repeated analysis for males and females separately and found a similar trend of SIRT1 reduction in both males and females with metabolic syndrome or insulin resistance, compared with non–metabolic syndrome and insulin sensitivity, respectively (supplementary Fig. 1). However caution should be paid in interpreting these results because the number of females in the non–metabolic syndrome and insulin-sensitive groups was very limited (n = 2).
SIRT1 expression and glucose homeostasis.
We then looked at differences in SIRT1 expression in subjects with altered glucose metabolism (IFG or IGT). In comparison with subjects with NGT (n = 41), subjects with pre-diabetes (n = 13) had significantly lower levels of SIRT1 gene (Fig. 1E) and protein expression (0.39 ± 0.05 vs. 0.59 ± 0.06 arbitrary units; P = 0.022). This significant association between SIRT1 and carbohydrate metabolism is corroborated by the observation of the direct correlation between SIRT1 gene expression and Si (r = 0.47; P < 0.001; Fig. 1F). A quite similar trend of SIRT1 reduction in pre-diabetic subjects versus subjects with NGT was found in both males and females (supplementary Fig. 1).
SIRT1 expression in metabolic syndrome cluster and subclinical atherosclerosis.
SIRT1 gene expression was significantly correlated with all parameters that define metabolic syndrome and are linked to insulin resistance: waist circumference (r = −0.43; P = 0.001), HDL cholesterol (r = 0.38; P = 0.007), triglycerides (r = −0.34; P = 0.014), systolic blood pressure (r = −0.29; P = 0.03), and fasting plasma glucose (r = −0.3; P = 0.04). To better describe the relationship between SIRT1 and metabolic syndrome, we first evaluated the association of individual metabolic syndrome components according to the revised ATP-III definition and SIRT1 gene expression. We found that each metabolic syndrome component was associated with a significant reduction of SIRT1 gene expression (Fig. 1G). Furthermore, when the level of SIRT1 expression was plotted against the number of metabolic syndrome components in each subject, there was a trend of SIRT1 downregulation with components clustering. Compared with subjects with no component, SIRT1 downregulation was statistically significant after α-adjusting when at least three components were present together, allowing the diagnosis of metabolic syndrome (Fig. 1I). Interestingly, among patients without metabolic syndrome, those belonging to the insulin-resistant group showed a significant SIRT1 downregulation compared with insulin-sensitive subjects (Fig. 1H). To further clarify these associations, we ran a stepwise multiple regression analysis, which showed that age and the number of metabolic syndrome components were significantly correlated with SIRT1 gene expression independently of sex, of all the parameters that define metabolic syndrome components and Si (supplementary Table 4).
Finally, as metabolic syndrome components are known cardiovascular risk factor, we looked for an association between SIRT1 downregulation and carotid IMT, a marker of early atherosclerosis: we found a significant negative correlation between SIRT1 gene expression and maximal carotid IMT (Fig. 1J).
SIRT1 expression in THP-1 cells.
Insulin resistance, elevated glucose, and systemic FFA levels are significant contributors of the pathophysiological aspects associated with metabolic syndrome. Furthermore, hepatic SIRT1 is an important factor in the regulation of glucose and lipid metabolism (20). Based on these previous data, and in the light of our in vivo observations, we hypothesized that high glucose and FFA levels might be involved in the cellular regulation of SIRT1 expression. Therefore, we investigated the effects of glucose, insulin, and palmitate acid in THP-1 cells, in vitro. As shown in Fig. 2A, gene expression of SIRT1 decreased significantly after 24 h of treatment with 20 mmol/l glucose, reaching the maximal reduction after 48 h (20 mmol/l mannitol was used as osmotic control). Consistently, SIRT1 protein level decreased significantly after 48 h of glucose treatment (Fig. 2B). A similar effect was also induced by 10 mmol/l glucose (not shown). Fatty acids are potent nutrient modulators of insulin resistance. Therefore, we incubated THP-1 cells with representative saturated (palmitate) and unsaturated (linolenic) fatty acids for 3 and 24 h. Treatment of THP-1 cells for 24 h with palmitate (500 μmol/l) markedly reduced SIRT1 gene (Fig. 2C) and protein (Fig. 2D) expression, whereas no effect was seen in linolenic acid (500 μmol/l)–treated THP-1 cells. Treatment of THP-1 cells with insulin (from 0.1 to 100 nmol/l) did not modify the expression of SIRT1 (data not shown). Osmotic control with mannitol (10 or 20 mmol/l) did not produce any significant effect on SIRT protein expression (supplementary Fig. 2).
FIG. 2.
Effects of glucose and FFAs on SIRT1 gene and protein expression in THP-1 cells. A and B: THP-1 cells were cultured under normal (5.5 mmol/l) or high (20 mmol/l) glucose for 3, 24, and 48 h. SIRT1 gene (A) and protein (B) expression analysis was performed by Q-PCR and Western blot, respectively. C and D: THP-1 cells were incubated in presence or absence of FFAs (500 μmol/l palmitic acid or 500 μmol/l linoleic acid) for 3 and 24 h. SIRT1 gene (C) and protein (D) expression analysis was performed by Q-PCR and Western blot, respectively. Data are expressed as mean of six experiments ± SE, *P < 0.05.
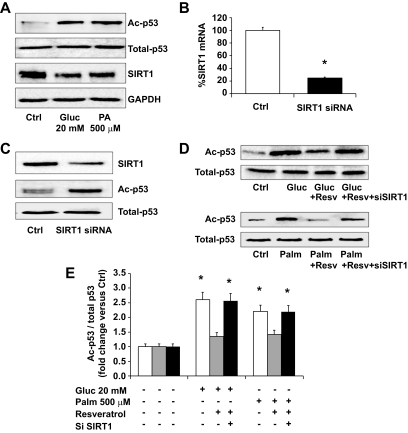
Effect of glucose and palmitic acid on p53 acetylation in THP-1 cells.
Previous studies have demonstrated that high glucose reduced SIRT1 expression leading to increased p53 acetylation (21). Therefore, we measured the effects of high glucose and palmitate on the acetylation of p53 in THP-1 cells. As shown in Fig. 3A, high level of glucose and palmitate increased acetylated p53 in comparison with control cells. To confirm the role of SIRT1 in the regulation of p53 acetylation induced by high glucose and palmitate, we knocked down SIRT1 with siRNA in THP-1 cells. As shown in Fig. 3B, SIRT1 protein content was reduced by ∼70%, and the levels of p53 acetylation were constitutively higher in siRNA SIRT1 cells than in control cells (Fig. 3C). Then, we treated the cells with resveratrol (250 μmol/l), a known SIRT1 activator, and measured the level of p53 acetylation in cells exposed to high glucose or palmitate. As shown in Fig. 3D and E, resveratrol decreased p53 acetylation in THP-1 cells treated with high glucose or palmitate, but not in siRNA SIRT1 cells. These data show that increased acetylation of p53 by high glucose and palmitate can be reversed by resveratrol, and that this effect is SIRT1 dependent.
FIG. 3.
Effect of resveratrol on glucose- and palmitate-induced p53 acetylation in THP-1 cells with and without SIRT1 knockdown. A: THP-1 cells were cultured under normal (5.5 mmol/l) or high (20 mmol/l) glucose for 48 h or with palmitic acid (500 μmol/l for 24 h). B and C: THP-1 cells were transiently transfected with siRNA specific for SIRT1. SIRT1 expression was evaluated by Q-PCR (B) and Western blot analysis (C). D: THP-1 cells were also cultured under normal (5.5 mmol/l) or high (20 mmol/l) glucose for 48 h or with palmitic acid (500 μmol/l) for 24 h in presence and absence of resveratrol (250 μmol/l) and siRNA SIRT1 cells. The level of p53 acetylation was determined by immunoprecipitation followed by immunoblot against acetylated lysine residues. Densitometric analysis of acetylated p53 was normalized for total p53 (E). Data are expressed as fold increase normalized to control and expressed as mean of three experiments ± SE, *P < 0.01.
It is well known that high glucose and fatty acids generate ROSs in various cells, including monocytes (22,23). Thus, we evaluated the effects of high glucose and palmitate on ROS production in THP-1 cells. Both 20 mmol/l glucose and palmitate increased ROS production and oxidative stress in THP-1 cells, and these effects were abolished by resveratrol (Fig. 4A and B). To confirm that an increase of ROSs may regulate SIRT1 protein level, we treated cells with H2O2 (250 μmol/l for 24 h): SIRT1 protein level was decreased and p53 acetylation dramatically increased, although resveratrol attenuated these effects (Fig. 4C and D).
FIG. 4.
Effects of resveratrol on glucose- and palmitate-induced ROS production, p53 acetylation, and JNK activation in THP-1. A and B: THP-1 cells were cultured under normal (5.5 mmol/l) or high (20 mmol/l) glucose for 3, 24, and 48 h (A) or with palmitate (500 μmol/l) for 3 and 24 h in presence and absence of resveratrol (250 μmol/l) (B). Intracellular ROS production was quantified using the fluorescent probe dichlorodihydrofluorescein diacetate acetyl ester. C and D: THP-1 cells were treated with H2O2 (250 μmol/l for 24 h), in presence and absence of resveratrol. The level of p53 acetylation was performed by immunoprecipitation followed by an immunoblot against acetylated lysine residues. E: THP-1 cells were cultured under normal (5.5 mmol/l) or high (20 mmol/l, 48 h) glucose or with palmitate (500 μmol/l for 24 h) in presence and absence of resveratrol (250 μmol/l) and in siRNA SIRT1 cells. The level of JNK phosphorylation (p-JNK) was measured by Western blot. F: Densitometric analysis of p-JNK after normalization for total JNK in THP-1 cells. Data are expressed as p-JNK/JNK normalized to control. G and H: Effects of high glucose and palmitate on NAMPT expression and NAD+ cellular content: THP-1 cells were cultured under normal (5.5 mmol/l) or high (20 mmol/l) glucose or with palmitate (500 μmol/l) for 3, 6, 24, and 48 h. NAMPT expression was expressed as fold change versus the control condition (5.5 mmol/l glucose and no palmitate) (G). NAD+ was quantified in THP-1 cells exposed to high glucose (20 mmol/l) or palmitate (500 μmol/l) for 24 h (H). The values are expressed as mean fold increase of 3–6 experiments over control ± SE, *P < 0.01 versus basal or control.
Effect of glucose and palmitic acid on JNK activation in THP-1 cells.
We investigated the effects of high glucose and palmitate on the activation of Jun NH2-terminal kinase (JNK). As shown in Fig. 4E, both high glucose and palmitate increased JNK phosphorylation. These effects were attenuated by activating SIRT1 with resveratrol. This phenomenon was abolished in siRNA SIRT1–treated cells, suggesting that it is SIRT1 dependent (Fig. 4F).
Effect of high glucose and palmitic acid on NAMPT expression.
Nicotinamide phosphoribosyltransferase (NAMPT) is the rate-limiting factor in NAD+ biosynthesis, and SIRT1 protein expression is regulated by the level of NAD+. Therefore, we tested whether high level of glucose or palmitate modulates NAMPT expression in THP-1 cells. Gene expression of NAMPT markedly decreased after 6-h high glucose and palmitate treatment (Fig. 4G). Accordingly, high glucose and palmitic acid reduced the levels of intracellular NAD+ in THP-1 cells in comparison with cells grown at normal level of glucose (Fig. 4H).
DISCUSSION
This set of both in vivo and in vitro studies offers important novel insights on the relationship among SIRT1, insulin resistance, and metabolic syndrome. The major result from the in vivo study is that gene and protein expression of SIRT1 in PBMCs is significantly reduced in relation to insulin resistance and metabolic syndrome. This result is corroborated by the direct correlation between SIRT1 expression and a dynamic measure of insulin sensitivity, as well as by the correlation between SIRT1 expression and the number of metabolic syndrome components. Although there is considerable disagreement on the underlying metabolic syndrome pathophysiology, clinical and experimental data support a link between SIRT1 and metabolic syndrome. Interestingly, the only clinical variable related to SIRT1 beyond metabolic syndrome components was age, which is physiologically related to SIRT1 function as a life-span determinant gene.
The link between SIRT1 and glucose homeostasis is substantiated by the SIRT1 downregulation observed in subjects with pre-diabetes, compared with subjects with normal glucose regulation. Thus, the expression of SIRT1 in PBMCs appears as a novel marker of insulin resistance, metabolic syndrome, and pre-diabetes. Obviously, we cannot equate that the biological regulation of SIRT1 in PBMCs parallels that in insulin-sensitive tissues; nonetheless, we anticipate that the gene and protein expression of SIRT1 in PBMCs may represent a potential and novel pathogenic pathway of metabolic syndrome. In support of this, a recent study demonstrated that SIRT1 gene expression in peripheral blood cells of obese subjects was modulated by caloric restriction, suggesting that SIRT1 may have an important role in different types of tissue (24,25). Other data from the literature support this view, because SIRT1 has been suggested to be involved in the regulation of glucose homeostasis: it controls hepatic glucose metabolism by interacting with PGC-1α, a transcriptional coactivator that controls glucose metabolism in the liver (6). SIRT1 increases lipolysis (11), stimulates FOXO and adiponectin gene expression (26), and is directly involved in intracellular insulin pathways by selective inhibition of insulin-induced tyrosine phosphorylation of insulin receptor substrate-2 (27). SIRT1 also improves insulin sensitivity under insulin-resistant conditions by repressing protein tyrosine phosphatase-1B (28). Mice overexpressing SIRT1 on a high-fat diet show lower lipid-induced inflammation along with better glucose tolerance (29). It should be noted that the systemic effects of SIRT1 may be more complex than expected: for instance, liver-targeted SIRT1 knockdown was shown to decrease basal hepatic glucose production and increase hepatic insulin sensitivity in type 2 diabetic rats (30). This paradoxical result, compared with other studies (29), is probably related to the tissue-specific versus the systemic effects of SIRT1 modulation, which differ in the regulation of some soluble mediators, such as adiponectin. Nonetheless, available experimental data collectively indicate that activation of SIRT1 may have a potential beneficial role in humans. These findings are clinically relevant, because decreased SIRT1 may be associated with a series of metabolic events predisposing to a shorter life span, as shown in experimental animal models. In Zucker fa/fa rats, hyperinsulinemic-euglycemic clamp studies demonstrate that SIRT1 activators improve whole-body glucose homeostasis and insulin sensitivity in adipose tissue, skeletal muscle, and liver (31).
Our data suggest that SIRT1 expression is decreased in subjects who are insulin resistant, specifically in those who are glucose intolerant, and particularly in those with several components of metabolic syndrome. A challenging hypothesis is that, in these subjects at risk for premature cardiovascular disease, SIRT1 determines life span and disease progression. We lend indirect support to this hypothesis by showing a significant negative correlation between SIRT1 gene expression and carotid IMT, an index of early atherosclerosis, supporting the hypothesis that low SIRT1 is proatherogenic. As recently shown by Cardellini et al, SIRT1 may play an important protective role in vascular biology (32).
Unfortunately, in vivo observations do not allow to define cause-effect relationships: thus, it is not clear whether low SIRT1 expression predisposes to metabolic syndrome and insulin resistance, plays a role in disease pathophysiology, or simply is an epiphenomenon of metabolic abnormalities. Therefore, an additional aim of the present study was to test the hypothesis that excessive substrate availability, such as that present in patients with metabolic syndrome, may directly influence SIRT1 in cells that may be involved in both atherosclerotic plaque formation and visceral fat inflammation.
The evidence that glucose and palmitic acid induce SIRT1 downregulation in THP-1 cells supports a model whereby biochemical factors acting in the setting of metabolic syndrome, such as glucose intolerance and impaired release of free fatty acids, are responsible for SIRT1 downregulation. Our data demonstrate also that the mechanisms linking high glucose and palmitate to SIRT1 impairment include reduction of NAMPT expression with consequent depletion of cellular NAD+ (a SIRT1 activator), together with increased generation of ROSs. As a consequence of SIRT1 downregulation in glucose- and palmitate-treated THP-1 cells, acetylation of p53 increased significantly, an event that is typically followed by transcription-independent proapoptotic signals. In addition, we show that SIRT1 downregulation leads to activation of the stress-sensing pathway of JNK, which has been related to macrophage infiltration into adipose tissue and to whole-body insulin sensitivity (33). These pathways triggered in monocytes by glucose and palmitate through SIRT1 downregulation may be important not only in the pathophysiology of metabolic syndrome but also in terms of cardiovascular disease. Our findings testify that the interaction between fatty acids and SIRT is reciprocal; it was previously shown that SIRTs are important regulators of lipid oxidation through activation of the transcriptional coactivator PGC-1α (6), although almost unknown is the regulation of SIRT expression by FFAs. Our in vitro study sheds light on the complex relationship between substrate availability and SIRT: we specifically found that a saturated FFA (palmitate) significantly decreases SIRT expression, whereas an unsaturated FFA (linoleate) did not. At present, we cannot affirm that this in vitro effect takes place also in vivo. Despite subjects with insulin resistance having a significantly lower suppression of FFA concentration during glucose load, we found no correlation between SIRT1 expression and FFA AUC. However, we cannot provide data on FFA plasma composition of our patients to test the relationship between circulating saturated fatty acids and SIRT expression.
Glucose and palmitate activated the p53 and JNK pathways, which have been implicated in both obesity (34,35) and atherosclerosis (36,37). Even if we cannot definitely rule out other pathways linking glucotoxicity and lipotoxicity to JNK and p53 activation (Fig. 5), SIRT1 appears to act as a transducer of negative metabolic signals conveyed by excess nutrients. Moreover, SIRTs are central modulators of signaling networks critical for maintaining vascular endothelial homeostasis, especially in the setting of diabetes (38–40). Thus, our in vitro studies confirm that SIRT1 could be a potential therapeutic target to improve the metabolic milieu and prevent cardiovascular complications. Herein, we demonstrate that resveratrol, a phytoalexin provided with antioxidant properties that is found in red wine, is able to limit the negative effects of glucose and palmitate by counteracting SIRT1 downregulation.
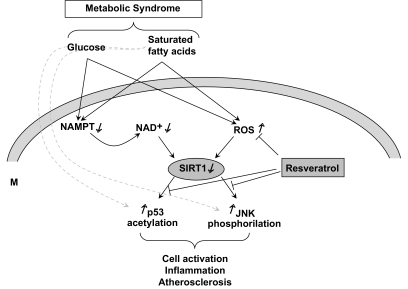
FIG. 5.
Proposed mechanism whereby glucose and palmitate downregulate SIRT1 and induce activation and inflammation in monocytes. According to the data presented, high glucose and palmitate impair expression and function of NAMPT, thus reducing cell NAD+ content. As SIRT1 is NAD+ dependent, this leads to reduction of expression and activity of SIRT1. In parallel, both high glucose and palmitate promote production of ROSs, which may themselves reduce SIRT1. SIRT1 downregulation is then responsible for high p53 acetylation and JNK activation, which are related to cell activation and inflammation. All of these pathways can be prevented by the antioxidant resveratrol. We cannot rule out alternative pathways by which glucose and palmitate activate JNK and p53 independently of SIRT1 (dashed lines).
We found no alteration of SIRT2, SIRT3, SIRT4, and SIRT5 in human metabolic syndrome, insulin resistance, or pre-diabetes in vivo, even if many relationships have been previously reported between these Sirtuins and metabolic disorders. For instance, it was recently shown that SIRT2, the most abundant Sirtuin in adipocytes, inhibits differentiation of these cells, promotes adipogenesis through modulation of FOXO1 activity, and may play a role in controlling adipose tissue mass and function (41). It has also been shown that in PBMCs, SIRT2 is responsive to caloric restriction (42). The lack of difference in SIRT2 expression in our study is related to the fact that caloric restriction is possibly a major stimulus for the change in its expression: indeed, our study subjects were all assessed after at least 12 h of fasting, a period that is probably too short to detect an increase in SIRT2 expression. We observed a reduction in SIRT6 and SIRT7 expression in metabolic syndrome, which did not reach statistical significance but deserves further investigation because SIRT6 has been recently described as a critical factor in mammalian aging, as its absence creates a remarkable phenotype in rodents that includes metabolic alterations, loss of subcutaneous fat, and premature death (43). Similarly to SIRT2, cellular SIRT6 levels are increased by calorie restriction as well, although it was shown that this increase appears to result from protein stabilization rather than augmented gene expression (44).
In conclusion, we provide, for the first time in humans, evidence that insulin resistance and metabolic syndrome affect SIRT1 gene and protein expression in PBMCs. Glucose and saturated fatty acids may be implicated in SIRT1 downregulation through induction of oxidative stress and depletion of NAD+. Although we cannot translate this observation to insulin-sensitive cells, we hypothesize that this relationship may be true also in tissues that play a relevant role in determining insulin resistance. In addition, expression of SIRT1 in circulating blood cells may represent a novel marker for a disturbed metabolism, as well as a pathogenic actor in monocyte-mediated atherosclerotic process, through p53 and JNK pathways. Interestingly, all of these pathways could be prevented by resveratrol (Fig. 5).
Supplementary Material
ACKNOWLEDGMENTS
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bertoni AG, Wong ND, Shea S, Ma S, Liu K, Preethi S, Jacobs DR, Jr, Wu C, Saad MF, Szklo M: Insulin resistance, metabolic syndrome, and subclinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 2007; 30: 2951– 2956 [DOI] [PubMed] [Google Scholar]
- 2.Imai S, Armstrong CM, Kaeberlein M, Guarente L: Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 2000; 403: 795– 800 [DOI] [PubMed] [Google Scholar]
- 3.Guarente L: Sirtuins as potential targets for metabolic syndrome. Nature 2006; 444: 868– 874 [DOI] [PubMed] [Google Scholar]
- 4.Westphal CH, Dipp MA, Guarente L: A therapeutic role for sirtuins in diseases of aging? Trends Biochem Sci 2007; 32: 555– 560 [DOI] [PubMed] [Google Scholar]
- 5.Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L: Mammalian SIRT1 represses forkhead transcription factors. Cell 2004; 116: 551– 563 [DOI] [PubMed] [Google Scholar]
- 6.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P: Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 2005; 434: 113– 118 [DOI] [PubMed] [Google Scholar]
- 7.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW: Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 2004; 23: 2369– 2380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA: Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 2004; 305: 390– 392 [DOI] [PubMed] [Google Scholar]
- 9.Chen D, Steele AD, Lindquist S, Guarente L: Increase in activity during calorie restriction requires Sirt1. Science 2005; 310: 1641. [DOI] [PubMed] [Google Scholar]
- 10.Jiang WJ: Sirtuins: novel targets for metabolic disease in drug development. Biochem Biophys Res Commun 2008; 373: 341– 344 [DOI] [PubMed] [Google Scholar]
- 11.Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW, Guarente L: Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature 2004; 429: 771– 776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haigis MC, Mostoslavsky R, Haigis KM, Fahie K, Christodoulou DC, Murphy AJ, Valenzuela DM, Yancopoulos GD, Karow M, Blander G, Wolberger C, Prolla TA, Weindruch R, Alt FW, Guarente L: SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell 2006; 126: 941– 954 [DOI] [PubMed] [Google Scholar]
- 13.Ahuja N, Schwer B, Carobbio S, Waltregny D, North BJ, Castronovo V, Maechler P, Verdin E: Regulation of insulin secretion by SIRT4, a mitochondrial ADP-ribosyltransferase. J Biol Chem 2007; 282: 33583– 33592 [DOI] [PubMed] [Google Scholar]
- 14.Libby P, Geng YJ, Aikawa M, Schoenbeck U, Mach F, Clinton SK, Sukhova GK, Lee RT: Macrophages and atherosclerotic plaque stability. Curr Opin Lipidol 1996; 7: 330– 335 [DOI] [PubMed] [Google Scholar]
- 15.Odegaard JI, Chawla A: Mechanisms of macrophage activation in obesity-induced insulin resistance. Nat Clin Pract Endocrinol Metab 2008; 4: 619– 626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fadini GP, Coracina A, Baesso I, Agostini C, Tiengo A, Avogaro A, de Kreutzenberg SV: Peripheral blood CD34+KDR+ endothelial progenitor cells are determinants of subclinical atherosclerosis in a middle-aged general population. Stroke 2006; 37: 2277– 2282 [DOI] [PubMed] [Google Scholar]
- 17.Dalla Man C, Caumo A, Cobelli C: The oral glucose minimal model: estimation of insulin sensitivity from a meal test. IEEE Trans Biomed Eng 2002; 49: 419– 429 [DOI] [PubMed] [Google Scholar]
- 18.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Jr, Stone NJ: National Heart, Lung, and Blood Institute, American College of Cardiology Foundation, American Heart Association Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004; 110: 227– 239 [DOI] [PubMed] [Google Scholar]
- 19.Breda E, Cavaghan MK, Toffolo G, Polonsky KS, Cobelli C: Oral glucose tolerance test minimal model indexes of beta-cell function and insulin sensitivity. Diabetes 2001; 50: 150– 158 [DOI] [PubMed] [Google Scholar]
- 20.Rodgers JT, Puigserver P: Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc Natl Acad Sci U S A 2007; 104: 12861– 12866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nemoto S, Fergusson MM, Finkel T: Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science 2004; 306: 2105– 2108 [DOI] [PubMed] [Google Scholar]
- 22.Thorlaksdottir AY, Jonsson JJ, Tryggvadottir L, Skuladottir GV, Petursdottir AL, Ogmundsdottir HM, Eyfjord JE, Hardardottir I: Positive association between DNA strand breaks in peripheral blood mononuclear cells and polyunsaturated fatty acids in red blood cells from women. Nutr Cancer 2007; 59: 21– 28 [DOI] [PubMed] [Google Scholar]
- 23.Avogaro A, Pagnin E, Calò L: Monocyte NADPH oxidase subunit p22(phox) and inducible hemeoxygenase-1 gene expressions are increased in type II diabetic patients: relationship with oxidative stress. J Clin Endocrinol Metab 2003; 88: 1753– 1759 [DOI] [PubMed] [Google Scholar]
- 24.Crujeiras AB, Parra D, Goyenechea E, Martínez JA: Sirtuin gene expression in human mononuclear cells is modulated by caloric restriction. Eur J Clin Invest 2008; 38: 672– 678 [DOI] [PubMed] [Google Scholar]
- 25.Finkel T, Deng CX, Mostoslavsky R: Recent progress in the biology and physiology of sirtuins. Nature 2009; 460: 587– 591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschöp MH: Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci U S A 2008; 105: 9793– 9798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J: The direct involvement of SirT1 in insulin-induced insulin receptor substrate-2 tyrosine phosphorylation. J Biol Chem 2007; 282: 34356– 34364 [DOI] [PubMed] [Google Scholar]
- 28.Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, Zhai Q: SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab 2007; 6: 307– 319 [DOI] [PubMed] [Google Scholar]
- 29.Ramsey KM, Mills KF, Satoh A, Imai S: Age-associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in beta cell-specific Sirt1-overexpressing (BESTO) mice. Aging Cell 2008; 7: 78– 88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erion DM, Yonemitsu S, Nie Y, Nagai Y, Gillum MP, Hsiao JJ, Iwasaki T, Stark R, Weismann D, Yu XX, Murray SF, Bhanot S, Monia BP, Horvath TL, Gao Q, Samuel VT, Shulman GI: SirT1 knockdown in liver decreases basal hepatic glucose production and increases hepatic insulin responsiveness in diabetic rats. Proc Natl Acad Sci U S A 2009; 106: 11288– 11293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH: Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 2007; 450: 712– 716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cardellini M, Menghini R, Martelli E, Casagrande V, Marino A, Rizza S, Porzio O, Mauriello A, Solini A, Ippoliti A, Lauro R, Folli F, Federici M: TIMP3 is reduced in atherosclerotic plaques from subjects with type 2 diabetes and increased by SirT1. Diabetes 2009; 58: 2396– 2401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blüher M, Bashan N, Shai I, Harman-Boehm I, Tarnovscki T, Avinaoch E, Stumvoll M, Dietrich A, Klöting N, Rudich A: Activated Ask1-MKK4-p38MAPK/JNK stress signaling pathway in human omental fat tissue may link macrophage infiltration to whole-body insulin sensitivity. J Clin Endocrinol Metab 2009; 94: 2507– 2515 [DOI] [PubMed] [Google Scholar]
- 34.Hotamisligil GS: Role of endoplasmic reticulum stress and c-Jun NH2-terminal kinase pathways in inflammation and origin of obesity and diabetes. Diabetes 2005; 54( Suppl. 2): S73– S78 [DOI] [PubMed] [Google Scholar]
- 35.Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, Lambert PD, Mataki C, Elliott PJ, Auwerx J: Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab 2008; 8: 347– 358 [DOI] [PubMed] [Google Scholar]
- 36.Mercer J, Bennett M: The role of p53 in atherosclerosis. Cell Cycle 2006; 5: 1907– 1909 [DOI] [PubMed] [Google Scholar]
- 37.Sumara G, Belwal M, Ricci R: “Jnking” atherosclerosis. Cell Mol Life Sci 2005; 62: 2487– 2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Potente M, Dimmeler S: NO targets SIRT1: a novel signaling network in endothelial senescence. Arterioscler Thromb Vasc Biol 2008; 28: 1577– 1579 [DOI] [PubMed] [Google Scholar]
- 39.Potente M, Dimmeler S: Emerging roles of SIRT1 in vascular endothelial homeostasis. Cell Cycle 2008; 7: 2117– 2122 [DOI] [PubMed] [Google Scholar]
- 40.Orimo M, Minamino T, Miyauchi H, Tateno K, Okada S, Moriya J, Komuro I: Protective role of SIRT1 in diabetic vascular dysfunction. Arterioscler Thromb Vasc Biol 2009; 29: 889– 894 [DOI] [PubMed] [Google Scholar]
- 41.Jing E, Gesta S, Kahn CR: SIRT2 regulates adipocyte differentiation through FoxO1 acetylation/deacetylation. Cell Metab 2007; 6: 105– 114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang F, Nguyen M, Qin FX, Tong Q: SIRT2 deacetylates FOXO3a in response to oxidative stress and caloric restriction. Aging Cell 2007; 6: 505– 514 [DOI] [PubMed] [Google Scholar]
- 43.Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR, Gellon L, Liu P, Mostoslavsky G, Franco S, Murphy MM, Mills KD, Patel P, Hsu JT, Hong AL, Ford E, Cheng HL, Kennedy C, Nunez N, Bronson R, Frendewey D, Auerbach W, Valenzuela D, Karow M, Hottiger MO, Hursting S, Barrett JC, Guarente L, Mulligan R, Demple B, Yancopoulos GD, Alt FW: Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 2006; 124: 315– 329 [DOI] [PubMed] [Google Scholar]
- 44.Kanfi Y, Shalman R, Peshti V, Pilosof SN, Gozlan YM, Pearson KJ, Lerrer B, Moazed D, Marine JC, de Cabo R, Cohen HY: Regulation of SIRT6 protein levels by nutrient availability. FEBS Lett 2008; 582: 543– 548 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.