Abstract
OBJECTIVE
Insulin resistance might play a role in the pathogenesis of polycystic ovarian syndrome (PCOS). The family of glycoprotein 130 (gp130) cytokines could influence insulin action. One of these cytokines is interleukin (IL)-6, which exerts a short-term insulin-sensitizing effect, whereas in a long-term period, it might induce insulin resistance. Some other gp130 activators are supposed to have beneficial metabolic effects. Gp130 is present in the circulation in the soluble form (sgp130), which inhibits intracellular gp130 signaling. The aim of the present study was to estimate the relation between sgp130 and insulin sensitivity in women with PCOS.
RESEARCH DESIGN AND METHODS
We studied 78 women with PCOS (35 lean and 43 obese) and 34 healthy women (18 lean and 16 obese). The euglycemic-hyperinsulinemic clamp and the measurements of serum sgp130, IL-6, soluble IL-6 receptor (sIL-6R), and sex hormones were performed.
RESULTS
Both obesity and PCOS were characterized by an increased sgp130 (P < 0.0001 and P = 0.0002, respectively). sIL-6R concentration was lower (P = 0.0036) in women with PCOS independently of obesity. Serum sgp130 was negatively correlated with insulin sensitivity when control and PCOS women were analyzed together (r = −0.36, P < 0.0001) and in the PCOS subjects separately (r = −0.34, P = 0.002). In multiple regression analysis, this correlation was significant after adjustment for BMI, waist, percent of body fat, postload glucose and insulin, triglycerides, and high-sensitive C-reactive protein.
CONCLUSIONS
Serum sgp130 is inversely and independently associated with insulin sensitivity in women with PCOS. An increased serum sgp130 in obesity and PCOS suggests an inhibition of intracellular gp130 signaling in insulin-resistant conditions.
Polycystic ovarian syndrome (PCOS) is a common endocrine disorder with a diverse and heterogeneous nature that is present in 5–10% of reproductive-age women. PCOS is characterized by hyperandrogenism and chronic anovulation (1,2). Insulin resistance is a common feature of PCOS and might be the cause of significant metabolic and cardiovascular complications observed in this condition (3).
The family of glycoprotein 130 (gp130) cytokines might influence insulin action (4). This family includes interleukin (IL)-6, IL-11, leukemia inhibitory factor, ciliary neurotrophic factor, oncostatin, and cardiotrophin-1 (5). Gp130 cytokines play a pivotal role in the regulation of the immune, hematopoietic, nervous, cardiovascular, and endocrine systems (5). These cytokines exert their actions through a heterodimeric or homodimeric receptor consisting of two membrane-bound glycoproteins: a cytokine binding subunit and a glycoprotein termed IL-6 transducer, or IL6ST, also known as gp130, which is responsible for signal transduction (6).
IL-6 was thought to be an insulin-desensitizing factor (7). However, it might have some beneficial metabolic actions, and it was hypothesized that it exerts a short-term insulin-sensitizing effect, whereas in the long-term period, IL-6 might induce insulin resistance (8). IL-6 increased glycogen synthesis and enhanced lipid oxidation via an AMP-activated protein kinase (AMPK)-dependent mechanism in skeletal muscle (9). Another gp130 cytokine, ciliary neurotrophic factor, prevented weight gain and stimulated AMPK in skeletal muscle (10). Therefore, it is supposed that gp130 activators might be useful in the treatment of obesity and insulin resistance (4). Gp130 is expressed in most cells of the body (6) and is present in the circulation in soluble form (sgp130), which inhibits intracellular gp130 signaling (11). The role of sgp130 in the pathogenesis of human insulin resistance has not been studied so far.
Soluble form of the IL-6R (sIL-6R) occurs in various body fluids and might induce gp130 dimerization and signaling when complexed with IL-6. The presence of sIL-6R leads to sensitization of IL-6–responsive cells toward the ligand (12). The IL-6/sIL-6R complex stimulates several types of cells that only express gp130 and are unresponsive to IL-6 alone. This process is called trans-signaling (13). Sgp130 exerts also inhibitory effects toward the IL-6/sIL-6R complex (14).
We hypothesized that serum spg130 might be altered in insulin-resistant conditions in humans and might be related to insulin sensitivity. To test this hypothesis, we studied serum sgp130, IL-6, and sIL-6R in relation to insulin sensitivity in lean and obese women with PCOS and BMI-matched healthy control subjects.
RESEARCH DESIGN AND METHODS
The study group consisted of 78 women with PCOS (35 lean, BMI <25 kg/m2, and 43 overweight or obese, BMI >25 kg/m2) and 34 healthy normally menstruating women (18 lean and 16 overweight or obese). The subjects' recruitment and diagnosis of PCOS according to Rotterdam criteria (15) was described in detail previously (16). Before entering the study, a physical examination and appropriate laboratory test were performed. A standard 75-g oral glucose tolerance test was performed, and all participants had normal glucose tolerance. None of the women had morbid obesity (BMI >40 kg/m2), cardiovascular disease, hypertension, infections, or other serious medical problems; all were nonsmokers, and they were not taking any anti-inflammatory drugs and drugs known to affect glucose and lipid metabolism. Studies were performed in the PCOS group 3–5 days after a spontaneous menses or independent of cycle phase in the presence of amenorrhea and in regularly cycling women during the early follicular phase (3–5 days) of their menstrual cycle. All analyses were performed after an overnight fast. The study protocol was approved by the ethics committee of the Medical University of Białystok, Poland. All participants gave written informed consent before entering the study. Anthropometric measurements were performed as previously described (16).
Insulin sensitivity.
Insulin sensitivity was evaluated by the euglycemic-hyperinsulinemic clamp technique as previously described (16). The rate of whole-body glucose uptake (M value) was calculated as the mean glucose infusion rate from 80 to 120 min, corrected for glucose space and normalized per kilogram of fat-free mass (M/FFM).
Biochemical analyses.
Plasma glucose was measured immediately by the enzymatic method using a glucose analyzer (YSI 2300 STAT Plus; Yellow Springs Instruments, Yellow Springs, OH). The serum insulin was measured with immunoradiometric assay with the sensitivity of 1 μIU/ml and intra- and interassay coefficients of variation (CVs) <2.2 and 6.5%, respectively (BioSource Europe, Nivelles, Belgium). Serum lipids were measured as previously described (16).
Before estimation of serum insulin, sgp130, IL-6, and sIL6-R, samples were kept frozen at −80°C. Serum sgp130 was measured with a quantitative sandwich enzyme immunoassay kit (ELISA) (R&D Systems, Minneapolis, MN) with the lowest detectable limit of 0.08 ng/ml and with intra- and interassay CVs below 5.5 and 5.2%, respectively. Serum high-sensitivity IL-6 (hsIL-6) and sIL-6R concentrations were determined with ELISA kits (R&D Systems). The minimum detectable concentration was 0.039 pg/ml for hsIL-6 and 6.5 pg/ml for sIL-6R. Intra- and interassay CVs were, respectively, <7.8 and 9.6% for hsIL-6 and <8.6 and 9.6% for sIL-6R. Serum high-sensitive C-reactive protein (hsCRP) was measured by means of particle enhanced immunonephelometry using a CardioPhase kit (Dade Behring, Marburg, Germany) with a sensitivity of 0.175 mg/l and intra- and interassay CVs <4.5 and 5.8%, respectively.
Serum luteinizing hormone, follicle-stimulating hormone, testosterone, and estradiol were measured by the chemiluminescence method (ACS Chirone 180), and serum sex hormone–binding globulin (SHBG) was measured by the immunoradiometric assay (ZenTech, Angleur, Belgium). The sensitivity and CVs for all these assays were identical, as reported previously (16). Free androgen index was calculated as serum testosterone (nmol/l) × 100/SHBG (nmol/l) ratio (17).
Statistical analysis.
The statistics were performed with STATISTICA 8.0 software. The variables, which did not have normal distribution (fasting and postload insulin, triglycerides, IL-6, sIL-6R, sgp130, SHBG, testosterone, free androgen index, estradiol, and hsCRP) were log-transformed before the analyses. For the purpose of the data presentation, these variables were transformed to absolute values in the results. The differences between the groups were estimated with factorial ANOVA, with PCOS status and obesity as categorical factors and the studied parameters as dependent variables. The relationships between variables were studied with the Pearson product-moment correlation analysis and with multiple regression analysis. The level of significance was accepted at P < 0.05.
RESULTS
The clinical characteristics of the studied groups is shown in Table 1. In the factorial ANOVA, we observed that the PCOS women had higher serum sgp130 concentrations than the control group (P = 0.0002). We also found that the obese women had higher serum sgp130 concentrations than the lean women (P < 0.0001). The interaction between PCOS status and obesity was not significant (P = 0.29), which suggests that an increase in sgp130 in PCOS was independent of obesity and an increase in sgp130 in obesity was independent of PCOS status. Additionally, PCOS women had significantly lower serum sIL-6R (P = 0.0036). In contrast, serum sIL-6R did not differ between lean and obese women (P = 0.93). Serum IL-6 and hsCRP did not differ between PCOS and control subjects, whereas they were increased in the obese group compared with the lean group (P = 0.007 and P < 0.001, respectively).
TABLE 1.
Clinical and biochemical characteristics of the studied groups
| Lean |
Obese |
P (obesity/PCOS) | |||
|---|---|---|---|---|---|
| Control | PCOS | Control | PCOS | ||
| n | 18 | 35 | 16 | 43 | |
| Age (years) | 26.33 ± 5.56 | 24.11 ± 3.94 | 27.44 ± 5.27 | 25.60 ± 5.57 | NS |
| BMI (kg/m2) | 22.19 ± 1.92 | 21.71 ± 1.81 | 30.66 ± 4.37 | 31.46 ± 4.34 | <0.001/NS |
| Waist (cm) | 73.72 ± 5.50 | 72.66 ± 6.95 | 94.13 ± 10.95 | 94.36 ± 12.57 | <0.001/NS |
| Body fat (%) | 21.97 ± 5.71 | 24.76 ± 6.84 | 37.79 ± 9.03 | 37.90 ± 10.84 | <0.001/NS |
| Fasting glucose (mg/dl) | 80.82 ± 6.20 | 80.22 ± 8.21 | 81.08 ± 6.98 | 86.00 ± 7.28 | NS |
| Postload glucose (mg/dl) | 81.31 ± 15.40 | 82.22 ± 15.31 | 92.68 ± 20.24 | 97.28 ± 23.09 | 0.0013/NS |
| Fasting insulin (μIU/ml) | 8.44 ± 3.21 | 11.80 ± 5.95 | 14.33 ± 5.30 | 16.14 ± 9.58 | <0.001/NS |
| Postload insulin (μIU/ml) | 31.28 ± 15.90 | 40.17 ± 25.88 | 48.37 ± 37.37 | 82.01 ± 92.34 | 0.014/NS |
| Cholesterol (mg/dl) | 186.68 ± 36.83 | 181.06 ± 36.16 | 173.25 ± 40.97 | 185.78 ± 38.66 | NS |
| Triglycerides (mg/dl) | 66.92 ± 21.44 | 70.40 ± 34.06 | 105.83 ± 103.47 | 103.27 ± 54.74 | 0.005/NS |
| HDL cholesterol (mg/dl) | 56.35 ± 12.73 | 62.99 ± 13.73 | 57.16 ± 9.02 | 50.23 ± 9.56 | 0.013/NS |
| LDL cholesterol (mg/dl) | 117.89 ± 38.26 | 102.89 ± 33.80 | 94.23 ± 28.77 | 114.70 ± 39.12 | NS |
| M/BW (mg · kg body wt−1 · min−1) | 8.85 ± 2.27 | 7.21 ± 2.86 | 5.91 ± 2.19 | 4.47 ± 2.37 | <0.001/0.003 |
| M/FFM (mg · kg FFM−1 · min−1) | 11.41 ± 3.06 | 9.54 ± 3.75 | 9.64 ± 3.33 | 7.07 ± 3.23 | 0.003/0.002 |
| hsIL-6 (pg/ml) | 0.74 ± 0.73 | 0.85 ± 0.83 | 0.85 ± 0.37 | 1.16 ± 0.74 | 0.007/NS |
| sIL-6R (ng/ml) | 48.14 ± 21.94 | 37.53 ± 14.65 | 46.15 ± 13.03 | 36.55 ± 11.96 | NS/0.003 |
| sgp130 (ng/ml) | 309.96 ± 42.31 | 367.01 ± 81.54 | 369.53 ± 61.03 | 402.03 ± 53.26 | <0.001/<0.001 |
| hsCRP (mg/l) | 0.47 ± 0.22 | 0.67 ± 0.50 | 1.20 ± 0.83 | 1.16 ± 0.74 | <0.001/NS |
Data are means ± SD unless otherwise indicated. P values are derived from factorial ANOVA. All interactions between PCOS status and obesity are nonsignificant. Postload glucose and insulin refer to value at 120 min of the oral glucose tolerance test.
Both the presence of PCOS and obesity were independently characterized by lower insulin sensitivity (P = 0.0021 and P = 0.0033, respectively; P = 0.62 for the interaction), lower serum SHBG (P = 0.011 and P < 0.0001, respectively; P = 0.22 for the interaction), and higher free androgen index (both P < 0.0001, P = 0.34 for the interaction). PCOS women also had higher serum luteinizing hormone and testosterone concentrations (both P < 0.0001) (Table 2).
TABLE 2.
Hormonal characteristics of the studied groups
| Lean |
Obese |
P (obesity/PCOS) | |||
|---|---|---|---|---|---|
| Control | PCOS | Control | PCOS | ||
| n | 18 | 35 | 16 | 43 | |
| Estradiol (pmol/l) | 299.4 ± 303.78 | 282.00 ± 267.18 | 163.98 ± 113.98 | 245.26 ± 242.32 | NS |
| Follicle-stimulating hormone (IU/l) | 5.99 ± 1.25 | 5.78 ± 1.48 | 5.56 ± 1.73 | 5.65 ± 1.39 | NS |
| Luteinizing hormone (IU/l) | 6.94 ± 5.56 | 10.21 ± 5.30 | 4.25 ± 2.24 | 8.93 ± 4.01 | NS/<0.001 |
| Testosterone (nmol/l) | 1.79 ± 0.38 | 2.93 ± 1.11 | 1.75 ± 0.51 | 2.83 ± 1.06 | NS/<0.001 |
| SHBG (nmol/l) | 89.51 ± 56.28 | 59.91 ± 46.74 | 41.50 ± 18.35 | 36.20 ± 17.96 | <0.001/0.011 |
| Free androgen index | 2.79 ± 1.73 | 6.95 ± 4.49 | 4.69 ± 1.70 | 9.74 ± 6.19 | <0.001/<0.001 |
Data are means ± SD unless otherwise indicated. P values are derived from factorial ANOVA. All interactions between PCOS status and obesity are nonsignificant.
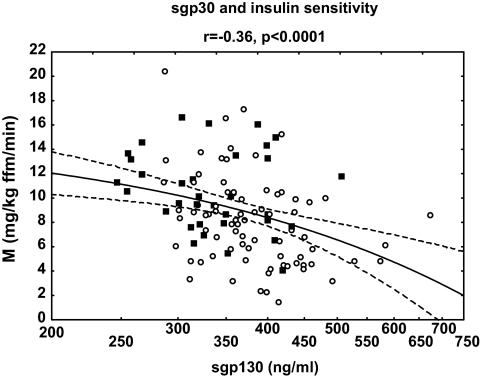
Cross-sectionally, serum sgp130 was related to BMI (r = 0.35, P < 0.001), waist (r = 0.36, P < 0.001), body fat (r = 0.28, P = 0.005), postload glucose and insulin (r = 0.29, P = 0.002, and r = 0.29, P = 0.002, respectively), triglycerides (r = 0.20, P = 0.032), hsCRP (r = 0.24, P = 0.012), and free androgen index (r = 0.28, P = 0.002). We also observed the significant inverse correlation of sgp130 with insulin sensitivity (r = −0.36, P < 0.0001) (Fig. 1) and SHBG (r = −0.23, P = 0.015). In the subgroup analysis, the correlation between serum sgp130 and insulin sensitivity was present in the PCOS subjects (r = −0.34, P = 0.002) but not in the control group (r = −0.16, P = 0.35).
FIG. 1.
Correlations between sgp130 and insulin sensitivity in the whole studied group (n = 112). Values of sgp130 are shown on a log-transformed scale. ○, PCOS group; ■, control group.
Using M value normalized for body weight (M/BW) instead of M/FFM increased the effect of obesity (Table 1) and the correlation with sgp130 was slightly stronger (r = −0.40, P < 0.001).
Serum sIL-6R was related to follicle-stimulating hormone in the entire study group (r = 0.23, P = 0.017) and in the subgroup of PCOS women (r = 0.24, P = 0.041).
In multiple regression analysis, we observed that the relationship between sgp130 and insulin sensitivity was independent of BMI, waist, percent of body fat, postload glucose and insulin, triglycerides, and hsCRP (all adjusted β values between −0.23 and −0.33, all P < 0.05), whereas correlations of sgp130 with SHBG and free androgen index disappeared after controlling for insulin sensitivity.
Exclusion of subjects with the highest values of postload insulin did not change any of the results.
DISCUSSION
The main finding of the present study is an increased serum sgp130 concentration in obesity and PCOS and an inverse relationship between sgp130 and insulin sensitivity in PCOS women.
So far, serum sgp130 has not been determined in obesity and PCOS. Escobar-Morreale et al. (18) did not find the difference in circulating sgp130 between control and hyperandrogenic women; however, almost 40% of subjects in the hyperandrogenic group did not have PCOS. The frontier between PCOS and hyperandrogenism is diffuse; however, because of the large heterogeneity of PCOS itself, differences in characteristics of the studied groups are likely to influence the results. There are studies showing the association between polymorphisms in IL-6 or IL-6R and gp130 genes with clinical characteristics of PCOS and hyperandrogenism (18,19). Regarding the studied polymorphism of the gp130 gene, it did not affect the circulating level of this factor. In another study, polymorphism at the gp130 locus was associated with traits of the metabolic syndrome; however, circulating sgp130 was not reported (20).
Serum sgp130 was inversely related to insulin sensitivity in PCOS women. As mentioned, it was hypothesized that the gp130 cytokine family might exert beneficial effects and might be useful in the treatment of obesity and insulin resistance (4). Most gp130 cytokines occur in circulation in small, usually undetectable, concentrations. It is possible that they might exert auto- and paracrine effects in insulin-sensitive tissues, as in experimental studies, where the ciliary neurotrophic factor was demonstrated to regulate metabolic processes in adipocytes and skeletal muscle cells (4,10). Given the fact that circulating sgp130 acts as an inhibitor of intracellular gp130 signaling (11), the correlation observed in our study might reflect this inhibitory effect.
The question arises as to whether an association between sgp130 and insulin sensitivity is specific only for PCOS. We did not observe a significant correlation in the control group. Other populations with different clinical characteristics should probably be investigated. However, sgp130 was also increased in obese insulin-resistant women without PCOS.
Serum sgp130 was also related to SHBG and free androgen index; however, these associations were no longer significant after controlling for insulin sensitivity. IL-6, sIL-6R, and sgp130 might also regulate other reproductive functions, such as granulosa cell survival, follicular growth, development of human preovulatory processes, and blastocyst implantation (21). We cannot exclude the possibility that sgp130 influences other features of PCOS phenotype, not assessed in the present study. Nevertheless, these are mainly local actions of sgp130. In our study, estimation of sgp130 serum concentration was performed during the 3–5 days of the menstrual cycle, and the influence of sgp130 on endometrium in the proliferative phase of the menstrual cycle seemed to be relatively weak (21).
There are studies that demonstrate an increase in circulating IL-6 and hsCRP in PCOS (22). However, our results on the lack of an increase in these parameters in PCOS are in agreement with other data (23,24).
To our knowledge, decrease in sIL-6R in PCOS was not reported previously. Polymorphisms in the IL-6R gene were associated with BMI and characteristics of the metabolic syndrome (18,25), but not with hyperandrogenism (18). The balance between sIL-6R and sgp130 in PCOS seems to be directed toward an inhibition of IL-6 action; however, the significance of this finding remains unclear.
In conclusion, our data indicate that serum sgp130 is inversely and independently associated with insulin sensitivity in women with PCOS. An increased serum sp130 in obesity and PCOS suggests an inhibition of intracellular gp130 signaling in insulin-resistant conditions.
ACKNOWLEDGMENTS
This study was supported by Grants 3-50815L and 3-50816L from the Medical University of Bialystok.
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Diamanti-Kandarakis E, Kouli CR, Bergiele AT, Filandra FA, Tsianateli TC, Spina GG, Zapanti ED, Bartzis MI: A survey of the polycystic ovary syndrome in the Greek island of Lesbos: hormonal and metabolic profile. J Clin Endocrinol Metab 1999; 84: 4006– 4011 [DOI] [PubMed] [Google Scholar]
- 2.Asunción M, Calvo RM, San Millán JL, Sancho J, Avila S, Escobar-Morreale HF: A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab 2000; 85: 2434– 2438 [DOI] [PubMed] [Google Scholar]
- 3.Carmina E, Lobo RA: Polycystic ovary syndrome (PCOS): arguably the most common endocrinopathy is associated with significant morbidity in women. J Clin Endocrinol Metab 1999; 84: 1897– 1899 [DOI] [PubMed] [Google Scholar]
- 4.Febbraio MA: gp130 receptor ligands as potential therapeutic targets for obesity. J Clin Invest 2007; 117: 841– 849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirano T, Matsuda T, Nakajima K: Signal transduction through gp130 that is shared among the receptors for the interleukin 6 related cytokine subfamily. Stem Cells 1994; 12: 262– 277 [DOI] [PubMed] [Google Scholar]
- 6.Taga T, Kishimoto T: gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol 1997; 15: 797– 819 [DOI] [PubMed] [Google Scholar]
- 7.Senn JJ, Klover PJ, Nowak IA, Mooney RA: Interleukin-6 induces cellular insulin resistance in hepatocytes. Diabetes 2002; 51: 3391– 3399 [DOI] [PubMed] [Google Scholar]
- 8.Carey AL, Febbraio MA: Interleukin-6 and insulin sensitivity: friend or foe? Diabetologia 2004; 47: 1135– 1142 [DOI] [PubMed] [Google Scholar]
- 9.Al-Khalili L, Bouzakri K, Glund S, Lönnqvist F, Koistinen HA, Krook A: Signaling specificity of interleukin-6 action on glucose and lipid metabolism in skeletal muscle. Mol Endocrinol 2006; 20: 3364– 3375 [DOI] [PubMed] [Google Scholar]
- 10.Watt MJ, Dzamko N, Thomas WG, Rose-John S, Ernst M, Carling D, Kemp BE, Febbraio MA, Steinberg GR: CNTF reverses obesity-induced insulin resistance by activating skeletal muscle AMPK. Nat Med 2006; 12: 541– 548 [DOI] [PubMed] [Google Scholar]
- 11.Narazaki M, Yasukawa K, Saito T, Ohsugi Y, Fukui H, Koishihara Y, Yancopoulos GD, Taga T, Kishimoto T: Soluble forms of the interleukin-6 signal-transducing receptor component gp130 in human serum possessing a potential to inhibit signals through membrane-anchored gp130. Blood 1993; 82: 1120– 1126 [PubMed] [Google Scholar]
- 12.Peters M, Jacobs S, Ehlers M, Vollmer P, Müllberg J, Wolf E, Brem G, Meyer zum Büschenfelde KH, Rose-John S: The function of the soluble interleukin 6 (IL-6) receptor in vivo: sensitization of human soluble IL-6 receptor transgenic mice towards IL-6 and prolongation of the plasma half-life of IL-6. J Exp Med 1996; 183: 1399– 1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones SA, Richards PJ, Scheller J, Rose-John S: IL-6 transsignaling: the in vivo consequences. J Interferon Cytokine Res 2005; 25: 241– 253 [DOI] [PubMed] [Google Scholar]
- 14.Rose-John S, Scheller J, Elson G, Jones SA: Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J Leuk Biol 2006; 80: 227– 236 [DOI] [PubMed] [Google Scholar]
- 15.The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group Revised 2003 consensus on diagnostic criteria and long term health risk related to polycystic ovary syndrome (PCOS). Hum Rep 2004; 19: 41– 47 [DOI] [PubMed] [Google Scholar]
- 16.Kowalska I, Straczkowski M, Nikolajuk A, Adamska A, Karczewska-Kupczewska M, Otziomek E, Wolczynski S, Gorska M: Serum visfatin in relation to insulin resistance and markers of hyperandrogenism in lean and obese women with polycystic ovary syndrome. Hum Rep 2007; 22: 1824– 1829 [DOI] [PubMed] [Google Scholar]
- 17.Vermeulen A, Verdonck L, Kaufman JM: A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999; 84: 3666– 3672 [DOI] [PubMed] [Google Scholar]
- 18.Escobar-Morreale HF, Calvo RM, Villuendas G, Sancho J, San Millan JL: Association of polymorphisms in the interleukin 6 receptor complex with obesity and hyperandrogenism. Obesity Res 2003; 11: 987– 996 [DOI] [PubMed] [Google Scholar]
- 19.Walch K, Grimm C, Zeillinger R, Huber JC, Nagele F, Hefler LA: A common interleukin-6 gene promoter polymorphism influences the clinical characteristics of women with polycystic ovary syndrome. Fertil Steril 2004; 81: 1638– 1641 [DOI] [PubMed] [Google Scholar]
- 20.Gottardo L, De Cosmo S, Zhang YY, Powers C, Prudente S, Marescotti MC, Trischitta V, Avogaro A, Doria A: A polymorphism at the IL6ST (gp130) locus is associated with traits of the metabolic syndrome. Obesity 2008; 16: 205– 210 [DOI] [PubMed] [Google Scholar]
- 21.Kawasaki F, Kawano Y, Kosay Hasan Z, Narahara H, Miyakawa I: The critical role of interleukin-6 and interleukin-6 soluble receptor in human follicular fluids. Clin Exp Med 2003; 3: 27– 31 [DOI] [PubMed] [Google Scholar]
- 22.González F, Rote NS, Minium J, Kirwan JP: Evidence of proatherogenic inflammation in polycystic ovary syndrome. Metabolism 2009; 58: 954– 962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Escobar-Morreale HF, Villuendas G, Botella-Carretero JI, Sancho J, San Millán JL: Obesity, and not insulin resistance, is the major determinant of serum inflammatory cardiovascular risk markers in pre-menopausal women. Diabetologia 2003; 46: 625– 633 [DOI] [PubMed] [Google Scholar]
- 24.Möhlig M, Spranger J, Osterhoff M, Ristow M, Pfeiffer AF, Schill T, Schlösser HW, Brabant G, Schöfl C: The polycystic ovary syndrome per se is not associated with increased chronic inflammation. Eur J Endocrinol 2004; 150: 525– 532 [DOI] [PubMed] [Google Scholar]
- 25.Esteve E, Villuendas G, Mallolas J, Vendrell J, Lopez-Bermejo A, Rodriguez M, Recasens M, Ricart W, San Millan JL, Escobar-Morreale HF, Richart C, Fernandez-Real JM: Polymorphisms in interleukin-6 receptor gene are associated with body mass index and with characteristics of the metabolic syndrome. Clin Endocrinol 2006; 65: 88– 91 [DOI] [PubMed] [Google Scholar]