Abstract
OBJECTIVE
Although intensive glycemic control achieved with insulin therapy increases the incidence of both moderate and severe hypoglycemia, clinical reports of cognitive impairment due to severe hypoglycemia have been highly variable. It was hypothesized that recurrent moderate hypoglycemia preconditions the brain and protects against damage caused by severe hypoglycemia.
RESEARCH DESIGN AND METHODS
Nine-week-old male Sprague-Dawley rats were subjected to either 3 consecutive days of recurrent moderate (25–40 mg/dl) hypoglycemia (RH) or saline injections. On the fourth day, rats were subjected to a hyperinsulinemic (0.2 units · kg−1 · min−1) severe hypoglycemic (∼11 mg/dl) clamp for 60 or 90 min. Neuronal damage was subsequently assessed by hematoxylin-eosin and Fluoro-Jade B staining. The functional significance of severe hypoglycemia–induced brain damage was evaluated by motor and cognitive testing.
RESULTS
Severe hypoglycemia induced brain damage and striking deficits in spatial learning and memory. Rats subjected to recurrent moderate hypoglycemia had 62–74% less brain cell death and were protected from most of these cognitive disturbances.
CONCLUSIONS
Antecedent recurrent moderate hypoglycemia preconditioned the brain and markedly limited both the extent of severe hypoglycemia–induced neuronal damage and associated cognitive impairment. In conclusion, changes brought about by recurrent moderate hypoglycemia can be viewed, paradoxically, as providing a beneficial adaptive response in that there is mitigation against severe hypoglycemia–induced brain damage and cognitive dysfunction.
Hypoglycemia is the major obstacle in achieving tight glycemic control in people with diabetes (1). Intensive insulin therapy increases the risk of iatrogenic hypoglycemia (2). Episodes of both moderate and severe hypoglycemia have long-term clinical consequences. Recurrent moderate hypoglycemia induces a maladaptive response that limits symptoms of hypoglycemia (hypoglycemia unawareness), limits the counterregulatory response to subsequent hypoglycemia (hypoglycemia-associated autonomic failure), and thus jeopardizes patient safety (1). By depriving the brain of glucose, more severe hypoglycemia causes brain damage in animal studies and leads to long-term impairments in learning and memory (3,4). However, studies examining the effect of severe hypoglycemia in humans are conflicting. Severe hypoglycemia has been shown to alter brain structure (5–7) and cause significant cognitive damage in many (5,7–12) but not all (13–16) studies. Reasons for the discrepancy between human and animal studies are unknown, but a major contributing factor may be the extent of glycemia control (including recurrent hypoglycemia) prior to the episode of severe hypoglycemia.
In other models of brain damage, such as ischemic stroke, brief, mild episodes of antecedent brain ischemia has been shown to cause a beneficial adaptation that protects the brain against a subsequent episode of more severe ischemia (a phenomena known as ischemic preconditioning) (17). In a similar fashion, antecedent, recurrent episodes of moderate hypoglycemia were hypothesized to protect the brain against damage caused by a subsequent episode of more severe hypoglycemia.
To investigate this hypothesis, recurrent moderately hypoglycemic (25–40 mg/dl) rats (RH rats) and control saline-injected rats (CON rats) were subjected to hyperinsulinemic, severe hypoglycemic clamps (10–15 mg/dl). One group of rats was killed 1 week after severe hypoglycemia to quantify brain damage, while a second group of rats was evaluated by behavioral and cognitive tests 6–8 weeks after the severe hypoglycemia. The results demonstrated that recurrent antecedent moderate hypoglycemia preconditioned the brain and protected it against neurological damage and cognitive defects induced by an episode of severe hypoglycemia.
RESEARCH DESIGN AND METHODS
Nine-week-old male Sprague-Dawley rats (Charles River Laboratories) were individually housed in a temperature- and light-controlled environment maintaining the animal's diurnal cycle (12 h light and 12 h dark) with an ad libitum standard rat chow diet. All studies were done in accordance with the Animal Studies Committee at the Washington University School of Medicine.
Implantation of arterial and venous catheters.
Micro-renathane (Braintree Scientific) catheters were inserted into the left carotid artery and into the right jugular vein of anesthetized rats (40–80 mg/kg ketamine with 5–8 mg/kg xylazine). To maintain patency, catheters were filled with 40% polyvinylpyrrolidone (Sigma) in heparin (1,000 units/ml; USP) (Baxter Healthcare Corporation).
Recurrent moderate hypoglycemia (hypoglycemic preconditioning).
One week after catheter implantation, recurrent moderate hypoglycemia was induced in nonfasted rats with injections of subcutaneous regular human insulin (Lilly) (6 units/kg on day 1, 5 units/kg on day 2, and 4 units/kg on day 3), while CON rats were given equal-volume saline injections for 3 consecutive days. Food was withheld, and tail-vein blood glucose values were measured hourly. For insulin-treated rats, recurrent hypoglycemia resulted in blood glucose levels of 25–40 mg/dl for 3 h. To terminate moderate hypoglycemia, rats were given a subcutaneous injection of dextrose (Hospira) and were allowed free access to food.
Hyperinsulinemic-severe hypoglycemia clamp.
Animals were fasted overnight after the third day of injections and the following morning were subjected to a hyperinsulinemic (0.2 units · kg−1 · min−1) severe hypoglycemic clamp (Fig. 1). Rats were awake, unrestrained, and had free access to water. Arterial blood glucose was measured every 15 min with Ascensia Contour glucose monitors (Bayer HealthCare), which are reported to have accurate blood glucose readings in the hypoglycemia range, although accuracy in the severe hypoglycema range has not been reported. Insulin and glucose were coinfused intravenously to lower blood glucose to 10–15 mg/dl, as this level of severe hypoglycemia was necessary to induce neuronal damage (3,18). Severe hypoglycemia (SH rats) was maintained between 10 and 15 mg/dl for either 60 min (CON-SH60, n = 6; RH-SH60, n = 10) or 90 min (CON-SH90, n = 20; RH-SH90, n = 18) for the CON and RH-treated rats, respectively. To terminate hypoglycemia, insulin infusion was stopped and infusions of dextrose were given until animals could maintain euglycemia. Additional blood samples were obtained during the basal period and 2 h into the hyperinsulinemic clamp when severe hypoglycemia had been reached for 30 min for epinephrine measurements, as determined by the single-isotope derivative method (19).
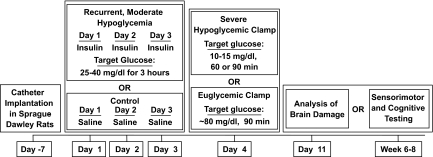
FIG. 1.
Experimental protocol. Arterial and venous catheters were implanted into 9-week-old Sprague-Dawley rats. After 1 week of recovery, animals were either given an insulin injection daily for 3 consecutive days to induce moderate hypoglycemia (25–40 mg/dl) or they were given saline injections as a control. On the fourth day, rats underwent a severe hypoglycemic (10–15 mg/dl) hyperinsulinemic (0.2 units · kg−1 · min−1) clamp for either 60 or 90 min or, alternatively, underwent a 90-min euglycemic (∼80 mg/dl) hyperinsulinemic (0.2 units · kg−1 · min−1) clamp. Animals were either killed 1 week later to assess neuronal damage by H-E and Fluoro-Jade B staining or underwent sensorimotor and cognitive testing 6–8 weeks following the clamp.
Tonic-clonic seizure-like behavior was visually noted by characteristic brief (5–10 s) neck extensions, tonic stretching, uncontrolled limb movements, and spontaneous spinning (18,20). The number of episodes of seizure-like behavior during the clamp was quantified for each rat and was later correlated with histological and cognitive findings.
Two other groups of rats were made either recurrently hypoglycemic or given saline injections as described above and, on the fourth day, underwent a 90-min hyperinsulinemic-euglycemic (0.2 units · kg−1 · min−1) clamp (CON-euglycemic [EUG], n = 9; RH-EUG, n = 11). These two additional groups served as euglycemic control rats treated in the same fashion except that they were not exposed to severe hypoglycemia.
The first grouping of rats that underwent hyperinsulinemic severe hypoglycemic clamps or hyperinsulinemic-euglycemic clamps was analyzed for brain damage. The second grouping of rats was subjected to the same hyperinsulinemic clamp protocols except that they underwent sensorimotor and behavioral testing (Fig. 1).
Histology.
One week after the severe hypoglycemic or euglycemic clamps, anesthetized rats were intracardially perfused with 0.01 mol/l PBS (Sigma) followed by 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA). Brains were immersed in 4% paraformaldehyde overnight and then cryoprotected in 30% sucrose. Beginning at 2.8 mm posterior to the bregma, coronal cryostat sections (20 μm) were collected on Superfrost coated slides (VWR). Four coronal sections, 120 μm apart, were analyzed for neuronal damage by Fluoro-Jade B (Chemicon International) and hematoxylin-eosin (H-E) (Sigma) staining according to the manufacturer's protocol. Fluoro-Jade B is a well-characterized stain for degenerating neurons (21). Fluorescent cells (Fluoro-Jade–positive cells) were quantified in both hemispheres of the cortex and of the hippocampal structures CA1 and dentate gyrus. For each region of interest, data are expressed as the average number of Fluoro-Jade B–positive (FJB+) cells per section. (CON-SH90, n = 9; RH-SH90, n = 8).
Behavioral testing.
Consistent with other protocol designs (4,22,23), histopathological outcomes were assessed 1 week following the hypoglycemic neuronal insult, while cognitive studies were performed 6–8 weeks later in a separate group of similarly treated rats. This later assessment of cognitive function is a more useful measure of clinical outcome and a better functional index of neuroprotection because it allows for a complete and integrated evaluation of ongoing damage and possible recovery (24). Because the Morris water maze test is a measure of hippocampal-dependent spatial learning/memory and because the rats that underwent 60 min of severe hypoglycemia had little damage in the hippocampus, cognitive testing was not performed in this group. Cognitive testing was performed in the rats that underwent 90 min of severe hypoglycemia because these animals had marked damage in the hippocampus. After a 6- to 8-week recovery from the severe hypoglycemic (CON-SH90, n = 11; RH-SH90, n = 9) and euglycemic (CON-EUG, n = 7; RH-EUG, n = 9) clamps, rats were transferred to the behavioral testing facility and allowed 1 week to acclimate before locomotor activity, sensorimotor measures, and Morris maze tests were performed under euglycemic conditions.
One-hour locomotor activity test and sensorimotor battery.
General locomotor activity and exploratory behavior were evaluated for 1 h using a computerized system (MotorMonitor; Kinder Scientific) of photobeam pairs to quantify ambulations (whole body movements) and rearing frequency. As previously described (25), the ledge, platform, 90°-inclined screen, and walking initiation tests were conducted to measure balance, strength, coordination, and initiation of movement.
Water maze cognitive testing.
Spatial learning and memory were assessed using the Morris water maze test similarly to previously published methods (25). Briefly, a computerized tracking program (Polytrack; San Diego Instruments) recorded the swim-path lengths and time required to find the platform. For the cued trials, rats were trained to swim to the submerged platform (1.5 cm below the surface) marked (cued) by a visible pole. Spatial learning capabilities of the rats were tested during the place trials. In the place trials, rats were trained to learn the position of a submerged and nonvisible platform that remained in the same location across all trials. To evaluate memory retention of the platform location, a probe trial was conducted 1 h after the last place trial, which involved removing the platform from the pool and quantifying rats' search behaviors for 30 s. Probe trial performance indexes included the following: the number of times a rat passed directly over the platform location (platform crossings), the time spent in the target quadrant versus the time spent in each of the other pool quadrants (spatial bias), and average proximity (distance to the platform location sampled and averaged across 1-s epochs throughout the trial).
Statistical analysis.
All data are expressed as means ± SEM. Statistical analyses were performed by either Student's t tests or ANOVA. Quantification of brain damage and behavioral assessments were made by investigators blinded to treatment conditions.
RESULTS
Recurrent hypoglycemia reduced cortical brain damage induced by 60 min of severe hypoglycemia.
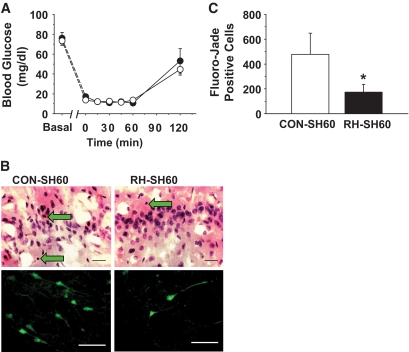
No significant differences in blood glucose were observed before, during, or after the 60-min severe hypoglycemic clamps between RH and CON rats (Fig. 2A). As expected, RH-SH60 rats had an attenuated epinephrine response to hypoglycemia compared with CON-SH60 rats (2001 ± 241 and 3,487 ± 474 pg/ml; P < 0.01) (supplementary Fig. 1, available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db09-1495/DC1). Importantly, RH-SH60 rats had 64% less neuronal damage, as assessed by the number of FJB+ cells, in the cortex than CON-SH60 rats (173 ± 64 vs. 479 ± 170 cells; P < 0.05) (Fig. 2B and C). Of note, 60 min of severe hypoglycemia did not induce significant damage in the hippocampus in either RH-SH60 or CON-SH60 rats.
FIG. 2.
Recurrent hypoglycemia attenuates brain damage after 60 min of severe hypoglycemia. A: Blood glucose levels are shown in rats subjected to a 60-min severe hypoglycemic (10–15 mg/dl) hyperinsulinemic (0.2 units · kg−1 · min−1) clamp. Blood glucose was not significantly different between CON-SH6 (open circles) (n = 6) and RH-SH60 (closed circles) (n = 10) rats during 60 min of severe hypoglycemia. B: Representative H-E (top panel) and Fluoro-Jade B–positive (bottom panel) staining of the cortex of CON-SH60 and RH-SH60 rats 1 week following 60 min of severe hypoglycemia. Neuronal damage is indicated by pyknotic cells (H-E staining; green arrows) or with Fluoro-Jade B–positive cells (green fluorescence). Scale bar = 100 μm. C: Quantification of Fluoro-Jade B staining in CON-SH60 (white bar) (n = 6) and RH-SH60 (black bar) (n = 10) rats. Following severe hypoglycemia, RH rats had significantly fewer degenerating cells in the cortex than CON rats (*P < 0.05, by Student's t test). (A high-quality digital representation of this figure is available in the online issue.)
Recurrent hypoglycemia attenuated cortical and hippocampal brain injury after 90 min of severe hypoglycemia.
To consistently induce hypoglycemic brain damage in the hippocampus, the above experiments were repeated except that the duration of severe hypoglycemia was extended to 90 min. The average blood glucose during 90 min of severe hypoglycemia was 10.9 ± 0.2 vs. 11.0 ± 0.3 mg/dl in the CON-SH90 and RH-SH90 rats, respectively (P = NS) (Fig. 3C). As an additional set of experimental controls, euglycemic-hyperinsulinemic clamps were also performed in RH-EUG (n = 2) or CON-EUG (n = 2) rats. Blood glucose was maintained at 76 ± 5 and 84 ± 6 mg/dl in the CON-EUG and RH-EUG rats, respectively (P = NS) (Fig. 3C).
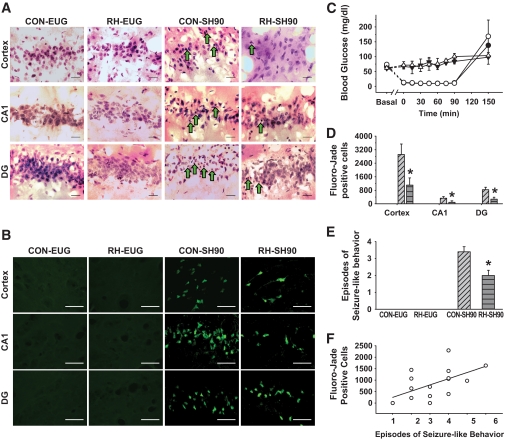
FIG. 3.
Recurrent hypoglycemia limits brain cell death 1 week following 90 min of severe hypoglycemia. A: Representative H-E staining of the cortex and hippocampal structures, CA1, and the dentate gyrus (DG), 1 week following 90-min severe hypoglycemic or euglycemic clamps in RH-SH90, RH-EUG, CON-SH90, and CON-EUG rats. Rats that underwent severe hypoglycemia had damaged neurons characterized by pyknotic nuclei (green arrows). Scale bar = 100 μm. B: Fluoro-Jade B–positive cells (green fluorescence) in the cortex, hippocampal CA1 region, and dentate gyrus of the same four treatment groups. Scale bar = 100 μm. C: Blood glucose was not significantly different between CON-SH90 (open circles) (n = 9) and RH-SH90 (closed circles) (n = 8) rats during 90 min of severe hypoglycemia. Blood glucose was clamped at equal levels of euglycemia in CON-EUG (open triangles) (n = 9) and RH-EUG (closed triangles) (n = 11) rats for 90 min. D: Following 90 min of severe hypoglycemia, the markedly increased number of FJB+ cells in the cortex, CA1, and dentate gyrus observed in the CON-SH90 rats (diagonal hatch) was significantly (*P < 0.05) reduced in RH-SH90 rats (gray horizontal hatch). Bars representing Fluoro-Jade B–positive cells in CON-EUG and RH-EUG groups are not visible in this figure because no appreciable brain damage was observed in euglycemic rats not exposed to severe hypoglycemia. E: CON-EUG and RH-EUG rats experienced no seizure-like behavior. Rats exposed to 90 min severe hypoglycemia exhibited seizure-like behavior, although RH-SH90 rats had significantly less seizure-like behavior than CON-SH90 rats (*P < 0.01). F: In rats that experienced severe hypoglycemia (RH-SH90 and CON-SH90), seizure-like behaviors positively correlated with the amount of Fluoro-Jade B cells in the hippocampus (R = 0.572; P < 0.05). (A high-quality digital representation of this figure is available in the online issue.)
Again validating the model of hypoglycemia-associated autonomic failure, RH reduced the epinephrine response to hypoglycemia (CON-SH90 3,175 ± 516 mg/dl and RH-SH90 2077 ± 426 pg/ml; P < 0.05) (supplementary Fig. 1). Severe hypoglycemia of 90 min induced significant cellular damage in the cortex, as evidenced by the presence of pyknotic cells observed with H-E staining (Fig. 3A) and the marked number of fluorescent cells with Fluoro-Jade B staining (Fig. 3B). Interestingly, 90 min of severe hypoglycemia induced sixfold-greater cortical neuronal damage than 60 min of severe hypoglycemia (Figs. 2C and 3D). Recurrent antecedent moderate hypoglycemia decreased cortical brain damage induced by 90 min of severe hypoglycemia by 62% (RH-SH90 1,107 ± 428 FJB+ cells and CON-SH90 2,918 ± 615 FJB+ cells; P < 0.05). Unlike 60 min of severe hypoglycemia, 90 min of severe hypoglycemia did induce hippocampal brain damage (Fig. 3). Recurrent antecedent hypoglycemia resulted in less hippocampal brain damage following 90 min of severe hypoglycemia compared with that in CON-SH90 rats (Fig. 3). Specifically, RH-SH90 rats had decreased FJB+ cells in the CA1 region by 74% (RH-SH90 88 ± 56 cells vs. CON-SH90 334 ± 91 cells; P < 0.05) and by 67% in the dentate gyrus (RH-SH90 274 ± 119 cells vs. CON-SH90 833 ± 148 cells; P < 0.05) compared with CON-SH90 (Fig. 3D). No damage was observed in the hypothalamus in either CON-SH90 or RH-SH90 rats (supplementary Fig. 2).
Interestingly, recurrent hypoglycemia also reduced the episodes of seizure-like behavior observed during severe hypoglycemia (RH-SH90 2.0 ± 0.3 vs. CON-SH90 3.4 ± 0.3; P < 0.01) (Fig. 3E). There was a significant correlation between the number of episodes of seizure-like behavior and number of FJB+ cells (R = 0.572; P < 0.05) (Fig. 3F).
In the absence of severe hypoglycemia, virtually no Fluoro-Jade–positive cells or pyknotic cells (H-E) were observed in the cortex or hippocampus of either the CON-EUG or RH-EUG groups (Fig. 3).
Preserved cognitive function in recurrently hypoglycemic rats.
General activity was not different between groups (supplementary Fig. 3). The severe hypoglycemic groups (both CON-SH90 and RH-SH90) exhibited significantly (P = 0.02) more rearings than the two groups of EUG rats (supplementary Fig. 3B). Data from the walking initiation, ledge, platform, and 90°-inclined screen were not significantly different between groups (supplementary Fig. 3C–F).
During the cue (Fig. 4A) and place (Fig. 4B) trials, the CON-SH90 rats performed worse than the other three groups in spite of having normal swimming speeds (supplementary Fig. 4). In the cue trials, CON-SH90 rats had significantly longer path lengths across the blocks of trials than the CON-EUG rats (P = 0.0002). Importantly, RH-SH90 rats had significantly shorter path lengths relative to the CON-SH90 rats (P = 0.0025), while no differences were observed between RH-SH90 and CON-EUG or between the two EUG control groups.
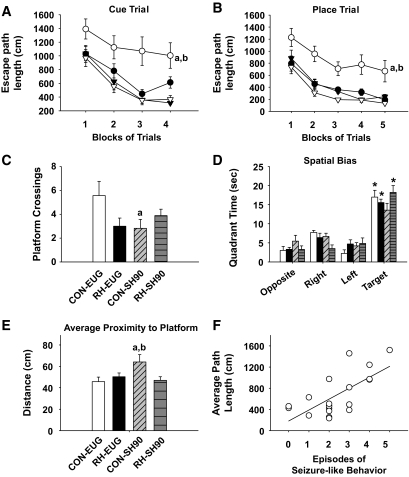
FIG. 4.
Antecedent recurrent hypoglycemia mitigated cognitive dysfunction induced by severe hypoglycemia. Morris water maze testing was performed 6–8 weeks following severe hypoglycemic or euglycemic clamps. A: During the cue trial, CON-SH90 rats (open circles) (n = 11) performed worse as evidenced by longer escape-path lengths than those of CON-EUG rats (open triangles) (n = 7) (aP = 0.002). Notably, rats exposed to recurrent moderate hypoglycemia before severe hypoglycemia (RH-SH90, n = 9 (closed circles) had shorter escape-path lengths than CON-SH90 rats (bP = 0.0025) and performed similarly to CON-EUG and RH-EUG rats (closed triangles) (n = 9). B: A similar pattern was observed during the place trials, where CON-SH90 rats had significantly higher escape-path lengths than CON-EUG (cP = 0.0001) and RH-SH90 (dP = 0.0006) rats. C: During the probe trial, CON-SH90 rats (diagonal hatch) had significantly fewer platform crossings than CON-EUG rats (white bar) (eP = 0.014). No significant differences were observed between CON-SH90 and RH-SH90 rats (gray horizontal hatch) or between CON-EUG and RH-EUG rats (black bar). D: RH-SH90, CON-EUG, and RH-EUG rats had a spatial bias toward the target quadrant while CON-SH90 rats did not (*P < 0.0025). E: During the probe trial, CON-SH90 rats showed an average proximity to the platform location that was significantly farther away than that of the CON-EUG rats (fP = 0.014). RH-SH90 rats swam significantly closer to the platform location than CON-SH90 rats (gP = 0.014)—similar to euglycemic controls. F: The number of episodes of seizure-like behaviors observed during severe hypoglycemia 6–8 weeks prior positively correlated with average path length during the place trials (R = 0.685; P < 0.001; n = 20).
During the place (spatial learning) trials, the CON-SH90 rats again showed significant performance deficits. CON-SH90 rats had significantly (P = 0.0001) longer path lengths across the blocks of trials than the CON-EUG rats (Fig. 4B). Notably, RH-SH90 rats had significantly shorter path lengths than CON-SH90 rats (P = 0.0006) (Fig. 4B). Again, no differences were observed between RH-SH90 and CON-EUG rats or between the two euglycemic groups.
During the probe trial, CON-SH90 rats made significantly fewer platform crossings relative to the CON-EUG rats (P = 0.014), though no differences in platform crossings between CON-SH90 and RH-SH90 rats were observed (Fig. 4C). However, with regard to spatial bias and average proximity to the platform location, RH-SH90 rats did have improved performance compared with CON-SH90 rats. In spatial bias analysis, RH-SH90, CON-EUG, and RH-EUG rats all exhibited spatial bias for the target quadrant; each group spent significantly more time in the target quadrant compared with the other pool quadrants (P < 0.0025). CON-SH90 rats did not show significant spatial bias (Fig. 4D). Further, CON-SH90 rats had significantly higher average proximity scores than CON-EUG (P = 0.014) and RH-SH90 (P = 0.014) rats. RH-SH90 rats performed similarly to CON-EUG rats (Fig. 4E). In summary, during the probe trial, severe hypoglycemia (CON-SH90 rats) significantly impaired all three tests of memory retention, and antecedent recurrent moderate hypoglycemia pretreatment (RH-SH90 rats) significantly improved memory performance on two out of three measures.
Interestingly, the number of episodes of seizure-like behavior during severe hypoglycemia positively correlated with performance during Morris water maze testing (Fig. 3F). Specifically, increases in the number of episodes of seizure-like behavior were associated with longer average path lengths (R = 0.685; P < 0.001) (Fig. 3F).
DISCUSSION
Given that severe hypoglycemia affects 40% of insulin-treated people with diabetes (26), concern regarding the hazardous potential for severe hypoglycemia to cause “brain damage” continues to be a very real barrier for realizing the full benefits of intensive glycemic control (27). Patients with the highest incidence of severe hypoglycemia are most often those who maintain intensive glycemic control and, hence, are likely to have had recurrent bouts of moderate hypoglycemia. In this study, recurrent moderate hypoglycemia preconditioned the brain and protected it against brain damage and cognitive dysfunction induced by severe hypoglycemia.
In these experiments, severe hypoglycemic brain injury was consistently induced with hyperinsulinemic-hypoglycemic (<15 mg/dl) clamps that carefully controlled the depth and duration of severe hypoglycemia and avoided the confounding effects of anesthesia (28–31). The amount and distribution of neuronal damage was markedly different between the 60- and 90-min clamp studies (Figs. 2 and 4). In spite of similar degrees of hypoglycemia (10–15 mg/dl), the extra 30 min of severe hypoglycemia induced a sixfold increase in cortical brain damage and markedly increased hippocampal brain damage (which was minimal in the 60-min clamp). These findings emphasize the importance of the duration of severe hypoglycemia, and not hypoglycemic nadir alone, as a critically important component in determining the extent of brain damage and cognitive dysfunction (22). Of note, the lack of brain-damaged cells in the euglycemic controls indicated that experimental conditions other than severe hypoglycemia (i.e., catheter implantation surgery, recurrent moderate hypoglycemia, hyperinsulinemic clamp, and glucose infusion) did not cause significant brain damage.
The most notable findings were that rats exposed to 3 days of recurrent moderate hypoglycemia had less brain injury associated with severe hypoglycemia in both the cortex and hippocampus. Thus, as with ischemic preconditioning (17), hypoglycemic preconditioning attenuated brain damage by 62–74%. Although hypoglycemia-induced neuronal damage in the hypothalamus has been noted (32), other studies (33) as well as this study observed no severe hypoglycemia-induced neuronal injury in the hypothalamus.
In spite of the marked degree of cortical neuronal damage induced by severe hypoglycemia, the rats had no meaningful deficit in sensorimotor function as measured by the locomotor activity and sensorimotor tests. Further supporting the absence of gross motor deficits following severe hypoglycemia was the observation of no differences between groups in swimming speeds (supplementary Fig. 4). Importantly, rats exposed to severe hypoglycemia showed no signs of sensorimotor impairments that could have affected interpretation of cognitive function as measured during the Morris water maze.
Cognitive assessment with water maze testing documented severe cognitive performance deficits induced by severe hypoglycemia, and these impairments were prevented by antecedent recurrent moderate hypoglycemia. Specifically, analysis of the escape path–length data showed that severe hypoglycemia significantly impaired performance relative to that of euglycemic controls during both the cued and place trials and that recurrent hypoglycemia completely prevented the impaired performance induced by severe hypoglycemia (Fig. 4). For the probe trial, three measures of memory performance were evaluated: platform crossings, spatial bias toward the target quadrant, and average proximity (Fig. 4). Severe hypoglycemia again induced significant memory impairment in all three measures. Antecedent recurrent hypoglycemia prevented these impairments in two of those measures (spatial bias and average proximity). Regarding platform crossings, recurrent hypoglycemia tended to improve performance but not significantly (RH-SH90 vs. CON-SH90 rats), indicating that recurrent hypoglycemia was unable to completely reverse the retention deficits concerning the exact location of the platform. However, analysis of the spatial bias and average proximity data demonstrated that recurrent hypoglycemia did preserve retention of a more general platform location. Specifically, RH-SH90 rats exhibited a spatial bias for the target quadrant, whereas CON-SH90 rats did not, and CON-SH90 rats had an average proximity that was farther away from the platform location than RH-SH90 rats and the euglycemic controls. These findings indicate that memory retention was impaired as a result of severe hypoglycemia relative to euglycemic controls in all probe trial variables and that recurrent hypoglycemia prevented severe hypoglycemia–induced impairments in two of three probe trial indexes.
Consistent with the notion that recurrent hypoglycemia induces an adaptive brain response is the observation that RH-SH90 rats had less seizure-like behavior during severe hypoglycemia (Fig. 3E), suggesting that the RH-treated brain better tolerated severe hypoglycemia. A novel finding of this study is that the number of episodes of seizure-like behavior observed during severe hypoglycemia also correlated with cognitive performance (Fig. 4F). As in the real-world setting, witnessed hypoglycemic seizures were defined clinically. In the absence of electroencephalogram monitoring, the effect of subclinical seizures (i.e., seizures not associated with noticeable motor activity) on brain damage and cognition could not be assessed. Nonetheless, in these experimental conditions, observable instances of seizure-like behavior correlated with the extent of neuronal damage and long-term cognitive function, and while not causative, the number of seizures during hypoglycemia was a marker for the extent of neuronal injury and was prognostic of long-term cognitive outcomes. Indeed, clinical studies support these findings because the presence of hypoglycemic seizures, even more than severe hypoglycemia per se, correlate more closely with impaired cognitive function (10,12).
Independent of episodes of severe hypoglycemia, previous studies have shown that recurrent moderate hypoglycemia can alter cognitive function. Recurrent moderate hypoglycemia did not cause neuronal damage in the hippocampus (as confirmed in this study) but has been shown to impair hippocampal long-term potentiation, a cellular mechanism believed to be involved in learning and memory (34). Conversely, recurrent hypoglycemia improved cognitive ability in rats tested in an euglycemic state (35,36). In the current study, recurrent moderate hypoglycemia-treated control rats not exposed to severe hypoglycemia did not have impaired or improved cognitive ability during Morris water maze testing. Since 2–3 weeks of scrupulous avoidance of hypoglycemia reverses the hypoglycemia unawareness associated with recurrent hypoglycemia (37,38), it is presumed that any effect of antecedent recurrent hypoglycemia on cognition may have dissipated during the 6–8 weeks' recovery prior to cognitive testing.
Although recurrent moderate hypoglycemia leads to maladaptive responses resulting in hypoglycemia unawareness and hypoglycemia-associated autonomic failure, the mechanism(s) by which recurrent hypoglycemia leads to these adaptations remains elusive. Similarly, the current experiments do not identify the mechanisms by which recurrent moderate hypoglycemia 1) protected against severe hypoglycemia–induced neuronal damage, 2) limited severe hypoglycemia–induced neurocognitive dysfunction, or 3) increased thresholds for hypoglycemic seizures. Putative mechanisms for these beneficial adaptations could include glycogen supercompensation (increased brain glycogen content above prehypoglycemic levels) (39–43). By keeping a higher level of stored fuel units, increased brain glycogen content has been shown to reduce hypoglycemic neuronal injury by maintaining brain electrical activity and forestalling electroencephalogram isoelectricity (44). Enhanced nutrient transport may also contribute to the neuroprotective effects of recurrent hypoglycemia (45,46). Monocarboxylate acid transport is increased during hypoglycemia in patients with well-controlled type 1 diabetes (45,46). Increased transport of monocarboxylate acids (e.g., lactate) could provide an alternative energy source that maintains neuronal function (4). Other possibilities that could account for the observed neuroprotective effect of recurrent hypoglycemia could be altered brain metabolism or neuronal activity (39,47–49). Recurrent hypoglycemia enhances the inhibitory neurotransmitter, γ-aminobutyric acid, which could reduce neuronal activity and limit excitotoxic damage (48). Further studies on the precise mechanisms of how recurrent hypoglycemia exerts its neuroprotective effects are warranted.
These studies demonstrate that recurrent moderate hypoglycemia preconditions and protects the brain against severe hypoglycemia–induced neuronal damage and its associated cognitive deficits. These intriguing findings suggest that recurrent bouts of moderate hypoglycemia that occur with intensive glycemic control might, paradoxically, render an individual more prone but less vulnerable to an episode of severe hypoglycemia. If the current data indicating a neuroprotective preconditioning effect of recurrent moderate hypoglycemia were to be extrapolated to the clinical setting, it could explain the apparent divergent findings between animal and clinical studies and may also explain the seemingly incongruous clinical findings that intensively treated patients who experience recurrent moderate and severe hypoglycemia may be paradoxically protected from severe hypoglycemia–induced brain damage and may not suffer from associated long-term cognitive damage (13,50).
Supplementary Material
ACKNOWLEDGMENTS
Research support from the National Institutes of Health (DK073683) and the Juvenile Diabetes Research Foundation (CDA 2-2004-541) and core grant support from Washington University's Diabetes Research and Training Center (DK020579), Clinical Nutrition Research Unit (DK056341), and Neuroscience Blueprint Center (NS057105) are gratefully acknowledged.
No potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in abstract form at the 68th Scientific Sessions of the American Diabetes Association, San Francisco, California, 6–10 June 2008.
The authors thank the laboratory of Dr. P. Cryer for performing the catecholamine determinations and Dr. K. Yamada for assistance with Fluoro-Jade staining.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Cryer PE: Diverse causes of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med 2004; 350: 2272– 2279 [DOI] [PubMed] [Google Scholar]
- 2.The Diabetes Control and Complications Trial Research Group Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes 1997; 46: 271– 286 [PubMed] [Google Scholar]
- 3.Auer RN: Hypoglycemic brain damage. Metab Brain Dis 2004; 19: 169– 175 [DOI] [PubMed] [Google Scholar]
- 4.Suh SW, Aoyama K, Matsumori Y, Liu J, Swanson RA: Pyruvate administered after severe hypoglycemia reduces neuronal death and cognitive impairment. Diabetes 2005; 54: 1452– 1458 [DOI] [PubMed] [Google Scholar]
- 5.Northam EA, Rankins D, Lin A, Wellard RM, Pell GS, Finch SJ, Werther GA, Cameron FJ: Central nervous system function in youth with type 1 diabetes 12 years after disease onset. Diabetes Care 2009; 32: 445– 450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perantie DC, Wu J, Koller JM, Lim A, Warren SL, Black KJ, Sadler M, White NH, Hershey T: Regional brain volume differences associated with hyperglycemia and severe hypoglycemia in youth with type 1 diabetes. Diabetes Care 2007; 30: 2331– 2337 [DOI] [PubMed] [Google Scholar]
- 7.Musen G, Lyoo IK, Sparks CR, Weinger K, Hwang J, Ryan CM, Jimerson DC, Hennen J, Renshaw PF, Jacobson AM: Effects of type 1 diabetes on gray matter density as measured by voxel-based morphometry. Diabetes 2006; 55: 326– 333 [DOI] [PubMed] [Google Scholar]
- 8.Bjorgaas M, Gimse R, Vik T, Sand T: Cognitive function in type 1 diabetic children with and without episodes of severe hypoglycaemia. Acta Paediatr 1997; 86: 148– 153 [DOI] [PubMed] [Google Scholar]
- 9.Hershey T, Lillie R, Sadler M, White NH: Severe hypoglycemia and long-term spatial memory in children with type 1 diabetes mellitus: a retrospective study. J Int Neuropsychol Soc 2003; 9: 740– 750 [DOI] [PubMed] [Google Scholar]
- 10.Kaufman FR, Epport K, Engilman R, Halvorson M: Neurocognitive functioning in children diagnosed with diabetes before age 10 years. J Diabetes Complications 1999; 13: 31– 38 [DOI] [PubMed] [Google Scholar]
- 11.Langan SJ, Deary IJ, Hepburn DA, Frier BM: Cumulative cognitive impairment following recurrent severe hypoglycaemia in adult patients with insulin-treated diabetes mellitus. Diabetologia 1991; 34: 337– 344 [DOI] [PubMed] [Google Scholar]
- 12.Rovet JF, Ehrlich RM: The effect of hypoglycemic seizures on cognitive function in children with diabetes: a 7-year prospective study. J Pediatr 1999; 134: 503– 506 [DOI] [PubMed] [Google Scholar]
- 13.Jacobson AM, Musen G, Ryan CM, Silvers N, Cleary P, Waberski B, Burwood A, Weinger K, Bayless M, Dahms W, Harth J: Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med 2007; 356: 1842– 1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kramer L, Fasching P, Madl C, Schneider B, Damjancic P, Waldhausl W, Irsigler K, Grimm G: Previous episodes of hypoglycemic coma are not associated with permanent cognitive brain dysfunction in IDDM patients on intensive insulin treatment. Diabetes 1998; 47: 1909– 1914 [DOI] [PubMed] [Google Scholar]
- 15.Strudwick SK, Carne C, Gardiner J, Foster JK, Davis EA, Jones TW: Cognitive functioning in children with early onset type 1 diabetes and severe hypoglycemia. J Pediatr 2005; 147: 680– 685 [DOI] [PubMed] [Google Scholar]
- 16.Wysocki T, Harris MA, Mauras N, Fox L, Taylor A, Jackson SC, White NH: Absence of adverse effects of severe hypoglycemia on cognitive function in school-aged children with diabetes over 18 months. Diabetes Care 2003; 26: 1100– 1105 [DOI] [PubMed] [Google Scholar]
- 17.Gidday JM: Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci 2006; 7: 437– 448 [DOI] [PubMed] [Google Scholar]
- 18.Bree AJ, Puente EC, Daphna-Iken D, Fisher SJ: Diabetes increases brain damage caused by severe hypoglycemia. Am J Physiol Endocrinol Metab 2009; 297: E194– E201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah SD, Clutter WE, Cryer PE: External and internal standards in the single-isotope derivative (radioenzymatic) measurement of plasma norepinephrine and epinephrine. J Lab Clin Med 1985; 106: 624– 629 [PubMed] [Google Scholar]
- 20.Del Campo M, Abdelmalik PA, Wu CP, Carlen PL, Zhang L: Seizure-like activity in the hypoglycemic rat: lack of correlation with the electroencephalogram of free-moving animals. Epilepsy Res 2009; 83: 243– 248 [DOI] [PubMed] [Google Scholar]
- 21.Schmued LC, Hopkins KJ: Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res 2000; 874: 123– 130 [DOI] [PubMed] [Google Scholar]
- 22.Suh SW, Aoyama K, Chen Y, Garnier P, Matsumori Y, Gum E, Liu J, Swanson RA: Hypoglycemic neuronal death and cognitive impairment are prevented by poly(ADP-ribose) polymerase inhibitors administered after hypoglycemia. J Neurosci 2003; 23: 10681– 10690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suh SW, Gum ET, Hamby AM, Chan PH, Swanson RA: Hypoglycemic neuronal death is triggered by glucose reperfusion and activation of neuronal NADPH oxidase. J Clin Invest 2007; 117: 910– 918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corbett D, Nurse S: The problem of assessing effective neuroprotection in experimental cerebral ischemia. Prog Neurobiol 1998; 54: 531– 548 [DOI] [PubMed] [Google Scholar]
- 25.Wong M, Wozniak DF, Yamada KA: An animal model of generalized nonconvulsive status epilepticus: immediate characteristics and long-term effects. Exp Neurol 2003; 183: 87– 99 [DOI] [PubMed] [Google Scholar]
- 26.ter Braak EW, Appelman AM, van de LM, Stolk RP, van Haeften TW, Erkelens DW: Clinical characteristics of type 1 diabetic patients with and without severe hypoglycemia. Diabetes Care 2000; 23: 1467– 1471 [DOI] [PubMed] [Google Scholar]
- 27.Cox DJ, Irvine A, Gonder-Frederick L, Nowacek G, Butterfield J: Fear of hypoglycemia: quantification, validation, and utilization. Diabetes Care 1987; 10: 617– 621 [DOI] [PubMed] [Google Scholar]
- 28.Alkire MT, Pomfrett CJ, Haier RJ, Gianzero MV, Chan CM, Jacobsen BP, Fallon JH: Functional brain imaging during anesthesia in humans: effects of halothane on global and regional cerebral glucose metabolism. Anesthesiology 1999; 90: 701– 709 [DOI] [PubMed] [Google Scholar]
- 29.Canabal DD, Potian JG, Duran RG, McArdle JJ, Routh VH: Hyperglycemia impairs glucose and insulin regulation of nitric oxide production in glucose-inhibited neurons in the ventromedial hypothalamus. Am J Physiol Regul Integr Comp Physiol 2007; 293: R592– R600 [DOI] [PubMed] [Google Scholar]
- 30.Jeong YB, Kim JS, Jeong SM, Park JW, Choi IC: Comparison of the effects of sevoflurane and propofol anaesthesia on regional cerebral glucose metabolism in humans using positron emission tomography. J Int Med Res 2006; 34: 374– 384 [DOI] [PubMed] [Google Scholar]
- 31.Nakao Y, Itoh Y, Kuang TY, Cook M, Jehle J, Sokoloff L: Effects of anesthesia on functional activation of cerebral blood flow and metabolism. Proc Natl Acad Sci U S A 2001; 98: 7593– 7598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tkacs NC, Pan Y, Raghupathi R, Dunn-Meynell AA, Levin BE: Cortical Fluoro-Jade staining and blunted adrenomedullary response to hypoglycemia after noncoma hypoglycemia in rats. J Cereb Blood Flow Metab 2005; 25: 1645– 1655 [DOI] [PubMed] [Google Scholar]
- 33.Tkacs NC, Dunn-Meynell AA, Levin BE: Presumed apoptosis and reduced arcuate nucleus neuropeptide Y and pro-opiomelanocortin mRNA in non-coma hypoglycemia. Diabetes 2000; 49: 820– 826 [DOI] [PubMed] [Google Scholar]
- 34.Yamada KA, Rensing N, Izumi Y, De Erausquin GA, Gazit V, Dorsey DA, Herrera DG: Repetitive hypoglycemia in young rats impairs hippocampal long-term potentiation. Pediatr Res 2004; 55: 372– 379 [DOI] [PubMed] [Google Scholar]
- 35.McNay EC, Sherwin RS: Effect of recurrent hypoglycemia on spatial cognition and cognitive metabolism in normal and diabetic rats. Diabetes 2004; 53: 418– 425 [DOI] [PubMed] [Google Scholar]
- 36.McNay EC, Williamson A, McCrimmon RJ, Sherwin RS: Cognitive and neural hippocampal effects of long-term moderate recurrent hypoglycemia. Diabetes 2006; 55: 1088– 1095 [DOI] [PubMed] [Google Scholar]
- 37.Dagogo-Jack S, Rattarasarn C, Cryer PE: Reversal of hypoglycemia unawareness, but not defective glucose counterregulation, in IDDM. Diabetes 1994; 43: 1426– 1434 [DOI] [PubMed] [Google Scholar]
- 38.Fanelli CG, Epifano L, Rambotti AM, Pampanelli S, Di Vincenzo A, Modarelli F, Lepore M, Annibale B, Ciofetta M, Bottini P: Meticulous prevention of hypoglycemia normalizes the glycemic thresholds and magnitude of most of neuroendocrine responses to, symptoms of, and cognitive function during hypoglycemia in intensively treated patients with short-term IDDM. Diabetes 1993; 42: 1683– 1689 [DOI] [PubMed] [Google Scholar]
- 39.Alquier T, Kawashima J, Tsuji Y, Kahn BB: Role of hypothalamic adenosine 5′-monophosphate-activated protein kinase in the impaired counterregulatory response induced by repetitive neuroglucopenia. Endocrinology 2007; 148: 1367– 1375 [DOI] [PubMed] [Google Scholar]
- 40.Brown AM, Sickmann HM, Fosgerau K, Lund TM, Schousboe A, Waagepetersen HS, Ransom BR: Astrocyte glycogen metabolism is required for neural activity during aglycemia or intense stimulation in mouse white matter. J Neurosci Res 2005; 79: 74– 80 [DOI] [PubMed] [Google Scholar]
- 41.Brucklacher RM, Vannucci RC, Vannucci SJ: Hypoxic preconditioning increases brain glycogen and delays energy depletion from hypoxia-ischemia in the immature rat. Dev Neurosci 2002; 24: 411– 417 [DOI] [PubMed] [Google Scholar]
- 42.Choi IY, Seaquist ER, Gruetter R: Effect of hypoglycemia on brain glycogen metabolism in vivo. J Neurosci Res 2003; 72: 25– 32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wender R, Brown AM, Fern R, Swanson RA, Farrell K, Ransom BR: Astrocytic glycogen influences axon function and survival during glucose deprivation in central white matter. J Neurosci 2000; 20: 6804– 6810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suh SW, Hamby AM, Swanson RA: Hypoglycemia, brain energetics, and hypoglycemic neuronal death. Glia 2007; 55: 1280– 1286 [DOI] [PubMed] [Google Scholar]
- 45.Boyle PJ, Kempers SF, O'Connor AM, Nagy RJ: Brain glucose uptake and unawareness of hypoglycemia in patients with insulin-dependent diabetes mellitus. N Engl J Med 1995; 333: 1726– 1731 [DOI] [PubMed] [Google Scholar]
- 46.Mason GF, Petersen KF, Levon V, Rothman DL, Shulman GI: Increased brain monocarboxylic acid transport and utilization in type 1 diabetes. Diabetes 2006; 55: 929– 934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chan O, Lawson M, Zhu W, Beverly JL, Sherwin RS: ATP-sensitive K(+) channels regulate the release of GABA in the ventromedial hypothalamus during hypoglycemia. Diabetes 2007; 56: 1120– 1126 [DOI] [PubMed] [Google Scholar]
- 48.Chan O, Cheng H, Herzog R, Czyzyk D, Zhu W, Wang A, McCrimmon RJ, Seashore MR, Sherwin RS: Increased GABAergic tone in the ventromedial hypothalamus contributes to suppression of counterregulatory responses after antecedent hypoglycemia. Diabetes 2008; 57: 1363– 1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dunn-Meynell AA, Routh VH, Kang L, Gaspers L, Levin BE: Glucokinase is the likely mediator of glucosensing in both glucose-excited and glucose-inhibited central neurons. Diabetes 2002; 51: 2056– 2065 [DOI] [PubMed] [Google Scholar]
- 50.Amiel SA: Hypoglycaemia in diabetes mellitus–protecting the brain. Diabetologia 1997; 40( Suppl. 2): S62– S68 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.