Abstract
OBJECTIVE
The prostacyclin analog, beraprost sodium (BPS), was examined for its potential to improve the symptoms of obesity-type diabetes (i.e., hyperglycemia, hyperinsulinemia, dyslipidemia, histopathologic changes, and diabetic complications).
RESEARCH DESIGN AND METHODS
Obese Zucker rats, an experimental model of genetic obesity-induced type 2 diabetes, were repeatedly administered BPS at oral doses of 0.2 or 0.6 mg · kg−1 · day−1 b.i.d. for 12 weeks, and serum chemistry, urinalysis, and histopathologic examination were performed.
RESULTS
BPS dose-dependently suppressed serum glucose, insulin, triglyceride, and cholesterol levels in obese animals. In oral glucose tolerance test, BPS suppressed the post–glucose-loading elevation of serum glucose in a dose-dependent manner. Urinary N-acetyl-β-D-glucosaminidase was significantly lower in BPS-treated obese animals compared with control animals, although no significant differences were observed in urinary protein levels between the BPS-treated groups and the control group. In addition, histopathologic examination revealed significant protective effects of BPS against renal disorder in obese animals. Histopathologically, BPS also inhibited the progression of hepatic steatosis, hypertrophy of adipose tissue, and pancreatic fibrosis. Furthermore, thermographic analysis of the hind limb sole skin surface indicated a significant increase in temperature in BPS-treated animals, compared with control animals, which was likely due to improved blood circulation by administration of BPS.
CONCLUSIONS
BPS suppressed the pathogenesis and development of diabetes and its complication, nephropathy, which was presumably accompanied by improving glucose intolerance and insulin resistance in obese Zucker rats.
Diabetes has become a major health concern worldwide. In fact, the number of patients with diabetes is still increasing and currently accounts for at least 5% of the adults in the world. Among them, ∼30% of diabetic patients develop nephropathy. The mechanisms underlying the pathogenesis of diabetes and subsequent nephropathy are extremely complex and have not yet been fully elucidated. Glucose intolerance and insulin resistance, however, commonly occur and are often accompanied by hyperglycemia and hyperinsulinemia in diabetic patients, especially in those with obesity-induced type 2 diabetes. These abnormalities are also regarded as risk factors leading to macrovascular and microvascular complications, such as myocardial infarction, nephropathy, retinopathy, and neuropathy (1).
Skeletal muscle, liver, and adipose tissues are insulin-responsive organs that are considered to be closely associated with glucose intolerance and insulin resistance. Among them, skeletal muscle glucose disposal is a primary defect that leads to the development of glucose intolerance and type 2 diabetes (2,3). In addition, glucose imbalance in diabetic patients is presumed to be closely associated with poor secretion of the endogenous vasodilator, NO, from endothelial cells and the resultant reduction of blood flow (4). This reduction in blood flow presumably triggers further resistance of the skeletal muscle to glucose disposal. It is well known that variety of activity and distribution were observed in NO synthase (NOS) according to its subtypes (5–8). Because there were contradictory reports for involvement of subtypes such as inducible NOS to diabetic condition in obese Zucker rats (9,10), the role of NOS subtypes was still unclear. However, in the case of endothelial NOS (eNOS), decrease in its activity would be closely related to the exacerbation of diabetic conditions (6).
Beraprost sodium (BPS), the stable prostacyclin analog, has potent vasodilating activity and activation effect of eNOS expression in the endothelium (11,12). Based on the vasodilating activity together with protection of the endothelium, anti-inflammatory antiplatelet activities, and increasing of eNOS production, BPS improves regional blood flow (13,14). Accordingly, BPS is expected to improve insulin resistance, at least in part, by improving blood flow in muscle and consequently to ameliorate other pathogenesis seen in type 2 diabetes. However, the potential of BPS against these pathologic conditions remains to be elucidated.
Obese Zucker rats are widely used as a useful experimental model for genetic obesity-induced type 2 diabetes because development of the disease shares many features with that in humans. Namely, these rats are obese and develop progressive insulin resistance, glucose intolerance, hyperinsulinemia, and hyperlipidemia (15–17). Therefore, to verify the hypothesis that BPS can improve insulin resistance and ameliorate the symptoms and complications of type 2 diabetes, we have used Zucker rats as an animal model.
The present report describes the changes in some serum chemistry parameters related to glucose and lipid metabolism and some urinary biomarkers, indicative of renal function, in male obese Zucker rats treated with repeated oral BPS. Histopathologic examination, focusing on changes in the pancreas, liver, adipose tissue, and kidney, and blood pressure and thermographic analyses of changes in blood flow are also described.
RESEARCH DESIGN AND METHODS
Animals.
Male obese and lean Zucker rats (ZUC-Leprfa/Leprfa rats) were purchased from Charles River Japan (Tokyo, Japan). Animals were housed in an animal room where the light cycle and temperature were maintained from 0700 to 1900, and 23 ± 2°C, respectively. Animals were allowed free access to drinking water and standard rat chow (MF; Oriental Yeast, Tokyo, Japan). All experiments were performed in compliance with the ethics standards for animal studies in Toray Industries.
Drug treatment.
BPS was administrated orally to obese Zucker rats at 0.2 or 0.6 mg · kg−1 · day−1 b.i.d. from 7 to 19 weeks of age. Animals in the control group were administered distilled water as a vehicle control, in place of BPS. For each animal, body weight was measured at the beginning of each week throughout the administration period. The body weight data were recorded as a parameter for monitoring the general condition of the animal and were used to determine the dosing volume administered to the animal each week.
Food consumption.
Food consumption was measured at the time of urinalysis.
Blood.
Blood was collected from animals once a week during the administration period via the tail artery under ether anesthesia. Serum fractions were obtained by centrifugation and used for the analysis of the following parameters with the respective enzyme assay kits: glucose (N-assay GLU-UL Nittobo), triglyceride (TG) (N-assay L TG-H Nittobo), and total cholesterol (Tcho) (N-assay L T-CHO-H Nittobo; all from Nittobo Medical, Tokyo, Japan). Insulin was analyzed using a commercially available ELISA kit (Rat Insulin ELISA; Mercodia, Uppsala, Sweden).
A1C was analyzed at 19 weeks of age using a commercially available kit (NycoCard HbA1c; AXIS-SHIELD PoC AS, Kimbolton, U.K.). The value below the lower limit of quantification (<3.0%) was treated as zero.
Urine.
At 7, 13, and 17 weeks of age, animals were housed individually in metabolic cages, and urine was collected in metabolic sampling bottles for 24 h. Urine was analyzed for the following parameters using commercially available enzyme assay kits: total protein (Micro TP Test Kit; Wako, Osaka, Japan) and N-acetyl-β-D-glucosaminidase (NAG) (NAG; N-assay L NAG Nittobo; Nittobo Medical).
OGTT.
At 18 weeks of age, after receiving the twice-daily administration of BPS for 11 weeks, animals were subjected to an oral glucose tolerance test (OGTT). Animals were administered the second daily dose and then fasted overnight (16 h). Animals were administered 2 g/kg of glucose, and blood samples were collected via the tail vein at 0, 0.5, 1, 2, and 4 h after the oral glucose load. The blood samples were analyzed for serum glucose and insulin, as described above.
Blood pressure.
Systolic blood pressure and heart rate were measured in conscious rats at 12 weeks of age using an automated noninvasive sphygmomanometer (BP-98A; Softron, Tokyo, Japan) equipped with a tail-cuff sensor.
Skin temperature.
At 19 weeks of age, after receiving the twice-a-day administration of BPS for 12 weeks, the skin temperature of the animals was assessed using a thermograph (Neo Thermo TVS-700; NIPPON AVIONICS, Tokyo, Japan). For each animal, the thermal image of the left hind leg was recorded at 0 and 2 h after the first administration of the day. The image was analyzed for temperature at about 4,000 points in the area corresponding to each hind leg, and the mean temperature was calculated using the software PE professional (NIPPON AVIONICS).
Processing tissues for histopathologic evaluation.
At the end of the administration period, animals were exsanguinated via the abdominal aorta under ether anesthesia. The kidney, pancreas, liver, and adipose tissue were removed from each animal, fixed in 10% neutral buffered formalin, and embedded in paraffin. Thin sections were prepared from the paraffin blocks and stained with hematoxylin-eosin (H-E) and periodic acid Schiff.
For the quantification of areas of lipid accumulation in the liver, H-E–stained images of the lean, control, and BPS groups were imported into a computer for analysis. In each liver, five high-power fields were randomly selected to extract porosity areas and the rate of lipid accumulation area was calculated in each section by an image analysis system (MacSCOPE; Mitani, Tokyo, Japan).
Glomerular lesions and tubulointerstitial fibrosis were evaluated semiquantitatively. In brief, glomerular lesions were graded according to the severity of lesions for each glomerulus, from 0 to 4+ as follows; 0: no lesion, 1+: expansion of the mesangial area was observed, but there was no glomerulosclerosis, 2+: sclerosis of less than 50%, 3+: 50–75% sclerosis, 4+: more than 75% of glomerular tuft. Tubulointerstitial fibrosis was graded according to the area of injury in 20 randomly selected high-power fields (×40) and were graded according to the area of alteration from 0 to 5+ as follows: 0: no lesion, 1+: <10%, 2+: 10–25%, 3+: 25–50%, 4+: 50–75%, 5+: >75% in each left kidney.
Statistical analysis.
Body weight, food consumption, serum and urine chemistry, blood pressure, heart rate, skin temperature, and histopathologic data were expressed as the mean ± SE. Statistical significance between BPS-treated group and control group was assessed using a two-way ANOVA, followed by parametric Williams test (body weight, food consumption, systolic blood pressure, heart rate, skin temperature, and hepatic steatosis) and nonparametric Williams test (serum and urinary parameter, and renal injury); and lean group and control obese group, by t test (body weight, food consumption, systolic blood pressure, heart rate, sole temperature, and hepatic steatosis) and Welch test (serum and urinary parameter, and renal injury). The probability value of P < 0.05 was considered statistically significant.
RESULTS
Body weight and food consumption.
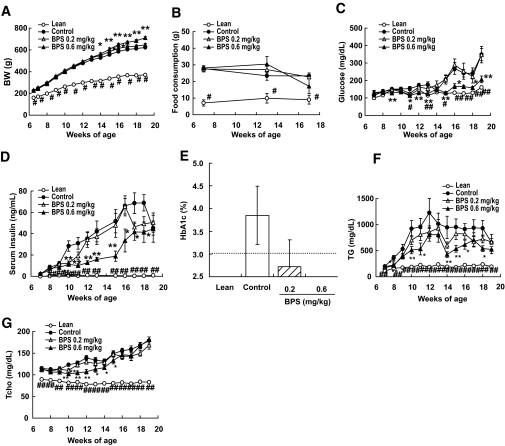
The body weight was significantly greater in obese Zucker rats in the control group than in that for lean rats throughout the administration period. In comparison, among the groups of obese rats, the body weight was significantly greater in the 0.6-mg/kg BPS group than in the control group from 14 weeks of age to the end of the administration period (Fig. 1A). In the 0.2-mg/kg BPS group, the body weight was only higher than the control group from 16 to 17 weeks of age. Food consumption was significantly high in obese Zucker rats in the control group than in that for lean rats, but there was no difference between control rats and BPS-treated rats (Fig. 1B).
FIG. 1.
Body weight (BW) (A), food consumption (B), serum glucose (C), insulin (D), A1C (E), TG (F), and total cholesterol (G) in control obese Zucker rats (●), obese Zucker rats treated with BPS (▵, 0.2 mg · kg−1 · day−1; ▴, 0.6), and Zucker lean rats (○) as line graph, and control obese Zucker rats (□), Zucker rats treated with BPS (▨, 0.2 mg · kg−1 · day−1; ■, 0.6), and Zucker lean rats ( ) as bar graph. Data are mean ± SE of 7–8 rats. *P < 0.05, **P < 0.01 versus control rats by parametric Williams test (body weight, food consumption) and nonparametric Williams test (glucose, insulin, TG, and total cholesterol). #P < 0.05, ##P < 0.01 versus control by t test (body weight, food consumption) and Welch test (glucose, insulin, TG, and total cholesterol).
) as bar graph. Data are mean ± SE of 7–8 rats. *P < 0.05, **P < 0.01 versus control rats by parametric Williams test (body weight, food consumption) and nonparametric Williams test (glucose, insulin, TG, and total cholesterol). #P < 0.05, ##P < 0.01 versus control by t test (body weight, food consumption) and Welch test (glucose, insulin, TG, and total cholesterol).
Hematologic examination.
Serum glucose was significantly higher in obese Zucker rats than in lean rats from 11 weeks of age until the end of the administration period. During this period, obese Zucker rats treated with 0.6 mg/kg BPS exhibited significantly lower serum glucose levels than the control Zucker rats (Fig. 1C). Serum insulin was markedly elevated in control untreated obese Zucker rats from 8 weeks of age, compared with lean rats. No such elevation was observed in obese Zucker rats in the 0.6-mg/kg BPS group throughout the administration period. Indeed, insulin levels were significantly lower in the 0.6-mg/kg BPS group compared with the control group (Fig. 1D). A1C tended to be dose dependently lowered by BPS treatment (Fig. 1E).
Regarding the parameters related to lipid metabolism, serum TGs were significantly higher in control untreated obese Zucker rats compared with lean animals throughout the administration period. Serum TG levels, however, were significantly lower in the 0.6-mg/kg BPS group compared with the control group (Fig. 1F). Similarly, serum Tcho was significantly higher in control Zucker rats than in lean animals throughout the administration period. Significantly lower Tcho levels were observed in the 0.6-mg/kg BPS group, compared with those in the control group, from 10 to 15 weeks of age, but not for other ages (Fig. 1G).
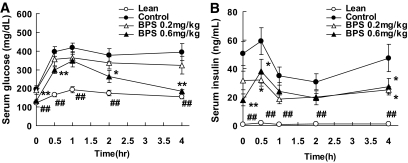
OGTT.
After the glucose loading, control untreated obese Zucker rats showed rapid and remarkable elevation of serum glucose, whereas serum glucose elevation was relatively slow and brief in lean rats (Fig. 2A). After reaching a plateau 0.5–1 h after loading, serum glucose was maintained at an essentially constant level in either control untreated obese or lean animals until 4 h after loading. Accordingly, serum glucose levels were significantly higher in control untreated obese animals than in lean rats. The 0.6-mg/kg BPS group, however, showed a less rapid and less remarkable serum glucose elevation, compared with the control group.
FIG. 2.
Time course changes in the level of serum glucose and insulin during OGTT in control obese Zucker rats (●), obese Zucker rats treated with BPS (▵, 0.2 mg · kg−1 · day−1; ▴, 0.6), and Zucker lean rats (○). Data are mean ± SE of 7–8 rats. *P < 0.05, **P < 0.01 versus control rats by nonparametric Williams test. ##P < 0.01 versus control by Welch test.
Serum insulin was remarkably higher in control untreated obese rats than in lean rats, even at the baseline, and throughout the 4-h postloading observation period (Fig. 2B). Compared with the control group, the 0.6-mg/kg BPS group showed significantly lower insulin levels at 0, 0.5, and 4 h after loading.
Urinalysis.
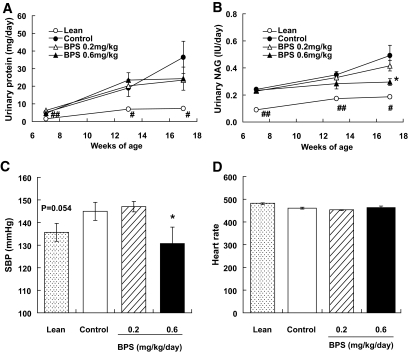
As for urinary protein, a remarkable elevation was observed in control untreated obese Zucker rats, whereas lean animals exhibited only a slight elevation (Fig. 3A). Thus, urinary protein was significantly higher than in lean rats from 7 weeks of age until the end of the administration period. BPS apparently suppressed the elevation of urinary protein level observed in obese Zucker rats during the administration period, although these values were not significantly different from the control group.
FIG. 3.
Urinary protein (A), NAG (B), systolic blood pressure (SBP) (C), and heart rate (D) in control obese Zucker rats (●), obese Zucker rats treated with BPS (▵, 0.2 mg · kg−1 · day−1; ▴, 0.6), and Zucker lean rats (○) as line graph, and control obese Zucker rats (□), Zucker rats treated with BPS (▨, 0.2 mg · kg−1 · day−1; ■, 0.6), and Zucker lean rats ( ) as bar graph. Data are mean ± SE of 7–8 rats. *P < 0.05 versus control rats by nonparametric (NAG) and parametric (systolic blood pressure) Williams test. #P < 0.05, ##P < 0.01 versus control by Welch test.
) as bar graph. Data are mean ± SE of 7–8 rats. *P < 0.05 versus control rats by nonparametric (NAG) and parametric (systolic blood pressure) Williams test. #P < 0.05, ##P < 0.01 versus control by Welch test.
As for urinary NAG, gradual elevations were observed in control untreated obese Zucker rats as well as in lean rats, although the levels were significantly higher in the former than in the latter group (Fig. 3B). BPS suppressed the elevation observed in obese Zucker rats, and a significant difference from the control group was detected in the 0.6-mg/kg BPS group at 17 weeks of age.
Serum creatinine level was not elevated in obese Zucker rats and there were no differences between each group (data not shown).
Blood pressure.
The systolic blood pressure of obese Zucker rats was higher than that of lean rats (P = 0.054) at 12 weeks of age. Although BPS inhibited the elevation of blood pressure (Fig. 3C), the heart rate was not different in each group (Fig. 3D).
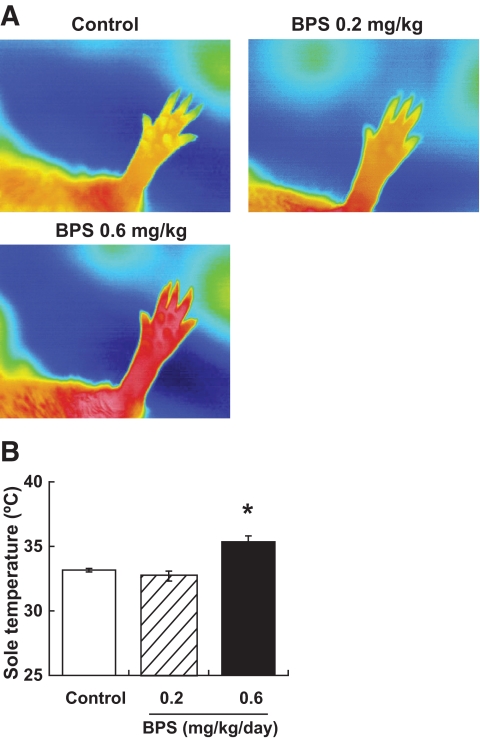
Skin temperature.
Immediately before the daily administration, thermographic image analysis revealed that there was no apparent change in the temperature in the area corresponding to the left hind leg among BPS-treated obese Zucker rats, control untreated obese Zucker rats, and lean rats (data not shown). At 2 h after administration, the skin temperature in the hind leg area was significantly higher in the 0.6-mg/kg BPS-treated group than in the control group (Fig. 4A and B).
FIG. 4.
The level of sole temperature after administration. A: Representative thermal images of each groups at 2 h after administration. B: Sole temperature at 2 h after administration in control obese Zucker rats (□) and obese Zucker rats treated with BPS (▨, 0.2 mg · kg−1 · day−1; ■, 0.6). Data are mean ± SE of four rats. *P < 0.05, versus control rats by parametric Williams test. (A high-quality digital representation of this figure is available in the online issue.)
Morphology.
Compared with the pancreatic islets in lean animals, control untreated obese Zucker rats exhibited obviously severe hypertrophic, lobed, and fibrotic changes. In obese rats treated repeatedly with BPS for 12 weeks, these changes were less extensive (Fig. 5).
FIG. 5.
Photomicrographs of H-E staining of the pancreas in control obese Zucker rats, obese Zucker rats treated with high-dose of BPS, and Zucker lean rat. (A high-quality digital representation of this figure is available in the online issue.)
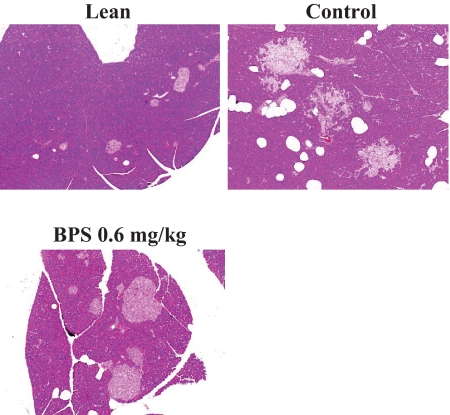
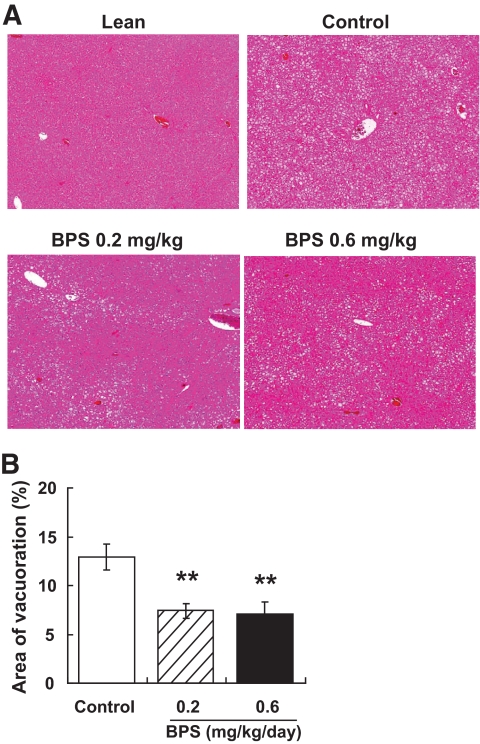
Severe hepatic steatosis, characterized by the accumulation of higher levels of lipid in hepatic intracellular vesicles and ballooning degeneration of hepatocytes, was evident in obese Zucker rats (Fig. 6A). By contrast, BPS treatment markedly reduced lipid accumulation and ballooning degeneration of hepatocytes (Fig. 6A and B).
FIG. 6.
Photomicrographs of H-E staining of the liver in control obese Zucker rats, obese Zucker rats treated with BPS, and Zucker lean rat (A) and quantitative analysis of vesicles in control obese Zucker rats (□) and obese Zucker rats treated with BPS (▨, 0.2 mg · kg−1 · day−1; ■, 0.6) (B). Data are mean ± SE of 7–8 rats. **P < 0.01 versus control by parametric Williams test. (A high-quality digital representation of this figure is available in the online issue.)
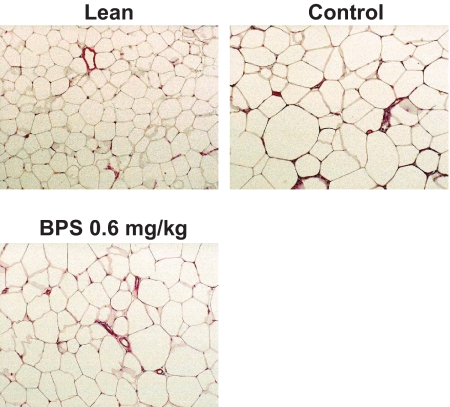
Adipocytes were obviously larger in obese rats compared with lean animals. Adipose tissues from BPS-treated rats, however, exhibited greater populations of much smaller adipocytes (Fig. 7).
FIG. 7.
Photomicrographs of H-E staining of the adipose tissues in control obese Zucker rats, obese Zucker rats treated with high-dose of BPS, and Zucker lean rat. (A high-quality digital representation of this figure is available in the online issue.)
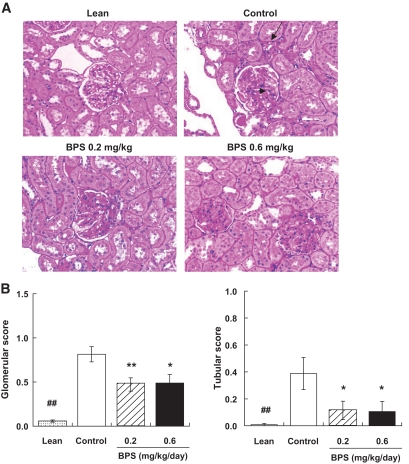
Compared with kidney tissues from lean rats, control untreated obese Zucker rats exhibited focal and segmental glomerulosclerosis and expansion of glomerular matrix (Fig. 8A, arrowhead). Furthermore, tubular damage such as tubular atrophy, interstitial fibrosis, thickening of the tubular basement membranes, and infiltration of inflammatory cells were also observed in obese Zucker rats (Fig. 8A, arrow). These changes were comparatively mild in kidney tissues from obese Zucker rats treated repeatedly with BPS. In fact, semiquantitative histopathologic analysis indicated that both glomerular and tubular abnormality scores were significantly lower in obese Zucker rats treated with BPS at either 0.2 or 0.6 mg/kg compared with in control untreated obese rats (Fig. 8B).
FIG. 8.
Photomicrographs of periodic acid Schiff staining of the kidney in control obese Zucker rats, obese Zucker rats treated with BPS, and Zucker lean rat (A) and semiquantitative analysis of glomerular (B, left) and tubular (B, right) injuries in control obese Zucker rats (□), obese Zucker rats treated with BPS (▨, 0.2 mg · kg−1 · day−1; ■, 0.6), and Zucker lean rats ( ). Data are mean ± SE of 7–8 rats. *P < 0.05, **P < 0.01 versus control by nonparametric Williams test. ##P < 0.01 versus control by Wilcoxon test. (A high-quality digital representation of this figure is available in the online issue.)
). Data are mean ± SE of 7–8 rats. *P < 0.05, **P < 0.01 versus control by nonparametric Williams test. ##P < 0.01 versus control by Wilcoxon test. (A high-quality digital representation of this figure is available in the online issue.)
DISCUSSION
In the present study, we examined the effects of the prostacyclin analog BPS to improve various diabetic markers in obese Zucker rats; an animal model whose pathologic condition resembles that seen in human genetic obesity-induced type 2 diabetes. Specifically, BPS inhibited the increases in blood glucose, insulin, TG, total cholesterol, and blood pressure, and ameliorated the development of glucose intolerance and insulin resistance. Histopathologically, BPS inhibited the progression of hepatic steatosis, hypertrophy of the adipose cells, pancreatic fibrosis, and renal damage. Thus, we revealed that BPS suppressed the pathogenesis and development of diabetes, which was presumably accompanied by improving glucose intolerance and insulin resistance.
Insulin resistance plays a central role in the development of diabetes. The muscles, liver, and adipocytes are deeply involved in the utilization of glucose as insulin-responsive organs, and are, thus, closely related to insulin resistance. The transport of glucose into muscles and the liver is decreased with increasing levels of glucose and lipids in blood (2,18–21). In adipocytes, the secretion of cytokines, such as tumor necrosis factor-α (TNF-α) and monocyte chemoattractant protein 1 (MCP-1), is increased, consistent with enlargement of adipocytes caused by excessive accumulation of adipose and with infiltration of inflammatory cells, such as macrophages. It is known that these changes may decrease insulin signaling (22,23).
When the action of insulin decreased through insulin resistance, abnormal metabolism of glucose and lipid is the result, furthering development of diabetes. Therefore, it is especially important to improve insulin resistance to prevent development of diabetes.
In this study, we demonstrated that BPS inhibited the development of diabetes in obese Zucker rats, probably by improving insulin resistance. Although the mechanisms underlying the findings of this study remain uncertain, we discussed the following hypothesis.
In patients with diabetes, the vasodilating response was decreased by reduced production of NO from the endothelium, and structural and functional disorder of capillary blood vessels and decreasing muscle blood flow was caused (24,25). In Zucker rats, in addition to insulin resistance, microvascular density, blood flow, and responsiveness of insulin-stimulated glucose metabolism in muscles (26,27), together with activity of prostacyclin (PGI2) synthase and eNOS in blood vessels (5), were decreased. In addition, endothelium-dependent relaxation was ameliorated together with enhanced eNOS activity and reduced superoxide anion release in the aorta of obese Zucker rats by polyphenol treatment (6). Based on these observations, it was considered that reduced production of NO by decreased eNOS activity together with microvascular disturbances would be closely related to exacerbated diabetic conditions, especially insulin resistance. Reduced PGI2 also might be involved in induction of insulin resistance, either directly or through lowered NO production.
BPS, a PGI2 derivative, exhibits direct actions on smooth muscle cells to elicit vasodilatation, and increase NO production by facilitating expression of eNOS mRNA in endothelial cells as well (11,12). It is also known that BPS can protect the endothelium from high levels of glucose and other cytotoxic agents (28). In addition, BPS ameliorated the decrease in NO production in the endothelial cells of streptozocin-induced diabetic model rats (29). Therefore, BPS is supposed to improve insulin resistance through its vasodilating activity, protective effects on vascular endothelial cells, and the facilitation of NO production, and, as a result, amelioration of blood flow in muscles. In this study, increased muscle blood flow was confirmed, based on the data regarding skin temperature obtained after BPS administration.
It is also known that diabetes may be improved by increasing fatty acid oxidation and energy consumption in muscles (30), and it was confirmed that insulin resistance was improved by increased thermogenesis and energy consumption in Zucker rats (31). Therefore, it is suggested that BPS improved insulin resistance by increased thermogenesis and energy metabolism, as a consequence of increasing blood flow in muscles.
Adipocytes also play a central role in energy metabolism, and can store energy, especially through the intake of free fatty acids, in an insulin-dependent manner. When the amount of accumulated fat is increased by hyperlipidemia, enlarged adipocytes and infiltrating macrophages produce cytokines, such as MCP-1 and TNF-α, which are considered to be closely related to the deterioration of insulin resistance. In this study, it was clarified that increases in blood TGs and enlargement of adipocytes were inhibited by BPS treatment. It was reported that serum levels of TNF-α and MCP-1 were increased in Zucker rats (32,33), and BPS could exhibit an inhibitory action on the production of MCP-1 and TNF-α in another models (34,35). Moreover, lipolytic activity was increased when production of PGI2 was increased in adipose tissues (36). Based on these facts, it was supposed that BPS improved insulin resistance by reducing the enlargement of adipocytes and production of developmental factors, such as MCP-1 and TNF-α.
In recent years, it has also been reported that BPS can enhance peroxisome proliferator–activated receptor-δ (37). GW501516, peroxisome proliferator–activated receptor-δ agonist, is considered to facilitate fatty acid oxidation in muscles, and improve insulin resistance and obesity (30). Thus, this action by BPS may be involved in the improvement of insulin resistance in Zucker rats.
In histopathologic examination, we clarified that BPS would have effects, not only to improve the functional disturbance of insulin resistance but also to improve tissue disturbance in the pancreas, liver, and kidney, which are characteristic of patients with diabetes. In this model, fibrosis, caused by deterioration of the pancreas when insulin secretion associated with insulin resistance was increased continuously, was improved by the administration of BPS. The possibility exists that both direct protective action of BPS on the pancreas, including the inhibition of vascular endothelial disorders, and indirect inhibitory action on increased insulin by improving insulin resistance may be involved in the improvement of fibrosis.
Regarding the liver, it is known that hepatic steatosis often occurs in patients with obesity-induced type 2 diabetes, and as mentioned above, insulin resistance is deeply involved. In this study, vacuoles, caused by the accumulation of adipose in hepatic cells, were observed in Zucker rats and were improved by BPS. Because BPS inhibits increases of blood glucose and TGs, and improves insulin resistance, these actions are considered to be comprehensively involved in the inhibition of the development of hepatic steatosis. In addition, the direct protective action of BPS on hepatic cells (38) may contribute to improvements in the hepatic steatosis.
Diabetic nephropathy is one of the major complications among patients with diabetes. It is important to inhibit the development of renal disorder for improving the prognosis of diabetic patients. In this study, characteristic morphologic changes such as segmental glomerulosclerosis, expansion of glomerular matrix, tubular damage such as tubular atrophy, interstitial fibrosis, thickening of the tubular basement membranes, and infiltration of inflammatory cells, which were also seen in human diabetic nephropathy, were observed, although nodular glomerulosclerosis was not observed up to 19 weeks of age in this study, and these changes were improved by the administration of BPS. In Zucker rats, decreased NOS (eNOS) expression in the kidney was observed concomitant with renal injury (39), and renal damage was caused when NO production was inhibited by administration of l-NG-nitro-l-arginine methyl ester (40). Thus, it was suggested that decreased production of NO would be involved in the development of renal disorder and there is the possibility that increased NO production upon administration of BPS may inhibit the development of nephropathy. In addition, because it is known that insulin resistance is involved in renal injury in this model (41), the improvement in insulin resistance by BPS also may contribute to the improvement of renal damage. In OLETF rats, another obesity-type diabetes model, renal disorder was also inhibited by BPS. By contrast, because blood glucose did not change prominently at the stage of OLETF rats, effect of BPS against blood glucose was unclear (42). Furthermore, it has been reported that in the renal failure model with no evidence of diabetes, BPS could inhibit the development of renal disorder (35,43). Therefore, there is the another possibility that in addition to the inhibitory effect on development observed in improvement of diabetes, the direct inhibitory action against renal disorder may be somehow involved in the inhibition of renal damage by BPS observed in Zucker rats. BPS maintained blood pressure over time in Zucker rats to the same level as that in lean rats. Because increased blood pressure and renal damage are observed in patients with diabetes (44), and these are correlated with each other (45,46), inhibition of blood pressure by BPS may be related to inhibition of renal disorder development.
Like other antidiabetic agents (47,48), diabetic conditions such as hyperglycemia and hyperlipidemia were ameliorated despite constant increasing of body weight by BPS treatment. Because food consumption was not increased and edema was not observed in BPS-treated rats, we supposed that mild increase in body weight of BPS-treated rats might be the ameliorating effect of glucose utilization by BPS treatment.
As for the involvement of increased NO production and eNOS activity for the beneficial effect of BPS, further investigation would be needed focusing on the local expression of eNOS and other NOS subtypes in the artery, kidney, and other related organs.
Taken together, using Zucker rats, an experimental model of genetic obesity-induced type 2 diabetes, we succeeded in revealing for the first time that BPS, a prostacyclin analog, would improve a variety of markers of obesity-type diabetes and nephropathy, one of diabetic complications, in addition to tissue disturbance in the liver, adipocytes, and pancreas. And it is considered that improvement of insulin resistance by BPS treatment may contribute to ameliorating these pathologic conditions.
ACKNOWLEDGMENTS
No potential conflicts of interest relevant to this article were reported.
We thank Dr. Osamu Hotta (Sendai Shakaihoken Hospital) for helpful discussion and Kenji Shirao for his excellent technical assistance.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1. Reaven GM: Role of insulin resistance in human disease (syndrome X): an expanded definition. Annu Rev Med 1993; 44: 121– 131 [DOI] [PubMed] [Google Scholar]
- 2. Zierath JR, Krook A, Wallberg-Henriksson H: Insulin action and insulin resistance in human skeletal muscle. Diabetologia 2000; 43: 821– 835 [DOI] [PubMed] [Google Scholar]
- 3. Crettaz M, Prentki M, Zaninetti D, Jeanrenaud B: Insulin resistance in soleus muscle from obese Zucker rats: involvement of several defective sites. Biochem J 1980; 186: 525– 534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dandona P, Aljada A: Advances in diabetes for the millennium: diabetes and the endothelium. Med Genet Med 2004; 6( Suppl.): 6. [PMC free article] [PubMed] [Google Scholar]
- 5. Du X, Edelstein D, Obici S, Higham N, Zou MH, Brownlee M: Insulin resistance reduces arterial prostacyclin synthase and eNOS activities by increasing endothelial fatty acid oxidation. J Clin Invest 2006; 116: 1071– 1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Agouni A, Lagrue-Lak-Hal AH, Mostefai HA, Tesse A, Mulder P, Rouet P, Desmoulin F, Heymes C, Martínez MC, Andriantsitohaina R: Red wine polyphenols prevent metabolic and cardiovascular alterations associated with obesity in Zucker fatty rats (Fa/Fa). PLoS One 2009; 4: e5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kuhad A, Sachdeva AK, Chopra K: Attenuation of renoinflammatory cascade in experimental model of diabetic nephropathy by sesamol. J Agric Food Chem 2009; 57: 6123– 6128 [DOI] [PubMed] [Google Scholar]
- 8. Liang JH, Li YN, Qi JS, Jia XX: Peroxynitrite-induced protein nitration is responsible for renal mitochondrial damage in diabetic rat. J Endocrinol Invest. 11 September 2009. [ Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9. Kawaguchi M, Koshimura K, Sohmiya M, Murakami Y, Gonda T, Kato Y: Effect of insulin on nitric oxide synthase-like immunostaining of arteries in various organs in Zucker diabetic fatty rats. Eur J Endocrinol 2001; 145: 343– 349 [DOI] [PubMed] [Google Scholar]
- 10. Song D, Kuo KH, Yao R, Hutchings SR, Pang CC: Inducible nitric oxide synthase depresses cardiac contractile function in Zucker diabetic fatty rats. Eur J Pharmacol 2008; 579: 253– 259 [DOI] [PubMed] [Google Scholar]
- 11. Niwano K, Arai M, Tomaru K, Uchiyama T, Ohyama Y, Kurabayashi M: Transcriptional stimulation of the eNOS gene by the stable prostacyclin analogue beraprost is mediated through cAMP-responsive element in vascular endothelial cells: close link between PGI2 signal and NO pathways. Circ Res 2003; 93: 523– 530 [DOI] [PubMed] [Google Scholar]
- 12. Niwano K, Arai M, Koitabashi N, Hara S, Watanabe A, Sekiguchi K, Tanaka T, Iso T, Kurabayashi M: Competitive binding of CREB and ATF2 to cAMP/ATF responsive element regulates eNOS gene expression in endothelial cells. Arterioscler Thromb Vasc Biol 2006; 26: 1036– 1042 [DOI] [PubMed] [Google Scholar]
- 13. Okuda Y, Sone H, Mizutani S, Asano M, Tsurushima Y, Ogawa M, Tada K, Asakura Y, Kawakami Y, Suzuki S, Yamashita K: Acute effect of beraprost sodium on lower limb circulation in patients with non-insulin-dependent diabetes mellitus-evaluation by color Doppler ultrasonography and laser cutaneous blood flowmetry. Prostaglandins 1996; 52: 375– 384 [DOI] [PubMed] [Google Scholar]
- 14. Aso Y, Tayama K, Takanashi K, Inukai T, Takemura Y: Changes in skin blood flow in type 2 diabetes induced by prostacyclin: association with ankle brachial index and plasma thrombomodulin levels. Metabolism 2001; 50: 568– 572 [DOI] [PubMed] [Google Scholar]
- 15. Kasiske BL, O'Donnell MP, Keane WF: The Zucker rat model of obesity, insulin resistance, hyperlipidemia, and renal injury. Hypertension 1992; 19( Suppl.): I110– I115 [DOI] [PubMed] [Google Scholar]
- 16. Ionescu E, Sauter JF, Jeanrenaud B: Abnormal oral glucose torelance in genetically obese (fa/fa) rats. Am J Physiol 1985; 248: E500– E506 [DOI] [PubMed] [Google Scholar]
- 17. Kurtz TW, Morris RC, Pershadsingh HA: The Zucker fatty rat as a genetic model of obesity and hypertension. Hypertension 1989; 13( Pt. 2): 896– 901 [DOI] [PubMed] [Google Scholar]
- 18. Virkamäki A, Korsheninnikova E, Seppälä-Lindroos A, Vehkavaara S, Goto T, Halavaara J, Häkkinen AM, Yki-Järvinen H: Intramyocellular lipid is associated with resistance to in vivo insulin actions on glucose uptake, antilipolysis, and early insulin signaling pathways in human skeletal muscle. Diabetes 2001; 50: 2337– 2343 [DOI] [PubMed] [Google Scholar]
- 19. Korach-André M, Gounarides J, Deacon R, Beil M, Sun D, Gao J, Laurent D: Age and muscle-type modulated role of intramyocellular lipids in the progression of insulin resistance in nondiabetic Zucker rats. Metabolism 2005; 54: 522– 528 [DOI] [PubMed] [Google Scholar]
- 20. Teachey MK, Taylor ZC, Maier T, Saengsirisuwan V, Sloniger JA, Jacob S, Klatt MJ, Ptock A, Kraemer K, Hasselwander O, Henriksen EJ: Interactions of conjugated linoleic acid and lipoic acid on insulin action in the obese Zucker rat. Metabolism 2003; 52: 1167– 1174 [DOI] [PubMed] [Google Scholar]
- 21. Kim JK, Fillmore JJ, Chen Y, Yu C, Moore IK, Pypaert M, Lutz EP, Kako Y, Velez-Carrasco W, Goldberg IJ, Breslow JL, Shulman GI: Tissue-specific overexpression of lipoprotein lipase causes tissue-specific insulin resistance. Proc Natl Acad Sci U S A 2001; 98: 7522– 7527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS: Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature 1997; 389: 610– 614 [DOI] [PubMed] [Google Scholar]
- 23. Sartipy P, Loskutoff DJ: Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci U S A 2003; 100: 7265– 7270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Menon RK, Grace AA, Burgoyne W, Fonseca VA, James IM, Dandona P: Muscle blood flow in diabetes mellitus: evidence of abnormality after exercise. Diabetes Care 1992; 15: 693– 695 [DOI] [PubMed] [Google Scholar]
- 25. Ding Y, Vaziri ND, Coulson R, Kamanna VS, Roh DD: Effects of simulated hyperglycemia, insulin, and glucagon on endothelial nitric oxide synthase expression. Am J Physiol Endocrinol Metab 2000; 279: E11– E17 [DOI] [PubMed] [Google Scholar]
- 26. Frisbee JC, Delp MD: Vascular function in the metabolic syndrome and the effects on skeletal muscle perfusion: lessons from the obese Zucker rat. Essays Biochem 2006; 42: 145– 161 [DOI] [PubMed] [Google Scholar]
- 27. Young ME, Leighton B: Evidence for altered sensitivity of the nitric oxide/cGMP signalling cascade in insulin-resistant skeletal muscle. Biochem J 1998; 329: 73– 79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matsumoto K, Morishita R, Tomita N, Moriguchi A, Yamasaki K, Aoki M, Matsumoto K, Nakamura T, Higaki J, Ogihara T: Impaired endothelial dysfunction in diabetes mellitus rats was restored by oral administration of prostaglandin I2 analogue. J Endocrinol 2002; 175: 217– 223 [DOI] [PubMed] [Google Scholar]
- 29. Yamashita T, Shikata K, Matsuda M, Okada S, Ogawa D, Sugimoto H, Wada J, Makino H: Beraprost sodium, prostacyclin analogue, attenuates glomerular hyperfiltration and glomerular macrophage infiltration by modulating ecNOS expression in diabetic rats. Diabetes Res Clin Pract 2002; 57: 149– 161 [DOI] [PubMed] [Google Scholar]
- 30. Tanaka T, Yamamoto J, Iwasaki S, Asaba H, Hamura H, Ikeda Y, Watanabe M, Magoori K, Ioka RX, Tachibana K, Watanabe Y, Uchiyama Y, Sumi K, Iguchi H, Ito S, Doi T, Hamakubo T, Naito M, Auwerx J, Yanagisawa M, Kodama T, Sakai J: Activation of peroxisome proliferator-activated receptor δ induces fatty acid beta-oxidation in skeletal muscle and attenuates metabolic syndrome. Proc Natl Acad Sci U S A 2003; 100: 15924– 15929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Burkey BF, Dong M, Gagen K, Eckhardt M, Dragonas N, Chen W, Grosenstein P, Argentieri G, de Souza CJ: Effects of pioglitazone on promoting energy storage, not expenditure, in brown adipose tissue of obese fa/fa Zucker rats: comparison to CL 316,243. Metabolism 2000; 49: 1301– 1308 [DOI] [PubMed] [Google Scholar]
- 32. Nishimatsu H, Suzuki E, Takeda R, Takahashi M, Oba S, Kimura K, Nagano T, Hirata Y: Blockade of endogenous proinflammatory cytokines ameliorates endothelial dysfunction in obese Zucker rats. Hypertens Res 2008; 31: 737– 743 [DOI] [PubMed] [Google Scholar]
- 33. Schäfer A, Pfrang J, Neumüller J, Fiedler S, Ertl G, Bauersachs J: The cannabinoid receptor-1 antagonist rimonabant inhibits platelet activation and reduces pro-inflammatory chemokines and leukocytes in Zucker rats. Br J Pharmacol 2008; 154: 1047– 1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fujiwara K, Nagasaka A, Nagata M, Yamamoto K, Imamura S, Oda N, Sawai Y, Hayakawa N, Suzuki A, Itoh M: A stable prostacyclin analogue reduces high serum TNF-alpha levels in diabetic patients. Exp Clin Endocrinol Diabetes 2004; 112: 390– 394 [DOI] [PubMed] [Google Scholar]
- 35. Yamada M, Sasaki R, Sato N, Suzuki M, Tamura M, Matsushita T, Kurumatani H: Amelioration by beraprost sodium, a prostacyclin analogue, of established renal dysfunction in rat glomerulonephritis model. Eur J Pharmacol 2002; 449: 167– 176 [DOI] [PubMed] [Google Scholar]
- 36. Chatzipanteli K, Rudolph S, Axelrod L: Coordinate control of lipolysis by prostaglandin E2 and prostacyclin in rat adipose tissue. Diabetes 1992; 41: 927– 935 [DOI] [PubMed] [Google Scholar]
- 37. Lin H, Lee JL, Hou HH, Chung CP, Hsu SP, Juan SH: Molecular mechanisms of the antiproliferative effect of beraprost, a prostacyclin agonist, in murine vascular smooth muscle cells. J Cell Physiol 2008; 214: 434– 441 [DOI] [PubMed] [Google Scholar]
- 38. Fujiwara K, Mochida S, Ohno A, Arai M, Matsui A, Masaki N, Hirata K, Tomiya T, Yamaoka M, Nagoshi S: Use of prostaglandin I2 analog in treatment of massive hepatic necrosis associated with endothelial cell injury and diffuse sinusoidal fibrin deposition. Dig Dis Sci 1995; 40: 41– 47 [DOI] [PubMed] [Google Scholar]
- 39. Li Z, Rodríguez-Iturbe B, Ni Z, Shahkarami A, Sepassi L, Vaziri ND: Effect of hereditary obesity on renal expressions of NO synthase, caveolin-1, AKt, guanylate cyclase, and calmodulin. Kidney Int 2005; 68: 2766– 2772 [DOI] [PubMed] [Google Scholar]
- 40. Baylis C, Mitruka B, Deng A: Chronic blockade of nitric oxide synthesis in the rat produces systemic hypertension and glomerular damage. J Clin Invest 1992; 90: 278– 281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Whaley-Connell A, DeMarco VG, Lastra G, Manrique C, Nistala R, Cooper SA, Westerly B, Hayden MR, Wiedmeyer C, Wei Y, Sowers JR: Insulin resistance, oxidative stress, and podocyte injury: role of rosuvastatin modulation of filtration barrier injury. Am J Nephrol 2008; 28: 67– 75 [DOI] [PubMed] [Google Scholar]
- 42. Watanabe M, Nakashima H, Mochizuki S, Abe Y, Ishimura A, Ito K, Fukushima T, Miyake K, Ogahara S, Saito T: Amelioration of diabetic nephropathy in OLETF rats by prostaglandin I2 analog, beraprost sodium. Am J Nephrol 2009; 30: 1– 11 [DOI] [PubMed] [Google Scholar]
- 43. Kushiro M, Shikata K, Sugimoto H, Shikata Y, Miyatake N, Wada J, Miyasaka M, Makino H: Therapeutic effects of prostacyclin analog on crescentic glomerulonephritis of rat. Kidney Int 1998; 53: 1314– 1320 [DOI] [PubMed] [Google Scholar]
- 44. Barit D, Cooper M E: Diabetic patients and kidney protection: an attainable target. J Hypertens 2008; 26: S3– S7 [DOI] [PubMed] [Google Scholar]
- 45. Siddiqui AH, Ali Q, Hussain T: Protective role of angiotensin II subtype 2 receptor in blood pressure increase in obese Zucker rats. Hypertension 2009; 53: 256– 261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhao X, Li LY: PPAR-alpha agonist fenofibrate induces renal CYP enzymes and reduces blood pressure and glomerular hypertrophy in Zucker diabetic fatty rats. Am J Nephrol 2008; 28: 598– 606 [DOI] [PubMed] [Google Scholar]
- 47. de Souza CJ, Eckhardt M, Gagen K, Dong M, Chen W, Laurent D, Burkey BF: Effects of pioglitazone on adipose tissue remodeling within the setting of obesity and insulin resistance. Diabetes 2001; 50: 1863– 1871 [DOI] [PubMed] [Google Scholar]
- 48. Riddle MC, Schneider J: Glimepiride Combination Group. Beginning insulin treatment of obese patients with evening 70/30 insulin plus glimepiride versus insulin alone. Diabetes Care 1998; 21: 1052– 1057 [DOI] [PubMed] [Google Scholar]