Abstract
The Banting Medal for Scientific Achievement Award is the American Diabetes Association's highest scientific award and honors an individual who has made significant, long-term contributions to the understanding of diabetes, its treatment, and/or prevention. The award is named after Nobel Prize winner Sir Frederick Banting, who codiscovered insulin treatment for diabetes.
Dr. Eisenbarth received the American Diabetes Association's Banting Medal for Scientific Achievement at the Association's 69th Scientific Sessions, June 5–9, 2009, in New Orleans, Louisiana. He presented the Banting Lecture, An Unfinished Journey—Type 1 Diabetes—Molecular Pathogenesis to Prevention, on Sunday, June 7, 2009.
The majority of individuals, but not all, developing what is routinely diagnosed as type 1 diabetes have the immune-mediated form of the disease (type 1A) that results from T cell–mediated specific β-cell destruction. Studies of the NOD mouse model suggest that the root cause of type 1 diabetes involves germline-encoded sequences forming trimolecular complexes consisting of the insulin peptide B:9-23 presented by the class II major histocompatibility complex (MHC) molecule I-Ag7 and recognized by T cell receptors having a specific germline-encoded α-chain sequence (TRAV-5D-4*04 Vα). Utilizing genetic, autoantibody, and metabolic parameters it is now possible to predict type 1A diabetes in humans, and immune therapy can delay, but not permanently prevent, destruction of β-cells. With an increasing incidence and an estimated 1 million individuals in the U.S. developing type 1A diabetes, safe prevention has become a major international goal. Achieving this goal may come from incremental modification of immune therapies currently being tested and/or may involve a deeper understanding of the autoimmune trimolecular complexes underlying the disorder's pathogenesis.
Type 1A diabetes is associated with both devastating chronic complications and acute life-threatening ketoacidosis and hypoglycemia (1–3). There are multiple pathways being pursued to “cure” this disease or at least dramatically ameliorate the burden it imposes on patients and their families. Continuous glucose monitoring is already improving the lives of many patients by providing “real time” information with alarms for hypo- and hyperglycemia (4,5). Multiple groups are now studying devices that will control insulin pumps, in particular turning off insulin delivery to prevent hypoglycemia (6). In developed countries, such devices will hopefully rapidly become the standard of care for patients with insulin-dependent diabetes.
Though many patients do not consider such mechanical devices, especially the current “first” generation of devices, as a true cure, these therapies will set the bar in evaluating immunologic therapies considered for prevention of diabetes and β-cell replacement. Thus, the bar will be high and hopefully ever higher over the next decade. At present, pancreatic (long term) (7) as well as islet transplantation (short term) (8,9) can cure type 1 diabetes but, for most patients, with unacceptable risks associated with immune suppression. It is likely that autoimmunity, in addition to alloimmunity, limits the therapeutic potential of either of these forms of transplantation (10).
The field addressing the immunology of type 1 diabetes has grown rapidly, with thousands of relevant publications. This review can only recognize a portion of that literature and will emphasize a relatively simple hypothesis that hopefully allows presentation with a clear focus: Autoimmune type 1 diabetes results from specific β-cell destruction due to chronic T cell targeting of insulin, and the major molecular determinants of such targeting are hardwired in the genome.
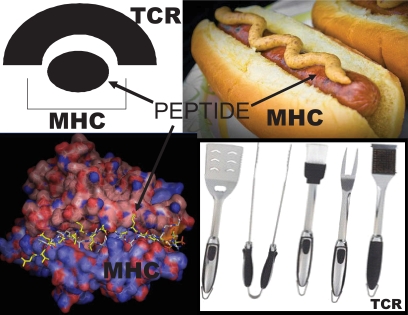
Though there are clear phenotypic differences, it is remarkable at a molecular level how similar the NOD mouse and human type 1 diabetes may be. I will first review the pathogenesis of disease in the NOD mouse (where it is easier to attempt to disprove the above specific hypothesis) and then in type 1 diabetes of humans, ending with an outline of the status of clinical trials. I believe the root cause of type 1 diabetes of the NOD mouse is three genome encoded sequences, which are shown in Fig. 1. The relevant sequences are thought to be:
The insulin peptide B:9-23 sequence (11);
The susceptible MHC I-Ag7 sequence (12);
A specific T cell receptor (TCR) Vα sequence (13).
FIG. 1.
Hypothesized “primary” pathogenic trimolecular complex of the NOD class II presenting molecule: I-Ag7/insulin peptide amino acids B chain 9-23/TCR with TRAV5D-4*04.
B:9-23 INSULIN PEPTIDE
The first component of the trimolecular complex essential for the development of diabetes in NOD mice is a peptide of insulin, namely amino acids 9 to 23. A growing body of evidence indicates autoimmunity directed at insulin is central to the pathogenesis of type 1A diabetes of the NOD mouse (11,14–21). The major advance in defining a role for T cells targeting insulin was the cloning of T cells directly from islets of NOD mice by Daniel and colleagues (22). They discovered that the majority of CD4 T cell clones derived from such islets reacted with insulin, and of those that reacted with insulin, more than 90% were stimulated by the B:9-23 peptide (23). These T cell clones were able to accelerate the development of diabetes in NOD mice and a subset was able to cause diabetes in immunodeficient mice. T cell clones and TCR transgenics (21) targeting islet autoantigens can produce or prevent diabetes. Though in this review I will emphasize CD4 T cells, CD8 T cells, including T cells targeting the insulin B:15-23 sequence, are of parallel importance. Class II alleles (and thus potentially CD4 T cells), however, predominate in determining diabetes risk of humans and animal models (24–26).
Mice have two insulin genes. Deleting the insulin 1 gene prevents the development of diabetes for 90% of NOD mice (27). Deleting the insulin 2 gene dramatically accelerates the development of diabetes (17,27). Both insulin genes are expressed in islets, while only insulin 2 is expressed in the thymus. It is likely that the toleragenic influence of small amounts of insulin produced within thymic epithelial cells (i.e., the induction of central tolerance) accounts for the different phenotypes of the insulin gene knockout NOD strains. In contrast, deleting other autoantigens, e.g., GAD65 (28), IGRP (15), IA-2 (29), and IA-2beta (30), does not alter the progression to diabetes of NOD mice.
To test whether targeting of insulin is essential for the development of diabetes we combined insulin 1 and insulin 2 knockouts. To prevent metabolic diabetes in the double knockouts, a preproinsulin transgene was introduced with a mutated sequence of insulin B:9-23 (31). The specific mutation was chosen (Tyrosine to Alanine at position B16 of insulin) because the anti-B:9-23 T cell clones created by Daniel and colleagues were not stimulated by this mutated peptide. NOD mice with only the mutated insulin do not develop diabetes and are protected, albeit not completely, from both expression of insulin autoantibodies and insulitis (31). Replacing the missing native insulin sequence with transplantation of islets with the native insulin sequence or immunization with native B:9-23 peptide, or a transgene coding for native insulin, restores development of insulin autoantibodies (32). These latter studies are complex in that though the transplanted islets have the native insulin sequence, the endogenous islets of the pancreas do not. Thus, to demonstrate induction of diabetes following transplantation of native B:9-23 islets into double-knockout mice, splenocytes from the mice are transferred into an immunodeficient mouse recipient that has the native B:9-23 sequence in its islets. Of note, islets with the native insulin sequence transplanted under the kidney capsule after 4 weeks of age induce insulin autoantibodies, and thus neither the specific anatomic location (i.e., pancreas) nor the neonatal presence of the inducing insulin B:9-23 epitope are critical to disease development (32).
Given the number of islet autoantigens recognized by T cells of the NOD mouse, an important question relates to the hierarchy of antigen recognition. Multiple investigators have demonstrated that techniques designed to induce “recessive” tolerance to insulin prevent diabetes (11,14–17). Recent studies by Krishnamurthy and coworkers demonstrate that the immune response to insulin is “upstream” of the response to the major islet autoantigen IGRP (15,33). Of note, though IGRP CD8-reactive T cells are very prominent in the NOD mouse model, mice with I-E promoter-driven proinsulin, introduced for the purpose of inducing tolerance to insulin, do not develop IGRP-reactive T cells. Even TCR transgenic mice targeting IGRP with blocked response to insulin do not spontaneously develop diabetes (33). Thus, the immune response to IGRP is both downstream of the immune response to proinsulin and not necessary for progression to diabetes. Whether responses to all islet autoantigens, except insulin, are dispensable cannot be answered at present in that several prominent autoreactive T cell clones target antigens that are either unknown or have not yet been knocked out in NOD mice (e.g., chromagranin, the target of BDC2.5 T cells) (34a).
Lack of a critical role of specific islet autoantigens, demonstrated by knockout experiments, does not rule out the importance of such autoantigens following intermolecular epitope spreading. Detection of autoantibodies or T cell responses to such autoantigens may also be important for diabetes prediction, and immunization with such autoantigens may still induce forms of dominant tolerance (34). Once NOD mice have developed diabetes, islet transplants with only the B16:A mutated insulin sequence are rapidly destroyed. This indicates that once autoimmunity is advanced, anti-islet autoimmunity directed at noninsulin molecules is sufficient for islet destruction, or that in the transplant setting recognition of the B16:A altered epitopes (or other epitopes of insulin) suffice to target β-cell destruction. Of note, in contrast to transplanted islets, islets within the pancreas with mutated B16:A sequence resist destruction following transfer of splenocytes from diabetic NOD mice. This suggests that though not completely protective in this setting, lack of the critical B:9-23 sequence remains important despite epitope spreading (32).
In contrast to antigen knockout experiments, where to date only knocking out the insulin genes influences the development of diabetes, multiple islet autoantigens and their peptides can be administered in a variety of ways to prevent the development of diabetes of NOD mice (34–36). Such dominant suppression of disease is typically associated with the enhancement of regulatory T cells. There is evidence for regulatory CD4 (37–40), CD8 (24,41), and even B lymphocytes (42). The insulin B:9-23 peptide, when administered by intranasal or subcutaneous injection (especially when given in incomplete Freund's adjuvant) prevents diabetes (43,44). Of note, despite prevention of diabetes, subcutaneous injection of the B:9-23 peptide induces insulin autoantibodies presumably by activating CD4 T cells, and these antibodies bind to insulin but not the immunizing peptide (indicated by absorption studies) (45). Even normal Balb/c mice show such a response to the insulin peptide B:9-23 (46). With the appropriate class II molecules (i.e., I-Ag7 of the NOD or related I-Ad of Balb/c mice that have the same I-A alpha chain sequence), the peptide induces insulin autoantibodies, presumably due to activation of anti-B:9-23 CD4 T cells. Despite induction of insulin autoantibodies, insulitis does not develop unless the innate immune system is activated (e.g., via poly-IC injection) (47).
I-Ag7
The TCRs of murine CD4 T cells recognize their target peptides presented in the groove of the antigen-presenting class II, I-E, and I-A molecules, and CD8 T cells target peptides in the groove of class I K, and D molecules (48,49). The homologous molecules of humans are DQ for I-A, DR for I-E (HLA-A, -B, and -C for mouse K and D). These presenting molecules are extremely polymorphic. The amino acid sequence lining the groove binds peptides and determines immune targeting. As in humans (50) and rat models of type 1 diabetes (51), class II molecules are critical for diabetes of the NOD mouse (12,52). The genetic effect is so large that it could be demonstrated with just eight diabetic mice, all being homozygous for the NODs unique major histocompatibility region (having I-Ag7 and lacking I-E) in a cross of NOD mice with a control strain (having I-Ak and I-Ek) (12,52). The class II molecules likely act by altering the TCR repertoire and enhancing the targeting of specific peptides by T cells. In particular, the I-Ag7 molecule, as well as human DR4-associated DQ8 and DR3-associated DQ2, have an unusual binding pocket (pocket 9) that lacks an aspartic acid (52,53). It has been hypothesized that such a lack of aspartic acid would favor presentation of autoantigenic peptides that have a charged residue that could bind in this unusual pocket 9. Recent studies in collaboration with the Kappler laboratory (B. Stadinski, unpublished data) suggest just the opposite, namely that the B:9-23 peptide recognized by pathogenic TCRs recognize the peptide in a low-affinity alternative register. Insulin is produced in the thymus of mouse and humans by specialized thymic epithelial cells and can result in deletion of insulin reactive T cells. It is likely that T cells with TCRs targeting B:9-23 in the thymus rarely encounter the peptide in this low-affinity register and thus escape central (i.e., thymic) deletion. These T cells that escape can then destroy β-cells in the pancreatic islets with their huge local concentration of insulin.
TCRs WITH TRAV5D-4*04 α-CHAIN SEGMENT
The third element of the trimolecular complex is the TCR made up of an α- and β-chain. TCRs are formed by random selection and recombination of gene segments (e.g., for the TCR α-chain, Vα and Jα TCR segments) that form billions of unique TCRs (48). The final TCR resembles a barrel with six fingers (the complementarity determining regions [CDRs]) extending from the barrel. Three CDR are provided by the TCR α-chain and three by its β-chain. It is these fingers that bind to the MHC-peptide complex and lead to the activation of T cells bearing the receptor. For classic recognition of a target peptide, all of the fingers (i.e., CDR1, CDR2, and CDR3 for both TCR α- and β-chains) are involved in recognition, and the CDR3 region is often predominant. The CDR1 and CDR2 elements are genome encoded sequences of both Vα and Vβ TCR segments. In contrast, the CDR3 α-chain element is formed at the interface of the randomly combining Vα and Jα gene segments, as well as V,D,J for the TCR β-chain. In addition, the joining is imprecise, with addition and subtraction of nucleotides leading to additional CDR3 sequence variation.
Given the specific and unique properties of I-Ag7 and the B:9-23 peptide related to development of autoimmune diabetes, it was perhaps to be expected that the pathogenic NOD anti-B:9-23 TCR would be unusual. Sequencing of the TCR from the clones discovered by Wegmann et al. revealed that the majority utilized a specific Vα segment, termed TRAV5D-4*04. (Note: Each unique Vα segment is given a number.) Unexpectedly, there was no conservation of what is termed the N-region of CDR3 (i.e., the region created by joining V and J plus nucleotide insertions and deletions) and no conservation of the TCR β-chain elements (54). We hypothesized that if we created mice with just this conserved Vα chain, allowing the mice to create multiple β-chains, we would induce islet autoimmunity (14). Using the technology of Vignali and colleagues (55), we have produced transgenic and retrogenic mice with multiple different α-chain sequences containing TRAV-5D-4*04 (i.e., Cα knockout background). The great majority of such mice develop insulin autoantibodies, and a subset develop diabetes (13,56). With the creation of TCR hybridomas from these mice, we estimate that ∼1 per 100 TCRs with a TRAV-5D-4*04 containing anti-B:9-23 α-chain respond to the B:9-23 peptide (56).
There is, as of yet, no crystal structure of the complete trimolecular complex discussed above (i.e., I-Ag7:peptide B:9-23:TCR with TRAV5D-4*04). We hypothesize that when such a structure is elucidated, it will reveal the anti-B:9-23 TCR bind at an unusual angle such that the primary contacts are the CDR1 and CDR2 portions of the TRAV5D-4*04 Vα-chain interacting with specific side chains of the B:9-23 peptide. The other CDR TCR elements will play a secondary role. Thus, we believe an accident of nature of NOD mice encoded in the genome sequences of the B:9-23 insulin peptide, the presenting I-Ag7 molecule, and the TCR Vα 5D-4 segment results in susceptibility to diabetes if tolerance mechanisms fail (14). The apparently low-affinity register in which the peptide needs to bind for recognition makes deletion of pathogenic anti-B:9-23 T cells in the thymus unlikely, and the extreme concentration of insulin in islet β-cells (i.e., approximately one-third of protein in β-cells is insulin) enhances peripheral immunological recognition. The simple rules for TCR recognition of insulin peptide B:9-23, dominated by a single Vα segment, presumably enhances the numbers of targeting T cells.
Two of the elements of the trimolecular complex targeting insulin are common to many mouse strains (TRAV5D-4 α-chain sequences) or all strains (insulin B:9-23 sequence). That said, I-Ag7 is a unique NOD contribution. Other genetic polymorphisms related to maintenance of tolerance (57) and environmental factors (or lack of protective environmental factors such as certain viral infections) influence activation of autoimmunity (58). Given what may be the very large number of T cells able to target the B:9-23 peptide, we believe the interaction between β-cells and the immune system of the NOD is similar to that of a swarm of bees. Once tolerance is broken, there are numerous individual T cells sharing common features of their TCRs, poised to target insulin.
The aforementioned hypothesis has not yet been subjected to a number of crucial tests. Thus, a crucial prediction is that knocking out the TRAV-5D-4*04 gene segment should prevent diabetes and insulin autoantibodies, similar to the effect of mutating the B:9-23 insulin peptide (11). In addition to genetic approaches to disprove the hypothesis that the shared Vα sequence is critical, development of therapeutics that are able to specifically block the above trimolecular complex are actively being explored.
We are taking two approaches to therapeutic targeting of the above NOD trimolecular complex. In collaboration with David Ostrov, who has defined a series of small molecules using a DOCKING program (59) that screens an NCI library of 140,000 small molecules designed to be “drug” candidates, we have identified small molecules that can modulate anti-B:9-23 TCR signaling (A. Michels, unpublished data). We believe families of small molecules binding to specific pockets of I-Ag7 will be able to both enhance and suppress TCR responses to the B:9-23 peptide, as well as alter specific cytokine responses of the dominant TRAV5D-4*04 B:9-23 T cells of NOD mice.
In addition, we have evidence that antibodies that recognize the B:9-23 peptide bound in the groove of I-Ag7 can block in vitro presentation of the B:9-23 peptide (L. Zhang, unpublished data). With this, the induction of such antibodies for their ability to prevent disease will soon be tested.
STAGES OF HUMAN TYPE 1 DIABETES
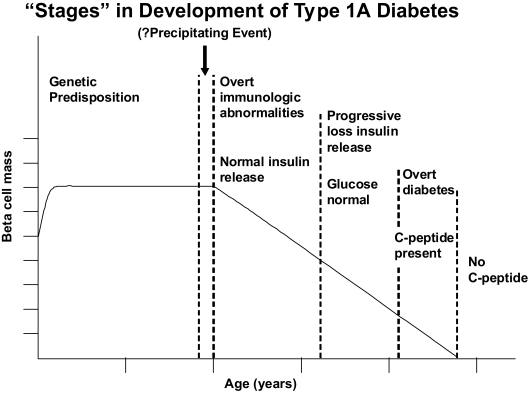
In 1986, we proposed a model of chronic autoimmune development of type 1A diabetes with disease pathogenesis divided into a series of stages (Fig. 2) (60,61). Technology to directly assess β-cell mass in humans is still lacking, and thus a useful debate continues as to whether there is a relapsing remitting course contributing to β-cell destruction and whether for some patients, the process of β-cell destruction is acute. In this model, the x-axis of Fig. 2 never specified exact times, with such a lack intentional, given inter-individual heterogeneity (even between monozygotic twins, both progressing to diabetes) (62). Different individuals progress at different rates to overt diabetes, and decades can elapse between the development of diabetes in one monozygotic twin and the development of islet autoantibodies in their twin mate (62). The model highlighted the potential to predict type 1A diabetes, a notion borne of multiple studies (63,64) as well as prevention trials (65,66).
FIG. 2.
Model of the development of type 1A diabetes highlighting chronic progressive nature of the disease. Modified and reprinted with permission from N Engl J Med 1986;314:1360–1368.
For some of the stages there has been dramatic progress, while for others progress has been more limited; for all stages, however, a research infrastructure (67) that holds the promise of addressing fundamental questions over the next decade has been put in place.
Stage 1: Genetic susceptibility.
Type 1A diabetes is usually polygenic in etiology, but there are two highly informative rare “monogenic” autoimmune syndromes associated with this disease: APS-1 (autoimmune polyendocrine syndrome type 1) (68) and IPEX syndrome (Immune Dysregulation, Polyendocrinopathy, Enteropathy, X-linked) (69).
IPEX syndrome results from mutations of the FoxP3 gene, a transcription factor that is essential for the development of regulatory T cells (70). In the absence of FoxP3, children develop overwhelming autoimmunity, and it is estimated that 80% develop type 1 diabetes. Diabetes can present as early as the first days of life. This syndrome clearly demonstrates the crucial role played by regulatory T cells and that most humans would develop type 1 diabetes unless pathogenic T cells are held in check. Bone marrow transplantation, by providing dominant regulatory T cells, is a consideration for children diagnosed with this fatal autoimmune syndrome (71). It is possible to generate antigen specific T cell lines expressing FoxP3 with regulatory properties, and this too is being explored as a potential therapy (38).
APS-1 is more common than IPEX syndrome but still rare, though increased in a few populations (e.g., Iranian, Jewish) (68). The syndrome is characterized by mucocutaneous candidiasis, Addison's disease, and hypoparathyroidism and results from mutations of the AIRE (autoimmune regulator) gene, another transcription factor. The disease is usually inherited in an autosomal recessive manner, but a single family with a dominant mutation has been described, and animal models for both have been created (72). The AIRE gene has a major role in enhancing the expression of peripheral antigens such as insulin within the thymic medullary epithelial cells (73,74). Tiny amounts of “peripheral” (i.e., not in the thymus) antigens such as insulin, antigens expressed within the thymus, are associated with negative selection of autoreactive T cells and protection from autoimmunity. Patients with APS-1 develop a series of autoimmune disorders over time and usually patients express more autoantibodies than specific diseases (68). Of note, 100% of these patients are reported to have autoantibodies reacting with interferon-α (75). Type 1 diabetes occurs in ∼18% of these patients, and development of diabetes is influenced by the dominantly protective HLA allele DQB1*0602 and insulin gene polymorphisms (76,77).
In combination, these two syndromes illustrate extremes of genetic determination of autoimmune diabetes when one or more pathways that are essential to maintenance of tolerance are disrupted. The genome of humans favors the development type 1 diabetes when mutations in genes controlling tolerance override the normal polygenic prevention of autoimmunity.
APS-2 syndrome is much more common than APS-1 (69). APS-2 syndrome is characterized by the occurrence of multiple autoimmune disorders in the same individual (e.g., type 1A diabetes, Addison's disease, thyroiditis, celiac disease, etc.) (78). It has a complex inheritance similar but not identical to type 1A diabetes (69,79). In particular, the highest risk HLA genotype (DR3/4-DQ2/8) for Addison's disease has DRB1*0404 and not DRB1*0401 with DQB1*0302 (80,81). Patients with type 1A diabetes are at increased risk for the development of the series of autoimmune disorders of the APS-2 syndrome and in particular Addison's disease, celiac disease, thyroid autoimmunity, and pernicious anemia (82). We routinely screen patients with type 1 diabetes for associated autoimmunity targeting the adrenal, intestine, and thyroid with measurement of 21 hydroxylase autoantibodies for Addison's disease (1.5% positive) and transglutaminase autoantibodies for celiac disease (10% positive) and with TSH determination. Within the 1st year after the diagnosis of type 1 diabetes, approximately one-third of patients already express autoantibodies reacting with one or more of these target organs (83).
Type 1A diabetes has become one of the best studied complex genetic disorders (84,85). Approximately 1 in 300 individuals in the U.S. develop type 1A diabetes versus approximately 1 in 20 first-degree relatives. Here too, we note that the risk of siblings and offspring of a father with type 1 diabetes is greater than the risk of offspring of a mother. With very long–term follow up, the majority of monozygotic twins of a patient with type 1 diabetes develop islet autoimmunity (i.e., >70%) and diabetes (i.e., >60%) (62). As many as 30 years can elapse between development of diabetes of the first twin and the second twin. In contrast, the risk of diabetes in dizygotic twins might not differ from that of siblings, with ∼5% developing diabetes (86).
Despite the strong genetic predisposition, as evidenced above, the great majority of individuals developing type 1A diabetes, >90%, do not have a first-degree relative with diabetes. Though 40% of individuals in the general Denver population have the high-risk HLA alleles DR3 or DR4, only 2.4% have the highest risk genotype, namely DR3 and DR4, and such heterozygous individuals make up ∼30% of children developing diabetes (87). Thus, HLA genotype, inherited from both parents, is most important for the development of type 1 diabetes, with susceptible alleles very common in the general population (88).
Similar to the NOD mouse model, the major genetic determinants of type 1A diabetes are polymorphisms of class II MHC genes DQ DR, and DP, in this order (50). The highest-risk genotype consists of a DR3 haplotype DRB1*0301-DQA1*0501-DQB1*0201 on one chromosome and a DR4 haplotype on the other (DRB1*0401-DQA1*0301-DQB1*0302, i.e., in shorthand DR3/4-DQ2/8) (88). Between 30 and 50% of children developing type 1A diabetes have this genotype (89). (Note: The younger the onset of disease, the greater the DR3/4-DQ2/8 genotype.) The absolute risk in the general population with this genotype is ∼5%. The DRB1*0403 allele decreases risk of DR4 haplotypes, and certain DP alleles also alter risk (e.g., decreased with DPB1*0402) (90). In addition, there are several extremely potent protective HLA alleles. In particular, DQB1*0602 occurs in ∼20% of the general population but in only 1% of patients developing type 1A diabetes.
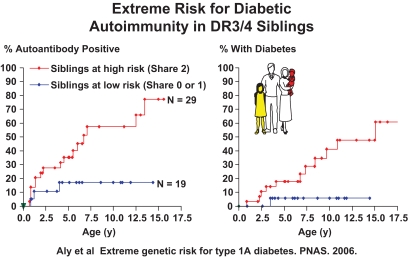
The DAISY study, headed by Marian Rewers, has HLA typed more than 30,000 newborns in Denver, Colorado, and followed prospectively from birth more than 1,000 general population newborns with HLA risk alleles, as well as 1,000 first-degree relatives independent of HLA (91). Siblings of a patient with type 1 diabetes have a diabetes risk that is several-fold that of offspring, despite both siblings and offspring sharing approximately one-half of their genome with their proband. There could be multiple genetic and environmental reasons for the higher risk of siblings, but the simplest explanation would be the presence of additional polymorphisms of genes within the MHC that increase risk for certain haplotypes beyond the sequence of HLA DR and DQ alleles. That this may be the case is suggested by the extreme risk of DR3/4-DQ2/8 siblings who shared both HLA haplotypes identical by descent with their sibling proband compared with DR3/4-DQ2/8 siblings sharing only one or no haplotype identical by descent (Fig. 3) (92). The risk of activating islet autoimmunity in the DAISY study for such siblings was as high as 70%, and such siblings make up a major portion of all of the DAISY children progressing to islet autoimmunity and then diabetes (92). With typing for DR DQ, and DP in the DAISY study, risks as high as 20% can be defined in the general population (90). Nevertheless, a risk of 20% of DR3/DR4 heterozygotes of the general population is much less than the 70% of DR3/4 siblings with MHC inherited identical by descent, suggesting that there remain additional polymorphisms of genes in the MHC to be discovered. We have evidence that one of the loci is at the far telomeric end of the MHC (93), and there is evidence that specific class I HLA alleles (or loci in linkage disequilibrium with these alleles) contribute to risk, in particular HLA-B39, present in ∼2–4% of patients, and HLA-A24 has been associated with earlier onset of type 1A diabetes (94,95). Of note, the presence of HLA B39 increases the risk of DR8 haplotypes to that conferred by DR4 haplotypes (J. Baschal, unpublished data).
FIG. 3.
Extreme risk of islet autoimmunity and diabetes for DR3/4-DQ2/DQ8 siblings who share both HLA haplotypes identical by descent with proband sibling.
Given the existence of almost total conservation of multiple haplotypes (96) for millions of base pairs across the MHC (the two best known conserved haplotypes: HLA-A1,B8,DR3 haplotype [97,98] and HLA-A30,B18,DR3 “Basque” haplotype [99]) makes the search for such non-HLA disease determinants in the MHC difficult (96). The Basque haplotype is higher risk than the A1,B8,DR3 haplotype even though both haplotypes have the same sequence for their DR and DQ alleles. Both haplotypes, given the presence of DR3, increase diabetes risk (100).
Given the dramatic protection provided by certain specific HLA alleles, the question has been raised as to whether provision of such alleles might be considered therapeutically or assessed in family planning. The alleles protecting and determining risk are presumably “normal” HLA alleles determining the targeting of specific self-molecules; for instance, the DR2-DQB1*0602 haplotype that provides dominant protection from type 1A diabetes is the highest risk haplotype for multiple sclerosis (101).
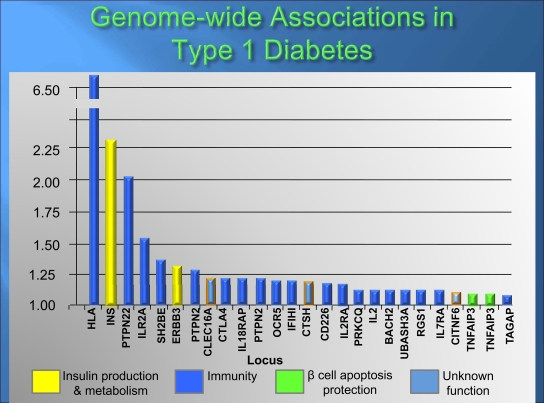
There has been an explosion in identifying non-MHC loci contributing to genetic risk, with >40 loci confirmed (Fig. 4) (84,102). All of these genetic loci together have a much smaller influence on diabetes risk than the MHC, with many having odds ratios (ORs) of <1.2. (Note: An OR of 1.0 indicates no risk, and the MHC OR is >6). After the MHC, the next most important locus is that of the insulin gene. Protective polymorphisms in a sequence 5′ of the insulin gene increases the amount of insulin expressed in the thymus and is associated with decreased diabetes risk, probably by increasing thymic deletion of T cells targeting insulin (103,104).
FIG. 4.
Results of genome-wide association studies in type 1 diabetes. Modified and reprinted with permission from N Engl J Med 2009;360:1646–1654.
The PTPN22 gene is the third most important locus (105–107). A change of a single amino acid (R640W) increases the risk of diabetes, with an OR of ∼2. The molecule (lyp) encoded by the PTPN22 gene is a lymphocyte-specific tyrosine phosphatase, and the disease-associated polymorphism increases inhibition of TCR signaling, likely through a gain of function (107). How such a gain of function contributes to diabetes risk is unknown, but the predominant hypothesis is that it may decrease negative selection of T cells in the thymus. Multiple other specific genes have been implicated including CTLA4 and CCR5 and the IL2 receptor, as well as multiple loci with one or more potentially relevant genes. A helicase (IFIh1) involved in interferon signaling has several rare variants associated with disease risk as well as common single nucleotide polymorphisms (108). Almost all of the identified loci appear to alter risk of diabetes by effects on the immune system (84). Interestingly, the genetic polymorphisms associated with type 2 diabetes do not generally influence the development of type 1A diabetes (109,110), though there are conflicting reports of an association of polymorphisms of TCF7L2 gene with latent autoimmune diabetes in adults (111,112). On balance, it would appear that the genetics of type 1A diabetes and type 2 diabetes are distinct, with type 1A diabetes an immune-mediated disorder.
The search for rare variants of genes contributing to type 1 diabetes risk has hardly begun, but it is likely that multiple such variants exist. In addition, it is likely that either single or more likely multiple loci within the MHC remain to be discovered, some of which may modulate risk more dramatically than currently discovered common variants outside of the MHC (92,94). Multiple common pathways underlie genetic susceptibility to type 1 diabetes including T cell targeting of peptide antigens (HLA class I and class II) in general, as well as targeting of proinsulin/insulin and, in particular, TCR signaling (e.g., PTPN22, CTLA4), tolerance maintenance (e.g., foxP3 and AIRE), and innate immune responses (e.g., IFIh1). It is now possible to identify individuals with high or extreme risk of type 1A diabetes related to defined HLA genotypes/haplotypes, with smaller contributions from the insulin locus and PTPN22 (113). Common SNPs of loci with ORs <1.2 do not facilitate genetic prediction but will hopefully identify important pathogenic mechanisms (114). Though very high–risk individuals can be identified with HLA analysis (e.g., DR3/4 heterozygotes), they comprise <50% of those developing type 1A diabetes, and there has been a trend over the last 50 years for the percentage of patients with DR3/4 to decrease (115,116). High-risk general population individuals may be appropriate for preventive trials similar to relatives of patients with type 1 diabetes, and their identification has the potential to prevent morbidity and mortality at the time of diabetes onset if genetic screening is combined with islet autoantibody determination (117).
Stage II: Triggering-environment.
The least amount of knowledge gains over the past two decades has, arguably, been made in defining environmental factors contributing to the development of type 1 diabetes. It is nevertheless clear that environmental determinants are important given the remarkable doubling of type 1 diabetes incidence in most Western countries over the past 20 years (118–120). This is too rapid a change for a common genetic disorder to be ascribed to genetic alteration (alone) of the population. A leading hypothesis to explain such an increase in incidence is the “hygiene” hypothesis, positing decreased infections increasing multiple immune-mediated disorders including asthma and diabetes (121,122). Both the NOD mouse and BB rat, when raised in a germ-free environment, have been reported to alter diabetes, with recent studies suggesting that intestinal microbiota in animal models modulates development of diabetes (123). Another hypothesis posits that insulin resistance somehow “accelerates” the pathogenesis of type 1 and type 2 diabetes and that type 1 and type 2 diabetes have similar genetic causes, though differing in terms of MHC alleles (124,125). Current genetic analysis as reviewed does not support this hypothesis (126), though insulin resistance associated with obesity influences progression to type 1 diabetes, presumably as insulin secretion fails (127).
It is noteworthy that certain medications clearly induce development of type 1A diabetes. In particular, interferon therapy is associated with diabetes in animal models (128), and in humans this treatment can induce islet autoantibodies and accelerate diabetes progression leading to ketoacidosis (129,130). Methimizole (131,132), penicillamine (133), and lipoic acid (134) (i.e., sulfhydryl-containing drugs) can induce autoantibodies, with titers of the antibodies, at times, high enough to influence metabolism (e.g., insulin autoimmune syndrome: Hirata disease associated with DRB1*0406) (131).
Other contenders for environmental factors are dietary, with reports that cows milk (135), early introduction of cereals (136,137), decreased levels of n-3 fatty acids (138), and vitamin D contribute to diabetes risk (139). Large current trials and prospective observational studies will hopefully rigorously test these hypotheses (67). Of note, both metabolomics (140) and analysis of mRNA arrays (141) are being applied to identify abnormalities potentially preceding the occurrence of anti-islet autoantibodies. Both a power and potential weakness of these studies is that the analysis of multiple parameters, and thus testing of thousands of hypotheses at the same time, will yield many false-positive results. Thus, replication studies will be crucial.
Many studies of viral infections inducing diabetes preceded the discovery that type 1A diabetes is a chronic autoimmune disease. Thus investigators searched for viral infection at the time of diabetes onset, including studies of pancreas from patients that died at diabetes onset. Congenital rubella infection and enteroviral infections have been studied in most detail, with at present a lack of clear consensus as to their importance (142,143). A major difficulty may relate to the induction of islet autoimmunity years prior to the development of diabetes and the likelihood that viruses triggering islet autoimmunity may be many, ubiquitous, and act over a very short time period. All of the preceding would be lessons learned from virus-induced autoimmune diabetes in rat models.
Perhaps the best animal model of the triggering of type 1A diabetes comes from the discovery that infection with the Kilham rat virus (KRV) induces diabetes in diabetes-resistant BB rats (144). BB-DR rats were bred to be a normal rat control strain and lack the lymphopenia of the BB original strain that spontaneously developed diabetes (51). The KRV infection was discovered to induce diabetes when a subset of BB-DR rats spontaneously developed diabetes and it was subsequently discovered that the strain had become infected with the virus. It is now known that multiple rat strains, all with the same class II MHC alleles (i.e., RT1-U) and specific TCR loci, develop diabetes (at varying frequency) when infected with several different viruses or when stimulated with immunologic inducers including toll-like receptor (TLR) agonists (145,146). In a similar manner, mice engineered to express the B7 molecule on the surface of islet β-cells develop diabetes when given poly-IC, a viral RNA mimic and activator of TLR3 (147). In this model, poly-IC induces interferon-α. Antibodies to interferon-α block diabetogenesis, and interferon itself can induce diabetes. The KRV does not infect islet β-cells and only needs to be transiently present to induce diabetes. If a virus induces diabetes in humans, we believe its discovery will depend upon monitoring individuals for acute infections and initial appearance of islet autoantibodies on a relatively short time scale, and the inducing virus(es) will be ubiquitous.
Stage III: Active autoimmunity.
The immunocytochemical (ICA) assay measures a combination of autoantibodies reacting with multiple defined autoantigens (except insulin), and its discovery was central to the search for defined molecular targets (148). The ICA assay is difficult to standardize and interpret given this heterogeneity and the need to utilize frozen human pancreatic sections as substrate.
The clearest indication of the presence of islet autoimmunity is the expression of islet autoantibodies (149). At present, four major islet autoantigens (i.e., insulin, GAD65, IA-2, and Znt8) have been identified, and assays for these autoantibodies have been validated in international workshops. ZnT8, the most recently defined autoantigen, is the islet-specific zinc transporter involved in transport of zinc into insulin secretary granules (150). It is of interest that a common polymorphism of ZnT8 that changes a single amino acid is a major target of anti-ZnT8 autoantibodies. Patients homozygous for specific ZnT8 variants more often make autoantibodies to their own genome encoded variant, demonstrating the autoimmune nature of the targeting (151).
The simplest rule for predicting type 1A diabetes is that expression of two or more of the four “biochemical” autoantibodies predict diabetes (63,152). It is currently unknown why such combinatorial prediction is so effective, but the null hypothesis is that it simply relates to statistical probabilities. With a combination of the binomial theorem and Bayes theorem, one can calculate positive and negative predictive values of combinatorial autoantibody testing. Specifically, one can derive the probability for instance of detecting one or more, or two or more, islet autoantibodies with the four assays currently utilized, with assays having a specificity set at the 99th percentile. Approximately 4% of normal individuals will be positive for one or more autoantibodies, while only 0.06% will be positive for two or more autoantibodies (i.e., 6 in 10,000 normal individuals). For assays with specificity at the 95th percentile, the corresponding false-positive frequencies are 19 and 1.4%. Both for research and clinical care, assays with false-positive rates of 5%, when multiple assays are combined, are very problematic and potentially underlie some confusion concerning expression of islet autoantibodies in populations at low risk for type 1A diabetes, such as patients clinically identified as having type 2 diabetes. In such populations, expression of two or more autoantibodies, set at the 95 percentile, remains reasonably specific (i.e., 1.4% “false positive”) but not the presence of one or more autoantibodies.
A “biologic” false-positive islet autoantibody result is not synonymous with lack of autoantibodies binding to the assayed autoantigen or confirmation, even over time, of the presence of the autoantibody. Thus, true autoantibodies may be present, but the epitope that they recognize, the level of the autoantibody, or the lack of expression of multiple autoantibodies is such that its presence may not indicate an increased diabetes risk. This is well illustrated by the isolated presence of low-affinity insulin autoantibodies, where most children with such autoantibodies do not progress to diabetes (64,153). Recently Hampe and colleagues have reported the presence of anti-idiotypic antibodies in normal individuals whose removal results in normal sera becoming positive for GAD65 autoantibodies (154). The experimental system is complex, with the potential for monoclonal human anti-GAD65 autoantibodies to be eluted off of beads. Further studies are needed to test this novel finding (154).
Similarly, lack of all islet autoantibodies cannot be equated with absence of type 1A diabetes (155). As children are followed to the development of diabetes, a wide fluctuation in autoantibodies over years is often observed, with one autoantibody falling and another autoantibody increasing. A small subset of autoantibody-positive children loses expression of all autoantibodies prior to diabetes onset. If a child presents with diabetes and all four standard biochemical autoantibodies are absent, consideration of monogenic forms of diabetes is in order, as rare forms of diabetes can greatly influence choice of therapy, e.g., sulfonylureas, or prognosis, e.g., DIDMOAD (diabetes insipidus, diabetes mellitus, optic atrophy, and deafness)/Wolfram syndrome (156). It is estimated that 10% of children with diabetes lacking all islet autoantibodies have definable monogenic forms of diabetes (A. Hattersley, personal communication).
Insulin autoantibodies are unique in that their levels and frequency of positivity are inversely related to the age at which diabetes develops (157). Within several weeks of the introduction of insulin therapy, almost all individuals express insulin antibodies that cannot at present be distinguished from insulin autoantibodies. Insulin autoantibodies are often, but not always, the first autoantibody to appear in children followed from birth, followed by the appearance of GAD65, and then IA-2 and ZnT8 (153,158,159). Standard ELISAs should, in general, be avoided for the measurement of these autoantibodies (160), though a novel GAD assay using plate-bound anti-GAD autoantibody rather than plate-bound antigen (as for standard ELISAs) performs well in international workshops (161).
There will likely be need for point-of-care screening assays for islet autoantibodies, and this need will be acute if preventive therapies are introduced into clinical practice. Most of the cost of screening for islet autoantibodies now relates to shipping samples to laboratories and the paperwork involved. If point-of-care screening assays were available, it would allow direct testing in a physicians office and referral for confirmation and staging of the relatively uncommon individual expressing one or more autoantibodies. At present, the insulin autoantibody is the most difficult for laboratories to master, and most fluid-phase radioassays for GAD65, IA-2, and ZnT8 perform well in international workshops (162).
Stage IV: Progressive metabolic abnormalities.
There are multiple ways to assess metabolic progression of patients developing diabetes. One of the most specific is the intravenous glucose tolerance test. Most patients within 1 year of diabetes have a 1 + 3 min insulin secretion following a bolus of glucose less than the first percentile of normal control subjects (163). This is also one of the least convenient methodologies utilizing an intravenous injection. Oral glucose tolerance testing with measurement of glucose is also highly predictive of deterioration (164). In studies such as DAISY, we now utilize fingerstick measurement of A1C, with the great majority (but not all) of children developing diabetes demonstrating a gradual rise of A1C in the normal range within the 1 to 2 years prior to overt diabetes (165). With rising A1C, an oral glucose tolerance is utilized to confirm diabetes.
Stage V: Overt diabetes.
The development of overt type 1A diabetes is often acute (166). This is perhaps not unexpected for an autoimmune organ-specific disease, and we see similar sudden dramatic increases in ACTH in patients expressing 21 hydroxylase autoantibodies, who develop overt Addison's disease. It is likely that the majority of patients presenting with type 1A diabetes have had the disease for months to a year prior to diagnosis; they present with high A1C and at times glucose >1,000 mg/dl (117). The children who die at onset frequently have a history that the first health care providers to evaluate the child missed the diagnosis of diabetes, and a several day delay in treatment can be fatal. Such acute presentations are prevented in children pre-identified for diabetes risk in studies such as DAISY (117). Once diabetes has developed, loss of C-peptide secretion is the primary parameter to follow further disease progression (167).
Stage VI: Insulin dependence.
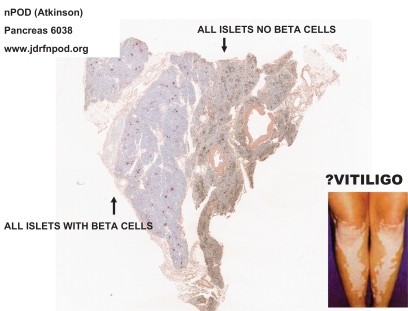
A controversy has arisen relative to the quantitative preservation of C-peptide and β-cells in patients with long-term type 1 diabetes, with important evidence of preservation in some insulin-expressing cells within the pancreas of some long-term patients with childhood-onset diabetes, as well as apoptosis of β-cells (168,169). Fortunately a large set of pancreases of cadaveric donors is rapidly becoming available for histologic analysis through the nPOD program headed by Mark Atkinson. An advantage of these pancreases is the availability of the whole pancreas for analysis of multiple regions of the organ. Pancreases from cadaveric donors with type 1 diabetes, as well as nondiabetic cadaveric donors, are being analyzed, and the slides are posted on a Web site for international scientific study (www.jdrfnpod.org). The slides can be viewed via desktop computer as if through a microscope. It is already apparent that a subset of childhood-onset patients with a clinical diagnosis of type 1 diabetes has large numbers of β-cells, with β-cells in 100% of their islets, but it is likely that these patients do not have type 1A diabetes (170), and their disease may be related to ketosis-prone forms of diabetes occurring in minority populations of the U.S (171). Most of the pancreases from patients with long-term childhood-onset diabetes in nPOD have no β-cells within islets. Approximately 10% have limited areas with insulin containing β-cells. This heterogeneity with lobular areas with β-cells remaining (reported decades ago by Foulis and coworkers studying recent-onset patients [172,173]) likely explains the slow progression to type 1A diabetes. It is reminiscent of the destruction of melanocytes in patches of skin of patients with vitiligo (Fig. 5).
FIG. 5.
nPOD pancreas 608 of cadaveric donor with long-term type 1 diabetes, showing lobular loss of β-cells. Picture provided by R. Gianani from nPOD Web site (www.jdrfnpod.org).
TRIALS FOR PREVENTION OF β-CELL DESTRUCTION
Though multiple interventions have failed to prevent either progression to type 1 diabetes or loss of β-cell function postdiagnosis, we are entering an era where several immunotherapies can almost certainly ameliorate β-cell loss (174,175), with several phase III trials underway or planned. Key information nevertheless remains lacking in terms of the long-term efficacy and safety and each therapeutic pathway, thus necessitating the need for further study. One can broadly divide therapies into those that are generally immunosuppressive and immunomodulatory versus antigen specific. The immunomodulatory/immunosuppressive trials have a higher probability of efficacy, based on current data, but likely involve greater risk. I will highlight just four therapies, each with some evidence of efficacy, given both the void of “positive” trials and paucity of large well-controlled studies.
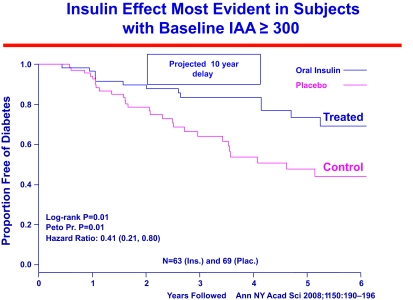
The National Institutes of Health (NIH) Diabetes Prevention Trial evaluated both parenteral insulin and oral insulin (to induce “oral tolerance”) in cytoplasmic islet autoantibody–positive relatives of patients with type 1 diabetes. Relatives were staged in terms of their expression of insulin autoantibodies, metabolic abnormalities, and presence of the protective HLA allele DQB1*0602. In addition, GAD65 and ICA512 autoantibodies were measured though not included in the prediction of disease risk. These large studies, as well as the ENDIT prevention study utilizing nicotinamide, demonstrated the ability to predict type 1 diabetes on a relatively large scale and to assign various levels of risk for disease (65,66,159,176,177). Overall, none of these studies influenced progression to diabetes, though in one subgroup of the oral insulin trial, those with high levels of insulin autoantibodies, oral insulin significantly slowed progression to diabetes (Fig. 6). Given the subgroup analysis, the NIH TrialNet group is now seeking to confirm whether oral insulin can ameliorate progression to diabetes of islet autoantibody–positive relatives expressing multiple islet autoantibodies as well as those having insulin autoantibodies.
FIG. 6.
A subgroup of islet autoantibody–positive relatives with the highest levels of insulin autoantibodies showed delayed progression to diabetes when treated with oral insulin to induce mucosal tolerance.
The primary outcome variable in trials at the onset of type 1 diabetes is preservation of C-peptide. GAD65 is a prominent target of the autoimmunity of humans, with GAD65 autoantibodies one of the best predictors of progression to diabetes. GAD65 in the adjuvant alum in a randomized placebo controlled small trial delayed loss of C-peptide, though without improving A1C or decreasing insulin requirements in the treated patients (178). Confirmatory studies of GAD65 vaccination are underway, including a new-onset TrialNet study, with plans for a prevention study.
Two studies, using single courses of two different anti-CD3 antibodies, significantly delayed the loss of C-peptide secretion (173,174). The anti-CD3 antibodies were modified to decrease cytokine release and limit acute toxicity. The delay in loss of C-peptide lasted for ∼6–12 months, followed by what appears to be resumption of C-peptide decline parallel to (though delayed) control subjects. Decreased insulin utilization was associated with decreased loss of C-peptide. There is evidence that in addition to acute and transient depletion of T cells, the therapy induces regulatory T cells and, in particular, evidence for regulatory CD8 T cells (41,179). Longer follow up and evaluation of a larger number of individuals in phase III trials will be essential to judge efficacy and potential toxicity.
A recent TrialNet study indicates that the anti–B-cell antibody, anti-CD20, as a single course, significantly delays loss of C-peptide, improves A1C, and decreases insulin requirement (180). Anti-CD20 essentially eliminates B-lymphocytes from the circulation for months to a year but does not target plasma cells. Thus, many antibodies are unchanged with this therapy, while selected autoantibodies are markedly inhibited (181). Anti-CD20 has been utilized as therapy for B-cell lymphoma and shows efficacy in multiple sclerosis and rheumatoid arthritis. Thus, there is considerably more experience with this therapy than with anti-CD3. Nevertheless, further study is essential (including more than a single drug course) to evaluate both efficacy and safety as a single agent in patients developing diabetes and those with new-onset diabetes. Newer anti-CD20 antibodies and a series of therapeutics targeting B-cells are in development. From animal studies there is evidence of regulatory B-cell induction, and B-cells themselves are likely important for the presentation of islet autoantigens (42).
To date none of the aforementioned therapies have demonstrated long-term permanent arrest of disease progression. It is remarkable that despite being such a slow destructive process, the autoimmunity underlying type 1A diabetes is so resistant to immunotherapy. It is likely that the therapies showing promise to date in the above trials do not eliminate the underlying T cell memory driving β-cell destruction, and that multiple courses of therapy or combinatorial therapies will need to be developed to achieve long-term immunologic remission (182). That said, long-term continuous immunosuppression is almost certainly not an option as a therapy for type 1 diabetes.
Approximately 1 in 300 randomly selected cadaveric donors from the general population express multiple islet autoantibodies (183,184), and thus it is likely that ∼1 million individuals in the U.S. are in the process of developing type 1 diabetes. As immunomodulatory/immunosuppressive therapies improve or, hopefully, antigen specific therapies are developed, we believe it is likely that chronic active insulitis will become a treatable entity similar to chronic active hepatitis. North American patients with new-onset diabetes and relatives of patients with type 1 diabetes can be evaluated for islet autoantibodies and participation in trials by calling 1-800-HALT-DM1 or accessing the TrialNet Web site (www.diabetestrialnet.org).
ACKNOWLEDGMENTS
This work is supported by grants from the National Institutes of Health (DK32083, DK55969, DK62718, AI50864, DK32493, DK064605), the Immune Tolerance Network, the Diabetes Endocrine Research Center (P30 DK57516), the American Diabetes Association, the Juvenile Diabetes Foundation, the Helmsley Foundation, the Children's Diabetes Foundation, and the Brehm Coalition.
G.S.E. has been a paid consultant for and has received stock options from Bayhill Therapeutics. No other potential conflicts of interest relevant to this article were reported.
I thank my many fellows, students, collaborators, and a world of investigators who generated the bulk of what I reviewed and the families who have been partners for decades in deciphering the natural history of type 1 diabetes. I thank Dr. Mark Atkinson for his excellent editing.
REFERENCES
- 1.Rewers A, Klingensmith G, Davis C, Petitti DB, Pihoker C, Rodriguez B, Schwartz ID, Imperatore G, Williams D, Dolan LM, Dabelea D: Presence of diabetic ketoacidosis at diagnosis of diabetes mellitus in youth: the Search for Diabetes in Youth Study. Pediatrics 2008; 121: e1258– e1266 [DOI] [PubMed] [Google Scholar]
- 2.Dahlquist G, Källén B: Mortality in childhood-onset type 1 diabetes: a population-based study. Diabetes Care 2005; 28: 2384– 2387 [DOI] [PubMed] [Google Scholar]
- 3.Makinen VP, Forsblom C, Thorn LM, Waden J, Kaski K, Ala-Korpela M, Groop PH: Network of vascular diseases, death and biochemical characteristics in a set of 4,197 patients with type 1 diabetes (The FinnDiane Study). Cardiovasc Diabetologia 2009; 8: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamborlane WV, Beck RW: Continuous glucose monitoring in type 1 diabetes mellitus. Lancet 2009; 373: 1744– 1746 [DOI] [PubMed] [Google Scholar]
- 5.Garg SK, Kelly WC, Voelmle MK, Ritchie PJ, Gottlieb PA, McFann KK, Ellis SL: Continuous home monitoring of glucose: improved glycemic control with real-life use of continuous glucose sensors in adult subjects with type 1 diabetes. Diabetes Care 2007; 30: 3023– 3025 [DOI] [PubMed] [Google Scholar]
- 6.Wadwa RP, Fiallo-Scharer R, Vanderwel B, Messer LH, Cobry E, Chase HP: Continuous glucose monitoring in youth with type 1 diabetes. Diabetes Technol Ther 2009; 1( Suppl. 11): S83– S91 [DOI] [PubMed] [Google Scholar]
- 7.Kandaswamy R, Sutherland DE: Pancreas versus islet transplantation in diabetes mellitus: How to allocate deceased donor pancreata? Transplant Proc 2006; 38: 365– 367 [DOI] [PubMed] [Google Scholar]
- 8.Nanji SA, Shapiro AM: Advances in pancreatic islet transplantation in humans. Diabetes Obes Metab 2006; 8: 15– 25 [DOI] [PubMed] [Google Scholar]
- 9.Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, Alejandro R, Ryan EA, DiMercurio B, Morel P, Polonsky KS, Reems JA, Bretzel RG, Bertuzzi F, Froud T, Kandaswamy R, Sutherland DE, Eisenbarth G, Segal M, Preiksaitis J, Korbutt GS, Barton FB, Viviano L, Seyfert-Margolis V, Bluestone J, Lakey JR: International trial of the Edmonton protocol for islet transplantation. N Engl J Med 2006; 355: 1318– 1330 [DOI] [PubMed] [Google Scholar]
- 10.Laughlin E, Burke G, Pugliese A, Falk B, Nepom G: Recurrence of autoreactive antigen-specific CD4+ T cells in autoimmune diabetes after pancreas transplantation. Clin Immunol 2008; 128: 23– 30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakayama M, Abiru N, Moriyama H, Babaya N, Liu E, Miao D, Yu L, Wegmann DR, Hutton JC, Elliott JF, Eisenbarth GS: Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature 2005; 435: 220– 223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hattori M, Buse JB, Jackson RA, Glimcher L, Dorf ME, Minami M, Makino S, Moriwaki K, Kuzuya H, Imura H, Seidman JG, Eisenbarth GS: The NOD mouse: recessive diabetogenic gene in the major histocompatibility complex. Science 1986; 231: 733– 735 [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi M, Jasinski J, Liu E, Li M, Miao D, Zhang L, Yu L, Nakayama M, Eisenbarth GS: Conserved T cell receptor alpha-chain induces insulin autoantibodies. Proc Natl Acad Sci U S A 2008; 105: 10090– 10094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Homann D, Eisenbarth GS: An immunologic homunculus for type 1 diabetes. J Clin Invest 2006; 116: 1212– 1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krishnamurthy B, Dudek NL, McKenzie MD, Purcell AW, Brooks AG, Gellert S, Colman PG, Harrison LC, Lew AM, Thomas HE, Kay TW: Responses against islet antigens in NOD mice are prevented by tolerance to proinsulin but not IGRP. J Clin Invest 2006; 116: 3258– 3265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaeckel E, Lipes MA, von Boehmer H: Recessive tolerance to preproinsulin 2 reduces but does not abolish type 1 diabetes. Nat Immunol 2004; 5: 1028– 1035 [DOI] [PubMed] [Google Scholar]
- 17.Thébault-Baumont K, Dubois-Laforgue D, Krief P, Briand JP, Halbout P, Vallon-Geoffroy K, Morin J, Laloux V, Lehuen A, Carel JC, Jami J, Muller S, Boitard C: Acceleration of type 1 diabetes mellitus in proinsulin 2-deficient NOD mice. J Clin Invest 2003; 111: 851– 857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukushima K, Abiru N, Nagayama Y, Kobayashi M, Satoh T, Nakahara M, Kawasaki E, Yamasaki H, Ueha S, Matsushima K, Liu E, Eguchi K: Combined insulin B:9-23 self-peptide and polyinosinic-polycytidylic acid accelerate insulitis but inhibit development of diabetes by increasing the proportion of CD4+Foxp3+ regulatory T cells in the islets in non-obese diabetic mice. Biochem Biophys Res Commun 2008; 367: 719– 724 [DOI] [PubMed] [Google Scholar]
- 19.Solvason N, Lou YP, Peters W, Evans E, Martinez J, Ramirez U, Ocampo A, Yun R, Ahmad S, Liu E, Yu L, Eisenbarth G, Leviten M, Steinman L, Garren H: Improved efficacy of a tolerizing DNA vaccine for reversal of hyperglycemia through enhancement of gene expression and localization to intracellular sites. J Immunol 2008; 181: 8298– 8307 [DOI] [PubMed] [Google Scholar]
- 20.Suri A, Levisetti MG, Unanue ER: Do the peptide-binding properties of diabetogenic class II molecules explain autoreactivity? Curr Opin Immunol 2008; 20: 105– 110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du W, Wong FS, Li MO, Peng J, Qi H, Flavell RA, Sherwin R, Wen L: TGF-beta signaling is required for the function of insulin-reactive T regulatory cells. J Clin Invest 2006; 116: 1360– 1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wegmann DR, Norbury-Glaser M, Daniel D: Insulin-specific T cells are a predominant component of islet infiltrates in pre-diabetic NOD mice. Eur J Immunol 1994; 24: 1853– 1857 [DOI] [PubMed] [Google Scholar]
- 23.Daniel D, Gill RG, Schloot N, Wegmann D: Epitope specificity, cytokine production profile and diabetogenic activity of insulin-specific T cell clones isolated from NOD mice. Eur J Immunol 1995; 25: 1056– 1062 [DOI] [PubMed] [Google Scholar]
- 24.Tsai S, Shameli A, Santamaria P: CD8+ T cells in type 1 diabetes. Adv Immunol 2008; 100: 79– 124 [DOI] [PubMed] [Google Scholar]
- 25.Wong FS, Karttunen J, Dumont C, Wen L, Visintin I, Pilip IM, Shastri N, Pamer EG, Janeway CA, Jr: Identification of an MHC class I-restricted autoantigen in type 1 diabetes by screening an organ-specific cDNA library. Nat Med 1999; 5: 1026– 1031 [DOI] [PubMed] [Google Scholar]
- 26.DiLorenzo TP, Serreze DV: The good turned ugly: immunopathogenic basis for diabetogenic CD8+ T cells in NOD mice. Immunol Rev 2005; 204: 250– 263 [DOI] [PubMed] [Google Scholar]
- 27.Moriyama H, Abiru N, Paronen J, Sikora K, Liu E, Miao D, Devendra D, Beilke J, Gianani R, Gill RG, Eisenbarth GS: Evidence for a primary islet autoantigen (preproinsulin 1) for insulitis and diabetes in the nonobese diabetic mouse. Proc Natl Acad Sci U S A 2003; 100: 10376– 10381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamamoto T, Yamato E, Tashiro F, Sato T, Noso S, Ikegami H, Tamura S, Yanagawa Y, Miyazaki JI: Development of autoimmune diabetes in glutamic acid decarboxylase 65 (GAD65) knockout NOD mice. Diabetologia 2004; 47: 221– 224 [DOI] [PubMed] [Google Scholar]
- 29.Kubosaki A, Miura J, Notkins AL: IA-2 is not required for the development of diabetes in NOD mice. Diabetologia 2004; 47: 149– 150 [DOI] [PubMed] [Google Scholar]
- 30.Kubosaki A, Gross S, Miura J, Saeki K, Zhu M, Nakamura S, Hendriks W, Notkins AL: Targeted disruption of the IA-2beta gene causes glucose intolerance and impairs insulin secretion but does not prevent the development of diabetes in NOD mice. Diabetes 2004; 53: 1684– 1691 [DOI] [PubMed] [Google Scholar]
- 31.Brimnes MK, Bonifaz L, Steinman RM, Moran TM: Influenza virus-induced dendritic cell maturation is associated with the induction of strong T cell immunity to a coadministered, normally nonimmunogenic protein. J Exp Med 2003; 198: 133– 144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakayama M, Beilke JN, Jasinski JM, Kobayashi M, Miao D, Li M, Coulombe MG, Liu E, Elliott JF, Gill RG, Eisenbarth GS: Priming and effector dependence on insulin B:9-23 peptide in NOD islet autoimmunity. J Clin Invest 2007; 117: 1835– 1843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krishnamurthy B, Mariana L, Gellert SA, Colman PG, Harrison LC, Lew AM, Santamaria P, Thomas HE, Kay TW: Autoimmunity to both proinsulin and IGRP is required for diabetes in nonobese diabetic 8.3 TCR transgenic mice. J Immunol 2008; 180: 4458– 4464 [DOI] [PubMed] [Google Scholar]
- 34.Kim SK, Tarbell KV, Sanna M, Vadeboncoeur M, Warganich T, Lee M, Davis M, McDevitt HO: Prevention of type I diabetes transfer by glutamic acid decarboxylase 65 peptide 206-220–specific T cells. Proc Natl Acad Sci U S A 2004; 101: 14204– 14209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34a.Stadinski BD, Delong T, Reisdorph N, Reisdorph R, Powell RL, Armstrong M, Piganelli JD, Barbour G, Bradley B, Crawford F, Marrack P, Mahata SK, Kappler JW, Haskins K: Chromogranin A is an autoantigen in type 1 diabetes. Nat Immunol 2010; 11: 225– 231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han B, Serra P, Amrani A, Yamanouchi J, Marée AF, Edelstein-Keshet L, Santamaria P: Prevention of diabetes by manipulation of anti-IGRP autoimmunity: high efficiency of a low-affinity peptide. Nat Med 2005; 11: 645– 652 [DOI] [PubMed] [Google Scholar]
- 36.Pop SM, Wong CP, He Q, Wang Y, Wallet MA, Goudy KS, Tisch R: The type and frequency of immunoregulatory CD4+ T cells govern the efficacy of antigen-specific immunotherapy in nonobese diabetic mice. Diabetes 2007; 56: 1395– 1402 [DOI] [PubMed] [Google Scholar]
- 37.Mukherjee R, Chaturvedi P, Qin HY, Singh B: CD4+CD25+ regulatory T cells generated in response to insulin B:9-23 peptide prevent adoptive transfer of diabetes by diabetogenic T cells. J Autoimmun 2003; 21: 221– 237 [DOI] [PubMed] [Google Scholar]
- 38.Tang Q, Bluestone JA: The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol 2008; 9: 239– 244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanazawa Y, Shimada A, Oikawa Y, Okubo Y, Tada A, Imai T, Miyazaki J, Itoh H: Induction of anti-whole GAD65 reactivity in vivo results in disease suppression in type 1 diabetes. J Autoimmun 2009; 32: 104– 109 [DOI] [PubMed] [Google Scholar]
- 40.Bresson D, Togher L, Rodrigo E, Chen Y, Bluestone JA, Herold KC, von Herrath M: Anti-CD3 and nasal proinsulin combination therapy enhances remission from recent-onset autoimmune diabetes by inducing Tregs. J Clin Invest 2006; 116: 1371– 1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ablamunits V, Bisikirska BC, Herold KC: Human regulatory CD8 T cells. Ann N Y Acad Sci 2008; 1150: 234– 238 [DOI] [PubMed] [Google Scholar]
- 42.Hu CY, Rodriguez-Pinto D, Du W, Ahuja A, Henegariu O, Wong FS, Shlomchik MJ, Wen L: Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J Clin Invest 2007; 117: 3857– 3867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simone EA, Wegmann DR, Eisenbarth GS: Immunologic “vaccination” for the prevention of autoimmune diabetes mellitus (type 1A). Diabetes Care 1999; 22( Suppl. 2): B7– B15 [PubMed] [Google Scholar]
- 44.Kobayashi M, Abiru N, Arakawa T, Fukushima K, Zhou H, Kawasaki E, Yamasaki H, Liu E, Miao D, Wong FS, Eisenbarth GS, Eguchi K: Altered B:9 23 insulin, when administered intranasally with cholera toxin adjuvant, suppresses the expression of insulin autoantibodies and prevents diabetes. J Immunol 2007; 179: 2082– 2088 [DOI] [PubMed] [Google Scholar]
- 45.Abiru N, Yu L, Wegmann D, Eisenbarth GS: Induction of autoantibodies to native insulin in normal and NOD derived mice following immunization with the immunodominant self-B-chain insulin peptide B:9-23 (Abstract). Diabetes 2000; 49( Suppl. 1): A232 [Google Scholar]
- 46.Abiru N, Maniatis AK, Yu L, Miao D, Moriyama H, Wegmann D, Eisenbarth GS: Peptide and MHC specific breaking of humoral tolerance to native insulin with the B:9-23 peptide in diabetes prone and normal mice. Diabetes 2001; 50: 1274– 1281 [DOI] [PubMed] [Google Scholar]
- 47.Devendra D, Miao D, Nakayama M, Eisenbarth GS, Liu E: Pancreatic autoimmunity induction with insulin B:9-23 peptide and viral mimics in the NZB mouse. Ann N Y Acad Sci 2006; 1079: 135– 137 [DOI] [PubMed] [Google Scholar]
- 48.Eisenbarth SG, Homann D: Primer immunology and autoimmunity. In Type I Diabetes: Molecular, Cellular and Clinical Immunology [article online], 2009. Available from http://www.uchsc.edu/misc/diabetes/books.html Accessed 20 February 2010
- 49.Pietropaolo M, Surhigh JM, Nelson PW, Eisenbarth GS: Primer: immunity and autoimmunity. Diabetes 2008; 57: 2872– 2882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Erlich H, Valdes AM, Noble J, Carlson JA, Varney M, Concannon P, Mychaleckyj JC, Todd JA, Bonella P, Fear AL, Lavant E, Louey A, Moonsamy P: Type 1 Diabetes Genetics Consortium. HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: analysis of the Type 1 Diabetes Genetics Consortium families. Diabetes 2008; 57: 1084– 1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wallis RH, Wang K, Marandi L, Hsieh E, Ning T, Chao GY, Sarmiento J, Paterson AD, Poussier P: Type 1 diabetes in the BB rat: a polygenic disease. Diabetes 2009; 58: 1007– 1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Todd JA, Acha-Orbea H, Bell JI, Chao N, Fronek Z, Jacob CO, McDermott M, Sinha AA, Timmerman L, Steinman L: A molecular basis for MHC class II–associated autoimmunity. Science 1988; 240: 1003– 1009 [DOI] [PubMed] [Google Scholar]
- 53.Corper AL, Stratmann T, Apostolopoulos V, Scott CA, Garcia KC, Kang AS, Wilson IA, Teyton L: A structural framework for deciphering the link between I-Ag7 and autoimmune diabetes. Science 2000; 288: 505– 511 [DOI] [PubMed] [Google Scholar]
- 54.Simone E, Daniel D, Schloot N, Gottlieb P, Babu S, Kawasaki E, Wegmann D, Eisenbarth GS: T cell receptor restriction of diabetogenic autoimmune NOD T cells. Proc Natl Acad Sci U S A 1997; 94: 2518– 2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burton AR, Vincent E, Arnold PY, Lennon GP, Smeltzer M, Li CS, Haskins K, Hutton J, Tisch RM, Sercarz EE, Santamaria P, Workman CJ, Vignali DA: On the pathogenicity of autoantigen-specific T cell receptors. Diabetes 2008; 57: 1321– 1330 [DOI] [PubMed] [Google Scholar]
- 56.Zhang L, Jasinski JM, Kobayashi M, Davenport B, Johnson K, Davidson H, Nakayama M, Haskins K, Eisenbarth GS: Analysis of T cell receptor beta chains that combine with dominant conserved TRAV5D-4*04 anti-insulin B:9-23 alpha chains. J Autoimmun 2009; 33: 42– 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wicker LS, Clark J, Fraser HI, Garner VE, Gonzalez-Munoz A, Healy B, Howlett S, Hunter K, Rainbow D, Rosa RL, Smink LJ, Todd JA, Peterson LB: Type 1 diabetes genes and pathways shared by humans and NOD mice. J Autoimmun 2005; 25( Suppl.): 29– 33 [DOI] [PubMed] [Google Scholar]
- 58.Rainbow DB, Esposito L, Howlett SK, Hunter KM, Todd JA, Peterson LB, Wicker LS: Commonality in the genetic control of type 1 diabetes in humans and NOD mice: variants of genes in the IL-2 pathway are associated with autoimmune diabetes in both species. Biochem Soc Trans 2008; 36: 312– 315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hernández Prada JA, Ferreira AJ, Katovich MJ, Shenoy V, Qi Y, Santos RA, Castellano RK, Lampkins AJ, Gubala V, Ostrov DA, Raizada MK: Structure-based identification of small-molecule angiotensin-converting enzyme 2 activators as novel antihypertensive agents. Hypertension 2008; 51: 1312– 1317 [DOI] [PubMed] [Google Scholar]
- 60.Eisenbarth GS: Type I diabetes mellitus: a chronic autoimmune disease. N Engl J Med 1986; 314: 1360– 1368 [DOI] [PubMed] [Google Scholar]
- 61.Atkinson MA, Eisenbarth GS: Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet 2001; 358: 221– 229 [DOI] [PubMed] [Google Scholar]
- 62.Redondo MJ, Jeffrey J, Fain PR, Eisenbarth GS, Orban T: Concordance for islet autoimmunity among monozygotic twins. N Engl J Med 2008; 359: 2849– 2850 [DOI] [PubMed] [Google Scholar]
- 63.Verge CF, Gianani R, Kawasaki E, Yu L, Pietropaolo M, Jackson RA, Chase HP, Eisenbarth GS: Prediction of type I diabetes in first-degree relatives using a combination of insulin, GAD, and ICA512bdc/IA-2 autoantibodies. Diabetes 1996; 45: 926– 933 [DOI] [PubMed] [Google Scholar]
- 64.Achenbach P, Schlosser M, Williams AJ, Yu L, Mueller PW, Bingley PJ, Bonifacio E: Combined testing of antibody titer and affinity improves insulin autoantibody measurement: Diabetes Antibody Standardization Program. Clin Immunol 2007; 122: 85– 90 [DOI] [PubMed] [Google Scholar]
- 65.Bingley PJ, Mahon JL, Gale EA: European Nicotinamide Diabetes Intervention Trial Group. Insulin resistance and progression to type 1 diabetes in the European Nicotinamide Diabetes Intervention Trial (ENDIT). Diabetes Care 2008; 31: 146– 150 [DOI] [PubMed] [Google Scholar]
- 66.Skyler JS, Krischer JP, Wolfsdorf J, Cowie C, Palmer JP, Greenbaum C, Cuthbertson D, Rafkin-Mervis LE, Chase HP, Leschek E: Effects of oral insulin in relatives of patients with type 1 diabetes: The Diabetes Prevention Trial–Type 1. Diabetes Care 2005; 28: 1068– 1076 [DOI] [PubMed] [Google Scholar]
- 67.Hagopian WA, Lernmark A, Rewers MJ, Simell OG, She JX, Ziegler AG, Krischer JP, Akolkar B: TEDDY–The Environmental Determinants of Diabetes in the Young: an observational clinical trial. Ann N Y Acad Sci 2006; 1079: 320– 326 [DOI] [PubMed] [Google Scholar]
- 68.Perheentupa J: Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J Clin Endocrinol Metab 2006; 91: 2843– 2850 [DOI] [PubMed] [Google Scholar]
- 69.Eisenbarth GS, Gottlieb PA: Autoimmune polyendocrine syndromes. N Engl J Med 2004; 350: 2068– 2079 [DOI] [PubMed] [Google Scholar]
- 70.Bacchetta R, Passerini L, Gambineri E, Dai M, Allan SE, Perroni L, Dagna-Bricarelli F, Sartirana C, Matthes-Martin S, Lawitschka A, Azzari C, Ziegler SF, Levings MK, Roncarolo MG: Defective regulatory and effector T cell functions in patients with FOXP3 mutations. J Clin Invest 2006; 116: 1713– 1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rao A, Kamani N, Filipovich A, Lee SM, Davies SM, Dalal J, Shenoy S: Successful bone marrow transplantation for IPEX syndrome after reduced-intensity conditioning. Blood 2007; 109: 383– 385 [DOI] [PubMed] [Google Scholar]
- 72.Su MA, Giang K, Zumer K, Jiang H, Oven I, Rinn JL, Devoss JJ, Johannes KP, Lu W, Gardner J, Chang A, Bubulya P, Chang HY, Peterlin BM, Anderson MS: Mechanisms of an autoimmunity syndrome in mice caused by a dominant mutation in Aire. J Clin Invest 2008; 118: 1712– 1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D: Projection of an immunological self shadow within the thymus by the aire protein. Science 2002; 298: 1395– 1401 [DOI] [PubMed] [Google Scholar]
- 74.Kont V, Laan M, Kisand K, Merits A, Scott HS, Peterson P: Modulation of Aire regulates the expression of tissue-restricted antigens. Molecular Immunology 2008; 45: 25– 33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meloni A, Furcas M, Cetani F, Marcocci C, Falorni A, Perniola R, Pura M, Bøe Wolff AS, Husebye ES, Lilic D, Ryan KR, Gennery AR, Cant AJ, Abinun M, Spickett GP, Arkwright PD, Denning D, Costigan C, Dominguez M, McConnell V, Willcox N, Meager A: Autoantibodies against type I interferons as an additional diagnostic criterion for autoimmune polyendocrine syndrome type I. J Clin Endocrinol Metab 2008; 93: 4389– 4397 [DOI] [PubMed] [Google Scholar]
- 76.Adamson KA, Cheetham TD, Kendall-Taylor P, Seckl JR, Pearce SH: The role of the IDDM2 locus in the susceptibility of UK APS1 subjects to type 1 diabetes mellitus. Int J Immunogenet 2007; 34: 17– 21 [DOI] [PubMed] [Google Scholar]
- 77.Maruyama T, Shimada A, Kasuga A, Kasatani T, Ozawa Y, Ishii M, Takei I, Suzuki Y, Kobayashi A, Takeda S: Analysis of MHC class II antigens in Japanese IDDM by a novel HLA-typing method, hybridization protection assay. Diabetes Res Clin Pract 1994; 23: 77– 84 [DOI] [PubMed] [Google Scholar]
- 78.Nerup J: Addison's disease—clinical studies: a report of 108 cases. Acta Endocrinol 1974; 76: 127– 141 [DOI] [PubMed] [Google Scholar]
- 79.Betterle C, Lazzarotto F, Presotto F: Autoimmune polyglandular syndrome type 2: the tip of an iceberg? Clin Exp Immunol 2004; 137: 225– 233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yu L, Brewer KW, Gates S, Wu A, Wang T, Babu SR, Gottlieb PA, Freed BM, Noble J, Erlich HA, Rewers MJ, Eisenbarth GS: DRB1*04 and DQ alleles: expression of 21-hydroxylase autoantibodies and risk of progression to Addison's disease. J Clin Endocrinol Metab 1999; 84: 328– 335 [DOI] [PubMed] [Google Scholar]
- 81.Myhre AG, Undlien DE, Løvås K, Uhlving S, Nedrebø BG, Fougner KJ, Trovik T, Sørheim JI, Husebye ES: Autoimmune adrenocortical failure in Norway: Autoantibodies and HLA class II associations related to clinical features. J Clin Endocrinol Metab 2002; 87: 618– 623 [DOI] [PubMed] [Google Scholar]
- 82.Barker JM: Clinical review: Type 1 diabetes-associated autoimmunity: natural history, genetic associations, and screening. J Clin Endocrinol Metab 2006; 91: 1210– 1217 [DOI] [PubMed] [Google Scholar]
- 83.Barker JM: Clinical review: Type 1 diabetes–associated autoimmunity: natural history, genetic associations and screening. J Clin Endocrinol Metab 2006; 91: 1210– 1217 [DOI] [PubMed] [Google Scholar]
- 84.Concannon P, Rich SS, Nepom GT: Genetics of type 1A diabetes. N Engl J Med 2009; 360: 1646– 1654 [DOI] [PubMed] [Google Scholar]
- 85.Hulbert EM, Smink LJ, Adlem EC, Allen JE, Burdick DB, Burren OS, Cassen VM, Cavnor CC, Dolman GE, Flamez D, Friery KF, Healy BC, Killcoyne SA, Kutlu B, Schuilenburg H, Walker NM, Mychaleckyj J, Eizirik DL, Wicker LS, Todd JA, Goodman N: T1DBase: integration and presentation of complex data for type 1 diabetes research. Nucleic Acid Res 2007; 35: D742– D746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Redondo MJ, Fain PR, Krischer JP, Yu L, Cuthbertson D, Winter WE, Eisenbarth GS: DPT-1 Study Group. Expression of beta-cell autoimmunity does not differ between potential dizygotic twins and siblings of patients with type 1 diabetes. J Autoimmun 2004; 23: 275– 279 [DOI] [PubMed] [Google Scholar]
- 87.Barker JM, Barriga KJ, Yu L, Miao D, Erlich HA, Norris JM, Eisenbarth GS, Rewers M: Diabetes Autoimmunity Study in the Young. Prediction of autoantibody positivity and progression to type 1 diabetes: Diabetes Autoimmunity Study in the Young (DAISY). J Clin Endocrinol Metab 2004; 89: 3896– 3902 [DOI] [PubMed] [Google Scholar]
- 88.Thomson G, Valdes AM, Noble JA, Kockum I, Grote MN, Najman J, Erlich HA, Cucca F, Pugliese A, Steenkiste A, Dorman JS, Caillat-Zucman S, Hermann R, Ilonen J, Lambert AP, Bingley PJ, Gillespie KM, Lernmark A, Sanjeevi CB, Rønningen KS, Undlien DE, Thorsby E, Petrone A, Buzzetti R, Koeleman BP, Roep BO, Saruhan-Direskeneli G, Uyar FA, Günoz H, Gorodezky C, Alaez C, Boehm BO, Mlynarski W, Ikegami H, Berrino M, Fasano ME, Dametto E, Israel S, Brautbar C, Santiago-Cortes A, Frazer de Llado T, She JX, Bugawan TL, Rotter JI, Raffel L, Zeidler A, Leyva-Cobian F, Hawkins BR, Chan SH, Castano L, Pociot F, Nerup J: Relative predispositional effects of HLA class II DRB1-DQB1 haplotypes and genotypes on type 1 diabetes: a meta-analysis. Tissue Antigens 2007; 70: 110– 127 [DOI] [PubMed] [Google Scholar]
- 89.Barker JM, Triolo TM, Aly TA, Baschal EE, Babu SR, Kretowski A, Rewers MJ, Eisenbarth GS: Two single nucleotide polymorphisms identify the highest-risk diabetes HLA genotype: potential for rapid screening. Diabetes 2008; 57: 3152– 3155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Baschal EE, Aly TA, Babu SR, Fernando MS, Yu L, Miao D, Barriga KJ, Norris JM, Noble JA, Erlich HA, Rewers MJ, Eisenbarth GS: HLA-DPB1*0402 protects against type 1A diabetic autoimmunity in the highest risk DR3-DQB1*0201/DR4-DQB1*0302 DAISY population. Diabetes 2007; 56: 2405– 2409 [DOI] [PubMed] [Google Scholar]
- 91.Rewers M, Bugawan TL, Norris JM, Blair A, Beaty B, Hoffman M, McDuffie RS, Jr, Hamman RF, Klingensmith G, Eisenbarth GS, Erlich HA: Newborn screening for HLA markers associated with IDDM: diabetes autoimmunity study in the young (DAISY). Diabetologia 1996; 39: 807– 812 [DOI] [PubMed] [Google Scholar]
- 92.Aly TA, Ide A, Jahromi MM, Barker JM, Fernando MS, Babu SR, Yu L, Miao D, Erlich HA, Fain PR, Barriga KJ, Norris JM, Rewers MJ, Eisenbarth GS: Extreme genetic risk for type 1A diabetes. Proc Natl Acad Sci U S A 2006; 103: 14074– 14079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aly TA, Baschal EE, Jahromi MM, Fernando MS, Babu SR, Fingerlin TE, Kretowski A, Erlich HA, Fain PR, Rewers MJ, Eisenbarth GS: Analysis of single nucleotide polymorphisms identifies major type 1A diabetes locus telomeric of the major histocompatibility complex. Diabetes 2008; 57: 770– 776 [DOI] [PubMed] [Google Scholar]
- 94.Nejentsev S, Howson JM, Walker NM, Szeszko J, Field SF, Stevens HE, Reynolds P, Hardy M, King E, Masters J, Hulme J, Maier LM, Smyth D, Bailey R, Cooper JD, Ribas G, Campbell RD, Clayton DG, Todd JA: Wellcome Trust Case Control Consortium. Localization of type 1 diabetes susceptibility to the MHC class I genes HLA-B and HLA-A. Nature 2007; 450: 887– 892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Howson JM, Walker NM, Clayton D, Todd JA: Confirmation of HLA class II independent type 1 diabetes associations in the major histocompatibility complex including HLA-B and HLA-A. Diabetes Obes Metab 2009; 11( Suppl. 1): 31– 45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Baschal EE, Aly TA, Jasinski JM, Steck AK, Noble JA, Erlich HA, Eisenbarth GS: Type 1 Diabetes Genetics Consortium. Defining multiple common “completely” conserved major histocompatibility complex SNP haplotypes. Clin Immunol 2009; 132: 203– 214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Raum D, Awdeh Z, Yunis EJ, Alper CA, Gabbay KH: Extended major histocompatibility complex haplotypes in type I diabetes mellitus. J Clin Invest 1984; 74: 449– 454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Aly TA, Eller E, Ide A, Gowan K, Babu SR, Erlich HA, Rewers MJ, Eisenbarth GS, Fain PR: Multi-SNP analysis of MHC region: remarkable conservation of HLA-A1–B8-DR3 haplotype. Diabetes 2006; 55: 1265– 1269 [DOI] [PubMed] [Google Scholar]
- 99.Bilbao JR, Calvo B, Aransay AM, Martin-Pagola A, Perez de Nanclares G, Aly TA, Rica I, Vitoria JC, Gaztambide S, Noble J, Fain PR, Awdeh ZL, Alper CA, Castaño L: Conserved extended haplotypes discriminate HLA-DR3-homozygous Basque patients with type 1 diabetes mellitus and celiac disease. Genes Immun 2006; 7: 550– 554 [DOI] [PubMed] [Google Scholar]
- 100.Baschal EE, Aly TA, Jasinski JM, Steck AK, Johnson KN, Noble JA, Erlich HA, Eisenbarth GS: The frequent and conserved DR3–B8-A1 extended haplotype confers less diabetes risk than other DR3 haplotypes. Diabetes Obes Metab 2009; 11( Suppl .1): 25– 30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.International Multiple Sclerosis Genetics Consortium. Hafler DA, Compston A, Sawcer S, Lander ES, Daly MJ, De Jager PL, de Bakker PI, Gabriel SB, Mirel DB, Ivinson AJ, Pericak-Vance MA, Gregory SG, Rioux JD, McCauley JL, Haines JL, Barcellos LF, Cree B, Oksenberg JR, Hauser SL: Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med 2007; 357: 851– 862 [DOI] [PubMed] [Google Scholar]
- 102.Grant SF, Qu HQ, Bradfield JP, Marchand L, Kim CE, Glessner JT, Grabs R, Taback SP, Frackelton EC, Eckert AW, Annaiah K, Lawson ML, Otieno FG, Santa E, Shaner JL, Smith RM, Skraban R, Imielinski M, Chiavacci RM, Grundmeier RW, Stanley CA, Kirsch SE, Waggott D, Paterson AD, Monos DS, DCCT/EDIC Research Group. Polychronakos C, Hakonarson H: Follow-up analysis of genome-wide association data identifies novel loci for type 1 diabetes. Diabetes 2009; 58: 290– 295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pugliese A, Zeller M, Fernandez A, Jr, Zalcberg LJ, Bartlett RJ, Ricordi C, Pietropaolo M, Eisenbarth GS, Bennett ST, Patel DD: The insulin gene is transcribed in the human thymus and transcription levels correlated with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nat Genet 1997; 15: 293– 297 [DOI] [PubMed] [Google Scholar]
- 104.Vafiadis P, Bennett ST, Todd JA, Nadeau J, Grabs R, Goodyer CG, Wickramasinghe S, Colle E, Polychronakos C: Insulin expression in human thymus is modulated by INS VNTR alleles at the IDDM2 locus. Nat Genet 1997; 15: 289– 292 [DOI] [PubMed] [Google Scholar]
- 105.Zoledziewska M, Perra C, Orrù V, Moi L, Frongia P, Congia M, Bottini N, Cucca F: Further evidence of a primary, causal association of the PTPN22 620W variant with type 1 diabetes. Diabetes 2008; 57: 229– 234 [DOI] [PubMed] [Google Scholar]
- 106.Bottini N, Musumeci L, Alonso A, Rahmouni S, Nika K, Rostamkhani M, MacMurray J, Meloni GF, Lucarelli P, Pellecchia M, Eisenbarth GS, Comings D, Mustelin T: A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat Genet 2004; 36: 337– 338 [DOI] [PubMed] [Google Scholar]
- 107.Vang T, Congia M, Macis MD, Musumeci L, Orrú V, Zavattari P, Nika K, Tautz L, Taskén K, Cucca F, Mustelin T, Bottini N: Autoimmune-associated lymphoid tyrosine phosphatase is a gain-of-function variant. Nat Genet 2005; 37: 1317– 1319 [DOI] [PubMed] [Google Scholar]
- 108.Liu S, Wang H, Jin Y, Podolsky R, Reddy MV, Pedersen J, Bode B, Reed J, Steed D, Anderson S, Yang P, Muir A, Steed L, Hopkins D, Huang Y, Purohit S, Wang CY, Steck AK, Montemari A, Eisenbarth G, Rewers M, She JX: IFIH1 polymorphisms are significantly associated with type 1 diabetes and IFIH1 gene expression in peripheral blood mononuclear cells. Hum Mol Genet 2009; 18: 358– 365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Field SF, Howson JM, Smyth DJ, Walker NM, Dunger DB, Todd JA: Analysis of the type 2 diabetes gene, TCF7L2, in 13,795 type 1 diabetes cases and control subjects. Diabetologia 2007; 50: 212– 213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yu J, Steck AK, Babu S, Yu L, Miao D, McFann K, Hutton J, Eisenbarth GS, Klingensmith G: Single nucleotide transcription factor 7-like 2 (TCF7L2) gene polymorphisms in antiislet autoantibody-negative patients at onset of diabetes. J Clin Endocrinol Metab 2009; 94: 504– 510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cervin C, Lyssenko V, Bakhtadze E, Lindholm E, Nilsson P, Tuomi T, Cilio CM, Groop L: Genetic similarities between latent autoimmune diabetes in adults, type 1 diabetes, and type 2 diabetes. Diabetes 2008; 57: 1433– 1437 [DOI] [PubMed] [Google Scholar]
- 112.Steck AK, Eisenbarth GS: Genetic similarities between latent autoimmune diabetes and type 1 and type 2 diabetes. Diabetes 2008; 57: 1160– 1162 [DOI] [PubMed] [Google Scholar]
- 113.Steck AK, Liu SY, McFann K, Barriga KJ, Babu SR, Eisenbarth GS, Rewers MJ, She JX: Association of the PTPN22/LYP gene with type 1 diabetes. Pediatr Diabetes 2006; 7: 274– 278 [DOI] [PubMed] [Google Scholar]
- 114.Bjørnvold M, Undlien DE, Joner G, Dahl-Jørgensen K, Njølstad PR, Akselsen HE, Gervin K, Rønningen KS, Stene LC: Joint effects of HLA INS, PTPN22 and CTLA4 genes on the risk of type 1 diabetes. Diabetologia 2008; 51: 589– 596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gillespie KM, Bain SC, Barnett AH, Bingley PJ, Christie MR, Gill GV, Gale EA: The rising incidence of childhood type 1 diabetes and reduced contribution of high-risk HLA haplotypes. Lancet 2004; 364: 1699– 1700 [DOI] [PubMed] [Google Scholar]
- 116.Vehik K, Hamman RF, Lezotte D, Norris JM, Klingensmith GJ, Rewers M, Dabelea D: Trends in high-risk HLA susceptibility genes among Colorado youth with type 1 diabetes. Diabetes Care 2008; 31: 1392– 1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Barker JM, Goehrig SH, Barriga K, Hoffman M, Slover R, Eisenbarth GS, Norris JM, Klingensmith GJ, Rewers M: DAISY study Clinical characteristics of children diagnosed with type 1 diabetes through intensive screening and follow-up. Diabetes Care 2004; 27: 1399– 1404 [DOI] [PubMed] [Google Scholar]
- 118.Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G: EURODIAB Study Group Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet 2009; 373: 2027– 2033 [DOI] [PubMed] [Google Scholar]
- 119.Harjutsalo V, Sjöberg L, Tuomilehto J: Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet 2008; 371: 1777– 1782 [DOI] [PubMed] [Google Scholar]
- 120.Writing Group for the SEARCH for Diabetes in Youth Study Group. Dabelea D, Bell RA, D'Agostino RB, Jr, Imperatore G, Johansen JM, Linder B, Liu LL, Loots B, Marcovina S, Mayer-Davis EJ, Pettitt DJ, Waitzfelder B: Incidence of diabetes in youth in the United States. JAMA 2007; 297: 2716– 2724 [DOI] [PubMed] [Google Scholar]
- 121.Bach JF: The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med 2002; 347: 911– 920 [DOI] [PubMed] [Google Scholar]
- 122.Gale EA: A missing link in the hygiene hypothesis? Diabetologia 2002; 45: 588– 594 [DOI] [PubMed] [Google Scholar]
- 123.Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, Hu C, Wong FS, Szot GL, Bluestone JA, Gordon JI, Chervonsky AV: Innate immunity and intestinal microbiota in the development of type 1 diabetes. Nature 2008; 455: 1109– 1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gale EA: To boldly go—or to go too boldly? The accelerator hypothesis revisited. Diabetologia 2007; 50: 1571– 1575 [DOI] [PubMed] [Google Scholar]
- 125.Fourlanos S, Harrison LC, Colman PG: The accelerator hypothesis and increasing incidence of type 1 diabetes. Curr Opin Endocrinol Diabetes Obes 2008; 15: 321– 325 [DOI] [PubMed] [Google Scholar]
- 126.Raj SM, Howson JM, Walker NM, Cooper JD, Smyth DJ, Field SF, Stevens HE, Todd JA: No association of multiple type 2 diabetes loci with type 1 diabetes. Diabetologia 2009; 52: 2109– 2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fourlanos S, Narendran P, Byrnes GB, Colman PG, Harrison LC: Insulin resistance is a risk factor for progression to type 1 diabetes. Diabetologia 2004; 47: 1661– 1667 [DOI] [PubMed] [Google Scholar]
- 128.Li Q, Xu B, Michie SA, Rubins KH, Schreriber RD, McDevitt HO: Interferon-alpha initiates type 1 diabetes in nonobese diabetic mice. Proc Natl Acad Sci U S A 2008; 105: 12439– 12444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schreuder TC, Gelderblom HC, Weegink CJ, Hamann D, Reesink HW, Devries JH, Hoekstra JB, Jansen PL: High incidence of type 1 diabetes mellitus during or shortly after treatment with pegylated interferon alpha for chronic hepatitis C virus infection. Liver Int 2008; 28: 39– 46 [DOI] [PubMed] [Google Scholar]
- 130.Weintrob N, Sprecher E, Israel S, Pinhas-Hamiel O, Kwon OJ, Bloch K, Abramov N, Arbel A, Josefsberg Z, Brautbar C, Vardi P: Type 1 diabetes environmental factors and correspondence analysis of HLA class II genes in the Yemenite Jewish community in Israel. Diabetes Care 2001; 24: 650– 653 [DOI] [PubMed] [Google Scholar]
- 131.Ito Y, Nieda M, Uchigata Y, Nishimura M, Tokunaga K, Kuwata S, Obata F, Tadokoro K, Hirata Y, Omori Y: Recognition of human insulin in the context of HLA-DRB1*0406 products by T cells of insulin autoimmune syndrome patients and healthy donors. J Immunol 1993; 151: 5770– 5776 [PubMed] [Google Scholar]
- 132.Uchigata Y, Hirata Y, Omori Y, Iwamoto Y, Tokunaga K: Worldwide differences in the incidence of insulin autoimmune syndrome (Hirata disease) with respect to the evolution of HLA-DR4 alleles. Hum Immunol 2000; 61: 154– 157 [DOI] [PubMed] [Google Scholar]
- 133.Vardi P, Brik R, Barzilai D, Lorber M, Scharf Y: Frequent induction of insulin autoantibodies by D-penicillamine in patients with rheumatoid arthritis. J Rheumatol 1992; 19: 1527– 1530 [PubMed] [Google Scholar]
- 134.Furukawa N, Miyamura N, Nishida K, Motoshima H, Taketa K, Araki E: Possible relevance of alpha lipoic acid contained in a health supplement in a case of insulin autoimmune syndrome. Diabetes Res Clin Pract 2007; 75: 366– 367 [DOI] [PubMed] [Google Scholar]
- 135.Akerblom HK, Virtanen SM, Ilonen J, Savilahti E, Vaarala O, Reunanen A, Teramo K, Hämäläinen AM, Paronen J, Riikjärv MA, Ormisson A, Ludvigsson J, Dosch HM, Hakulinen T, Knip M: National TRIGR Study Groups. Dietary manipulation of beta cell autoimmunity in infants at increased risk of type 1 diabetes: a pilot study. Diabetologia 2005; 48: 829– 837 [DOI] [PubMed] [Google Scholar]
- 136.Poole JA, Barriga K, Leung DY, Hoffman M, Eisenbarth GS, Rewers M, Norris JM: Timing of initial exposure to cereal grains and the risk of wheat allergy. Pediatrics 2006; 117: 2175– 2182 [DOI] [PubMed] [Google Scholar]
- 137.Ziegler AG, Schmid S, Huber D, Hummel M, Bonifacio E: Early infant feeding and risk of developing type 1 diabetes-associated autoantibodies. JAMA 2003; 290: 1721– 1728 [DOI] [PubMed] [Google Scholar]
- 138.Norris JM, Yin X, Lamb MM, Barriga K, Seifert J, Hoffman M, Orton HD, Barón AE, Clare-Salzler M, Chase HP, Szabo NJ, Erlich H, Eisenbarth GS, Rewers M: Omega-3 polyunsaturated fatty acid intake and islet autoimmunity in children at increased risk for type 1 diabetes. JAMA 2007; 298: 1420– 1428 [DOI] [PubMed] [Google Scholar]
- 139.Stene LC, Joner G: Norwegian Childhood Diabetes Study Group. Use of cod liver oil during the first year of life is associated with lower risk of childhood-onset type 1 diabetes: a large, population-based, case-control study. Am J Clin Nutr 2003; 78: 1128– 1134 [DOI] [PubMed] [Google Scholar]
- 140.Oresic M, Simell S, Sysi-Aho M, Näntö-Salonen K, Seppänen-Laakso T, Parikka V, Katajamaa M, Hekkala A, Mattila I, Keskinen P, Yetukuri L, Reinikainen A, Lähde J, Suortti T, Hakalax J, Simell T, Hyöty H, Veijola R, Ilonen J, Lahesmaa R, Knip M, Simell O: Dysregulation of lipid and amino acid metabolism precedes islet autoimmunity in children who later progress to type 1 diabetes. J Exp Med 2008; 205: 2975– 2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang X, Jia S, Geoffrey R, Alemzadeh R, Ghosh S, Hessner MJ: Identification of a molecular signature in human type 1 diabetes mellitus using serum and functional genomics. J Immunol 2008; 180: 1929– 1937 [DOI] [PubMed] [Google Scholar]
- 142.Salminen KK, Vuorinen T, Oikarinen S, Helminen M, Simell S, Knip M, Ilonen J, Simell O, Hyöty H: Isolation of enterovirus strains from children with preclinical type 1 diabetes. Diabet Med 2004; 21: 156– 164 [DOI] [PubMed] [Google Scholar]
- 143.Graves PM, Rotbart HA, Nix WA, Pallansch MA, Erlich HA, Norris JM, Hoffman M, Eisenbarth GS, Rewers M: Prospective study of enteroviral infections and development of beta-cell autoimmunity: Diabetes Autoimmunity Study in the Young (DAISY). Diabetes Res Clin Pract 2003; 59: 51– 61 [DOI] [PubMed] [Google Scholar]
- 144.Guberski DL, Thomas VA, Shek WR, Like AA, Handler ES, Rossini AA, Wallace JE, Welsh RM: Induction of type I diabetes by Kilham's rat virus in diabetes-resistant BB/Wor rats. Science 1991; 254: 1010– 1013 [DOI] [PubMed] [Google Scholar]
- 145.Blankenhorn EP, Descipio C, Rodemich L, Cort L, Leif JH, Greiner DL, Mordes JP: Refinement of the Iddm4 diabetes susceptibility locus reveals TCRVbeta4 as a candidate gene. Ann N Y Acad Sci 2007; 1103: 128– 131 [DOI] [PubMed] [Google Scholar]
- 146.Zipris D, Lien E, Nair A, Xie JX, Greiner DL, Mordes JP, Rossini AA: TLR9-signaling pathways are involved in Kilham rat virus-induced autoimmune diabetes in the biobreeding diabetes-resistant rat. J Immunol 2007; 178: 693– 701 [DOI] [PubMed] [Google Scholar]
- 147.Devendra D, Jasinski J, Melanitou E, Nakayama M, Li M, Hensley B, Paronen J, Moriyama H, Miao D, Eisenbarth GS, Liu E: Interferon-α as a mediator of polyinosinic:polycytidylic acid–induced type 1 diabetes. Diabetes 2005; 54: 2549– 2556 [DOI] [PubMed] [Google Scholar]
- 148.Bottazzo GF, Florin-Christensen A, Doniach D: Islet-cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet 1974; 2: 1279– 1283 [DOI] [PubMed] [Google Scholar]
- 149.Yu L, Eisenbarth GS: Humoral autoimmunity in type 1 diabetes: cellular, molecular and clinical immunology [article online], 2009. Available from http://www.uchsc.edu/misc/diabetes/books.html Accessed 2 February 2010
- 150.Wenzlau JM, Moua O, Sarkar SA, Yu L, Rewers M, Eisenbarth GS, Davidson HW, Hutton JC: SlC30A8 is a major target of humoral autoimmunity in type 1 diabetes and a predictive marker in prediabetes. Ann N Y Acad Sci 2008; 1150: 256– 259 [DOI] [PubMed] [Google Scholar]
- 151.Wenzlau JM, Liu Y, Yu L, Moua O, Fowler KT, Rangasamy S, Walters J, Eisenbarth GS, Davidson HW, Hutton JC: A common nonsynonymous single nucleotide polymorphism in the SLC30A8 gene determines ZnT8 autoantibody specificity in type 1 diabetes. Diabetes 2008; 57: 2693– 2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bingley PJ, Christie MR, Bonifacio E, Bonfanti R, Shattock M, Fonte MT, Bottazzo GF, Gale EA: Combined analysis of autoantibodies improves prediction of IDDM in islet cell antibody-positive relatives. Diabetes 1994; 43: 1304– 1310 [DOI] [PubMed] [Google Scholar]
- 153.Achenbach P, Koczwara K, Knopff A, Naserke H, Ziegler AG, Bonifacio E: Mature high-affinity immune responses to (pro)insulin anticipate the autoimmune cascade that leads to type 1 diabetes. J Clin Invest 2004; 114: 589– 597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Oak S, Gilliam LK, Landin-Olsson M, Törn C, Kockum I, Pennington CR, Rowley MJ, Christie MR, Banga JP, Hampe CS: The lack of anti-idiotypic antibodies, not the presence of the corresponding autoantibodies to glutamate decarboxylase, defines type 1 diabetes. Proc Natl Acad Sci U S A 2008; 105: 5471– 5476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang J, Miao D, Babu S, Yu J, Barker J, Klingensmith G, Rewers M, Eisenbarth GS, Yu L: Prevalence of autoantibody-negative diabetes is not rare at all ages and increases with older age and obesity. J Clin Endocrinol Metab 2007; 92: 88– 92 [DOI] [PubMed] [Google Scholar]
- 156.Murphy R, Ellard S, Hattersley AT: Clinical implications of a molecular genetic classification of monogenic beta-cell diabetes. Nat Clin Pract Endocrinol Metab 2008; 4: 200– 213 [DOI] [PubMed] [Google Scholar]
- 157.Vardi P, Ziegler AG, Mathews JH, Dib S, Keller RJ, Ricker AT, Wolfsdorf JI, Herskowitz RD, Rabizadeh A, Eisenbarth GS: Concentration of insulin autoantibodies at onset of type I diabetes: inverse log-linear correlation with age. Diabetes Care 1988; 11: 736– 739 [DOI] [PubMed] [Google Scholar]
- 158.Achenbach P, Ziegler AG: Diabetes-related antibodies in euglycemic subjects. Best Pract Res Clin Endocrinol Metab 2005; 19: 101– 117 [DOI] [PubMed] [Google Scholar]
- 159.Achenbach P, Bonifacio E, Williams AJ, Ziegler AG, Gale EA, Bingley PJ: ENDIT Group: Autoantibodies to IA-2beta improve diabetes risk assessment in high-risk relatives. Diabetologia 2008; 51: 488– 92 [DOI] [PubMed] [Google Scholar]
- 160.Liu E, Eisenbarth GS: Accepting clocks that tell time poorly: fluid-phase versus standard ELISA autoantibody assays. Clin Immunol 2007; 125: 120– 126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Brooking H, Ananieva-Jordanova R, Arnold C, Amoroso M, Powell M, Betterle C, Zanchetta R, Furmaniak J, Smith BR: A sensitive non-isotopic assay for GAD65 autoantibodies. Clin Chim Acta 2003; 331: 55– 59 [DOI] [PubMed] [Google Scholar]
- 162.Bingley PJ, Bonifacio E, Mueller PW: Diabetes Antibody Standardization Program: first assay proficiency evaluation. Diabetes 2003; 52: 1128– 1136 [DOI] [PubMed] [Google Scholar]
- 163.Barker JM, McFann K, Harrison LC, Fourlanos S, Krischer J, Cuthbertson D, Chase HP, Eisenbarth GS: DPT-1 Study Group. Pre-type 1 diabetes dysmetabolism: maximal sensitivity achieved with both oral and intravenous glucose tolerance testing. J Pediatr 2007; 150: 31– 36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sosenko JM, Palmer JP, Rafkin-Mervis L, Krischer JP, Cuthbertson D, Matheson D, Skyler JS: Glucose and C-peptide changes in the perionset period of type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care 2008; 31: 2188– 2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Stene LC, Barriga K, Hoffman M, Kean J, Klingensmith G, Norris JM, Erlich HA, Eisenbarth GS, Rewers M: Normal but increasing hemoglobin A1c levels predict progression from islet autoimmunity to overt type 1 diabetes: Diabetes Autoimmunity Study in the Young (DAISY). Pediatr Diabetes 2006; 7: 247– 253 [DOI] [PubMed] [Google Scholar]
- 166.Allison JP, Havran WL: The immunobiology of T cells with invariant gamma delta antigen receptors. Annu Rev Immunol 1991; 9: 679– 705 [DOI] [PubMed] [Google Scholar]
- 167.Palmer JP, Fleming GA, Greenbaum CJ, Herold KC, Jansa LD, Kolb H, Lachin JM, Polonsky KS, Pozzilli P, Skyler JS, Steffes MW: C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve beta-cell function: report of an ADA workshop, 21–22 October 2001. Diabetes 2004; 53: 250– 264 [DOI] [PubMed] [Google Scholar]
- 168.Meier JJ, Bhushan A, Butler AE, Rizza RA, Butler PC: Sustained beta cell apoptosis in patients with long-standing type 1 diabetes: indirect evidence for islet regeneration? Diabetologia 2005; 48: 2221– 2228 [DOI] [PubMed] [Google Scholar]
- 169.Butler PC, Meier JJ, Butler AE, Bhushan A: The replication of beta cells in normal physiology, in disease and for therapy 1. Nat Clin Pract Endocrinol Metab 2007; 3: 758– 768 [DOI] [PubMed] [Google Scholar]
- 170.Gianani R, Campbell-Thompson M, Sarkar SA, Wasserfall C, Pugliese A, Solis JM, Kent S, Hering B, West E, Steck A, Bonner-Weir S, Atkinson MA, Coppieters K, von Herrath M, Eisenbarth GS: Dimorphic histopathology of long-standing childhood-onset diabetes. Diabetologia 2010. [ Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 171.Balasubramanyam A, Nalini R, Hampe CS, Maldonado M: Syndromes of ketosis-prone diabetes mellitus. Endocr Rev 2008; 29: 292– 302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Foulis AK, Clark A: Pathology of the pancreas in diabetes mellitus. In Joslin's Diabetes Mellitus 13th ed Kahn CR, Weir GC: Eds. Philadelphia, Lea & Febiger, 1994, p. 265– 281 [Google Scholar]
- 173.Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG: Analysis of islet inflammation in human type 1 diabetes. Clin Exp Immunol 2009; 155: 173– 181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Herold KC, Gitelman SE, Masharani U, Hagopian W, Bisikirska B, Donaldson D, Rother K, Diamond B, Harlan DM, Bluestone JA: A single course of anti-CD3 monoclonal antibody hOKT3γ1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes 2005; 54: 1763– 1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, Gorus F, Goldman M, Walter M, Candon S, Schandene L, Crenier L, De Block C, Seigneurin JM, De Pauw P, Pierard D, Weets I, Rebello P, Bird P, Berrie E, Frewin M, Waldmann H, Bach JF, Pipeleers D, Chatenoud L: Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med 2005; 352: 2598– 2608 [DOI] [PubMed] [Google Scholar]
- 176.Skyler JS, Brown D, Chase HP, Collier E, Cowie C, Eisenbarth GS, Fradkin J, Grave G, Greenbaum C, Jackson RA, Kaufman FR, Krischer JP, Marks JB, Palmer JP, Ricker A, Schatz DA, Wilson D, Winter WE, Wolfsdorf J, Zeidler A, Dickler H, Eastman RC, Maclaren NK, Malone JI, Robertson PR, Skyler JS, Krischer JP, Wolfsdorf J, Cowie C, Palmer JP, Greenbaum C, Cuthbertson D, Rafkin-Mervis LM, Kaufman FR, Chase HP, Palmer JP, Chase HP, Cowie C, Fradkin J, Eisenbarth GS, Greenbaum C, Herold K, Kaufman FR, Krischer JP, Marks JB, Rafkin-Mervis L, Schatz DA, Skyler JS, Aneju B, Conboy D, Cook R, Dennis MA, Finney L, Harris S, Matheson D, McCulloch-Olsen M, Smith T, Valenzuela J, Vega N, Crofford OB, DeMets D, Lachin JM, Nerup J, Rossini A, Schiffrin A, Steffes M, Tsiatis A, Zinman B: Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med 2002; 346: 1685B– 1691B [DOI] [PubMed] [Google Scholar]
- 177.Bingley PJ, Gale EA: European Nicotinamide Diabetes Intervention Trial (ENDIT) Group. Progression to type 1 diabetes in islet T cell antibody-positive relatives in the European Nicotinamide Diabetes Intervention Trial: the role of additional immune, genetic and metabolic markers of risk. Diabetologia 2006; 49: 881– 890 [DOI] [PubMed] [Google Scholar]
- 178.Ludvigsson J, Faresjö M, Hjorth M, Axelsson S, Chéramy M, Pihl M, Vaarala O, Forsander G, Ivarsson S, Johansson C, Lindh A, Nilsson NO, Aman J, Ortqvist E, Zerhouni P, Casas R: GAD treatment and insulin secretion in recent-onset type 1 diabetes. N Engl J Med 2008; 359: 1909– 1920 [DOI] [PubMed] [Google Scholar]
- 179.Herold KC, Gitelman S, Greenbaum C, Puck J, Hagopian W, Gottlieb P, Sayre P, Bianchine P, Wong E, Seyfert-Margolis V, Bourcier K, Bluestone JA: Immune Tolerance Network ITN007AI Study Group. Treatment of patients with new onset type 1 diabetes with a single course of anti-CD3 mAb Teplizumab preserves insulin production for up to 5 years. Clin Immunol 2009; 132: 166– 173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, Becker DJ, Gitelman SE, Goland R, Gottlieb PA, Marks JB, McGee PF, Moran AM, Raskin P, Rodriguez H, Schatz DA, Wherrett D, Wilson DM, Lachin JM, Skyler JS: Type 1 Diabetes TrialNet Anti-CD20 Study Group. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med 2009; 361: 2143– 2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.El Fassi D, Banga JP, Gilbert JA, Padoa C, Hegedüs L, Nielsen CH: Treatment of Graves' disease with rituximab specifically reduces the production of thyroid stimulating autoantibodies. Clin Immunol 2009; 130: 252– 258 [DOI] [PubMed] [Google Scholar]
- 182.Bresson D, von Herrath M: Moving towards efficient therapies in type 1 diabetes: to combine or not to combine? Autoimmun Rev 2007; 6: 315– 322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Maniatis AK, Yu L, Miao D, Nelson K, Eisenbarth GS: Rapid assays for detection of anti-islet autoantibodies: implications for organ donor screening. J Autoimmun 2001; 16: 71– 76 [DOI] [PubMed] [Google Scholar]
- 184.Gianani R, Putnam A, Still T, Yu L, Miao D, Gill RG, Beilke J, Supon P, Valentine A, Iveson A, Dunn S, Eisenbarth GS, Hutton J, Gottlieb P, Wiseman A: Initial results of screening of non-diabetic organ donors for expression of islet autoantibodies. J Clin Endocrinol Metab 2006; 91: 1855– 1861 [DOI] [PubMed] [Google Scholar]