Abstract
OBJECTIVE
In metazoans, target of rapamycin complex 1 (TORC1) plays the key role in nutrient- and hormone-dependent control of metabolism. However, the role of TORC1 in regulation of triglyceride storage and metabolism remains largely unknown.
RESEARCH DESIGN AND METHODS
In this study, we analyzed the effect of activation and inhibition of the mammalian TORC1 (mTORC1) signaling pathway on the expression of adipose triglyceride lipase (ATGL), hormone-sensitive lipase (HSL), lipolysis, lipogenesis, and lipid storage in different mammalian cells.
RESULTS
Activation of mTORC1 signaling in 3T3-L1 adipocytes by ectopic expression of Rheb inhibits expression of ATGL and HSL at the level of transcription, suppresses lipolysis, increases de novo lipogenesis, and promotes intracellular accumulation of triglycerides. Inhibition of mTORC1 signaling by rapamycin or by knockdown of raptor stimulates lipolysis primarily via activation of ATGL expression. Analogous results have been obtained in C2C12 myoblasts and mouse embryonic fibroblasts with genetic ablation of tuberous sclerosis 2 (TSC2) gene. Overexpression of ATGL in these cells antagonized the lipogenic effect of TSC2 knockout.
CONCLUSIONS
Our findings demonstrate that mTORC1 promotes fat storage in mammalian cells by suppression of lipolysis and stimulation of de novo lipogenesis.
Target of rapamycin (TOR; mTOR in mammalian cells) is an evolutionarily conserved serine/threonine protein kinase that is distributed between the two functionally distinct protein complexes, TORC1 and TORC2 (1). mTORC1, which is composed of mTOR, raptor, mLST8, and PRAS40, represents the central sensor of energy- and hormone-dependent metabolic regulation in metazoans (2,3). Activity of mTORC1 is controlled by the low-molecular-weight GTPase Rheb and GTPase-activating protein complex, which consists of two subunits: tuberous sclerosis 1 (TSC1) and TSC2 (3).
It is believed that activation of mTORC1 promotes anabolic processes, such as protein biosynthesis, and inhibits the ultimate catabolic process, autophagy. On the contrary, lack of nutrients/energy rapidly suppresses mTORC1 and mTORC1-controlled anabolic processes and activates autophagy (2,3).
Autophagy may represent the last line of defense against starvation (2,4). At the same time, mammalian organisms usually accumulate significant (in the case of humans, often, excessive) energy stores that eliminate the need for autophagy as a source of energy under non–life-threatening conditions. Therefore, we have hypothesized that prior to activation of autophagy, inhibition of the mTORC1 pathway should activate less traumatic catabolic processes that specifically serve to generate energy for vitally important cell functions. By the same token, activation of the TSC-mTORC1 pathway may facilitate energy storage when nutrients are unlimited. As in eukaryotes, energy is stored primarily in the form of fat, we decided to study the effect of the mTORC1 pathway on the central energy-generating process, lipolysis.
Our results strongly suggest that mTORC1, indeed, inhibits lipolysis and stimulates lipogenesis in adipocytes and other mammalian cells. We believe that our results are consistent with previously published data. Thus, in mice, genetic ablation of the mTORC1 substrate, S6K1, produces lean phenotype with elevated levels of free fatty acids in the blood under high-fat diet, suggesting that S6K1 may have an antilipolytic effect (5,6). On the contrary, knockdown of 4E-BP1 and 4E-BP2 leads to increased adiposity and reduced lipolysis (7).
In humans, hyperactivation of mTORC1 signaling in patients with tuberous sclerosis often leads to the development of hamartomas of the kidneys called angiomyolipomas (8). From the pathophysiological standpoint, the most essential element of angiomyolipomas is fat, suggesting that hyperactivation of the mTORC1-mediated pathway may contribute to accumulation of fat in vivo. In addition, a recent study has demonstrated that the majority of tuberous sclerosis patients have foci of fat in the myocardium that are readily detectable by computed tomography (9). These authors have concluded that such fatty foci represent an essential characteristic of tuberous sclerosis (9). Moreover, it is well known that the use of the mTORC1 inhibitor, rapamycin, in clinical practice (to prevent organ rejection in transplant patients, or for other purposes) often results in dyslipidemia, which is currently considered a prominent side effect of the drug (10,11). We believe that our results may shed light on the molecular mechanism of all these phenomena.
Furthermore, the regulatory link between mTORC1 and lipolysis is evolutionarily conserved. Thus, in Drosophila, overexpression of dTOR increases fat content (12), whereas hypomorphic mutation of dTOR has the opposite effect (13). In fact, inhibition of dTOR signaling increases the expression of the lipase called Brummer (13), which represents an analog of the mammalian enzyme that has several names (desnutrin, PNPLA2, TTS2.2, iPLA2ζ), but we will refer to it as ATGL for adipose triglyceride lipase. ATGL is responsible for the bulk of triacylglycerol hydrolase activity in cells (14). Importantly, ATGL represents the rate-limiting lipolytic enzyme in mammals (15–20), flies (21), and yeast (22). Furthermore, ATGL controls the size of lipid droplets in basal adipocytes (23). We show here that the mTORC1-mediated pathway suppresses lipolysis and promotes fat accumulation in mammalian cells primarily by inhibiting transcription of ATGL.
RESEARCH DESIGN AND METHODS
Antibodies.
Polyclonal antibodies against ATGL and hormone-sensitive lipase (HSL) were gifts from Dr. A. Greenberg (Tufts University); against LPL, a gift from Dr. S. Fried (Boston University School of Medicine); and against Ap2, a gift from Dr. S. Farmer (Boston University School of Medicine). A rabbit polyclonal antibody against cellugyrin was described previously (24). Polyclonal anti–peroxisome proliferator–activated receptor-γ and C/EBPα were from Santa Cruz Biotechnology (Santa Cruz, CA). Polyclonal antibodies against perilipin, raptor, and all phospho-specific antibodies were from Cell Signaling Technology (Beverly, MA). Monoclonal antibody against adiponectin was from Abcam (Cambridge, MA). Monoclonal anti–HA tag antibody was from Covance (Berkeley, CA).
Cell culture.
Preparation of 3T3-L1 and C2C12 cells stably expressing Rheb was described previously (25,26). 3T3-L1 preadipocytes were cultured, differentiated, and maintained as described previously (24). TSC2−/− mouse embryonic fibroblasts (MEFs) and littermate control wild-type MEFs (27) as well as C2C12 myoblasts were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10 and 20% FBS, respectively, in 2 mmol/l l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin.
Animal experiments.
All experiments with animals were performed under the approved Institutional Animal Care and Use Committee protocol. Male C57BL6 mice (10 weeks old; Charles River) were injected intraperitoneally daily with 5 mg/kg rapamycin or vehicle (5 μl DMSO in 500 μl sterile PBS) for 2 days. Mice were killed 16 h after the last injection by CO2 inhalation followed by cervical dislocation; epididymal fat fads were isolated, cut into small pieces, and incubated in DMEM with 2% fatty acid–free BSA at 37°C for 2 h. Glycerol release in the media was assayed as described below. Fat pads were homogenized in PBS with 1% Triton X-100 and standard inhibitors of proteases and phosphatases, and protein concentration was measured. Tissue homogenates were also analyzed for protein expression by Western blotting.
Transient transfections and reporter gene assays.
Transient transfections with cDNA were performed using Lipofectamine 2000 (Invitrogen Life Technologies, Grand Island, NY) according to the manufacturer's instructions. Briefly, ∼50% confluent MEFs were transfected with 500 ng of luciferase and 100 ng of enhanced green fluorescent protein (EGFP) cDNA in a 6-well plate format. All experiments were performed in triplicate. After 48 h of transfection, cells were harvested in Reporter Lysis Buffer (Promega). Luciferase activity was determined in whole-cell lysates using the Promega luciferase assay kit and expressed as relative light units. Expression of EGFP was measured fluorometrically. Firefly luciferase was normalized by EGFP fluorescence to correct for transfection efficiency.
Small interfering RNA (siRNA) against the sequence ATTACATTCACAGACTCGGGC of mouse raptor and scrambled siRNA were obtained from Integrated DNA Technologies (Coralville, IA). Transfection of siRNAs was performed with the help of the DeliverX Plus delivery kit (Panomics) according to instructions of the manufacturer.
RNA extraction and quantitative PCR.
Total RNA was extracted using TRIzol reagent (Invitrogen Life Technologies) and remaining DNA was removed with DNase using DNA-free kit (Ambion). Reverse transcription of 1 μg of total RNA was performed with random primers using ABI High-Capacity cDNA Reverse Transcription Kits. cDNA was diluted 1:10 (vol/vol) with water treated with diethyl pyrocarbonate. TaqMan reaction was carried out in a 384-well plate format in total volume of 20 μl, containing 9 μl diluted cDNA, 10 μl 2× TaqMan Universal PCR Master Mix, and 1 μl probe. Gene expression was determined by standard curve method and normalized either by glyceraldehyde-3-phosphate dehydrogenase (GAPDH), β-actin, or 36B4 internal control genes. Accuracy of RNA quantification was optimized by DNase treatment of the samples, no DNA, and no Taq controls. Primer and probe sequences are available upon request.
Immunofluorescence.
MEFs were grown in a 60-mm dish and transfected with HA-tagged ATGL expression construct. Cells were allowed to grow for 3 days after confluence, then lifted up by trypsin for 10 min at 37°C, reseeded onto Nunc LAB-TEK II 4-well chamber slides, and used for staining. Cells were fixed with 4% paraformaldehyde in PBS for 30 min. Fixed cells were washed with PBS, permeabilized with 0.2% Triton X-100 for 5 min, blocked with PBS with 5% donkey serum and 5% BSA, and stained with primary anti-HA monoclonal antibody and cyanin 3–conjugated donkey anti-mouse IgG (Jackson ImmunoResearch). Each incubation with antibody lasted for 60 min at room temperature and was followed by six quick washes with PBS. For visualization of fat droplets, cells were incubated with Nile red (1:1,000 vol/vol in 75% glycerol from 0.5 mg/ml stock in acetone) for another 30 min. Vectashield with DAPI (Vector Laboratories, Burlingame, CA) was used for mounting cells on slides that were then examined by fluorescence microscopy using Olympus IX70. Pictures were taken with the help of the Image-Pro 4.5 program.
Lipolysis assay.
Differentiated 3T3-L1 adipocytes were incubated in Phenol red–free DMEM with 2% fatty acid–free BSA for 2 h at 37°C in the presence or in the absence of 10 μmol/l isoproterenol. Glycerol content in the media was measured colorimetrically at 540 nm using the Triglyceride (GPO) Reagent Set (Pointe Scientific, Canton, MI) against a set of glycerol standards. Cells were then washed with cold PBS and lysed in 1% Triton X-100 buffer, and the protein concentration was determined and used to normalize glycerol release. All the experiments were carried out in triplicates.
Lipogenesis assay.
3T3-L1 adipocytes were incubated with 1 μCi of 14C-acetic acid (Perkin Elmer, Waltham, MA) for 24 h and total intracellular lipids were extracted with hexane and 2-propanol (3:2 vol/vol) mixture. Solvents were dried under nitrogen gas, and the pellet was resuspended in toluene. Incorporation of [1,2-14C]-acetic acid into lipid phase was assayed either by scintillation counting or by separating on a thin-layer chromatography plate (Whatman) using hexane/diethyl ester/acetic acid (70:30:1 vol/vol/vol) as mobile phase after autoradiography. All the experiments were carried out in triplicate and normalized by protein concentration in samples.
Measurement of triglycerides.
Cells were lysed in PBS containing 1% Nonidet P-40 and whole-cell lysates were analyzed for triglyceride content by Triglyceride (GPO) Reagent Set according to the manufacturer's instructions against a set of triolein standards. Intracellular triglycerides were normalized by protein concentrations.
Oil red O staining.
Cells were washed with PBS, fixed with 3.7% formaldehyde solution for 1 h, and stained with oil red O for 1 h using a 60:40 (vol/vol) dilution in water of a 0.5% stock solution in isopropanol. Cells were then washed twice with water and visualized under the microscope.
Triglyceride lipase assay.
Cells were homogenized in HES buffer (250 mmol/l sucrose, 20 mmol/l HEPES, 1 mmol/l dithiothreitol, 1 mmol/l EDTA, pH7.4), and cell lysates were centrifuged at 4°C for 20 min at 16000g. Triglyceride lipase activity was measured in 100 μg of supernatants by addition of 100 μl of substrate that was prepared by sonicating [1-14C]-triolein (70,000 cpm/reaction) in 100 mmol/l potassium phosphate, 2 mmol/l EDTA, pH 7.4, 1% BSA, 20 μmol/l phosphatidyl inositol/phosphatidyl serine (1:1 wt/wt), and 1 mmol/l dithiothreitol. Reaction mixtures were incubated at 37°C for 30 min, terminated by addition of 3.25 ml of methanol/chloroform/heptane (10:9:7), and extracted with 1 ml of 0.1 mol/l potassium carbonate and 0.1 mol/l boric acid, pH 9.5. Liberated fatty acid was quantified in 700 μl of the upper phase by liquid scintillation counting.
Oxygen consumption.
The rate of change of dissolved O2 in medium (bicarbonate-free DMEM with 10% FBS supplemented with 5 mmol/l glucose) immediately surrounding adipocytes derived from 3T3-L1 adipocytes cultured in custom 24-well plates (50,000 cells/well) was measured in a Seahorse Bioscience instrument (model XF24). Genomic DNA was extracted from each well using Pico Pure DNA extraction (Arcturus) and was quantified and used to normalize the O2 consumption level/well.
Gel electrophoresis and Western blotting.
Proteins were separated in SDS-polyacrylamide gels and transferred to Immobilon-P membranes (Millipore, Bedford, MA) in 25 mmol/l Tris, 192 mmol/l glycine. After transfer, the membrane was blocked with 10% nonfat milk in PBS with 0.5% Tween 20 for 2 h. The blots were probed overnight with specific primary antibodies at 4°C followed by 1-h incubation at room temperature with horseradish peroxidase–conjugated secondary antibodies (Sigma). Protein bands were detected with the enhanced chemiluminescence substrate kit (PerkinElmer Life Sciences, Boston, MA) using a Kodak Image Station 440CF (Eastman Kodak, Rochester, NY).
Statistics.
Student paired two-tailed t test was used to evaluate the statistical significance of the results.
RESULTS AND DISCUSSION
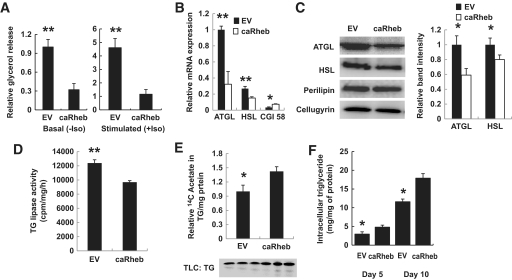
To determine the effect of mTORC1 on lipolysis, we have created a line of 3T3-L1 adipocytes stably overexpressing moderate levels of constitutively active S16H Rheb (hereafter referred to as caRheb). As is described in our recent study (25), expression of caRheb leads to the activation of the mTORC1-mediated signaling pathway but apparently does not change the level of cell differentiation as indicated by similar levels of expression of peroxisome proliferator–activated receptor-γ, C/EBPα, and perilipin (supplementary Fig. 1, available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db09-1602/DC1). Oxygen consumption of caRheb-expressing adipocytes is also not different from control (supplementary Fig. 2). However, the rate of basal and isoproterenol-stimulated lipolysis in these cells is decreased correspondingly threefold and fivefold in comparison with empty vector–infected cells (Fig. 1A).
FIG. 1.
mTORC1 promotes accumulation of triglycerides and downregulates expression of ATGL and HSL in 3T3–L1 adipocytes. A: Empty vector–infected (EV) and caRheb 3T3–L1 adipocytes on day 7 of differentiation were incubated in Phenol red–free DMEM with 2% fatty acid–free BSA without (basal) or with (stimulated) 10 μmol/l isoproterenol (Iso) for 2 h. Glycerol was measured in media aliquots in triplicate and normalized by protein concentration in whole-cell lysates. Data are presented relative to nonstimulated EV cells and expressed as mean ± SD. B: Levels of ATGL, HSL, and CGI-58 mRNA in differentiated (day 7) EV (black bars)– and caRheb (white bars)–expressing 3T3–L1 adipocytes were determined in triplicate by quantitative PCR and normalized by 36B4 mRNA levels. Data were expressed as mean ± SD relative to the expression levels in EV cells. C: 3T3–L1 adipocytes stably expressing Rheb or empty vector–transfected control adipocytes were homogenized, and total lysates (50 μg) were analyzed by Western blotting for ATGL, HSL, and perilipin. Irrelevant protein cellugyrin was used as loading control. Right panel: Relative band intensities for ATGL and HSL were determined and normalized to cellugyrin in three independent experiments using ImageJ software. Data were expressed as mean ± SD relative to the expression levels in EV cells. D: Triglyceride (TG) lipase activity in EV-infected or caRheb cells was measured in vitro in triplicate as described in the research design and methods section. E: 3T3–L1 adipocytes stably expressing Rheb or empty vector–transfected control adipocytes were treated with 1 μCi [1,2-14C]-acetic acid for 24 h, and the total lipids were extracted and separated on thin-layer chromatography in triplicate. Incorporation of 14C-labeled acetic acid into TGs was determined by autoradiography (bottom panel). Top panel: Relative spot intensities for triglycerides were measured with the help of ImageJ software and normalized to total protein. Data were expressed as mean ± SD relative to the expression levels in EV cells. F: Accumulation of intracellular triglycerides in empty vector–infected (EV) 3T3–L1 adipocytes and in cells stably expressing Rheb (caRheb) was measured on days 5 and 10 of differentiation. All panels: *P < 0.05; **P < 0.001.
It is now appreciated that lipolysis is a complicated multistep process. The complete hydrolysis of triglycerides to glycerol and free fatty acids is performed jointly by triacylglyceride, diacylglyceride, and monoacylglyceride lipases (14,28). Recently discovered enzyme ATGL (16–18), together with its protein cofactor CGI-58 (29), seems to be responsible for the bulk of triacylglycerol hydrolase activity in various cells, although may not represent the only triacylglycerol hydrolase in adipocytes (30). At the same time, ATGL has low affinity to diacylglycerides and monoacylglycerides (14,28). The major diacylglyceride lipase in adipocytes is HSL. Monoacylglyceride products of HSL are hydrolyzed by constitutively active monoacylglyceride lipase (14,28).
Using quantitative PCR, we found that the levels of mRNA for ATGL and HSL were significantly decreased in Rheb-overexpressing adipocytes (Fig. 1B). Note that ATGL mRNA was more abundant in 3T3-L1 adipocytes than HSL mRNA, and the inhibitory effect of caRheb on the expression of ATGL mRNA was greater than its effect on HSL mRNA. The protein levels of ATGL and HSL were also decreased by ∼40 and 20% correspondingly (Fig. 1C). As expected, reduced expression of ATGL was accompanied by a comparable decrease in the total triacylglycerol hydrolase activity in vitro (Fig. 1D).
Recent results suggest that mTORC1 may stimulate lipogenesis in mammalian cells (rev. in 31). In agreement with these data, we have also found that activation of mTORC1 in adipocytes stimulates de novo lipogenesis (Fig. 1E). Quantitative analysis of data shown in Fig. 1A and E demonstrates that mTORC1-mediated inhibition of basal lipolysis (from 16.4 ± 1.3 nm · mg−1 · h−1 in empty vector cells to 6.1 ± 0.78 in caRheb cells) may contribute to overall lipid accumulation to a greater extent, than stimulation of de novo lipogenesis (from 0.82 ± 0.12 nm · mg−1 · h−1 in empty vector cells to 1.93 ± 0.38 in caRheb cells), so, in this study, we decided to concentrate on the former phenomenon.
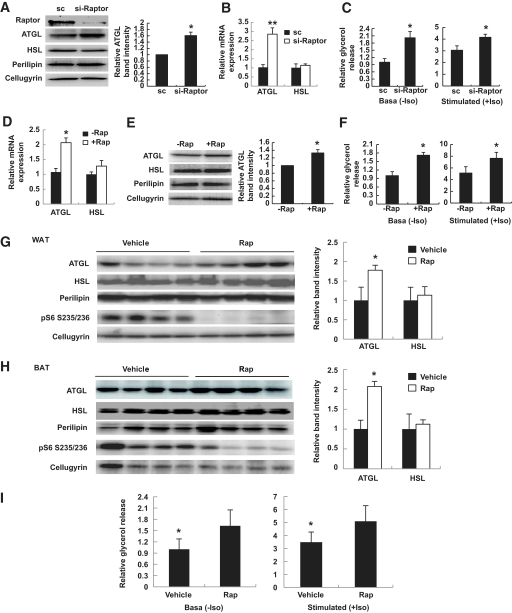
In the next experiment, we knocked down the crucial mTORC1 component, raptor, with the help of siRNA (Fig. 2A). Knockdown of raptor stimulated expression of ATGL mRNA (Fig. 2B) and protein (Fig. 2A) but had virtually no effect on the expression of HSL. Still, knockdown of raptor caused significant activation of both basal and isoproterenol-stimulated lipolysis (Fig. 2C). Treatment of 3T3-L1 adipocytes with the mTORC1 inhibitor, rapamycin, for 24 h had a similar positive effect on the expression of ATGL (but not HSL) and on the rate of lipolysis (Fig. 2D–F). In agreement with in vitro results, administration of rapamycin to mice in vivo also increased expression of ATGL and ex vivo lipolysis in both white and brown adipose tissue (Fig. 2G–I). Expression of HSL was not affected by rapamycin treatment in vivo in a statistically significant fashion. Thus, activation of mTORC1 suppresses expression of both ATGL and, less so, HSL (Fig. 1B and C), whereas inhibition of mTORC1 specifically increases expression of ATGL and does not affect expression of HSL (Fig. 2). This suggests that mTORC1 may regulate lipolysis primarily via ATGL.
FIG. 2.
Inhibition of mTORC1 signaling increases ATGL expression and lipolysis in adipocytes both in vitro and in vivo. A: 3T3–L1 adipocytes on day 7 of differentiation were transfected with 30 nmol/l scrambled (sc) or siRNA directed against mouse Raptor (si-Raptor). After 48 h, cells were homogenized and total lysates (50 μg) were analyzed by Western blotting. Right panel: Relative band intensities for ATGL were determined and normalized to cellugyrin. B: Levels of ATGL and HSL mRNA in sc (black bars) and si-Raptor cells (white bars) were determined by quantitative PCR and normalized by GAPDH mRNA levels. Data were expressed as mean ± SD relative to the expression levels in sc cells. **P < 0.001. C: Glycerol release in the media was determined in sc and si-Raptor cells for 2 h. Data were expressed as mean ± SD relative to nonstimulated sc cells. D: 3T3–L1 adipocytes on day 7 of differentiation were incubated with 100 nmol/l rapamycin (+Rap, white bars) or vehicle (−Rap, black bars) for 24 h. Levels of ATGL and HSL mRNA were determined by quantitative PCR and normalized by GAPDH mRNA levels. Data were expressed as mean ± SD relative to the expression levels in −Rap cells. E: Whole-cell lysates of rapamycin-treated and not treated 3T3-L1 adipocytes were analyzed by Western blotting (50 μg of total protein per lane). Right panel: Relative band intensities for ATGL were determined and normalized to cellugyrin. F: Glycerol release in the media was determined in rapamycin-treated and not treated 3T3-L1 adipocytes for 2 h. Data are presented relative to nonstimulated cells not treated with rapamycin and expressed as mean ± SD. G and H: Epididymal fat pads (white adipose tissue, WAT) and interscapular brown adipose tissue (BAT) were isolated from mice injected with Rap or vehicle for 2 days as described in the research design and methods section (n = 4–10), and total homogenates were analyzed by Western blotting (50 μg of total protein per lane). Right panels: Intensities of ATGL and HSL bands were normalized by cellugyrin and expressed as mean ± SD relative to the vehicle-injected animals. I: Lipolysis was measured ex vivo using the epididymal fat pads from mice injected with vehicle solution or rapamycin (Rap) for 2 h. All panels: *P < 0.05.
Recently, Polak et al. (32) reported that knockout of raptor in adipocytes decreased accumulation of triglycerides in these cells both in vitro and in vivo, which is consistent with our observations. Polak et al. (32) attributed this effect to elevated energy expenditure due to mitochondrial uncoupling. Our results demonstrate that mTORC1 may control the size of lipid stores in adipocytes by adjusting the rates of lipogenesis and lipolysis. Furthermore, we suggest that inhibition of ATGL expression may represent a major factor in mTORC1-induced triglyceride accumulation.
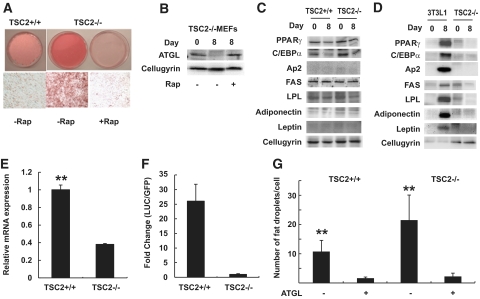
To confirm the link between the mTORC1-mediated pathway and ATGL expression in a different cell type, we used TSC2−/− MEFs that exhibit an elevated mTORC1 activity (27) together with wild-type (TSC2+/+) MEFs. As shown in Fig. 3A, culturing for 8 days after confluence leads to a significant accumulation of fat in TSC2−/− MEFs in comparison with wild-type cells. Addition of rapamycin to the media prevented fat accumulation. The levels of the ATGL protein in TSC2−/− MEFs reversely correlated with the level of fat accumulation and were significantly decreased on day 8 after confluence, whereas rapamycin reversed the loss of ATGL (Fig. 3B).
FIG. 3.
Genetic ablation of TSC2 promotes accumulation of triglycerides and decreases expression of ATGL in MEFs. A: Wild-type (TSC2+/+) and TSC2−/− MEFs were cultured in DMEM with 10% FBS for 8 days after confluence with (+Rap) or without (−Rap) 100 nmol/l rapamycin. Cells were then fixed in formaldehyde and stained with oil red O. Representative photographs of the plates (top panels) and respective micrographs (bottom panels) are shown. B: Western blot analysis of the ATGL protein in total cell lysates (50 μg) prepared from TSC2−/− MEFs immediately after confluence (day 0) or after 8 days of confluence (day 8) with or without 100 nmol/l rapamycin (Rap). Cellugyrin was used as loading control. C: Whole-cell lysates were prepared from TSC2+/+ and TSC2−/− MEFs immediately after confluence (day 0) or after 8 days of confluence (day 8) and analyzed by Western blotting. D: Total lysates obtained from TSC2−/− MEFs immediately after confluence (day 0) or after 8 days of confluence (day 8) were analyzed by Western blotting. 3T3-L1 preadipocytes (day 0) and differentiated 3T3-L1 adipocytes (day 8) served as negative and positive controls, respectively. Cellugyrin was used as a loading control. E: ATGL mRNA was measured in TSC2+/+ and TSC2−/− MEFs by quantitative PCR on the day of confluence. Gene expression was normalized to GAPDH mRNA levels, and data were presented as mean ± SD relative to ATGL expression in TSC2+/+ MEFs. F: TSC2+/+ and TSC2−/− MEFs were transiently transfected with −2979/+21 LUC ATGL promoter construct together with EGFP. After 48 h, cells were washed three times in cold PBS and harvested in the reporter lysis buffer. Luciferase activity in cell lysates was assayed as described in the research design and methods section and normalized by GFP fluorescence. Data are presented for triplicate samples as mean ± SD. G: TSC2+/+ and TSC2−/− MEFs were transfected with HA-tagged ATGL expression construct and allowed to grow for 3 days after confluence. Then, cells were replated onto 4-well chamber slides, fixed with 4% paraformaldehyde, permeabilized with 0.2% Triton X-100, and stained with primary anti-HA monoclonal antibody and cyanin 3–conjugated donkey anti-mouse IgG. Lipid droplets were stained with Nile red. The figure shows quantification of the number of fat droplets in 100 transfected (+ATGL) and nontransfected (−ATGL) cells. All panels: **P < 0.001. (A high-quality color representation of this figure is available in the online issue.)
Theoretically, it is possible that knockout of TSC2 in MEFs activates the differentiation program in these cells and converts them into adipocytes (33,34). Note, however, that, unlike Zhang et al. (34), we cultured MEFs not in the differentiation media but simply in DMEM. Under these conditions, accumulation of fat in postconfluent TSC2−/− MEFs was not accompanied by any significant increase in adipose markers (Fig. 3C), which, in addition, were expressed in these cells at much lower levels than in differentiated 3T3-L1 adipocytes (Fig. 3D). At the same time, similar to the situation with Rheb-transfected 3T3-L1 adipocytes, genetic ablation of TSC2 significantly decreased the level of ATGL mRNA in MEFs (Fig. 3E).
We then expressed the luciferase reporter construct containing ∼3 kb mouse ATGL promoter (35) in wild-type and TSC2−/− MEFs. As shown in Fig. 3F, the reporter construct was expressed much more efficiently in wild-type than in TSC2−/− MEFs. These results strongly suggest that mTORC1 signaling is implemented in the transcriptional control of ATGL expression.
To address the question of whether inhibition of ATGL expression can account for mTORC1-mediated increase in fat accumulation, we transiently expressed HA epitope–tagged ATGL in wild-type and TSC2−/− MEFs (for cell images, see supplementary Fig. 3). In agreement with data shown in Fig. 3A, nontransfected TSC2−/− MEFs accumulate more fat than wild-type cells (Fig. 3G). Ectopic expression of ATGL inhibited accumulation of triglycerides in lipid droplets both in wild-type and TSC2−/− MEFs (Fig. 3G; supplementary Fig. 3). We suggest, therefore, that mTORC1-dependent inhibition of ATGL promotes lipid accumulation in mammalian cells.
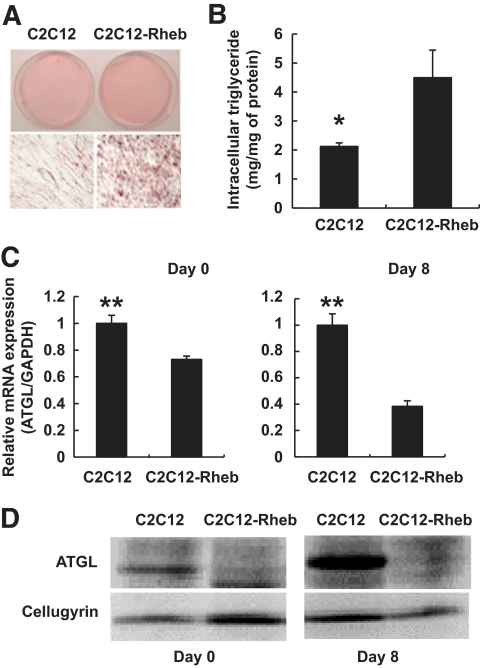
It has long been known that deposition of triglycerides in skeletal muscle is reversely correlated with their insulin sensitivity, although triglycerides per se may not affect insulin signaling, and lipotoxicity is more likely attributed to metabolic products of TGs (36–38). As skeletal muscles have a relatively low, but still detectable level of ATGL expression (35,39), we decided to determine whether the mTORC1-mediated pathway controls accumulation of triglycerides in myocytes as well. For that, we used C2C12 myoblasts stably transfected with HA epitope–tagged Rheb (26). As we have reported earlier (26), insulin signaling in these cells is attenuated (see also supplementary Fig. 4). In addition, Rheb-expressing C2C12 myoblasts demonstrate increased lipid deposition (Fig. 4A and B) and decreased ATGL mRNA (Fig. 4C) and protein (Fig. 4D) in comparison with empty vector–transfected cells.
FIG. 4.
Activation of mTORC1 promotes accumulation of triglycerides and decreases expression of ATGL in C2C12 myoblasts. A: C2C12 myoblasts stably transfected with empty vector (C2C12) or wild-type Rheb (C2C12-Rheb) were grown in DMEM supplemented with 20% FBS for 8 days after confluence. Cells were fixed and stained with oil red O. A representative photograph of the plates (top panel) and respective microphotographs (bottom panel) are shown. B: Accumulation of intracellular triglycerides in empty vector–infected C2C12 cells and in cells stably expressing Rheb (Rheb) was measured on day 8 after confluence. *P < 0.05. C: Expression of ATGL was determined by quantitative PCR, normalized to GAPDH, and expressed relative to the expression levels in C2C12 myoblasts on days 0 and 8 after confluence. **P < 0.001. D: Levels of ATGL protein in whole-cell lysates were determined by Western blotting on days 0 and 8 after confluence (50 μg of total protein per lane). (A high-quality color representation of this figure is available in the online issue.)
Previous work has shown that suppression of ATGL in 3T3-L1 adipocytes with siRNA decreases both basal and isoproterenol-stimulated lipolysis (39), and genetic ablation of ATGL in mice leads to triglyceride deposition in multiple tissues (19). In addition, mutations of ATGL are associated with neutral lipid storage disease in humans, an autosomal recessive disorder characterized by accumulation of triglyceride droplets and myopathy (40). These data as well as results described above suggest that mTORC1-mediated downregulation of ATGL represents an important factor in the overall control of lipid homeostasis that may underlie the molecular nature of metabolic disease.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by research grants DK-52057 and DK-56736 from the National Institutes of Health and Research Award from American Diabetes Association to K.V.K.
No potential conflicts of interest relevant to this article were reported.
We thank Dr. B. D. Manning for his gift of TSC2+/+ and TSC2−/− cells, Drs. R. Lamb and J. Procter for their gifts of cDNA, and Dr. A. Greenberg for his gift of antibody.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bhaskar PT, Hay N: The two TORCs and Akt. Dev Cell 2007; 12: 487– 502 [DOI] [PubMed] [Google Scholar]
- 2.Wullschleger S, Loewith R, Hall MN: TOR signaling in growth and metabolism. Cell 2006; 124: 471– 484 [DOI] [PubMed] [Google Scholar]
- 3.Huang J, Manning BD: The TSC1-TSC2 complex: a molecular switchboard controlling cell growth. Biochem J 2008; 412: 179– 190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klionsky DJ: Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol 2007; 8: 931– 937 [DOI] [PubMed] [Google Scholar]
- 5.Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, Thomas G: Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 2004; 431: 200– 205 [DOI] [PubMed] [Google Scholar]
- 6.Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, Woods A, Robinson IC, Schuster E, Batterham RL, Kozma SC, Thomas G, Carling D, Okkenhaug K, Thornton JM, Partridge L, Gems D, Withers DJ: Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science 2009; 326: 140– 144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Bacquer O, Petroulakis E, Paglialunga S, Poulin F, Richard D, Cianflone K, Sonenberg N: Elevated sensitivity to diet-induced obesity and insulin resistance in mice lacking 4E-BP1 and 4E-BP2. J Clin Invest 2007; 117: 387– 396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bissler JJ, Kingswood JC: Renal angiomyolipomata. Kidney Int 2004; 66: 924– 934 [DOI] [PubMed] [Google Scholar]
- 9.Adriaensen ME, Schaefer-Prokop CM, Duyndam DA, Zonnenberg BA, Prokop M: Fatty foci in the myocardium in patients with tuberous sclerosis complex: common finding at CT. Radiology 2009; 253: 359– 363 [DOI] [PubMed] [Google Scholar]
- 10.Kasiske BL, de Mattos A, Flechner SM, Gallon L, Meier-Kriesche HU, Weir MR, Wilkinson A: Mammalian target of rapamycin inhibitor dyslipidemia in kidney transplant recipients. Am J Transplant 2008; 8: 1384– 1392 [DOI] [PubMed] [Google Scholar]
- 11.Morrisett JD, Abdel-Fattah G, Hoogeveen R, Mitchell E, Ballantyne CM, Pownall HJ, Opekun AR, Jaffe JS, Oppermann S, Kahan BD: Effects of sirolimus on plasma lipids, lipoprotein levels, and fatty acid metabolism in renal transplant patients. J Lipid Res 2002; 43: 1170– 1180 [PubMed] [Google Scholar]
- 12.Teleman AA, Chen YW, Cohen SM: Drosophila Melted modulates FOXO and TOR activity. Dev Cell 2005; 9: 271– 281 [DOI] [PubMed] [Google Scholar]
- 13.Luong N, Davies CR, Wessells RJ, Graham SM, King MT, Veech R, Bodmer R, Oldham SM: Activated FOXO-mediated insulin resistance is blocked by reduction of TOR activity. Cell Metab 2006; 4: 133– 142 [DOI] [PubMed] [Google Scholar]
- 14.Zechner R, Kienesberger PC, Haemmerle G, Zimmermann R, Lass A: Adipose triglyceride lipase and the lipolytic catabolism of cellular fat stores. J Lipid Res 2009; 50: 3– 21 [DOI] [PubMed] [Google Scholar]
- 15.Smirnova E, Goldberg EB, Makarova KS, Lin L, Brown WJ, Jackson CL: ATGL has a key role in lipid droplet/adiposome degradation in mammalian cells. EMBO Rep 2006; 7: 106– 113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, Zechner R: Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 2004; 306: 1383– 1386 [DOI] [PubMed] [Google Scholar]
- 17.Villena JA, Roy S, Sarkadi-Nagy E, Kim KH, Sul HS: Desnutrin, an adipocyte gene encoding a novel patatin domain-containing protein, is induced by fasting and glucocorticoids: ectopic expression of desnutrin increases triglyceride hydrolysis. J Biol Chem 2004; 279: 47066– 47075 [DOI] [PubMed] [Google Scholar]
- 18.Jenkins CM, Mancuso DJ, Yan W, Sims HF, Gibson B, Gross RW: Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J Biol Chem 2004; 279: 48968– 48975 [DOI] [PubMed] [Google Scholar]
- 19.Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, Heldmaier G, Maier R, Theussl C, Eder S, Kratky D, Wagner EF, Klingenspor M, Hoefler G, Zechner R: Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science 2006; 312: 734– 737 [DOI] [PubMed] [Google Scholar]
- 20.Bezaire V, Mairal A, Ribet C, Lefort C, Girousse A, Jocken J, Laurencikiene J, Anesia R, Rodriguez AM, Ryden M, Stenson BM, Dani C, Ailhaud G, Arner P, Langin D: Contribution of adipose triglyceride lipase and hormone-sensitive lipase to lipolysis in hMADS adipocytes. J Biol Chem 2009; 284: 18282– 18291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grönke S, Mildner A, Fellert S, Tennagels N, Petry S, Müller G, Jäckle H, Kühnlein RP: Brummer lipase is an evolutionary conserved fat storage regulator in Drosophila. Cell Metab 2005; 1: 323– 330 [DOI] [PubMed] [Google Scholar]
- 22.Kurat CF, Natter K, Petschnigg J, Wolinski H, Scheuringer K, Scholz H, Zimmermann R, Leber R, Zechner R, Kohlwein SD: Obese yeast: triglyceride lipolysis is functionally conserved from mammals to yeast. J Biol Chem 2006; 281: 491– 500 [DOI] [PubMed] [Google Scholar]
- 23.Miyoshi H, Perfield JW, II, Obin MS, Greenberg AS: Adipose triglyceride lipase regulates basal lipolysis and lipid droplet size in adipocytes. J Cell Biochem 2008; 105: 1430– 1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Z, Kandror KV: Translocation of small preformed vesicles is responsible for the insulin activation of glucose transport in adipose cells: evidence from the in vitro reconstitution assay. J Biol Chem 2002; 277: 47972– 47975 [DOI] [PubMed] [Google Scholar]
- 25.Chakrabarti P, Anno T, Manning BD, Luo Z, Kandror KV: The Mammalian target of rapamycin complex 1 regulates leptin biosynthesis in adipocytes at the level of translation: the role of the 5′-untranslated region in the expression of leptin messenger ribonucleic acid. Mol Endocrinol 2008; 22: 2260– 2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tzatsos A, Kandror KV: Nutrients suppress phosphatidylinositol 3-kinase/Akt signaling via raptor-dependent mTOR-mediated insulin receptor substrate-1 phosphorylation. Mol Cell Biol 2006; 26: 63– 76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang H, Cicchetti G, Onda H, Koon HB, Asrican K, Bajraszewski N, Vazquez F, Carpenter CL, Kwiatkowski DJ: Loss of Tsc1/Tsc2 activates mTOR and disrupts PI3K-Akt signaling through downregulation of PDGFR. J Clin Invest 2003; 112: 1223– 1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duncan RE, Ahmadian M, Jaworski K, Sarkadi-Nagy E, Sul HS: Regulation of lipolysis in adipocytes. Annu Rev Nutr 2007; 27: 79– 101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lass A, Zimmermann R, Haemmerle G, Riederer M, Schoiswohl G, Schweiger M, Kienesberger P, Strauss JG, Gorkiewicz G, Zechner R: Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab 2006; 3: 309– 319 [DOI] [PubMed] [Google Scholar]
- 30.Wei E, Gao W, Lehner R: Attenuation of adipocyte triacylglycerol hydrolase activity decreases basal fatty acid efflux. J Biol Chem 2007; 282: 8027– 8035 [DOI] [PubMed] [Google Scholar]
- 31.Laplante M, Sabatini DM: An emerging role of mTOR in lipid biosynthesis. Curr Biol 2009; 19: R1046– R1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Polak P, Cybulski N, Feige JN, Auwerx J, Rüegg MA, Hall MN: Adipose-specific knockout of raptor results in lean mice with enhanced mitochondrial respiration. Cell Metab 2008; 8: 399– 410 [DOI] [PubMed] [Google Scholar]
- 33.Yeh WC, Bierer BE, McKnight SL: Rapamycin inhibits clonal expansion and adipogenic differentiation of 3T3–L1 cells. Proc Natl Acad Sci U S A 1995; 92: 11086– 11090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang HH, Huang J, Duvel K, Boback B, Wu S, Squillace RM, Wu CL, Manning BD: Insulin stimulates adipogenesis through the Akt-TSC2-mTORC1 pathway. PLoS One 2009; 4: e6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim JY, Tillison K, Lee JH, Rearick DA, Smas CM: The adipose tissue triglyceride lipase ATGL/PNPLA2 is downregulated by insulin and TNF-alpha in 3T3–L1 adipocytes and is a target for transactivation by PPARgamma. Am J Physiol Endocrinol Metab 2006; 291: E115– E127 [DOI] [PubMed] [Google Scholar]
- 36.Kienesberger PC, Lee D, Pulinilkunnil T, Brenner DS, Cai L, Magnes C, Koefeler HC, Streith IE, Rechberger GN, Haemmerle G, Flier JS, Zechner R, Kim YB, Kershaw EE: Adipose triglyceride lipase deficiency causes tissue-specific changes in insulin signaling. J Biol Chem 2009; 284: 30218– 30229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGarry JD: Banting Lecture 2001: Dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 2002; 51: 7– 18 [DOI] [PubMed] [Google Scholar]
- 38.Savage DB, Petersen KF, Shulman GI: Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev 2007; 87: 507– 520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kershaw EE, Hamm JK, Verhagen LA, Peroni O, Katic M, Flier JS: Adipose triglyceride lipase: function, regulation by insulin, and comparison with adiponutrin. Diabetes 2006; 55: 148– 157 [PMC free article] [PubMed] [Google Scholar]
- 40.Fischer J, Lefèvre C, Morava E, Mussini JM, Laforêt P, Negre-Salvayre A, Lathrop M, Salvayre R: The gene encoding adipose triglyceride lipase (PNPLA2) is mutated in neutral lipid storage disease with myopathy. Nat Genet 2007; 39: 28– 30 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.