Abstract
OBJECTIVE
Vascular endothelial cells (VECs) downregulate their rate of glucose uptake in response to hyperglycemia by decreasing the expression of their typical glucose transporter GLUT-1. Hitherto, we discovered critical roles for the protein calreticulin and the arachidonic acid–metabolizing enzyme 12-lipoxygenase in this autoregulatory process. The hypothesis that 4-hydroxydodeca-(2E,6Z)-dienal (4-HDDE), the peroxidation product of 12-lipoxygenase, mediates this downregulatory mechanism by activating peroxisome proliferator–activated receptor (PPAR) δ was investigated.
RESEARCH DESIGN AND METHODS
Effects of 4-HDDE and PPARδ on the glucose transport system and calreticulin expression in primary bovine aortic endothelial cells were evaluated by pharmacological and molecular interventions.
RESULTS
Using GW501516 (PPARδ agonist) and GSK0660 (PPARδ antagonist), we discovered that high-glucose–induced downregulation of the glucose transport system in VECs is mediated by PPARδ. A PPAR-sensitive luciferase reporter assay in VECs revealed that high glucose markedly increased luciferase activity, while GSK0660 abolished it. High-performance liquid chromatography analysis showed that high-glucose incubation substantially elevated the generation of 4-HDDE in VECs. Treatment of VECs, exposed to normal glucose, with 4-HDDE mimicked high glucose and downregulated the glucose transport system and increased calreticulin expression. Like high glucose, 4-HDDE significantly activated PPARδ in cells overexpressing human PPAR (hPPAR)δ but not hPPARα, -γ1, or -γ2. Moreover, silencing of PPARδ prevented high-glucose–dependent alterations in GLUT-1 and calreticulin expression. Finally, specific binding of PPARδ to a PPAR response element in the promoter region of the calreticulin gene was identified by utilizing a specific chromatin immunoprecipitation assay.
CONCLUSIONS
Collectively, our data show that 4-HDDE plays a central role in the downregulation of glucose uptake in VECs by activating PPARδ.
Hyperglycemia is a major and independent risk factor in the development of cardiovascular disease and atherosclerosis in diabetes (1,2). Vascular endothelial cell (VEC) dysfunction precedes the development of atherosclerotic plaques (3–6). These adverse functions in VECs result from impaired carbohydrate metabolism, mitochondrial dysfunction, oxidative stress, excessive protein glycation, malfolding of proteins, and altered expression of various genes (3,7).
For reasons not well understood, some diabetic patients never develop long-term vascular complications (8,9). We discovered an autoregulatory mechanism that protected VECs against deleterious effects of hyperglycemia by downregulating the level of their principal glucose transporter GLUT-1 mRNA and protein and its plasma membrane abundance (10–12). We linked this protective mechanism to an augmented expression of the enzyme 12-lipoxygenase (12-LO), which produces 12-hydroxyeicosatetraenoic acid (12-HETE) from arachidonic acid. Pharmacological inhibition of 12-LO completely blocked this downregulatory interaction, suggesting a critical role for 12-HETE in this process. We also found that GLUT-1 downregulation resulted from destabilization and degradation of GLUT-1 mRNA via a specific interaction with the protein calreticulin (13), whose expression was significantly increased in VECs and blood vessels under hyperglycemic conditions (13).
The 12-LO metabolite 12-hydroperoxyeicosatetraenoic acid (12-HpETE) is effectively reduced by glutathione peroxidase (GPx) to 12-HETE (14). Reactive oxygen species (ROS), which are produced under hyperglycemic conditions (7), inactivate GPx and slow this reaction. This renders 12-HpETE susceptible to radical induced peroxidation and a chain-breaking reaction to generate the corresponding reactive hydroxyalkenal, 4-hydroxydodeca-(2E, 6Z)-dienal (4-HDDE) (15,16).
High levels of 4-hydroxyalkenals are cytotoxic due to covalent adduct formation with macromolecules. Yet, these lipid peroxidation products function at low concentrations as signaling molecules (17,18). For instance, 4-hydroxy-2E-nonenal (4-HNE), the peroxidation product of 15-HpETE, binds to and activates the nuclear receptor peroxisome proliferator–activated receptor (PPAR) δ in 3T3-L1 preadipocytes (19).
The present study aimed at investigating the hypothesis that 12-HETE and/or 4-HDDE, the oxidation and peroxidation products of 12-HpETE, respectively, interact with PPARδ and regulate calreticulin expression to operate the downregulatory machinery of the glucose transport system in VECs.
RESEARCH DESIGN AND METHODS
Glucose-free Dulbecco's modified Eagle's medium was from Life Technologies (Grand Island, NY). Biological Industries (Beth-Haemek, Israel) supplied antibiotics, bovine fibronectin, FCS, and soybean trypsin inhibitor. Calbiochem (Darmstadt, Germany) supplied GW501516 and 4-HNE. 4-HDDE was synthesized as described (16). Sigma-Aldrich (Rehovot, Israel) supplied 12-HETE, WY14643, baicalein, cytochalasin B, GSK0660, l-glucose, troglitazone, and the anti–α-tubulin antibody. The following polyclonal antibodies were used: rabbit anti-calreticulin (Stressgen Biotechnologies, Victoria, BC, Canada); rabbit anti–GLUT-1 (courtesy of Dr. H.-G. Joost, the Institute of Human Nutrition, Bergholz-Rehbrücke, Germany); anti-(C terminus) GLUT-3 and -GLUT-4 (Millipore, Billerica, MA); anti-PPARα, -PPARγ, and -PPARδ (Cayman Chemicals, Ann Arbor, MI); anti-PPARδ (H-74; Santa Cruz Biotechnology, Santa Cruz, CA); and horseradish peroxidase–conjugated anti-rabbit IgG (Jackson ImmunoResearch, West Grove, PA). Abcam (Cambridge, U.K.) supplied the chromatin immunoprecipitation (ChIP) assay kit. American Radiolabeled Chemicals (St. Louis, MO) supplied 2-[1,2-3H(N)]deoxy-d-glucose (1.48 TBq/mmol). Mirus Bio-Corporation (Madison, WI) supplied the TransIT-LT1reagent and Biontex Laboratories (Munich, Germany) the Metafectene. Promega (Madison, WI) supplied the dual-luciferase reporter assay system, ligase, Pfu DNA polymerase, pGEM T-easy plasmid, random primers, restriction enzymes, and RNA polymerase. RNasin was from TaKaRa (Tokyo, Japan). Real-time PCR reagents were purchased from Applied Biosystems (Carlsbad, CA). ReddyMix PCR Master was from Thermo Scientific (Epsom, Surrey, U.K.). The PPARδ small-interfering RNA (siRNA) was from Dharmacon (Lafayette, CO). The pcDNA3 and pEGFP-N1 plasmids and the MCF10 cDNA library were kindly provided by Dr. R. Hertz and Dr. R. Reich, respectively (The Hebrew University, Jerusalem, Israel). The pSVPORT1-hRXR vector and the 3×PPAR response element (PPRE)-TK-luciferase plasmid were donated by Dr. B.M. Spiegelman (Dana Farber Cancer Institute, Boston, MA). The plasmids pCMX-hPPARγ1 and pCMX-hPPARγ2 and the respective empty plasmids were kindly provided by Dr. R. Evans (Howard Hughes Medical Institute, La Jolla, CA). The pSG5 and pSG5-hPPARα vectors were prepared in Dr. B. Staels' laboratory. All primers were synthesized by Sigma-Aldrich (Rehovot, Israel). Organic solvents were from Frutarom (Haifa, Israel) and Mallinckrodt Baker (Deventer, Holland).
Cell cultures.
Primary cultures of bovine aortic endothelial cells were prepared and characterized as described previously (20). EA.hy926 cells were cultured and maintained as described (21).
Hexose uptake assay.
The [H3]dGlc uptake assay in VEC cultures was conducted as previously described (22). The nonspecific [H3]dGlc uptake that was determined in the presence of 10 μmol/l cytochalasin B in the uptake mixture was <4% of the total uptake. Cell numbers were determined microscopically in a hemocytometer following cell detachment with a trypsin-EDTA solution for endothelial cells (Sigma-Aldrich, Rehovot, Israel). Soybean trypsin inhibitor (50 μg/ml) was used to stop the reaction. Trypan blue exclusion tests showed <1–3% dead cells following trypsinization.
Western blot analyses.
Cell lysates were prepared, and Western blot analyses of GLUT-1 calreticulin, PPARδ, and α-tubulin were performed as previously described (22) or according to the antibody suppliers' protocols. Cell surface biotinylation of VECs and determination of plasma membrane–associated GLUT-1 were carried as described (23).
Real-time PCR analysis.
RNA isolation, cDNA synthesis, and real-time PCR analyses of calreticulin and GLUT-1 mRNA in VECs were performed as described by Totary-Jain et al. (13), using the same primers. Both mRNA levels were normalized to 18S rRNA.
Cell transient transfections.
VEC cultures, at 60% confluency, were cotransfected with DNA complexed to TransIT-LT1reagent in 2 ml growth medium, according to the manufacturer's instructions. The DNA consisted of 165 ng pSG5-hPPARα, pCMX-hPPARγ1, pCMX-hPPARγ2, pcDNA-hPPARδ, or the corresponding empty plasmids. It also contained the following plasmids: pSVPORT1-hRXR (82.5 ng), 3×PPRE-TK-luciferase reporter (500 ng), the renilla luciferase (100 ng), and pEGFP-N1 (82.5 ng). The latter served to assess of the yield of transfection by fluorescence microscopy (>80%; excitation, 490 nm; emission, 515 nm). After 24 h, the cultures were washed, received fresh medium, and incubated for additional 24 h. Firefly luciferase–induced luminescence was determined with the dual-luciferase reporter assay in a Mithras LB-940 luminometer and normalized to Renilla luciferase activity as an internal control, according to the kit's instructions (Berthold Technologies, Bad Wilbad, Germany).
Extraction of polar lipids and high-performance liquid chromatography analysis.
Polar lipid extraction from culture media or plasma and high-performance liquid chromatography (HPLC) analysis were performed according to Zanardi et al. (24), with some modifications. Briefly, VEC cultures in 15-cm plates were incubated for 24 h with serum-free media and the indicated additions. The media were then collected, cleared by centrifugation, and loaded on prewashed Supelclean LC-18 SPE tubes (6 ml/1gr; Supelco, Bellefonte, PA) and washed with 15 ml cold water and petroleum ether (boiling range 40–60°C). The polar lipid fraction was then eluted with 3 ml cold methanol, dried under N2, dissolved in 300 μl cold methanol, filtered through a Teflon syringe filter (0.45 μmol/l; National Scientific, Rockwood, TN), sealed under N2, and kept at −70°C. HPLC analysis was performed in a Merck Hitachi machine with a Supelcosil LC-18-DB column (5 μmol/l particle size, 25 cm × 10 mm; Supelco, Bellefonte, PA) connected to an ultraviolet detector (295 nm). For 4-HDDE, the elution (1.0 ml/min) started with a gradient of acetonitrile:water (42:58), flowed by a linear gradient that progressed over 25 min to 100% acetonitrile. For 4-HNE detection, the initial ratio in the mixture was 30:70. Pure 4-HDDE and 4-HNE were used to resolve their respective peaks at 10.0 and 4.2 min. The recovery of the standards was 85–90%. Clarity-Lite software was used to analyze and quantify HPLC data.
ChIP.
VECs at 5.5 mmol/l glucose were transfected with pcDNA-hPPARδ, pSVPORT1-hRXR, and pEGFP-N1 plasmids, as described above, and incubated for 24 h. The cells were then treated with 100 nmol/l of GW501516 or with DMSO and incubated for additional 24 h. Cell lysates were then prepared, sonicated, fractionated, and immunoprecipitated with anti-PPARδ (H-74) and anti–histone H3 (positive control, ab-1791; Abcam, Cambridge, U.K.) and taken for PCR with specific primers, according to the protocol of the ChIP assay kit as described in the online appendix (available at http://diabetes.diabetesjournals.org/cgi/content/full/db09-1207/DC1).
PPARδ silencing with siRNA.
Subconfluent bovine aortic endothelial cultures were transfected with 100 nmol/l of the siRNA 5′-AAAATGATGTCACTGAAGGGC-3′ targeted to bovine PPARδ, using metafectene according to the manufacturer's instructions. Control cells were treated with metafectene only. Cells were harvested 72 h after transfection, and lysates were prepared and used for Western blot analyses.
Statistical analysis.
Results are given as means ± SE. Statistical analyses were performed using the nonparametric Mann-Whitney test.
RESULTS
Effects of PPAR agonists on the rate of glucose uptake in VECs.
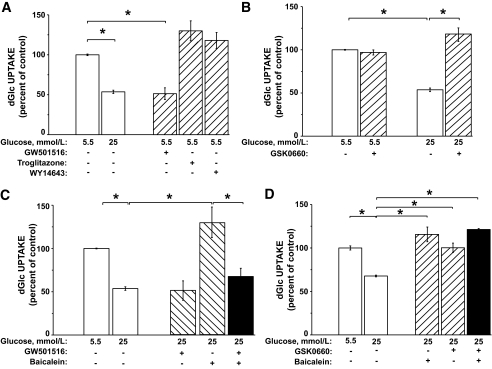
The potential role of PPARα, -γ, or -δ in the regulation of glucose uptake in VECs was elucidated by studying the capacity of their respective selective agonists, WY14643, troglitazone, and GW501516 (25), to downregulate the rate of glucose uptake in VECs under normal glucose levels (Fig. 1A). The control treatment (25 mmol/l glucose) shows the characteristic high-glucose–induced ∼50% reduction in the rate of hexose uptake in comparison to the 5.5 mmol/l glucose incubation (10). GW501516, but not troglitazone or WY14643, mimicked high glucose and comparably downregulated the uptake rate in cells under normal glucose, indicating that only PPARδ participates in the downregulatory response. Maximal effect of GW501516 was obtained between 1 and 100 nmol/l and within 36–48 h (supplemental Fig. S1A and B, which can be found in the online appendix. The vehicle (DMSO) had no significant effect on the rate of glucose uptake.
FIG. 1.
Activation of PPARδ reduces the rate of hexose transport in VECs. Confluent VEC cultures were exposed to 5.5 or 25 mmol/l glucose for 48 h. A: The 5.5 mmol/l cultures were treated without or with GW501516 (100 nmol/l), troglitazone (30 μmol/l), or WY14643 (60 μmol/l). B: Both 5.5 and 25 mmol/l glucose cultures were incubated for 48 h in the absence or presence of 1 μmol/l of GSK0660. C: VEC cultures were incubated at 5.5 or 25 mmol/l glucose and treated with GW501516 (100 nmol/l) and/or baicalein (80 μmol/l), as indicated. The latter was present during the last 10 h of incubation. D: VEC cultures were incubated at 5.5 or 25 mmol/l glucose for 48 h without or with GSK0660 (1 μmol/l, 48 h) and or baicalein (80 μmol/l, last 10 h). At the end of incubations, cells were washed and taken for the standard [3H]dGlc uptake assay. The respective rates of dGlc uptake at 5.5 mmol/l glucose (A: 70 ± 8, B: 54 ± 5, C: 96 ± 1, and D: 65 ± 8 pmol dGlc/106 cells/min) were taken as 100%. *P < 0.05 for differences from the respective controls (n = 4).
Figure 1B shows that the PPARδ antagonist GSK0660 (26) effectively prevented the downregulation of glucose uptake induced by 25 mmol/l glucose in VECs, whereas the basal high rate of glucose uptake in cells at 5.5 mmol/l glucose was not altered. Supplemental Fig. S1C confirms the specificity and the competitive nature of GSK0660-induced inhibition of PPARδ.
Baicalein, a specific 12-LO inhibitor, was used to test the hypothesis that 12-LO metabolites and PPARδ cooperate in the downregulatory response. Fig. 1C shows that the inhibition of 12-LO with baicalein prevented high-glucose–induced downregulation of glucose uptake. Yet, GW501516 downregulated the uptake in the presence of baicalein. No synergistic interactions between GSK0660 and baicalein in high-glucose VEC cultures were found, suggesting that 12-LO and PPARδ act in sequence (Fig. 1D). The cellular content of PPARδ was not altered by the ambient glucose, GW501516, or baicalein (supplemental Fig. S2). Supplemental Fig. S3 indicates that the slight increase in the osmotic pressure in the high-glucose–containing culture medium had no effect on the glucose transport system.
GW501516 reduces GLUT-1 expression and plasma membrane abundance in VECs.
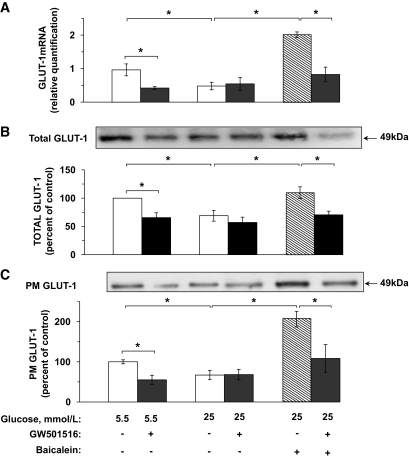
The data in Fig. 2A–C show that GW501516 reduced GLUT-1 mRNA and protein levels and plasma membrane abundance in cells exposed to 5.5 mmol/l glucose to the downregulated levels induced by the 25 mmol/l glucose incubation. Moreover, GW501516 also prevented baicalein-mediated upregulation of GLUT-1 expression and its subcellular distribution in cells exposed to high glucose, thus linking 12-LO metabolites to PPARδ in the mechanism of the downregulation of GLUT-1 expression. Supplemental Fig. S4 shows that the cell content of GLUT-3 and GLUT-4 in VECs was not altered by high glucose or GW501516.
FIG. 2.
GW501516 decreases the level of GLUT-1 mRNA and protein as well as its plasma membrane abundance in VECs.VEC cultures were treated with GW501516 and baicalein as described under the legend to Fig. 1C. Cells were then processed and taken for real-time PCR analysis of GLUT-1 mRNA (A), Western blot analysis of GLUT-1 (B), or for cell surface biotinylation to determine the relative plasma membrane (PM) abundance of GLUT-1 (C). *P < 0.05, for differences from the respective controls (n = 4).
High glucose activates PPARδ in VECs.
The hypothesis that high glucose levels activate PPARδ was further investigated in VECs that were transfected with a PPRE-luciferase reporter vector (3×PPRE-TK-luciferase). Figure 3A shows a 1.5-fold increase in luciferase activity in cells exposed to 25 mmol/l glucose in comparison with the low-glucose incubation. GSK0660 abolished this stimulatory effect, suggesting a PPARδ-dependent transcription of the luciferase gene. The control experiment in supplemental Fig. S5 shows that GSK0660 fully inhibited GW501516-induced transcription of luciferase in the same assay. VECs overexpressing the various hPPAR isotypes (α, γ1, γ2, or δ), hRXR, and the PPRE-luciferase reporter expression plasmids were used. Control cells received the empty plasmids. All four hPPAR isotypes were successfully overexpressed (supplemental Fig. S6). Figure 3B shows that high glucose increased luciferase activity 1.3- to 1.5-fold in VECs that were transfected with all empty plasmids. This effect, which was also found in VECs transfected with the reporter construct only (Fig. 3A), is attributed to the activation of an endogenous PPAR by high glucose. The observation that high glucose markedly increased luciferase activity (2.6-fold) only in cells overexpressing hPPARδ suggests that PPARδ is this endogenous receptor that is activated under hyperglycemic conditions.
FIG. 3.
High glucose selectively activates PPARδ in VECs. A: VEC cultures were transfected with the 3×PPRE-TK-luciferase reporter and the Renilla luciferase plasmids and incubated at 2 or 25 mmol/l glucose for 48 h. GSK0660 (1 μmol/l) was present as indicated during the last 24 h of incubation. P < 0.05 in comparison with the *2 or **25 mmol/l glucose control incubations (n = 4). B: VEC cultures were transfected with the following expression plasmids: pSG5-hPPARα or pCMX-hPPARγ1 or pCMX-hPPARγ2 or pCDNA-hPPARδ. Control cells were transfected with the corresponding empty plasmids. Cells were also cotransfected with pSVPORT-hRXR, pEGFP-N1 plasmid, 3×PPRE-TK-luciferase reporter plasmid, and the Renilla luciferase plasmid. The cells were incubated for 48 h at 2 or 25 mmol/l glucose and then harvested, lysed, and taken for the luciferase activity assay. Results are given as the relative luciferase activity in comparison with the 2 mmol/l glucose incubation. *P < 0.05 (n = 4).
12-HETE activates PPARδ in VECs.
High glucose increases 12-LO expression in VECs and consequently augments the synthesis and secretion of 12-HETE in VECs (10). When tested in the present luciferase reporter assay, 12-HETE (3 μmol/l, 24 h) increased luciferase activity 1.70 ± 0.03-fold (n = 3) in hPPARδ-expressing VECs, in comparison with control cells. This is most likely an underestimated value because 12-HETE is prone to a rapid oxidation in the open air (t1/2 = 10–12 min [10]). This degradation is most likely the reason for the failure of exogenously added 12-HETE to downregulate the rate of hexose transport in VEC cultures (10).
4-HDDE activates PPARδ and modulates the rate of hexose transport in VECs.
Radical-induced peroxidation of the 12-LO and 15-LO products, 12-HpETE and 15-HpETE, respectively, generate the corresponding biologically active aldehydes 4-HDDE and 4-HNE (16,18). It has been reported that 15-HETE and 4-HNE activate PPARδ (19). We asked whether 4-HDDE could activate PPARδ in VECs. Figure 4A shows a remarkable 4.3 ± 0.1-fold increase in hPPARδ-dependent, but not hPPARα-, hPPARγ1-, or hPPARγ2-dependent, stimulation of the reporter luciferase activity in cells treated with 100 nmol/l 4-HDDE. Like high glucose and GW501516 (Figs. 1 and 2), 4-HDDE (50 nmol/l) significantly reduced the rate of hexose uptake (Fig. 4B) and the cell content of GLUT-1 mRNA and protein (Fig. 4C and D) in VECs under 5.5 mmol/l glucose. The upregulatory effect induced by baicalein in high-glucose cultures was also reversed by 4-HDDE (Fig. 4B–D). The inhibitor GSK0660 abolished 4-HDDE–induced downregulation of the rate of hexose transport in VECs exposed to 5.5 mmol/l glucose (Fig. 4E). The concentrations of 4-HDDE used in these experiments (50–100 nmol/l) did not compromise VEC viability (supplemental Fig. S7). The similar effects of 4-HDDE and GW501516 suggest that the former is an endogenous ligand for PPARδ.
FIG. 4.
4-HDDE specifically activates PPARδ and downregulates the glucose transport system in VECs. A: VEC cultures that had been maintained at 5.5 mmol/l glucose were transfected with the various hPPAR expression vectors and other plasmids and incubated as described in the legend to Fig. 3B. Cultures received the indicated concentration of 4-HDDE during the last 24 h of incubation. The cells were then lysed, and the relative luciferase activity was determined and standardized to the untreated control. *P < 0.05, for differences from the untreated cells (n = 4). B–D: Confluent VEC cultures were incubated for 48 h with 5.5 or 25 mmol/l glucose in the absence or presence of 4-HDDE (50 nmol/l). Baicalein (80 μmol/l) was added to cultures for the last 10 h of incubation. The cells were then washed, lysed, and taken for the standard [3H]dGlc uptake assay (B), real-time PCR analysis of GLUT-1 mRNA (C), or Western blot analysis of total GLUT-1 (D). The rate of dGlc uptake at 5.5 mmol/l glucose (79 ± 3 pmol dGlc/106 cells/min) was taken as 100%. *P < 0.05, for differences from the respective controls (n = 4). E: Confluent VEC cultures were incubated at 5.5 or 25 mmol/l glucose for 48 h in the absence or presence of 4-HDDE (50 nmol/l) and or GSK0660 (1 μmol/l), as indicated. At the end of incubation the cells were washed and taken for the standard [3H]dGlc uptake assay. The rate of dGlc uptake at 5.5 mmol/l glucose (65 ± 8 pmol/106 cells/min) was taken as 100%. *P < 0.05, for differences from the respective controls (n = 4).
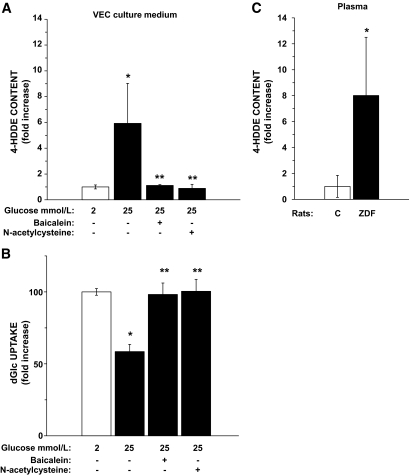
Extraction of polar lipid and HPLC analyses were performed to measure 4-HDDE production in VECs exposed to 2 or 25 mmol/l glucose. High glucose increased the secretion of 4-HDDE 5.9 ± 3.1-fold higher than at the low-glucose incubation (Fig. 5A), while baicalein blocked this effect, confirming that 4-HDDE is indeed derived from a 12-LO metabolite. The initial step in lipid peroxidation is a hydrogen atom abstraction by ROS (16); therefore, antioxidants can attenuate this process (27). Figure 5A and B shows that the antioxidant N-acetylcysteine completely prevented high-glucose–dependent generation of 4-HDDE and the downregulation of hexose transport. Fig. 5C depicts a comparable increase in 4-HDDE generation in the plasma of hyperglycemic Zucker diabetic rats (details on the animals are given in the online appendix). Representative HPLC tracings and 4-HDDE peaks in culture medium and plasma extracts are shown in supplemental Fig. S8. Noteworthy, similar HPLC analyses of the same extracts did not reveal significant differences in 4-HNE levels between the low- and high-glucose incubations (data not shown).
FIG. 5.
4-HDDE level is increased in high-glucose VEC cultures and in the plasma of Zucker diabetic fatty (ZDF) rats. A: Media (18 ml) of 2 or 25 mmol/l glucose VECs, without or with 80 μmol/l of baicalein or 1 mmol/l of N-acetylcysteine, were collected after 48 h of incubation, extracted, and taken for HPLC analysis of polar lipids. The average level of 4-HDDE in the 2 mmol/l medium extracts was taken as 1 unit. P < 0.05, for differences from the *2 or **25 mmol/l glucose incubations (n = 4). B: VEC cultures at 25 mmol/l glucose were treated similarly and taken at the end of the 48-h incubation period to the standard [3H]dGlc uptake assay. The rate of uptake at 5.5 mmol/l glucose (70 ± 1 pmol dGlc/106 cells/min) was taken as 100%. P < 0.05, for differences from the *2 or **25 mmol/l incubations (n = 4). C: The levels of 4-HDDE in extracts of plasma samples from ZDF and normoglycemic rats (respective blood glucose levels were 354 ± 28 and 92 ± 6 mg/dl, n = 4) were determined by HPLC, and the value of the average levels of 4-HDDE in the plasma of the nondiabetic rats was taken as 1 unit. *P < 0.05, for differences from the respective controls (n = 4).
PPARδ regulates calreticulin expression in VECs.
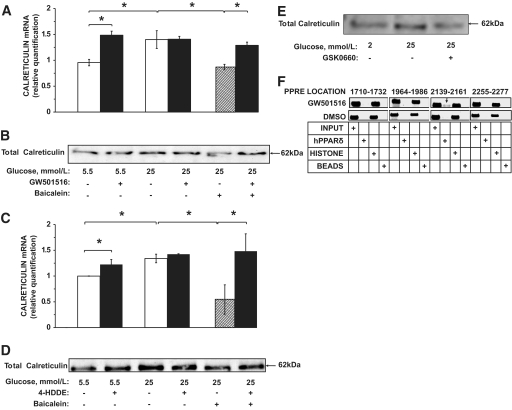
Calreticulin, whose expression is significantly increased in VECs exposed to high glucose, destabilizes GLUT-1 mRNA (13). Figures 6A–D confirm previous findings (13) on high-glucose–induced increased expression of calreticulin mRNA and protein levels in VECs under high glucose. This effect was eliminated in the presence of baicalein, suggesting that 12-LO metabolites participate in the regulation of calreticulin expression. Both GW501516 (Figs. 6A and B) and 4-HDDE (Figs. 6C and D) increased the expression levels of calreticulin mRNA and protein in VECs exposed to 5.5 mmol/l glucose. Both compounds also reversed the effect of baicalein and restored calreticulin mRNA and protein expression to the levels measured under the high-glucose incubation. Inversely, the inhibition of PPARδ with GSK0660 prevented high-glucose–induced increased expression of calreticulin in VECs (Fig. 6E). These data suggest that PPARδ participates in the regulation of calreticulin expression in VECs.
FIG. 6.
PPARδ regulates calreticulin expression in VECs. Confluent VEC cultures were treated with GW501516 (100 nmol/l) (A and B) or 4-HDDE (50 nmol/l) (C and D) as described in the legends to Figs. 1A and 4B, respectively. Cells were then processed for real-time PCR analysis of calreticulin mRNA (A and C) or Western blot analysis of calreticulin (B and D). *P < 0.05, for differences from the respective controls (n = 4). E: Confluent VEC cultures were treated with GSK0660 (1 μmol/l) as indicated for 48 h. At the end of incubation the cells were processed for Western blot analysis of calreticulin. F: VEC overexpressing hPPARδ and hRXR, which were prepared as described in the legend to Fig. 3B, were treated with GW501516 or the vehicle and processed for PPARδ-ChIP analysis as described in research design and methods.
The potential of PPARδ to interact with PPREs in the calreticulin gene was determined. We identified four PPRE sequences in the promoter region of the bovine calreticulin gene (3,000 bp upstream of the coding sequence) located at 5′-1,710–1,732, 1964–1986, 2,139–2,161, and 2,255–2,277 bp. The ChIP assay was used to determine whether PPARδ binds to these elements. DNA samples from VECs overexpressing hPPARδ and hRXR and treated with 100 nmol/l GW501516 or the vehicle were immunoprecipitated with an anti-PPARδ antibody and taken for the respective PCR analyses. Figure 6F depicts a specific binding interaction of PPARδ with the PPRE located at 5′-2139–2,161 bp in this promoter region.
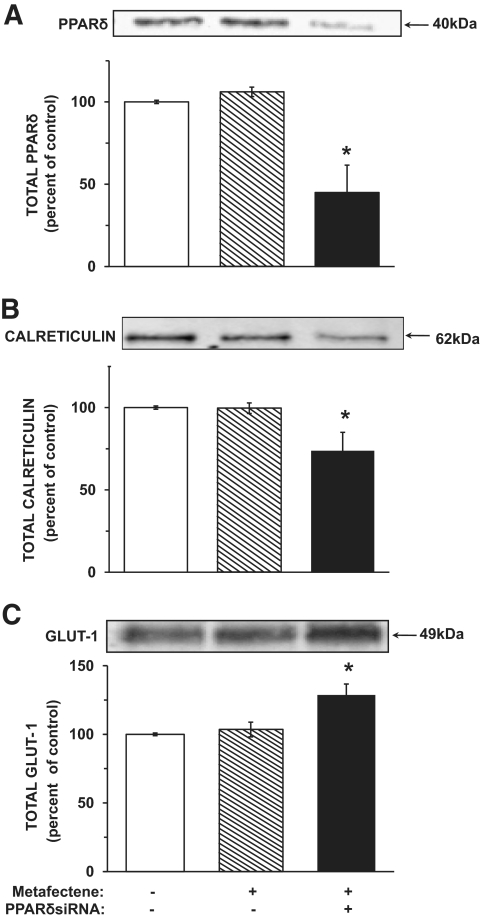
PPARδ expression in VECs was silenced with a specific siRNA, and the expression levels of calreticulin and GLUT-1 were determined (Fig. 7). A 50–60% reduction in PPARδ expression in VECs exposed to 25 mmol/l led to a 30–40% decrease in the content of calreticulin and a 30–40% increase in GLUT-1 expression in comparison with the respective controls. Similar effects were observed in VECs exposed to 5.5 mmol/l glucose (data not shown).
FIG. 7.
Partial silencing of PPARδ reduces calreticulin and increases GLUT-1 expression in VECs. Subconfluent cultures of VECs maintained at 25 mmol/l glucose were transfected with siRNA targeted to the bovine PPARδ mRNA, as described under research design and methods and incubated for 72 h. The cultures were then washed, lysed, and taken for Western blot analyses of PPARδ (A), calreticulin (B), and GLUT-1 (C). *P < 0.05, for differences from the metafectene controls (n = 4).
Finally, we used the EA.hy926 cell line to determine whether human-derived endothelial cells regulate the glucose transport system like bovine VECs. This cell line, which was derived from primary cultures of human umbilical cord vascular endothelial cells (21), has been shown in many studies to preserve markers and functions of the primary cells. High glucose downregulated the rate of glucose transport and total GLUT-1 level and increased calreticulin expression in a PPARδ-dependent manner in these cells in (supplemental Fig. S9A–C). Exposure of EA.hy926 cells to normal glucose conditions and 4-HDDE mimicked the effect of high glucose and reduced the rate of hexose transport, while GSK0660 prevented this effect (Fig. S9D). These data suggest that both bovine and human VECs use a similar mechanism to downregulate the glucose transport system.
DISCUSSION
Two key factors that mediate high-glucose–induced downregulation of the glucose transport system in bovine aortic endothelial cells and in the human-derived EA.hy926 cells have been identified: the lipid peroxidation product 4-HDDE and its cognate nuclear receptor PPARδ. The latter increases the expression of the protein calreticulin that was shown before to destabilize GLUT-1 mRNA (13).
The augmented production of 4-HDDE results from high-glucose–induced 12-LO expression and activity and glucose-derived ROS. The mechanism responsible for the increased expression of 12-LO is not yet known. Numerous studies have proven that hyperglycemia promotes the generation of ROS (3,7). We showed before an augmented production of ROS in bovine aortic endothelial cell primary cultures under high-glucose conditions (28). Two observations confirm the role of ROS in the generation of 4-HDDE from 12-LO metabolites. First, the inhibition of 12-LO activity with baicalein significantly reduced 4-HDDE secretion from VECs under high-glucose conditions. Second, the antioxidant N-acetylcysteine blocked 4-HDDE production in VECs under similar conditions. The capacity of baicalein and N-acetylcysteine to prevent high-glucose–induced downregulation of glucose uptake is attributed to the impeded production of 4-HDDE. Collectively, these data indicate that high-glucose–induced overexpression of 12-LO and overproduction of ROS underlie the augmented generation of 4-HDDE.
The physiological, pathophysiological, or cytotoxic effects of 4-hydroxyalkenals depend on their absolute concentrations. Esterbauer et al. (18) concluded that at concentration >20 μmol/l, 4-HNE exhibited cytotoxic effects due to its chemical reactivity and the formation of stable adducts with macromolecules. Bacot et al. (16,29) linked 4-hydroxyalkenals to membrane disorders due to the formation of stable adducts with ethanolamine phospholipids. It is important to emphasize that the effective concentrations of 4-HDDE (1–100 nmol/l) used in the present study were not cytotoxic to VECs. The present study shows that a moderate production of ROS, sufficient to initiate the generation of nontoxic levels of 4-HDDE in VECs, is required for an effective cellular defense against harmful effects of hyperglycemia. Notwithstanding, an excessive and uncontrolled oxidative stress in VECs may lead to the production of cytotoxic levels of 4-hydroxyalkenals. Many studies have indeed suggested that an excessive 4-hydroxyalkenal production underlies various pathological conditions (30,31).
Tissue-specific production of 4-hydroxyalkenals correlates with the particular pattern of expression of the various lipoxygenases and synthesis of their corresponding HpETEs. For example, the peroxidation products 4-HHE and 4-HNE, but not 4-HDDE, were found in the diabetic rat retina, which predominantly expresses 5-LO and 15-LO (29,32). Our present findings on 4-HDDE– and PPARδ-dependent downregulation of the glucose transport system in human VECs correlate well with previous reports on high-glucose–induced expression of 12-LO in human VECs (4).
We found before that high glucose levels increased the expression of 12-LO but not of 15-LO in VECs (10). Subsequently, high glucose promotes the generation of 4-HDDE from 12-HpETE but not of 4-HNE, which is derived from 15-HpETE. Nevertheless, it has been shown before that 4-HNE also activates PPARδ (19). Using the luciferase reporter assay we found that the potency of 4-HDDE to activate the system was 200-fold higher than that of 4-HNE (50 nmol/l vs. 10 μmol/l). These disparate potencies suggest that the binding affinity of 4-HDDE to the ligand binding domain (LBD) in PPARδ is markedly higher than that of 4-HNE. This may reflect the increased hydrophobicity of the 4-HDDE in comparison with 4-HNE (logP values 3.48 and 2.45, respectively, calculated with the Molinspiration Chemoinformatics software). X-ray analyses of crystal structures of the PPARδ LBD identified a network of hydrogen bonds with His413, Tyr427, His287, and Thr253 that are involved in the binding interaction of eicosapentanoic acid (33). His413 has also been implicated in a hydrogen-bonding interaction with the 4-hydroxy group of 4-HNE (19). The specific molecular binding kinetics and affinity of 4-HDDE to the PPARδ LBD remain to be analyzed.
Many studies over the last decade have assigned PPARδ key metabolic regulatory functions (34). For instance, it augments lipogenesis and glycolysis in the liver (35), increases fatty acid oxidation in adipocytes (36), and increases oxidative metabolism in skeletal muscles (37,38). Of interest are recent studies on protective effects of PPARδ ligands against the development of atherosclerosis by regulating lipid homeostasis, decreasing the expression of inflammatory genes, and attenuating macrophage migration. We speculate that 4-HDDE–activated PPARδ may mediate some of these effects.
This study links PPARδ activation to the transcription of the calreticulin gene. We have previously found that the final step in the downregulation of GLUT-1 expression in VECs is calreticulin-mediated destabilization of the transporter mRNA (13). Because calreticulin is a multifunctional protein (39), it is feasible that PPARδ-regulated transcription of calreticulin may affect some of these functions (e.g., calcium storage, cell adhesion, chaperoning of malfolded proteins). The present study has identified a PPRE in the promoter of the calreticulin gene that seems to interact with PPARδ. The precise nature of this interaction and its contribution to the assembly of an active transcription complex of calreticulin need further investigations.
Recently, Gross and Staels (40) have stressed the need for the development of novel PPARα and PPARγ agonists for the treatment of type 2 diabetes. We suggest that novel PPARδ agonists may reduce the risk of dysfunctional endothelial cells in blood vessels by downregulating the rate of glucose uptake and protecting them from the deleterious effects of an increased influx of glucose. Clearly, 4-HDDE is not considered a potential pharmacological agonist due to its severe side effects when present at concentrations that allow irreversible covalent binding and cross-linking of macromolecules. Nonetheless, molecular and chemical analyses of the interaction of 4-HDDE with the PPARδ LBD may provide a platform for a rational design of potent and safe PPARδ agonists.
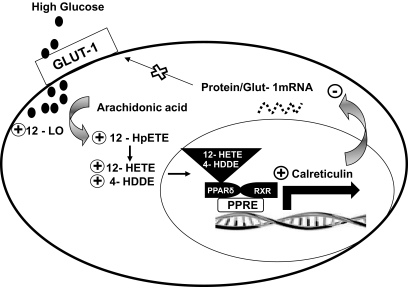
Finally, our previous and current findings on the autoregulation of the glucose transport system in VECs are summarized in the model shown in Fig. 8. High glucose levels increase the expression of the arachidonic acid, metabolizing enzyme 12-LO, which leads to an augmented production of 12-HpETE and its immediate metabolite 12-HETE. Concomitantly, high-glucose–derived ROS initiates the peroxidation of 12-HpETE and the generation of 4-HDDE. This molecule, and possibly 12-HETE, interact specifically with and activate PPARδ, which in turn binds to a PPRE in the promoter region of the calreticulin gene and augments its transcription. Calreticulin binds to a specific 10-nucleotide sequence located in the 3′-untranslated region of GLUT-1 mRNA, destabilizing and rendering it susceptible to degradation. Consequently, the cell content of GLUT-1 protein and its plasma membrane abundance are significantly reduced and downregulation of glucose uptake ensues.
FIG. 8.
A model for high-glucose–induced downregulation of the glucose transport system in VECs.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the German-Israel Foundation for Scientific Research and Development (I-750-165.2/2002), the Israel Ministry of Health, the Yedidut Foundation Mexico, the David R. Bloom Center for Pharmacy at the Hebrew University, The Dr. Adolf and Klara Brettler Center for Research in Molecular Pharmacology and Therapeutics at The Hebrew University and COST (European Cooperation in Science and Technology) Actions BM0602 and B35.
Y.R. Y.S.-M., G.C., E.A., and A.G. received fellowships from The Hebrew University Center for Diabetes Research. S.S. is affiliated with the David R. Bloom Center for Pharmacy and The Dr. Adolf and Klara Brettler Center for Research in Molecular Pharmacology and Therapeutics at The Hebrew University.
No potential conflicts of interest relevant to this article were reported.
We thank Drs. R. Hertz, M. Armoni, and T. Kahan for helpful comments and discussions.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Milicevic Z, Raz I, Beattie SD, Campaigne BN, Sarwat S, Gromniak E, Kowalska I, Galic E, Tan M, Hanefeld M: Natural history of cardiovascular disease in patients with diabetes: role of hyperglycemia. Diabetes Care 2008; 31( Suppl. 2): S155– S160 [DOI] [PubMed] [Google Scholar]
- 2.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR: Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321: 405– 412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu Y, Lyons TJ: A lethal tetrad in diabetes: hyperglycemia, dyslipidemia, oxidative stress, and endothelial dysfunction. Am J Med Sci 2005; 330: 227– 232 [DOI] [PubMed] [Google Scholar]
- 4.Patricia MK, Kim JA, Harper CM, Shih PT, Berliner JA, Natarajan R, Nadler JL, Hedrick CC: Lipoxygenase products increase monocyte adhesion to human aortic endothelial cells. Arterioscler Thromb Vasc Biol 1999; 19: 2615– 2622 [DOI] [PubMed] [Google Scholar]
- 5.Roth T, Podesta F, Stepp MA, Boeri D, Lorenzi M: Integrin overexpression induced by high glucose and by human diabetes: potential pathway to cell dysfunction in diabetic microangiopathy. Proc Natl Acad Sci U S A 1993; 90: 9640– 9644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stenina OI: Regulation of vascular genes by glucose. Curr Pharm Des 2005; 11: 2367– 2381 [DOI] [PubMed] [Google Scholar]
- 7.Brownlee M: Negative consequences of glycation. Metabolism 2000; 49: 9– 13 [DOI] [PubMed] [Google Scholar]
- 8.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Jr, Stone NJ: Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol 2004; 44: 720– 732 [DOI] [PubMed] [Google Scholar]
- 9.National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106: 3143– 3421 [PubMed] [Google Scholar]
- 10.Alpert E, Gruzman A, Totary H, Kaiser N, Reich R, Sasson S: A natural protective mechanism against hyperglycaemia in vascular endothelial and smooth-muscle cells: role of glucose and 12-hydroxyeicosatetraenoic acid. Biochem J 2002; 362: 413– 422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alpert E, Gruzman A, Riahi Y, Blejter R, Aharoni P, Weisinger G, Eckel J, Kaiser N, Sasson S: Delayed autoregulation of glucose transport in vascular endothelial cells. Diabetologia 2005; 48: 752– 755 [DOI] [PubMed] [Google Scholar]
- 12.Sasson S, Eckel J: Disparate effects of 12-lipoxygenase and 12-hydroxyeicosatetraenoic acid in vascular endothelial and smooth muscle cells and in cardiomyocytes. Arch Physiol Biochem 2006; 112: 119– 129 [DOI] [PubMed] [Google Scholar]
- 13.Totary-Jain H, Naveh-Many T, Riahi Y, Kaiser N, Eckel J, Sasson S: Calreticulin destabilizes glucose transporter-1 mRNA in vascular endothelial and smooth muscle cells under high-glucose conditions. Circ Res 2005; 97: 1001– 1008 [DOI] [PubMed] [Google Scholar]
- 14.Vericel E, Rey C, Calzada C, Haond P, Chapuy PH, Lagarde M: Age-related changes in arachidonic acid peroxidation and glutathione-peroxidase activity in human platelets. Prostaglandins 1992; 43: 75– 85 [DOI] [PubMed] [Google Scholar]
- 15.Guichardant M, Chantegrel B, Deshayes C, Doutheau A, Moliere P, Lagarde M: Specific markers of lipid peroxidation issued from n-3 and n-6 fatty acids. Biochem Soc Trans 2004; 32: 139– 140 [DOI] [PubMed] [Google Scholar]
- 16.Bacot S, Bernoud-Hubac N, Baddas N, Chantegrel B, Deshayes C, Doutheau A, Lagarde M, Guichardant M: Covalent binding of hydroxy-alkenals 4-HDDE, 4-HHE, and 4-HNE to ethanolamine phospholipid subclasses. J Lipid Res 2003; 44: 917– 926 [DOI] [PubMed] [Google Scholar]
- 17.Chen ZH, Niki E: 4-hydroxynonenal (4-HNE) has been widely accepted as an inducer of oxidative stress. Is this the whole truth about it or can 4-HNE also exert protective effects? IUBMB Life 2006; 58: 372– 373 [DOI] [PubMed] [Google Scholar]
- 18.Esterbauer H, Schaur RJ, Zollner H: Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med 1991; 11: 81– 128 [DOI] [PubMed] [Google Scholar]
- 19.Coleman JD, Prabhu KS, Thompson JT, Reddy PS, Peters JM, Peterson BR, Reddy CC, Vanden Heuvel JP: The oxidative stress mediator 4-hydroxynonenal is an intracellular agonist of the nuclear receptor peroxisome proliferator-activated receptor-beta/delta (PPARbeta/delta). Free Radic Biol Med 2007; 42: 1155– 1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaiser N, Sasson S, Feener EP, Boukobza-Vardi N, Higashi S, Moller DE, Davidheiser S, Przybylski RJ, King GL: Differential regulation of glucose transport and transporters by glucose in vascular endothelial and smooth muscle cells. Diabetes 1993; 42: 80– 89 [DOI] [PubMed] [Google Scholar]
- 21.Edgell CJ, McDonald CC, Graham JB: Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc Natl Acad Sci U S A 1983; 80: 3734– 3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sasson S, Gorowits N, Joost HG, King GL, Cerasi E, Kaiser N: Regulation by metformin of the hexose transport system in vascular endothelial and smooth muscle cells. Br J Pharmacol 1996; 117: 1318– 1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sasson S, Kaiser N, Dan-Goor M, Oron R, Koren S, Wertheimer E, Unluhizarci K, Cerasi E: Substrate autoregulation of glucose transport: hexose 6-phosphate mediates the cellular distribution of glucose transporters. Diabetologia 1997; 40: 30– 39 [DOI] [PubMed] [Google Scholar]
- 24.Zanardi E, Jagersma CG, Ghidini S, Chizzolini R: Solid phase extraction and liquid chromatography-tandem mass spectrometry for the evaluation of 4-hydroxy-2-nonenal in pork products. J Agric Food Chem 2002; 50: 5268– 5272 [DOI] [PubMed] [Google Scholar]
- 25.Oliver WR, Jr, Shenk JL, Snaith MR, Russell CS, Plunket KD, Bodkin NL, Lewis MC, Winegar DA, Sznaidman ML, Lambert MH, Xu HE, Sternbach DD, Kliewer SA, Hansen BC, Willson TM: A selective peroxisome proliferator-activated receptor delta agonist promotes reverse cholesterol transport. Proc Natl Acad Sci U S A 2001; 98: 5306– 5311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shearer BG, Steger DJ, Way JM, Stanley TB, Lobe DC, Grillot DA, Iannone MA, Lazar MA, Willson TM, Billin AN: Identification and characterization of a selective peroxisome proliferator-activated receptor beta/delta (NR1C2) antagonist. Mol Endocrinol 2008; 22: 523– 529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yajima D, Motani H, Hayakawa M, Sato Y, Sato K, Iwase H: The relationship between cell membrane damage and lipid peroxidation under the condition of hypoxia-reoxygenation: analysis of the mechanism using antioxidants and electron transport inhibitors. Cell Biochem Funct 2009 [DOI] [PubMed] [Google Scholar]
- 28.Altman H, Alpert E, Sasson S: Do glucose-derived reactive oxygen species contribute to the autoregulation of glucose transport in vascular endothelial and smooth muscle cells? In Cellular Dysfunction in Atherosclerosis and Diabetes: Reports From Bench to Bedside Simionescu M, Sima A, Popov D: Eds. Bucharest, Romania, Romanian Academy Publishing House, 2004, p. 274– 282 [Google Scholar]
- 29.Bacot S, Bernoud-Hubac N, Chantegrel B, Deshayes C, Doutheau A, Ponsin G, Lagarde M, Guichardant M: Evidence for in situ ethanolamine phospholipid adducts with hydroxy-alkenals. J Lipid Res 2007; 48: 816– 825 [DOI] [PubMed] [Google Scholar]
- 30.Comporti M: Lipid peroxidation and biogenic aldehydes: from the identification of 4-hydroxynonenal to further achievements in biopathology. Free Radic Res 1998; 28: 623– 635 [DOI] [PubMed] [Google Scholar]
- 31.Zarkovic N: 4-hydroxynonenal as a bioactive marker of pathophysiological processes. Mol Aspects Med 2003; 24: 281– 291 [DOI] [PubMed] [Google Scholar]
- 32.Guichardant M, Bacot S, Moliere P, Lagarde M: Hydroxy-alkenals from the peroxidation of n-3 and n-6 fatty acids and urinary metabolites. Prostaglandins Leukot Essent Fatty Acids 2006; 75: 179– 182 [DOI] [PubMed] [Google Scholar]
- 33.Willson TM, Brown PJ, Sternbach DD, Henke BR: The PPARs: from orphan receptors to drug discovery. J Med Chem 2000; 43: 527– 550 [DOI] [PubMed] [Google Scholar]
- 34.Reilly SM, Lee CH: PPAR delta as a therapeutic target in metabolic disease. FEBS Lett 2008; 582: 26– 31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee CH, Olson P, Hevener A, Mehl I, Chong LW, Olefsky JM, Gonzalez FJ, Ham J, Kang H, Peters JM, Evans RM: PPARdelta regulates glucose metabolism and insulin sensitivity. Proc Natl Acad Sci U S A 2006; 103: 3444– 3449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang YX, Lee CH, Tiep S, Yu RT, Ham J, Kang H, Evans RM: Peroxisome-proliferator-activated receptor delta activates fat metabolism to prevent obesity. Cell 2003; 113: 159– 170 [DOI] [PubMed] [Google Scholar]
- 37.Luquet S, Lopez-Soriano J, Holst D, Fredenrich A, Melki J, Rassoulzadegan M, Grimaldi PA: Peroxisome proliferator-activated receptor delta controls muscle development and oxidative capability. FASEB J 2003; 17: 2299– 2301 [DOI] [PubMed] [Google Scholar]
- 38.Wang YX, Zhang CL, Yu RT, Cho HK, Nelson MC, Bayuga-Ocampo CR, Ham J, Kang H, Evans RM: Regulation of muscle fiber type and running endurance by PPARdelta. PLoS Biol 2004; 2: e294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson S, Michalak M, Opas M, Eggleton P: The ins and outs of calreticulin: from the ER lumen to the extracellular space. Trends Cell Biol 2001; 11: 122– 129 [DOI] [PubMed] [Google Scholar]
- 40.Gross B, Staels B: PPAR agonists: multimodal drugs for the treatment of type-2 diabetes. Best Pract Res Clin Endocrinol Metab 2007; 21: 687– 710 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.