Abstract
OBJECTIVE
The aim of this study was to determine whether the type 2 diabetes–associated T-allele of transcription factor 7-like 2 (TCF7L2) rs7903146 associates with impaired insulin secretion to compensate for insulin resistance induced by bed rest.
RESEARCH DESIGN AND METHODS
A total of 38 healthy young Caucasian men were studied before and after bed rest using the hyperinsulinemic-euglycemic clamp technique combined with indirect calorimetry preceded by an intravenous glucose tolerance test. The TCF7L2 rs7903146 was genotyped using allelic discrimination performed with an ABI 7900 system. The genetic analyses were done assuming a dominant model of inheritance.
RESULTS
The first-phase insulin response (FPIR) was significantly lower in carriers of the T-allele compared with carriers of the CC genotype before bed rest, with and without correction for insulin resistance. The incremental rise of FPIR in response to insulin resistance induced by bed rest was lower in carriers of the T-allele (P < 0.001). Fasting plasma glucagon levels were significantly lower in carriers of the T-allele before and after bed rest. While carriers of the CC genotype developed increased hepatic insulin resistance, the TCF7L2 rs7903146 did not influence peripheral insulin action or the rate of lipolysis before or after bed rest.
CONCLUSIONS
Healthy carriers of the T-allele of TCF7L2 rs7903146 exhibit a diminished increase of insulin secretion in response to intravenous glucose to compensate for insulin resistance as induced by bed rest. Reduced paracrine glucagon stimulation may contribute to the impairment of β-cell function in the carriers TCF7L2 rs7903146 T-allele associated with increased risk of type 2 diabetes.
Type 2 diabetes is caused by a complicated interplay between genetic and environmental factors acting on glucose and fat metabolism involving multiple defects of peripheral (muscle) and hepatic insulin action, insulin secretion, adipose tissue metabolism, whole-body lipolysis, and possibly a range of additional metabolic defects in a number of other organs (1). Nevertheless, the manifestation of overt diabetes is a direct result of a metabolic state when pancreatic insulin secretion fails to compensate for insulin resistance.
The transcription factor 7-like 2 (TCF7L2) is a member of the T-cell transcription factor family, which, through regulation of cell proliferation and differentiation, plays a critical role in the WNT signaling pathway (2). During embryonic growth in humans, WNT signaling is required for the development and maturation of the pancreas, including the islets of Langerhans (3). Recent studies (4–9) have established TCF7L2 as the most significant type 2 diabetes susceptibility gene so far. Thus, each T-allele of TCF7L2 rs7903146 increases type 2 diabetes risk with an odds ratio of 1.37, even in the presence of a reduced body weight (4,6,10–14). The diabetogenic impact of the rs7903146 TCF7L2 variant or its linked causative variant appears to be mediated through decreased insulin secretion and/or through defects in insulin processing, reduced effects of glucagon-like peptide (GLP)-1, and increased hepatic glucose production (15–17).
Recently, Wegner et al. (18) showed that the T-allele of TCF7L2 rs7903146 risk associated with an absolute and relative impairment of insulin secretion and with increased peripheral insulin sensitivity in elderly twins. Similarly, in a study of young and healthy glucose tolerant men, Pilgaard et al. (19) demonstrated that the same variant associated with reduced insulin secretion relative to the glucose level during a mixed-meal test and with an elevated rate of endogenous glucose production in the basal state as well as following in vivo insulin infusion. In the present study, we performed detailed metabolic characterization of young healthy men to determine whether the T-allele of TCF7L2 rs7903146 is associated with impaired insulin secretion and/or insulin action in response to 9 days of bed rest.
RESEARCH DESIGN AND METHODS
A total of 38 healthy young Caucasian men completed the study. Subjects were recruited from a cohort of young men with low birth weight and normal birth weight via the Danish National Birth Registry. Low birth weight was defined as the lowest 10 percentile, and the control subjects were recruited from the 50–75 birth weight percentiles. Twenty-one subjects were carriers of the T-allele of TCF7L2 rs7903146 (combined TT and CT alleles), and 17 subjects were carriers of the CC genotype. The number of subjects with low birth weight was 11 in the group of carriers of the T-allele and 10 in carriers of the CC genotype. No impact of, or association between, birth weight and genotype were observed.
Ethics approval.
The study was approved by the regional ethical committee (ref. no. 01-262546), and all procedures were performed in accordance with the guidelines of the Declaration of Helsinki. Informed written consent was obtained from all the subjects before participation.
Experimental protocol control period.
Subjects were requested to abstain from strenuous physical activity and from consuming alcohol 3 days prior to examination. To ensure standardized conditions, all subjects were provided with an isocaloric nutritionally standardized diet 3 days prior to the first study day and during the bed rest, with adjusted caloric content to ensure weight stability. Body composition as well as fat-free mass and fat mass was determined by dual-energy X-ray absorptiometry scan as previously described (20).
Bed rest challenge.
All subjects were admitted to the Steno Diabetes Center for 9 days and were not permitted to deviate from a half-recumbent position during this period. Toilet visits were limited to 15 min per day. Study subjects were allowed to use a laptop, watch television, and to read in the bed.
Blood samples for measurements of fasting plasma insulin and fasting plasma C-peptide were taken at the 1st, 2nd, 3rd, 5th, 7th, and 9th day of bed rest. Body weight of all subjects was recorded every morning throughout the intervention to ensure weight stability.
Hyperinsulinemic-euglycemic clamp combined with stable isotope infusion and indirect calorimetry.
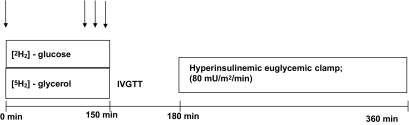
Identical in vivo experiments were performed before and after the bed rest intervention, as previously described in details (20). A schematic presentation of the experimental day is presented in Fig. 1.
FIG. 1.
Schematic presentation of the experimental day(s). Whole-body glucose metabolism was measured by a hyperinsulinemic-euglycemic clamp technique. Steady-state measurements of plasma glucose and plasma glycerol enrichments were performed during the basal period (before the insulin stimulation) to determine hepatic glucose production and whole-body lipolysis rate. Arrows show points for collecting of blood samples for basal-state determination of stable isotope kinetics.
Analytical procedures.
Blood samples for plasma insulin, plasma C-peptide, and for glucose and glycerol enrichment determination were performed as previously described (20). Glucagon concentrations were measured after extraction of plasma with 70% ethanol (vol/vol, final concentration). The antibody used was directed against the C-terminus of the glucagon molecule and therefore mainly measured glucagon of pancreatic origin (21). Standard was human glucagon, and tracer was monoiodinated human glucagon (both gifts from NovoNordisk, Bagsværd, Denmark). Sensitivity and detection limit is <1 pmol/l, intra-assay coefficient of variation <6% at 20–30 pmol/l, and recovery of standard, added to plasma before extraction, ∼100% when corrected for losses inherent in the plasma extraction procedure. Plasma triglyceride concentration was determined with Triglyceride GPO-PAP (Roche Diagnostics, Mannheim, Germany). Total and HDL cholesterol were analyzed with an enzymatic colorimetric test (Roche Diagnostics). LDL cholesterol was calculated from the Friedewald formula (22), and VLDL cholesterol was calculated as plasma triglycerides divided by 2.2. Glucose and glycerol enrichment samples were measured as previously described in details (23,24).
Genotyping.
Genomic DNA was extracted from blood using conventional methods. TCF7L2 rs7903146 was genotyped using allelic discrimination performed with an ABI 7900 system (KBioscience, Herts, U.K.) as previously described (18). The overall genotyping success rate was >96%.
Calculations.
Intravenous glucose tolerance test (β-cell test). The incremental first-phase insulin response (FPIR) during the intravenous glucose tolerance test (IVGTT) was calculated as the incremental area under the curve (AUC) (AUCinsulin [0–10 min] − AUCbasal [insulin 0–10]). The second-phase insulin secretion during IVGTT was calculated as AUCinsulin (10–30 min). The total AUC was calculated using a trapezoidal method for glucose and insulin during 30 min as (AUCinsulin [0–30 min]/AUCglucose [0–30 min]).
The disposition index expressing the inverse hyperbolic relationship between insulin secretion and insulin action is an estimate of the “true” in vivo pancreatic β-cell insulin secretion capacity. The peripheral disposition index (Di-peripheral) was calculated as (FPIR × M) and the hepatic disposition index (Di-hepatic) as (FPIR/hepatic insulin resistance [HIR]). The HIR was calculated as the product of fasting plasma insulin concentration and the basal hepatic glucose production as previously described (25).
Hyperinsulinemic-euglycemic clamp and indirect calorimetry.
Glucose infusion rates were calculated as the mean of steady-state glucose infusion rates during the predefined insulin-stimulated steady-state period from 330 to 360 min. Basal and insulin-stimulated rates of glucose and lipid oxidation as well as nonoxidative glucose oxidation rate were calculated according to the methods of Frayn (26).
Stable isotope tracer calculations.
Tracer-to-tracee ratios for both glucose and glycerol were calculated as previously described (20).
Statistics.
Statistical analysis was performed with the SAS statistical analysis package (version 9.1; SAS Institute, Cary, NC). Due to the low number of subjects, TT and CT genotypes were pooled and a dominant model was applied. The selection of the dominant model for this locus is arbitrary and one of convenience, as most of the evidence published on rs7903146 indicates an additive model of risk transmission. One-way ANOVA analyses were performed to test for differences between groups before and after bed rest. The paired-sample t test was used to detect statistically significant differences within groups in response to bed rest. The Kolmogorov-Smirnov test was used to test whether data were normally distributed and whether logarithmic transformation of non–normally distributed data rendered them normally distributed. P values of <0.05 were considered as significant, and data are presented as means ± SD. In a post hoc power calculation with the first-phase insulin secretion data as the end point, we had an 80% chance of detecting differences between carriers of the T-allele and carriers of the CC genotype of ∼66% after bed rest with a group 1 size of 21 subjects and a group 2 size of 17 subjects.
RESULTS
Clinical characteristics of study participants.
As presented in Table 1, we found no significant differences in age, weight, height, systolic and diastolic blood pressure, maximal oxygen uptake (Vo2max), total fat mass, fat percentage, trunk fat mass–to–total fat mass ratio, leg fat mass–to–total fat mass ratio, plasma triglycerides, plasma cholesterol, and plasma concentration of other lipoproteins between TT/CT carriers and CC carriers prior to bed rest. We demonstrated a significant decrease in Vo2max, plasma total cholesterol, and plasma HDL concentrations in carriers of the CC but not the TT/CT genotypes following 9 days of bed rest. However, we observed a similar BMI in carriers of the T-allele compared with carriers of the CC genotype before and after bed rest.
TABLE 1.
Clinical characteristics of male study participants according to the TCF7L2 rs7903146 genotype before and after bed rest
| rs7903146 | Before bed rest |
After bed rest |
||
|---|---|---|---|---|
| TT/CT | CC | TT/CT | CC | |
| n | 21 | 17 | 21 | 17 |
| Age (years) | 25.6 ± 2.0 | 25.2 ± 2 | 25.6 ± 2.0 | 25.2 ± 2.1 |
| Weight (kg) | 75.6 ± 11.7 | 82.4 ± 11.8 | 76.8 ± 10.4 | 81.5 ± 12.2 |
| Height (m) | 1.81 ± 0.06 | 1.82 ± 0.06 | 1.81 ± 0.06 | 1.82 ± 0.06 |
| BMI (kg/m2) | 23.0 ± 2.7 | 24.4 ± 3.1 | 23.2 ± 2.5 | 24.5 ± 3.0 |
| Vo2 max (ml · min−1 · kg−1) | 43.0 ± 6.8 | 43.7 ± 7.8 | 42.9 ± 7.1 | 40.7 ± 7.8* |
| Systolic blood pressure (mmHg) | 125 ± 10 | 129 ± 14 | 124 ± 11 | 127 ± 10 |
| Diastolic blood pressure (mmHg) | 69 ± 8 | 70 ± 10 | 70 ± 5 | 70 ± 8 |
| Waist-to-hip ratio | 0.85 ± 0.05 | 0.86 ± 0.05 | 0.85 ± 0.06 | 0.87 ± 0.06 |
| Total fat mass (kg) | 12.6 ± 6.6 | 16.7 ± 8.3 | 12.9 ± 6.9 | 17.2 ± 8.8 |
| Whole-body fat percentage (%) | 16.5 ± 6.5 | 19.8 ± 7.5 | 16.2 ± 6.6 | 20.3 ± 7.8 |
| Trunk fat mass-to-total fat mass ratio | 0.50 ± 0.04 | 0.51 ± 0.04 | 0.50 ± 0.05 | 0.52 ± 0.05 |
| Leg fat mass-to-total fat mass ratio† | 0.36 ± 0.04 | 0.35 ± 0.03 | 0.35 ± 0.04 | 0.35 ± 0.04 |
| Percent trunk fat mass-to-leg fat mass ratio | 1.41 ± 0.29 | 1.48 ± 0.30 | 1.46 ± 0.35 | 1.53 ± 0.33 |
| Triglycerides (mmol/l) | 0.9 ± 0.3 | 1.1 ± 0.9 | 0.9 ± 0.3 | 1.2 ± 0.6 |
| Cholesterol (mmol/l) | 3.8 ± 0.6 | 4.2 ± 1.0 | 3.8 ± 1.0 | 3.8 ± 1.0* |
| HDL (mmol/l) | 1.2 ± 0.2 | 1.3 ± 0.5 | 1.2 ± 0.2 | 1.1 ± 0.4* |
| LDL (mmol/l) | 2.1 ± 0.5 | 2.3 ± 1.0 | 2.2 ± 0.6 | 2.0 ± 0.6 |
| VLDL (mmol/l) | 0.4 ± 0.2 | 0.5 ± 0.4 | 0.4 ± 0.2 | 0.5 ± 0.3 |
Data are means ± SD.
*Significant difference before versus after bed rest; P < 0.05. †Log-transformed data.
No differences were observed between TT/CT and CC carriers with regard to age, weight, height, systolic and diastolic blood pressure, Vo2max, total fat mass, fat percentage, trunk fat mass–to–total fat mass ratio, leg fat mass–to–total fat mass or plasma concentrations of triglycerides, total cholesterol, LDL, and VLDL after bed rest.
Impact of the T-allele of rs7903146 on insulin secretion during IVGTT.
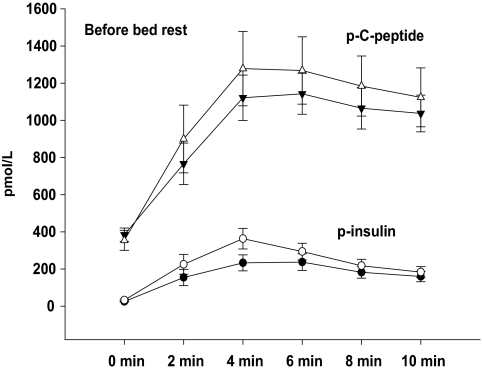
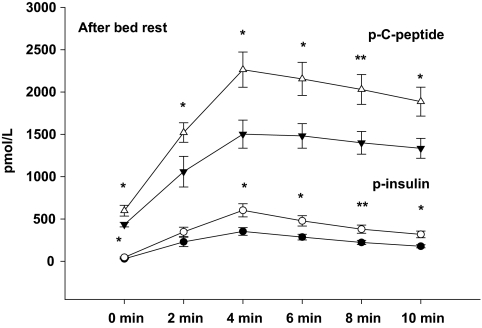
As presented in Table 2, first-phase insulin secretion (FPIR) was significantly diminished in carriers of the T-allele compared with carriers of the CC genotype before as well as after 9 days of bed rest. The decreased FPIR in carriers of the T-allele remained significant after correction for BMI before (P = 0.01) and after (P = 0.0001) bed rest. We showed a significantly lower insulin secretion during the time period from 10 to 30 min after glucose infusion (i.e., AUC10–30 min), to some extent reflecting a reduced late or second-phase insulin secretion in carriers of the T-allele compared with carriers of the CC genotype after bed rest (P < 0.001). The estimates of second-phase insulin secretion data are included also in Table 2. The AUC during IVGTT, AUC0–10 min, for plasma insulin and C-peptide were similar in TT/CT and CC genotype carriers before bed rest (Fig. 2). However, we unmasked a significantly lower the AUC during IVGTT, AUC0–10 min, for plasma insulin and C-peptide in carriers of the T-allele compared with carriers of the CC genotype after bed rest (Fig. 3).
TABLE 2.
Data on IVGTT, hyperinsulinemic-euglycemic clamp, and indirect calorimetry in male study participants according to TCF7L2 rs7903146 genotype before and after bed rest
| rs7903146 | Before bed rest |
After bed rest |
||
|---|---|---|---|---|
| TT/CT | CC | TT/CT | CC | |
| n | 21 | 17 | 21 | 17 |
| Plasma glucose (mmol/l) | ||||
| Basal | 4.6 ± 0.4 | 4.6 ± 0.5 | 4.6 ± 0.4 | 4.6 ± 0.4 |
| Insulin-stimulated state | 5.0 ± 0.2 | 5.0 ± 0.2 | 5.0 ± 0.4 | 5.1 ± 0.4 |
| Plasma insulin (pmol/l)* | ||||
| Basal | 26 ± 14 | 33 ± 23 | 30 ± 11 | 47 ± 27†‡ |
| Insulin-stimulated state | 745 ± 151 | 848 ± 258 | 821 ± 186 | 837 ± 192 |
| Plasma glucagon (pmol/l) | ||||
| Basal | 6.7 ± 2.9 | 10.5 ± 4.3§ | 6.7 ± 3.6 | 9.4 ± 4.0† |
| HOMA-IR+ (10−6 × mmol−1 · l−1 · mmol−1 · l−1)* | ||||
| Basal | 5.3 ± 3.2 | 6.9 ± 5.2 | 6.1 ± 2.5 | 9.7 ± 5.8†‡ |
| M value (mg · min−1 · kg fat-free mass−1) | ||||
| Insulin-stimulated state | 14.0 ± 1.9 | 14.0 ± 1.8 | 11.0 ± 1.7‡ | 10.0 ± 2.5‡ |
| Glucose oxidation rate (mg · min−1 · kg−1 fat-free mass) | ||||
| Basal | 1.6 ± 0.4 | 1.4 ± 0.4 | 2.3 ± 0.9‡ | 2.8 ± 0.7‡ |
| Insulin-stimulated state | 4.2 ± 0.6 | 4.3 ± 0.6 | 4.5 ± 0.6 | 4.2 ± 0.7 |
| Fat oxidation rate (mg · min−1 · kg−1 fat-free mass) | ||||
| Basal | 1.0 ± 0.3 | 1.1 ± 0.4 | 0.7 ± 0.4‡ | 0.5 ± 0.3‡ |
| Insulin-stimulated state | 0.1 ± 0.2 | 0.2 ± 0.4 | −0.1 ± 0.3 | 0.1 ± 0.3 |
| Nonoxidative glucose oxidation rate (mg · min−1 · kg−1 fat-free mass) | ||||
| Insulin-stimulated state | 9.9 ± 1.7 | 9.7 ± 2.0 | 6.5 ± 1.5‡ | 6.4 ± 1.8‡ |
| FPIR (pmol−1 · l−1 · min−1)* | 1,559 ± 1,330 | 2,122 ± 1,464§ | 2,108 ± 1,339‡ | 3,503 ± 1,670†‡ |
| Second-phase insulin response (pmol−1 · l−1 · min−1)* | 2,671 ± 2,954 | 2,618 ± 602 | 2,632 ± 1,012 | 4,960 ± 2,778†‡ |
| AUCtotal (pmol−1 · l−1 · min−1)* | 4,469 ± 3,905 | 5,032 ± 2,985 | 5,026 ± 2,190 | 8,946 ± 4,063†‡ |
| Di-peripheral (10−3 × pmol · ml−1 · min−1 · mg−1 · min−1 · kg fat-free mass−1)* | ||||
| Insulin-stimulated state | 21.9 ± 20.4 | 30.4 ± 21.5§ | 24.0 ± 16.2 | 34.3 ± 17.7† |
| Di-hepatic (pmol · l−1 · min−1 · μmol−1 · min−1 · kg fat-free mass−1 · pmol−1 · l−1)* | ||||
| Insulin-stimulated state | 6.6 ± 6.4 | 14.2 ± 18.1‖ | 7.8 ± 5.6 | 9.4 ± 6.0 |
Data are means ± SD.
*Log-transformed data.
†Significant difference between the TT/CT and CC groups after bed rest, P < 0.05.
‡Significant difference before versus after bed rest; P < 0.05. Significant difference between the TT/CT and CC groups before bed rest,
§P < 0.05 and
‖P = 0.05.
FIG. 2.
AUC0–10 min for plasma insulin during IVGTT before bed rest. Data are presented as means ± SE in carriers of the risk T-allele (●) and carriers of low-risk CC genotype (○) and AUC0–10 min for plasma C-peptide during IVGTT before bed rest. Data are presented as means ± SE in carriers of the risk T-allele (▼) and carriers of low-risk CC genotype (△).
FIG. 3.
AUC0–10 min for plasma insulin during IVGTT after bed rest. Data are presented as means ± SE in carriers of the risk T-allele (●) and carriers of low-risk CC genotype (○) and AUC0–10 min for plasma C-peptide during IVGTT after bed rest. Data are presented as means ± SE in carriers of the risk T-allele (▼) and carriers of low-risk CC genotype (△). *P < 0.05; **P < 0.01.
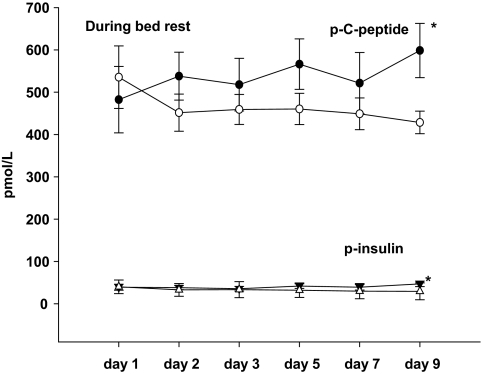
Fasting plasma insulin and C-peptide levels were significantly decreased in the TT/CT genotype as compared with the CC genotype carriers on day 9 during the bed rest experiments (Fig. 4). Furthermore, carriers of the T-allele had significantly reduced fasting plasma glucagon concentrations in the basal state before and after bed rest compared with carriers of the CC allele, as presented in Table 2. FPIR increased significantly in response to bed rest in both groups, but the increment in FPIR was significantly lower in carriers of the T-allele compared with carriers of the CC genotype (P < 0.001).
FIG. 4.
Plasma insulin concentration during 9 days of bed rest; *P < 0.05. Data are presented as means ± SE in carriers of the risk T-allele (△) and carriers of low-risk CC genotype (▼). PANOVAday1 = 0.93; Pday2 = 0.41; Pday3 = 0.75; Pday5 = 0.15; Pday7 = 0.24; Pday9 = 0.02. Plasma C-peptide concentration during 9 days of bed rest; *P < 0.05. Data are presented as means ± SE in carriers of the risk T-allele (○) and carriers of low-risk CC genotype (●). PANOVAday1 = 0.63; Pday2 = 0.24; Pday3 = 0.39; Pday5 = 0.14; Pday7 = 0.39; Pday9 = 0.02.
The total AUC (AUCtotal) was significantly lower in carriers of the T-allele compared with carriers of the CC genotype after bed rest. Furthermore, in response to bed rest we demonstrated a significant increase in AUCtotal in CC carriers but not in carriers of the risk T-allele (Table 2).
Impact of the T-allele of rs7903146 on hepatic and peripheral insulin sensitivity.
As seen in Table 2, fasting plasma insulin and glucose concentrations were similar between genotypes before as well as after bed rest. The peripheral (muscle) insulin action, measured by the hyperinsulinemic-euglycemic clamp, was significantly decreased in response to bed rest without differences between the genotype groups. Despite decreased peripheral insulin action in all subjects in response to bed rest, carriers of the T-allele tended to be more insulin sensitive after bed rest as determined by the homeostasis model assessment (HOMA) index. Furthermore, carriers of the T-allele demonstrated a similar decrease in the M value, as measured by the hyperinsulinemic-euglycemic clamp technique, compared with carriers of the CC genotype.
Estimates of insulin secretion adjusted for peripheral insulin sensitivity (i.e., peripheral and hepatic disposition index) were lower in carriers of the T-allele before bed rest. Notably, the peripheral disposition index, but not the hepatic disposition index, was also significantly decreased in carriers of the T-allele compared with carriers of the CC genotype after bed rest.
As presented in Table 3, carriers of the CC genotype, but not carriers of the T-allele, developed a significant increase in the HIR index (P < 0.01) in response to bed rest. However, we found no significant differences in rate of appearance of glucose and glycerol between genotype groups neither before nor after bed rest.
TABLE 3.
The rate of hepatic insulin action and whole-body lipolysis in male study participants according to TCF7L2 rs7903146 genotype in response to bed rest
| rs7903146 | Before bed rest |
After bed rest |
||
|---|---|---|---|---|
| TT/CT | CC | TT/CT | CC | |
| n | 21 | 17 | 21 | 17 |
| Ra glucose (μmol · min−1 · kg fat-free mass−1) | 10.5 ± 2.3 | 8.9 ± 3.7 | 10.7 ± 3.3 | 11.2 ± 3.4 |
| HIR index* | 267 ± 156 | 282 ± 165 | 310 ± 123 | 540 ± 369†‡ |
| Ra glycerol (μmol · min−1 · kg fat-free mass−1)* | 3.9 ± 2.8 | 3.3 ± 2.0 | 2.7 ± 1.6 | 3.0 ± 2.0 |
Data are means ± SD.
*Log-transformed data.
†Significant difference between TT/CT and CC group after bed rest, P < 0.05.
‡Significant difference before versus after bed rest; P < 0.05. Ra glucose, glucose rate of appearance; Ra glycerol, glycerol rate of appearance of glycerol.
Impact of the T-allele of rs7903146 T-allele on gaseous exchange measurements.
Basal glucose oxidation increased and basal fat oxidation decreased significantly in response to bed rest in the both genotype groups as presented in Table 2. The glucose and fat oxidation rates during insulin infusion were not significantly affected by bed rest in any of the genotype groups. The insulin-stimulated nonoxidative glucose metabolism decreased significantly in carriers of the CC genotype as well as in carriers of the T-allele (all P < 0.01), with no differences between the groups before and after bed rest.
DISCUSSION
Previous studies (27–29) have demonstrated that the mechanisms by which the T-allele of TCF7L2 rs7903146 is a marker for increased risk of type 2 diabetes most probably involve impairment at various steps of insulin biosynthesis and release. The most important finding from the present study is that young healthy carriers of the risk T-allele exhibit a diminished compensatory increase in glucose-stimulated plasma insulin and plasma C-peptide secretion during 9 days of bed rest, indicating a greater vulnerability to bed rest compared with carriers of the low-risk CC genotype.
Previous results from the Diabetes Prevention Program as well as from the Finnish Diabetes Prevention Study suggested an increased risk of type 2 diabetes in less physically active carriers of the TCF7L2 rs7903146 (28,30). In this study, we document that this significant adverse gene-environment interaction mechanistically may be caused by an inability of the carriers of the T-allele of TCF7L2 rs7903146 to increase insulin secretion to compensate for insulin resistance when exposed to physical inactivity. The extent to which other lifestyle factors, including diet, sleep deprivation, stress, etc., may interact with distinct susceptibility genotypes increasing the risk of developing overt type 2 diabetes remain to be documented.
It is generally recognized that in vivo insulin secretion should be corrected for the ambient degree of whole-body insulin sensitivity and expressed as a disposition index, taking into account the ability of the normal pancreatic β-cell to adapt insulin secretion to the level of insulin sensitivity (31). Lyssenko et al. (29) demonstrated reduced insulin secretion after correction for the degree of insulin action in response to oral glucose ingestion in glucose-tolerant carriers of the T-allele of TCF7L2 rs7903146, while no difference in the absolute insulin secretion capacity (AUCinsulin OGTT and AUCinsulin IVGTT) was seen between different genotype carriers. In addition, in glucose-tolerant individuals, no difference in arginin-stimulated insulin secretion was demonstrated between carriers of the CC genotype and carriers of the T-allele (29).
In the present extensive metabolic study, we unmasked a disproportionately reduced insulin secretion in response to an intravenous glucose infusion after bed rest in young healthy carriers of the T-allele of TCF7L2 rs7903146 compared with carriers of the CC genotype (Fig. 3). Furthermore, we demonstrated an impaired insulin secretion in carriers of the T-allele when corrected for the ambient level of peripheral insulin sensitivity before and, in particular, after bed rest (Table 2). Our finding provides direct evidence for an impaired pancreatic insulin secretion relative to the level of peripheral insulin resistance in young healthy carriers of the risk T-allele. Importantly, we show that carriers of the T-allele of TCF7L2 rs7903146 were unable to increase pancreatic insulin secretion to compensate appropriately for peripheral insulin resistance as induced by physical inactivity, providing proof of concept for an adverse interaction between genotype and an important lifestyle determinant such as physical inactivity on risk of developing type 2 diabetes. Furthermore, we confirmed our previous finding of significantly lower fasting plasma glucagon levels in carriers of the T-allele of TCF7L2 rs7903146 prior to and after bed rest (19). As discussed in this report by Pilgaard et al. (19), this may be explained by reduced expression of proglucagon in the α-cells or by altered posttranslational processing of proglucagon to glucagon. Importantly, our confirmation of reduced plasma glucagon in the context of a documented reduced insulin secretion in response to intravenous glucose supports the idea of impaired paracrine glucagon stimulation playing a significant role in the development of impaired β-cell function in carriers of the T-allele of TCF7L2 rs7903146.
Carriers of the T-allele of TCF7L2 have an increased risk of nonalcoholic liver steatosis and fibrosis (32), and other studies (18,19,29) report that the T-allele of TCF7L2 is associated with an increased rate of endogenous glucose production in the basal state and during insulin infusion. In this study, the slightly elevated rate of hepatic glucose production in carriers of the T-allele (P = 0.10) at baseline did not reach statistical significance. Furthermore, carriers of the T-allele exhibited a significantly, and paradoxically, decreased HIR index compared with carriers of the CC genotype after bed rest (Table 3). This interesting reversal of phenotype according to hepatic glucose production and hepatic insulin sensitivity is most likely to be explained by the clear separation of the fasting plasma insulin and C-peptide levels in the two study groups with increasing duration of bed rest (Fig. 4). These curves illustrate the unmasking effects of bed rest on a significant type 2 diabetes abnormality of reduced insulin secretion, even in the fasting state, in carriers of the T-allele. In that situation, the apparently increased hepatic insulin sensitivity in the carriers of the T-allele is due only to reduced fasting plasma insulin levels and the question may therefore be raised to which extent this calculated sensitivity index is a true biological phenomenon or a result of an inadequate estimate of hepatic insulin sensitivity calculated from peripheral and not portal plasma insulin levels. However, the parallel differences of circulating plasma insulin as well as plasma C-peptide levels suggest that this is not only a result of altered hepatic insulin extraction. Although the lower fasting plasma glucagon levels may contribute to the lower HIR in carriers of the T-allele after bed rest, this is unlikely to be the full explanation. Thus, in agreement with the data from Pilgaard et al. (19), fasting plasma glucagon levels were reduced to the same extent in carriers of the T-allele prior to bed rest when HIR was not reduced in these subjects.
Wegner et al. (18) demonstrated a reduced insulin secretion in response to intravenous glucose in the absolute sense as well as an increased peripheral insulin sensitivity with no difference in the peripheral disposition index in elderly carriers of the T-allele of TCF7L2 rs7903146 in a twin study. We speculated that the relatively increased peripheral insulin action in the elderly nondiabetic twins carrying the T-allele might be the result of an increase of peripheral (muscle) insulin action to compensate for a primary and long-lasting genuine impairment of insulin secretion (18). Along with this line of thinking, the relatively decreased HOMA, as well as peripheral insulin action, after bed rest in carriers of the T-allele of TCF7L2 rs7903146 may not be unexpected (Table 2). Although the conventional view is that the pancreatic insulin secretion senses and compensates for the ambient degree of both hepatic and peripheral insulin action, the present results support the view that the plasma insulin level, per se, is playing a significant role for the ambient degree of insulin action and that the compensatory interaction between insulin action and insulin secretion might work in both ways to preserve the metabolic homeostasis. In support of this view, we recently demonstrated that increased circulating insulin levels precedes the development of peripheral insulin resistance in response to short-term high-fat feeding in vivo in young and healthy men (33).
When including hepatic insulin action in the calculation of insulin secretion relative to insulin action, we found a lower insulin secretion hepatic disposition index in carriers of the T-allele prior to, but not after, bed rest. The similar level of insulin secretion hepatic disposition index after bed rest between carriers of different genotypes of TCF7L2 is likely to be caused by the elevated HIR in carriers of the CC genotype in response to bed rest.
Previous studies (19,34,35) suggested that the decreased insulin secretion in carriers of the T-allele might be explained by a reduced gut hormone incretin effect and, more specifically, a reduced insulinotropic effect of GLP-1. The finding of impaired insulin secretion in response to an IVGTT in carriers of the T-allele, bypassing the impact of gut incretin hormones, does not support the view that an impaired incretin effect may fully explain the decreased insulin secretion in carriers of the T-allele. Thus, the accumulated physiological evidence suggests that carriers of the T-allele of TCF7L2 rs7903146 exhibit reduced insulin secretion as a result of a combination of impaired incretin effect and impaired intrinsic pancreatic β-cell function and/or β-cell mass. To this end, impaired paracrine effect of glucagon to stimulate insulin secretion may count as a part of the intrinsic pancreatic β-cell dysfunction.
Correction for BMI being nonsignificantly lower in carriers of the at risk T-allele of TCF7L2 rs7903146 did not change the finding of a significantly reduced insulin secretion as well as a higher in vivo insulin action in these subjects as compared with carriers of the CC genotype after bed rest. In previous studies from our group (18,19), we also found trends toward a lower BMI in carriers of the T-allele, which in turn theoretically could be due to reduced insulin secretion.
The detailed and significant number of metabolic parameters measured in the study subjects were not corrected for multiple comparisons, which would have resulted in loss of statistical power and less significant differences between the two groups. The rationale for not correcting for multiple comparisons includes the fact that previous studies (19,28–30) have suggested different degrees of evidence for the different defects of metabolism with defective insulin secretion, increased hepatic glucose production, and reduced plasma glucagon levels being some of the most prominent defects previously reported in carriers of the T-allele of the TCF7L2 rs7903146. Indeed, these defects were the ones most likely to become unmasked by exposure to insulin resistance as induced by physical inactivity.
In another recent publication from our group (20), we reported that insulin-resistant first-degree relatives of patients with type 2 diabetes were more susceptible to develop HIR when exposed to bed rest as compared with control subjects without first-degree relatives. To avoid further genetic admixture of type 2 diabetes susceptibility genes in this study, subjects with first-degree relatives were not included. However, it is interesting that the currently most important type 2 diabetes susceptibility gene TCF7L2 is indeed very common among subjects without a genetic predisposition to diabetes and furthermore that the phenotypic appearance, including the response to bed rest, differs markedly between subjects with first-degree relatives and carriers of the most significant known susceptibility gene, with the latter representing a primary defective pancreatic insulin secretion. This suggests that other more significant, and currently unknown, insulin resistance genes are involved or alternatively that environmental factors clustering in families of patients with type 2 diabetes plays a more significant role in the phenotypic appearance.
In conclusion, young healthy men who are carriers of the type 2 diabetes–associated T-allele of TCF7L2 rs7903146 and who are exposed to 9 days of physical inactivity develop an insulin response to intravenous glucose that is insufficient to compensate for the induced insulin resistance.
ACKNOWLEDGMENTS
This study was funded by a European Union 6th Framework EXGENESIS Grant and supported by the Danish Strategic Research Council. A.C.A. was granted PhD scholarships from the Academy of Muscle Biology, Exercise, and Health Research and The Ministry of Science, Technology, and Innovation, Copenhagen, Denmark.
No potential conflicts of interest relevant to this article were reported.
The data presented in this manuscript are a part of a larger study on the influence of physical inactivity in young healthy subjects with and without risk of later development of type 2 diabetes, including subjects with low birth weight and first-degree relatives of patients with type 2 diabetes. Parts of the study have been published elsewhere (20). This work is initiated and funded by the European Union Framework VI, EXGENESIS project.
The authors thank Lars Sander Koch, Marianne Modest, and Nina Pluszek for eminent and dedicated technical assistance during experiments and when analyzing samples. The authors express their gratitude to all the young men who participated in this study.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Vaag A: On the pathophysiology of late onset non-insulin dependent diabetes mellitus: current controversies and new insights. Danish Med Bull 1999; 46: 197– 234 [PubMed] [Google Scholar]
- 2.Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA: Inhibition of adipogenesis by Wnt signaling. Science 2000; 289: 950– 953 [DOI] [PubMed] [Google Scholar]
- 3.Papadopoulou S, Edlund H: Attenuated Wnt signaling perturbs pancreatic growth but not pancreatic function. Diabetes 2005; 54: 2844– 2851 [DOI] [PubMed] [Google Scholar]
- 4.Grant SF, Thorleifsson G, Reynisdottir I, Benediktsson R, Manolescu A, Sainz J, Helgason A, Stefansson H, Emilsson V, Helgadottir A, Styrkarsdottir U, Magnusson KP, Walters GB, Palsdottir E, Jonsdottir T, Gudmundsdottir T, Gylfason A, Saemundsdottir J, Wilensky RL, Reilly MP, Rader DJ, Bagger Y, Christiansen C, Gudnason V, Sigurdsson G, Thorsteinsdottir U, Gulcher JR, Kong A, Stefansson K: Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet 2006; 38: 320– 323 [DOI] [PubMed] [Google Scholar]
- 5.Wiltshire S, Hattersley AT, Hitman GA, Walker M, Levy JC, Sampson M, O'Rahilly S, Frayling TM, Bell JI, Lathrop GM, Bennett A, Dhillon R, Fletcher C, Groves CJ, Jones E, Prestwich P, Simecek N, Rao PV, Wishart M, Bottazzo GF, Foxon R, Howell S, Smedley D, Cardon LR, Menzel S, McCarthy MI: A genomewide scan for loci predisposing to type 2 diabetes in a U.K. population (the Diabetes UK Warren 2 Repository): analysis of 573 pedigrees provides independent replication of a susceptibility locus on chromosome 1q. Am J Hum Genet 2001; 69: 553– 569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, Balkau B, Heude B, Charpentier G, Hudson TJ, Montpetit A, Pshezhetsky AV, Prentki M, Posner BI, Balding DJ, Meyre D, Polychronakos C, Froguel P: A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 2007; 445: 881– 885 [DOI] [PubMed] [Google Scholar]
- 7.Vionnet N, Hani EH, Dupont S, Gallina S, Francke S, Dotte S, De Matos F, Durand E, Leprêtre F, Lecoeur C, Gallina P, Zekiri L, Dina C, Froguel P: Genomewide search for type 2 diabetes-susceptibility genes in French whites: evidence for a novel susceptibility locus for early-onset diabetes on chromosome 3q27-qter and independent replication of a type 2-diabetes locus on chromosome 1q21–q24. Am J Hum Genet 2000; 67: 1470– 1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frayling TM: Genome-wide association studies provide new insights into type 2 diabetes aetiology. Nat Rev Genet 2007; 8: 657– 662 [DOI] [PubMed] [Google Scholar]
- 9.Mancarella R, Lupi R, Lyssenko V, Ling C, Del GS, Bugliani M, Torri S, Galli M, D'Aleo V, Marchetti P, Groop L, Del PS: TCF7L2 gene increase risk for future type 2 diabetes: Study on diabetic isolated islets. Diabetologia 2007; 50: S77 [Google Scholar]
- 10.Cauchi S, Meyre D, Dina C, Choquet H, Samson C, Gallina S, Balkau B, Charpentier G, Pattou F, Stetsyuk V, Scharfmann R, Staels B, Fruhbeck G, Froguel P: Transcription factor TCF7L2 genetic study in the French population: expression in human β-cells and adipose tissue and strong association with type 2 diabetes. Diabetes 2006; 55: 2903– 2908 [DOI] [PubMed] [Google Scholar]
- 11.Cauchi S, Meyre D, Choquet H, Dina C, Born C, Marre M, Balkau B, Froguel P: TCF7L2 variation predicts hyperglycemia incidence in a French general population: the data from an epidemiological study on the Insulin Resistance Syndrome (DESIR) study. Diabetes 2006; 55: 3189– 3192 [DOI] [PubMed] [Google Scholar]
- 12.Cauchi S, Choquet H, Gutierrez-Aguilar R, Capel F, Grau K, Proenca C, Dina C, Duval A, Balkau B, Marre M, Potoczna N, Langin D, Horber F, Sorensen TI, Charpentier G, Meyre D, Froguel P: Effects of TCF7L2 polymorphisms on obesity in European populations. Obesity (Silver Spring) 2008; 16: 476– 482 [DOI] [PubMed] [Google Scholar]
- 13.Cauchi S, El AY, Choquet H, Dina C, Krempler F, Weitgasser R, Nejjari C, Patsch W, Chikri M, Meyre D, Froguel P: TCF7L2 is reproducibly associated with type 2 diabetes in various ethnic groups: a global meta-analysis. J Mol Med 2007; 85: 777– 782 [DOI] [PubMed] [Google Scholar]
- 14.Helgason A, Palsson S, Thorleifsson G, Grant SF, Emilsson V, Gunnarsdottir S, Adeyemo A, Chen Y, Chen G, Reynisdottir I, Benediktsson R, Hinney A, Hansen T, Andersen G, Borch-Johnsen K, Jorgensen T, Schafer H, Faruque M, Doumatey A, Zhou J, Wilensky RL, Reilly MP, Rader DJ, Bagger Y, Christiansen C, Sigurdsson G, Hebebrand J, Pedersen O, Thorsteinsdottir U, Gulcher JR, Kong A, Rotimi C, Stefansson K: Refining the impact of TCF7L2 gene variants on type 2 diabetes and adaptive evolution. Nat Genet 2007; 39: 218– 225 [DOI] [PubMed] [Google Scholar]
- 15.Loos RJF, Franks PW, Francis RW, Barroso I, Gribble FM, Savage DB, Ong KK, O'Rahilly S, Wareham NJ: TCF7L2 polymorphisms modulate proinsulin levels and β-cell function in a British europid population. Diabetes 2007; 56: 1943– 1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirchhoff K, Machicao F, Haupt A, Schafer SA, Tschritter O, Staiger H, Stefan N, Haring HU, Fritsche A: Polymorphisms in the TCF7L2, CDKAL1 and SLC30A8 genes are associated with impaired proinsulin conversion. Diabetologia 2008; 51: 597– 601 [DOI] [PubMed] [Google Scholar]
- 17.Stolerman ES, Manning AK, McAteer JB, Fox CS, Dupuis J, Meigs JB, Florez JC: TCF7L2 variants are associated with increased proinsulin/insulin ratios but not obesity traits in the Framingham Heart Study. Diabetologia 2009; 52: 614– 620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wegner L, Hussain MS, Pilgaard K, Hansen T, Pedersen O, Vaag A, Poulsen P: Impact of TCF7L2 rs7903146 on Insulin Secretion and Action in Young and Elderly Danish Twins. J Clin Endocrinol Metab 2008; 93: 4013– 4019 [DOI] [PubMed] [Google Scholar]
- 19.Pilgaard K, Jensen CB, Schou JH, Lyssenko V, Wegner L, Brons C, Vilsboll T, Hansen T, Madsbad S, Holst JJ, Volund A, Poulsen P, Groop L, Pedersen O, Vaag AA: The T allele of rs7903146 TCF7L2 is associated with impaired insulinotropic action of incretin hormones, reduced 24 h profiles of plasma insulin and glucagon, and increased hepatic glucose production in young healthy men. Diabetologia 2009; 52: 1298– 307 [DOI] [PubMed] [Google Scholar]
- 20.Alibegovic AC, Hojbjerre L, Sonne MP, Van HG, Stallknecht B, Dela F, Vaag A: Impact of nine days of bed rest on hepatic and peripheral insulin action, insulin secretion and whole body lipolysis in healthy young male offspring of patients with type 2 diabetes. Diabetes 2009; 58: 2749– 56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holst JJ: Evidence that enteroglucagon (II) is identical with the C-terminal sequence (residues 33–69) of glicentin. Biochem J 1982; 207: 381– 388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson R, McNutt P, MacMahon S, Robson R: Use of the Friedewald formula to estimate LDL-cholesterol in patients with chronic renal failure on dialysis. Clin Chem 1997; 43: 2183– 2184 [PubMed] [Google Scholar]
- 23.Plomgaard P, Bouzakri K, Krogh-Madsen R, Mittendorfer B, Zierath JR, Pedersen BK: Tumor necrosis factor-alpha induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes 2005; 54: 2939– 2945 [DOI] [PubMed] [Google Scholar]
- 24.van Hall G, Sacchetti M, Radegran G, Saltin B: Human skeletal muscle fatty acid and glycerol metabolism during rest, exercise and recovery. J Physiol 2002; 543: 1047– 1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bajaj M, Suraamornkul S, Romanelli A, Cline GW, Mandarino LJ, Shulman GI, DeFronzo RA: Effect of a sustained reduction in plasma free fatty acid concentration on intramuscular long-chain fatty Acyl-CoAs and insulin action in type 2 diabetic patients. Diabetes 2005; 54: 3148– 3153 [DOI] [PubMed] [Google Scholar]
- 26.Frayn KN: Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol 1983; 55: 628– 634 [DOI] [PubMed] [Google Scholar]
- 27.Damcott CM, Pollin TI, Reinhart LJ, Ott SH, Shen H, Silver KD, Mitchell BD, Shuldiner AR: Polymorphisms in the transcription factor 7-like 2 (TCF7L2) gene are associated with type 2 diabetes in the Amish: replication and evidence for a role in both insulin secretion and insulin resistance. Diabetes 2006; 55: 2654– 2659 [DOI] [PubMed] [Google Scholar]
- 28.Florez JC, Jablonski KA, Bayley N, Pollin TI, de Bakker PIW, Shuldiner AR, Knowler WC, Nathan DM, Altshuler D: TCF7L2 polymorphisms and progression to diabetes in the Diabetes Prevention Program. N Engl J Med 2006; 355: 241– 250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lyssenko V, Lupi R, Marchetti P, Del GS, Orho-Melander M, Almgren P, Sjogren M, Ling C, Eriksson KF, Lethagen AL, Mancarella R, Berglund G, Tuomi T, Nilsson P, Del PS, Groop L: Mechanisms by which common variants in the TCF7L2 gene increase risk of type 2 diabetes. J Clin Invest 2007; 117: 2155– 2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, Kuusisto J, Vanttinen M, Kuulasmaa T, Lindstrom J, Tuomilehto J, Uusitupa M, Laakso M: Variants of transcription factor 7-like 2 (TCF7L2) gene predict conversion to type 2 diabetes in the Finnish Diabetes Prevention Study and are associated with impaired glucose regulation and impaired insulin secretion. Diabetologia 2007; 50: 1192– 1200 [DOI] [PubMed] [Google Scholar]
- 31.Bergman RN, Finegood DT, Kahn SE: The evolution of beta-cell dysfunction and insulin resistance in type 2 diabetes. Eur J Clin Invest 2002; 32( Suppl. 3): 35– 45 [DOI] [PubMed] [Google Scholar]
- 32.Musso G, Gambino R, Pacini G, Pagano G, Durazzo M, Cassader M: Transcription factor 7-like 2 polymorphism modulates glucose and lipid homeostasis, adipokine profile, and hepatocyte apoptosis in NASH. Hepatology 2009; 49: 426– 435 [DOI] [PubMed] [Google Scholar]
- 33.Brons C, Jensen CB, Storgaard H, Hiscock N, White A, Appel J, Jacobsen S, Nilsson E, Larsen C, Astrup A, Quistorff B, Vaag A: Impact of short-term high-fat feeding on glucose and insulin metabolism in young healthy men. J Physiol 2009; 587: 2387– 97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nauck MA, Meier JJ: The enteroinsular axis may mediate the diabetogenic effects of TCF7L2 polymorphisms. Diabetologia 2007; 50: 2413– 2416 [DOI] [PubMed] [Google Scholar]
- 35.Schafer SA, Tschritter O, Machicao F, Thamer C, Stefan N, Gallwitz B, Holst JJ, Dekker JM, 't Hart LM, Nijpels G, van Haeften TW, Haring HU, Fritsche A: Impaired glucagon-like peptide-1-induced insulin secretion in carriers of transcription factor 7-like 2 (TCF7L2) gene polymorphisms. Diabetologia 2007; 50: 2443– 2450 [DOI] [PMC free article] [PubMed] [Google Scholar]