Abstract
OBJECTIVE
Transient receptor potential (TRP) channel–induced cation influx activates human monocytes, which play an important role in the pathogenesis of atherosclerosis. In the present study, we investigated the effects of high glucose–induced oxidative stress on TRP channel expression in human monocytes.
RESEARCH DESIGN AND METHODS
Human monocytes were exposed to control conditions (5.6 mmol/l d-glucose), high glucose (30 mmol/l d-glucose or l-glucose), 100 μmol/l peroxynitrite, or high glucose in the presence of the superoxide dismutase mimetic tempol (100 μmol/l). TRP mRNA and TRP protein expression was measured using quantitative real-time RT-PCR and quantitative in-cell Western assay, respectively. Calcium influx and intracellular reactive oxygen species were measured using fluorescent dyes.
RESULTS
Administration of high d-glucose significantly increased reactive oxygen species. High d-glucose or peroxynitrite significantly increased the expression of TRP canonical type 1 (TRPC1), TRPC3, TRPC5, TRPC6, TRP melastatin type 6 (TRPM6), and TRPM7 mRNA and TRPC3 and TRPC6 proteins. High d-glucose plus tempol or high l-glucose did not affect TRP expression. Increased oxidative stress by lipopolysaccharide or tumor necrosis factor-α increased TRP mRNA expression, whereas the reduction of superoxide radicals using diphenylene iodonium significantly reduced TRP mRNA expression. Increased TRPC3 and TRPC6 protein expression was accompanied by increased 1-oleoyl-2-acetyl-sn-glycerol–induced calcium influx, which was blocked by the TRPC inhibitor 2-aminoethoxydiphenylborane. TRPC6 mRNA was significantly higher in monocytes from 18 patients with type 2 diabetes compared with 28 control subjects (P < 0.05).
CONCLUSIONS
High d-glucose–induced oxidative stress increases TRP expression and calcium influx in human monocytes, pointing to a novel pathway for increased activation of monocytes and hence atherosclerosis in patients with diabetes.
Cardiovascular complications due to atherosclerotic disease are a frequent cause of morbidity and mortality in patients with diabetes (1). Epidemiologic studies and preliminary intervention studies have shown that hyperglycemia is a direct and independent risk factor for cardiovascular disease (2). Atherogenesis has been considered to be an inflammatory disease with accumulation of monocytes within the artery wall (3). Monocytes are transitional cells, with a short half-life due to rapid differentiation into macrophages, and they are rapidly recruited to sites of inflammation (4,5). Monocyte activation, adhesion to the endothelium, and transmigration into the subendothelial space are key events in early pathogenesis of atherosclerosis. The mechanisms by which high glucose supports monocyte-associated atherosclerosis are only partially known. Mononuclear blood cells from patients with diabetes show increased generation of reactive oxygen species because of chronic high glucose levels (6–8). An increased activation of monocytes from patients with diabetes is associated with elevated protein kinase C activity and increased cytosolic calcium concentrations (9–11). Elevated transmembrane calcium influx may be mediated by increased transient receptor potential canonical (TRPC) channels. Until now, only few studies addressed transient receptor potential (TRP) expression under diabetic high glucose conditions. One study observed TRPC type 1 (TRPC1), TRPC4, and TRPC6 regulation and impaired capacitative calcium entry in vessels of diabetic patients compared with nondiabetic human vessels (12). As TRPC channels have been identified in several cell types including peripheral blood monocytes (13,14), the present study was aimed at elucidating the effects of high glucose and oxidative stress on TRP expression and their functional relevance in mediating calcium influx.
RESEARCH DESIGN AND METHODS
Preparation of cells.
Human monocytes were obtained from heparinized blood of healthy control subjects. All subjects gave written informed consent, and the study was approved by the local ethics committee. Monocytes were separated using superparamagnetic polystyrene beads coated with a primary monoclonal antibody specific for the CD14 membrane antigen expressed on human monocytes (Invitrogen, Groningen, Germany) and resuspended in Hanks balanced solution containing (in mmol/l) NaCl 136, KCl 5.40, CaCl2 1, KH2PO4 0.44, Na2HPO4 0.34, HEPES 10, pH 7.4. Monocytes were counted in Neubauer chamber and adjusted in each experiment.
To evaluate the effects of high glucose and oxidative stress on TRP channel expression, monocytes were exposed to 5.6 mmol/l d-glucose (control), 30 mmol/l d-glucose, or 100 μmol/l peroxynitrite (ONOO) for 4 h. Additional experiments were performed using 30 mmol/l l-glucose or 30 mmol/l d-glucose in the presence of the superoxide dismutase mimetic tempol (TMP, 100 μmol/l). We also evaluated the effects of lipopolysaccharide-induced oxidative stress, tumor necrosis factor-α (TNF-α)-induced activation of NADPH oxidase, and production of superoxide radicals as well as reduction of superoxide radicals using diphenylene iodonium. Next, the effects of selective inhibition of phosphatidylcholine-specific phospholipase C by tricyclodecan-9-yl xanthogenate (D609) or the inhibition of phospholipase C activation by 1-[6-[((17β)-3-methoxyestra-1,3,5 [10]-trien-17-yl)amino[hexyl]-1H-pyrrole-2,5-dione (U73122) on TRP expression in monocytes.
RNA isolation and reverse transcriptase.
Total RNA was isolated from monocytes using the RNeasy mini kit including RNase-free DNase set (Qiagen, Hilden, Germany). Using the Transcriptor first-strand cDNA synthesis kit (Roche Diagnostics, Mannheim, Germany), cDNA was synthesized from 2 μg of total RNA using oligo dT (12–18) and 5 units avian myeloblastosis virus reverse transcriptase at 50°C for 60 min, followed by heating to 85°C for 5 min.
Quantitative real-time RT-PCRs.
Quantitative (q) real-time RT-PCRs for TRPC1, TRPC3, TRPC5, TRPC6, TRP melastatin type 6 (TRPM6), TRPM7, TNF-α, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were performed using a LightCycler-FastStart DNA Master SYBR Green I Kit (Roche Diagnostics). The primers were as follows: TRPC1 (Reference Sequence [RefSeq] database accession number: NM_003304.4), forward, 5′TGCTTACCAAACTGCTGGTG3′; reverse, 5′AACTGTTTTGCCGTTTGACC3′. TRPC3 (NM_003305), forward, 5′GACTTTGGGATGCTGTCCAT3′; reverse, 5′GTACGCAATCCGAGAGAAGC3′. TRPC5 (NM_012471.2), forward, 5′CCACCAGCTATCAGATAAGG3′; reverse, 5′CGAAACAAGCCACTTATACC3′. TRPC6 (NM_004621), forward, 5′GCCAATGAGCATCTGGAAAT3′; reverse, 5′TGGAGTCACATCATGGGAGA3′. TRPM6 (NM_017662.4), forward, 5′TGATTCCTCTCGGAGTGAACAGCAC3′; reverse, 5′TTATGATTGGCACCTGGAGTCCTTG3′. TRPM7 (NM_017672.3), forward, 5′CTTATGAAGAGGCAGGTCATGG3′; reverse, 5′CATCTTGTCTGAAGGACTG3′. TNF-α (NM_000594.2), forward, 5′CCCAGGGACCTCTCTCTAATC3′; reverse, 5′ATGGGCTACAGGCTTGTCACT3′. GAPDH (NM_002046), forward, 5′AACTGCTTAGCACCCCTGGC3′; reverse, 5′ATGACCTTGCCCACAGCCTT3′. The expected and observed sizes of the PCR products were (in bp) TRPC1 243; TRPC3 249; TRPC5 161; TRPC6 243; TRPM6 347; TRPM7 214; TNF-α 84; and GAPDH 200.
qRT-PCR was performed using 2 μg RNA. LightCycler-FastStart DNA Master SYBR Green I Kit and 500 nmol/l of each primer were used in a final volume of 20 μl. The reaction was initiated at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 10 s; annealing for 10 s at 68°C (TRPM6), at 60°C (TRPC3, TRPM7, TNF-α), at 57°C (TRPC1, TRPC6), or at 54°C (TRPC5, GAPDH); and extension at 72°C for 15 s. Melting curve analysis was performed from 65°C to 95°C with a heating rate of 0.1°C/s. Data were recorded on a LightCycler 2.0 Instrument using LightCycler Software Version 4.0 (Roche Diagnostics).
The relative quantification method was used whereby the change in expression of the target genes (TRPC1, TRPC3, TRPC5, TRPC6, TRPM6, TRPM7, TNF-α) relative to the housekeeping gene (GAPDH) was calculated.
Immunofluorescence assay of TRPC channel protein.
For the identification of TRPC channel proteins, quantitative in-cell Western assays of human monocytes were performed using the Odyssey infrared imaging system (Licor Biosciences, Bad Homburg, Germany). Human monocytes in 96-well plates were incubated with rabbit anti-human TRPC3 or TRPC6 antibodies (1:1,000; Alomone Labs, Jerusalem, Israel) for 2 h, washed, and incubated with IRDye800-infrared fluorescent dye-conjugated sheep anti-rabbit antibodies (1:1,000; Biomol, Hamburg, Germany) overnight at 4°C. Imaging was performed at 810-nm emission with an excitation wavelength of 780 nm. Control experiments were performed with omission of primary or secondary antibodies. Conventional immunoblotting was performed as described previously by our group (13).
Measurements of cytosolic calcium using fluorescent dye technique.
For ratiometric imaging experiments, monocytes were loaded with 2 μmol/l of the calcium-sensitive, cell-permeable, intracellular fluorescence dye 1-[2-(5-carboxyoxazol-2-yl)-6-aminobenzofuran-5-oxy]-2-(2′-amino-5′-methyl-phenoxy)-ethane-N,N,N′N′-tetra acetic acid penta acetoxymethyl ester (fura-2/AM; Merck Biosciences, Darmstadt, Germany) at room temperature for 60 min and washed to remove extraneous dye. Fluorescence measurements were performed in a temperature-controlled 96-well fluorescent plate reader at 37°C (Fluoroskan Ascent Fluorometer; Thermo LabSystems Oy, Helsinki, Finland) at 510-nm emission with excitation wavelengths of 340 and 380 nm. Baseline fluorescence was measured for 10 min, and stable fluorescence readings were obtained throughout. Calcium influx was activated after administration of membrane-permeable 1-oleoyl-2-acetyl-sn-glycerol. We used the membrane-permeable 2-aminoethoxydiphenylborane (2-APB) to inhibit calcium influx through TRPC channels as previously described (15,16).
Measurements of reactive oxygen species using fluorescent dye technique.
Monocytes were incubated with the dye 2′,7′-dichlorofluorescein diacetate (DCF-DA, 50 μmol/l) for 60 min and then were washed and resuspended in Hanks' balanced salt solution. DCF-DA is a nonpolar compound that readily diffuses into cells, where it is hydrolyzed to the nonfluorescent polar derivative DCFH and thereby trapped within the cells. In the presence of reactive oxygen species, DCFH is oxidized to the highly fluorescent DCF, which was monitored spectrophotometrically at 530 nm with an excitation wavelength of 485 nm.
Statistics.
All values are reported as mean ± SEM of at least four independent experiments. Comparisons between groups were analyzed using nonparametric Mann-Whitney test (GraphPad Prism 5.0; GraphPad Software, La Jolla, CA). Data from multiple groups were analyzed using the nonparametric Kruskal-Wallis and Dunn multiple comparison post hoc test. The numbers given indicate the number of separate experiments. Each sample was tested in duplicate. Two-sided P values <0.05 were considered to indicate statistical significance. Where error bars do not appear on the figure, error was within the symbol size.
RESULTS
High d-glucose increased reactive oxygen species in human monocytes.
The induction of reactive oxygen species by high glucose was investigated using the dye DCF-DA. Compared with control conditions (d-glucose, 5.6 mmol/l), the administration of 30 mmol/l d-glucose for 90 min significantly increased reactive oxygen species from 1.0 ± 0.01 to 1.84 ± 0.17 arbitrary units [AU] (P < 0.01). Furthermore, the effect of d-glucose was blocked by the concurrent administration of the superoxide dismutase mimetic TMP (100 μmol/l) to 0.78 ± 0.07 AU (P < 0.01 compared with d-glucose alone).
Detection of TRP mRNA using qRT-PCR.
To investigate whether high glucose–induced oxidative stress may affect TRP channels, we first analyzed TRP mRNA in human monocytes from healthy control subjects using qRT-PCR. The melting curve analysis showed the presence of one single peak in monocytes after 4-h treatment under control conditions, with high glucose or ONOO. The expected and observed product sizes using gel electrophoresis were (in bp) TRPC1 243; TRPC3 249; TRPC5 161; TRPC6 243; TRPM6 347; TRPM7 214; and GAPDH 200.
High d-glucose and oxidative stress increased TRP mRNA in monocytes.
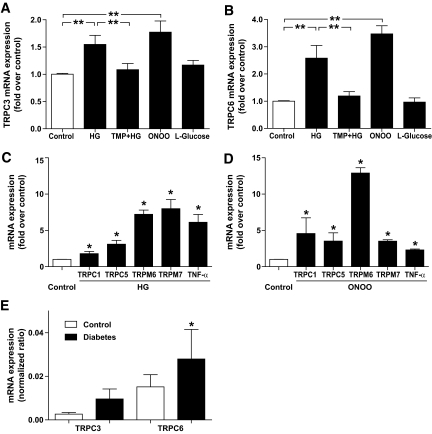
We determined the effects of high glucose and oxidative stress on TRPC3 and TRPC6 mRNA in human monocytes. As shown in Fig. 1, compared with control conditions with 5.6 mmol/l d-glucose, administration of high d-glucose (30 mmol/l) significantly increased TRPC3 mRNA by 1.55-fold (P < 0.01; n = 6) and TRPC6 mRNA by 2.58-fold (P < 0.01; n = 12). The administration of high l-glucose (30 mmol/l) did not significantly affect TRPC3 or TRPC6 mRNA. Concurrent administration of superoxide dismutase mimetic TMP (final concentration 100 μmol/l) blocked the stimulating effect of high d-glucose on TRPC3 and TRPC6 mRNA expression, indicating that high d-glucose causes its effects by increased oxidative stress. Compared with control conditions, oxidative stress induced by 100 μmol/l ONOO also significantly increased the TRPC3 mRNA by 1.77-fold (P < 0.01; n = 15) and TRPC6 mRNA by 3.47-fold (P < 0.01; n = 11).
FIG. 1.
Changes of mRNA expression of (A) TRPC3, (B) TRPC6, (C and D) TRPC1, TRPC5, TRPM6, TRPM7, and TNF-α induced by high glucose and ONOO. Exposure of monocytes to high glucose (HG; 30 mmol/l d-glucose) or ONOO (100 μmol/l) for 4 h significantly increased the TRPC3 and TRPC6 mRNA expression. The effect of high glucose is attenuated by superoxide dismutase mimetic TMP (100 μmol/l). Administration of l-glucose showed no significant effect. Control (open bar) indicates control glucose (5.6 mmol/l d-glucose). The expression of each factor is normalized to GAPDH expression. Data are mean ± SEM from at least four to six independent experiments. *P < 0.05; **P < 0.01 compared with control conditions. E: Bar graph showing TRPC3 mRNA and TRPC6 mRNA expression (normalized ratio) in monocytes from 28 age-matched control subjects (open bars) and from 18 patients with type 2 diabetes (filled bars). *P < 0.05 compared with control.
The administration of high d-glucose significantly increased mRNA from TRPC1, TRPC5, TRPM6, or TRPM7 by 1.81-, 3.10-, 7.22-, or 7.99-fold compared with control conditions, respectively (each P < 0.05; n = 4). The administration of ONOO significantly increased mRNA from TRPC1, TRPC5, TRPM6, or TRPM7 by 4.57-, 3.53-, 12.91-, or 3.51-fold, respectively (each P < 0.05; n = 4). We now investigated whether upregulated TRP expression was associated with elevated monocyte inflammatory gene expression. Indeed, after the administration of high d-glucose or ONOO and upregulation of TRP channels, mRNA of the inflammatory cytokine TNF-α was also significantly increased by 6.11- or 2.32-fold (each P < 0.05; n = 4), respectively.
Next, we evaluated whether induction of oxidative stress by several pathways may affect TRPC expression. Induction of oxidative stress using lipopolysaccharide significantly increased TRPC3 mRNA expression in monocytes from 1.00 ± 0.48 to 9.60 ± 0.40 (each n = 5; P < 0.05) and TRPC6 mRNA expression from 1.00 ± 0.23 to 3.90 ± 0.11 (each n = 5; P < 0.05). It is known that TNF-α–induced activation of NADPH oxidase increases superoxide radicals (17). TNF-α significantly increased TRPC3 mRNA expression in monocytes from 1.00 ± 0.01 to 1.58 ± 0.21 (each n = 4; P < 0.05) and TRPC6 mRNA expression from 1.00 ± 0.00 to 1.32 ± 0.08 (each n = 4; P < 0.05). In agreement with these findings, the reduction of superoxide radicals using diphenylene iodonium (18) significantly reduced TRPC3 mRNA expression in monocytes from 1.00 ± 0.06 to 0.52 ± 0.05 (each n = 5; P < 0.05) and TRPC6 mRNA expression from 1.00 ± 0.12 to 0.36 ± 0.03 (each n = 5; P < 0.05). These data indicate that TRPC3 and TRPC6 mRNA are regulated by oxidative stress, in particular by superoxide radicals.
We also investigated a possible role of phospholipase C on glucose-induced TRPC expression. Compared with control conditions, the inhibition of phosphatidylcholine-specific phospholipase C by D609, or the inhibition of phospholipase C activation by U73122, did not significantly affect high d-glucose–induced TRPC3 mRNA expression (control 1.00 ± 0.01; high d-glucose 1.57 ± 0.10 [P < 0.05 compared with control]; high d-glucose plus D609 1.47 ± 0.03 [not significant compared with high d-glucose alone]; high d-glucose plus U73122 1.95 ± 0.03 [not significant compared with high d-glucose alone]; each n = 4). It is known that TRPC-induced calcium influx is regulated by phospholipase C. However, phospholipase C seems not to be involved in glucose-induced upregulation of TRPC mRNA expression.
To evaluate whether increased TRPC mRNA is a feature of diabetes, we compared TRPC1, TRPC3, TRPC5, and TRPC6 mRNA in monocytes from 18 patients with type 2 diabetes (10 women, 8 men; mean age 57 ± 5 years; systolic blood pressure 133 ± 3 mmHg; diastolic blood pressure 79 ± 2 mmHg; serum sodium 135 ± 1 mmol/l; serum potassium 4.4 ± 0.1 mmol/l; hemoglobin 11.2 ± 0.5 g/dl; A1C 7.5 ± 0.3%) and from 28 age-matched control subjects (10 women, 18 men; mean age 56 ± 4 years; systolic blood pressure 132 ± 4 mmHg; diastolic blood pressure 81 ± 2 mmHg; serum sodium 136 ± 1 mmol/l; serum potassium 4.4 ± 0.2 mmol/l; hemoglobin 12.3 ± 0.5 g/dl; A1C 5.6 ± 0.1%). TRPC6 mRNA was significantly higher in monocytes from patients with type 2 diabetes compared with control subjects (TRPC6 normalized ratio 0.028 ± 0.014 [n = 18] vs. 0.015 ± 0.006 [n = 28]; P < 0.05; Fig. 1E). On the other hand, TRPC1, TRPC3, or TRPC5 mRNA was not significantly different between the two groups (TRPC1 0.025 ± 0.012 vs. 0.025 ± 0.006 [P = 0.62]; TRPC3 0.010 ± 0.005 vs. 0.003 ± 0.001 [P = 0.40]; TRPC5 0.007 ± 0.004 vs. 0.003 ± 0.001 [P = 0.83]). These data support the view that increased TRPC6 expression is a characteristic feature of peripheral blood cells from patients with type 2 diabetes.
High d-glucose and oxidative stress increased TRPC3 and TRPC6 protein expression in monocytes.
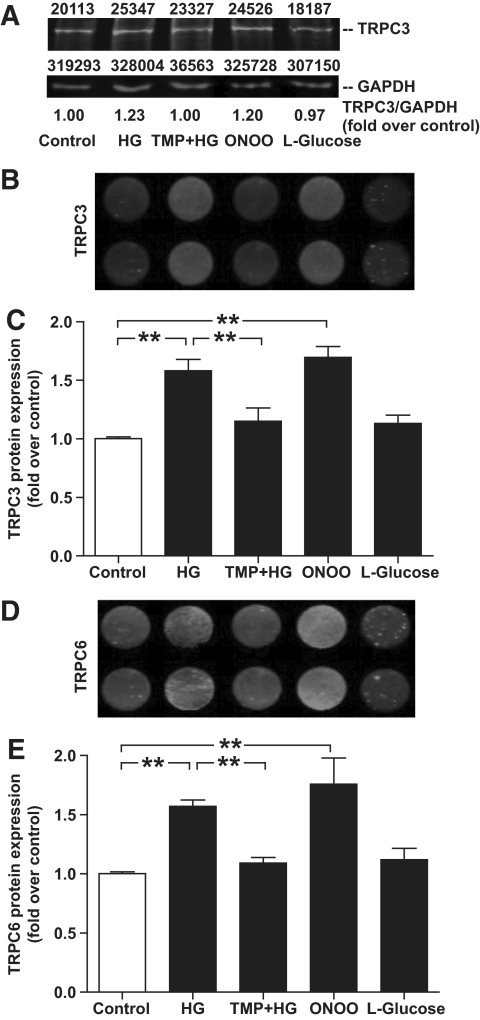
TRPC protein expression in monocytes was quantified using a quantitative in-cell Western assay. As shown in Fig. 2, compared with control conditions with 5.6 mmol/l d-glucose, administration of high d-glucose (30 mmol/l) significantly increased TRPC3 protein expression by 1.58-fold (P < 0.01; n = 10) and TRPC6 protein expression by 1.57-fold (P < 0.01; n = 8). The administration of high l-glucose (30 mmol/l) did not significantly affect TRPC3 or TRPC6 protein expression. Concurrent administration of TMP (100 μmol/l) blocked the stimulating effect of high d-glucose on TRPC3 and TRPC6 protein expression.
FIG. 2.
Quantitative in-cell Western assay of TRPC3 and TRPC6 channel protein expression in monocytes treated with HG (30 mmol/l d-glucose) in the absence and presence of TMP (100 μmol/l), ONOO (100 μmol/l), l-glucose (30 mmol/l), or control (5.6 mmol/l d-glucose). A: Representative conventional Western assay (TRPC3 protein and GAPDH protein for loading control with top values indicating densitometric analysis of blots). The TRPC3/GAPDH ratio (fold over control) is also indicated. B and D: Representative in-cell Western assays and (C and E) summary data are shown. Data are means ± SEM from at least eight independent experiments. **P < 0.01 compared with control conditions.
Compared with control conditions, oxidative stress induced by 100 μmol/l ONOO also significantly increased the TRPC3 protein expression by 1.70-fold (P < 0.01; n = 9) and TRPC6 protein expression by 1.8-fold (P < 0.01; n = 9).
High d-glucose–induced oxidative stress promoted increased TRPC-mediated calcium influx.
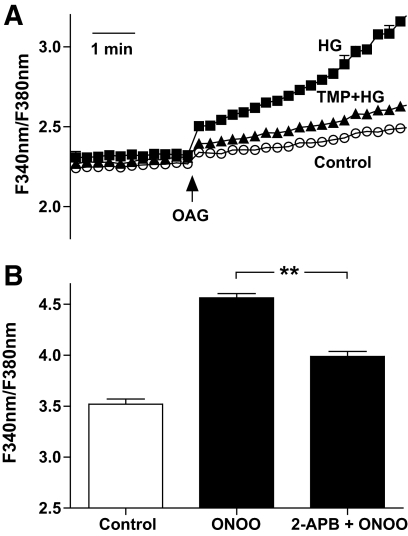
Cytosolic calcium was measured in fura-2–loaded monocytes. 1-Oleoyl-2-acetyl-rac-glycerol (OAG) increased cytosolic calcium to 2.41 ± 0.01 (n = 15), whereas OAG in the presence of high d-glucose increased cytosolic calcium to 2.79 ± 0.03 (n = 14; P < 0.01). Furthermore, it should be noted that concurrent preincubation of cells with TMP (100 μmol/l) and high d-glucose normalized OAG-induced calcium influx (2.49 ± 0.02; n = 13; not significant compared with control). Representative traces are shown in Fig. 3A. These data indicate that high d-glucose–associated oxidative stress promotes increased TRPC-mediated calcium influx.
FIG. 3.
Recordings of fura-2 fluorescence in human monocytes. A: OAG-induced calcium influx was measured under control conditions (5.6 mmol/l d-glucose, ○) and in the presence of high glucose (30 mmol/l d-glucose) without (■) and with (▲) TMP (100 μmol/l). B: Summary data showing intracellular calcium levels after treatment of monocytes with high d-glucose for 4 h (control) stimulated with ONOO in the absence and presence of the membrane-permeable TRPC blocker, 2-APB. **P < 0.01.
After treatment of monocytes with high d-glucose for 4 h, the administration of ONOO subsequently increased intracellular calcium levels. In the presence of a membrane-permeable TRPC blocker, 2-APB, the ONOO-induced calcium increase was significantly attenuated (3.99 ± 0.05 vs. 4.56 ± 0.04; each n = 15; P < 0.01 compared with ONOO alone; Fig. 3B).
DISCUSSION
Our present data show that high d-glucose–induced oxidative stress causes increased TRP expression and calcium influx in human monocytes. An increased calcium influx through TRP channels may be responsible for an increased activation of monocytes and enhanced atherosclerosis in patients with diabetes.
The presence of high glucose levels is a characteristic feature of diabetes and is considered a major pathogenic factor for increased atherosclerosis and consecutive diseases including cardiovascular disease (19). Both an increased activation of monocytes and an increased generation of reactive oxygen species in monocytes have been described in patients with diabetes (6,20). The production of mitochondrial superoxide has been implicated as a major underlying mechanism linking high glucose and cellular dysfunction (21,22). In the present study, we confirmed that high glucose increases reactive oxygen species in monocytes. The high glucose–induced increase of reactive oxygen species was blocked by the superoxide dismutase mimetic TMP.
Activated monocytes in patients with diabetes are characterized by increased calcium concentrations (9–11). According to our present results, increased TRPC channel expression in monocytes may be responsible for an increased calcium influx after high glucose–induced oxidative stress. We observed that high d-glucose–induced oxidative stress increased the expression of both TRPC3 and TRPC6 mRNA and TRPC3 and TRPC6 channel proteins in monocytes. The stimulating effect of high glucose could be blocked by concurrent administration of TMP, supporting the relevance of increased oxidative stress after high d-glucose. Recently, Shanmugam et al. (23) showed that exposure of monocytes to high glucose increased the expression of several inflammatory cytokines, including TNF-α in an oxidant stress–dependent manner. Our present results support these findings, indicating that high glucose–induced upregulation of TRP expression was associated with enhanced mRNA levels of the inflammatory cytokine TNF-α. We observed that high glucose elevates the expression of TNF-α even higher than ONOO. The increased TNF-α response after high glucose compared with ONOO indicates that irrespective of oxidative stress, other pathways (e.g., the polyol pathway) contribute to increased inflammatory response in diabetes (24). Taken together, high glucose–induced oxidative stress activates monocytes by enhancing inflammatory cytokines and TRP channel expression promoting transmembrane calcium influx. Indeed, our experiments using the fluorescent dye fura-2 showed that high glucose–induced oxidative stress promotes increased TRP-mediated calcium influx.
Balzer et al. (25) suggested that TRPC channels are the molecular basis of oxidant-activated cation channels in endothelial cells. Poteser et al. (26) reported that redox modulation of TRPC channels is responsible for oxidative stress–induced changes in cytosolic calcium signaling. The present study extends their findings, showing that oxidative stress increased the expression of both TRPC3 and TRPC6 mRNA and proteins. Therefore, high glucose–induced oxidative stress in patients with diabetes may activate monocytes both by interaction with TRPC channels and by enhanced TRPC expression. The present study indicates that induction of oxidative stress by several pathways may affect TRP expression. Induction of oxidative stress using lipopolysaccharide- or TNF-α–induced activation of NADPH oxidase significantly increased TRPC3 mRNA expression, whereas the reduction of superoxide radicals using diphenylene iodonium significantly reduced TRPC3 mRNA expression. In the present study, we also confirmed that high d-glucose–induced oxidative stress increases TRPM6 and TRPM7 mRNA expression in agreement with data from Yamamoto et al. (27), who showed the regulation of TRPM expression by oxidative stress.
To evaluate whether increased TRPC mRNA is a feature of diabetes, we also compared TRPC mRNA in monocytes from patients with type 2 diabetes and control subjects. We observed increased TRPC6 mRNA in monocytes from patients with type 2 diabetes. Increased TRPC6 protein expression has already been reported in platelets from patients with diabetes compared with control subjects (28), supporting the view that increased TRPC6 expression is a characteristic feature of peripheral blood cells from patients with diabetes. Furthermore, Hu et al. (29) showed increased TRPC6 expression in the adrenal medulla from Ossabaw miniature pigs with pre-diabetic metabolic syndrome. The effects of high glucose could be cell specific. For example, Graham et al. (30) showed that high glucose downregulates TRPC6 in cultured mesangial cells. However, differences might be explained in part by use of native blood cells and cultured cells. In our study, inhibition of phospholipase C did not affect TRPC mRNA expression. However, several pathways including phospholipase C, protein kinase C, or phosphatidylinositol 3-kinase may modulate TRP channel protein expression and function, as suggested by recent literature (31–33).
In conclusion, the present study shows that high glucose–induced oxidative stress increases TRPC3 and TRPC6 channel expression and calcium influx in human monocytes. These data point to a novel pathway for an increased activation of monocytes and hence atherosclerosis in patients with diabetes.
ACKNOWLEDGMENTS
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N: Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med 2004; 141: 413– 420 [DOI] [PubMed] [Google Scholar]
- 2.Ceriello A: Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes 2005; 54: 1– 7 [DOI] [PubMed] [Google Scholar]
- 3.Ross R: Atherosclerosis: an inflammatory disease. N Engl J Med 1999; 340: 115– 126 [DOI] [PubMed] [Google Scholar]
- 4.Geissmann F, Jung S, Littman DR: Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 2003; 19: 71– 82 [DOI] [PubMed] [Google Scholar]
- 5.Wu H, Gower RM, Wang H, Perrard XY, Ma R, Bullard DC, Burns AR, Paul A, Smith CW, Simon SI, Ballantyne CM: Functional role of CD11c+ monocytes in atherogenesis associated with hypercholesterolemia. Circulation 2009; 119: 2708– 2717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orie NN, Zidek W, Tepel M: Increased intracellular generation of reactive oxygen species in mononuclear leukocytes from patients with diabetes mellitus type 2. Exp Clin Endocrinol Diabetes 2000; 108: 175– 180 [DOI] [PubMed] [Google Scholar]
- 7.Hiramatsu K, Arimori S: Increased superoxide production by mononuclear cells of patients with hypertriglyceridemia and diabetes. Diabetes 1988; 37: 832– 837 [DOI] [PubMed] [Google Scholar]
- 8.Mohanty P, Hamouda W, Garg R, Aljada A, Ghanim H, Dandona P: Glucose challenge stimulates reactive oxygen species (ROS) generation by leucocytes. J Clin Endocrinol Metab 2000; 85: 2970– 2973 [DOI] [PubMed] [Google Scholar]
- 9.Cipolletta C, Ryan KE, Hanna EV, Trimble ER: Activation of peripheral blood CD14+ monocytes occurs in diabetes. Diabetes 2005; 54: 2779– 2786 [DOI] [PubMed] [Google Scholar]
- 10.Ceolotto G, Gallo A, Miola M, Sartori M, Trevisan R, Del Prato S, Semplicini A, Avogaro A: Protein kinase C activity is acutely regulated by plasma glucose concentration in human monocytes in vivo. Diabetes 1999; 48: 1316– 1322 [DOI] [PubMed] [Google Scholar]
- 11.Caimi G, Canino B, Ferrara F, Montana M, Meli F, Catania A, Lopresti R: Leukocyte flow properties, polymorphonuclear membrane fluidity, and cytosolic Ca2+ content in subjects with vascular atherosclerotic disease with and without noninsulin-dependent diabetes mellitus. Angiology 1996; 47: 757– 763 [DOI] [PubMed] [Google Scholar]
- 12.Chung AW, Au Yeung K, Chum E, Okon EB, van Breemen C: Diabetes modulates capacitative calcium entry and expression of transient receptor potential canonical channels in human saphenous vein. Eur J Pharmacol 2009; 613: 114– 118 [DOI] [PubMed] [Google Scholar]
- 13.Liu D, Scholze A, Zhu Z, Krueger K, Thilo F, Burkert A, Streffer K, Holz S, Harteneck C, Zidek W, Tepel M: Transient receptor potential channels in essential hypertension. J Hypertens 2006; 24: 1105– 1114 [DOI] [PubMed] [Google Scholar]
- 14.Thilo F, Scholze A, Liu DY, Zidek W, Tepel M: Association of transient receptor potential canonical type 3 (TRPC3) channel transcripts with proinflammatory cytokines. Arch Biochem Biophys 2008; 471: 57– 62 [DOI] [PubMed] [Google Scholar]
- 15.Iwasaki H, Mori Y, Hara Y, Uchida K, Zhou H, Mikoshiba K: 2-Aminoethoxydiphenyl borate (2-APB) inhibits capacitative calcium entry independently of the function of inositol 1,4,5-trisphosphate receptors. Receptors Channels 2001; 7: 429– 439 [PubMed] [Google Scholar]
- 16.Prakriya M, Lewis RS: Potentiation and inhibition of Ca(2+) release activated Ca(2+) channels by 2-aminoethyldiphenyl borate (2-APB) occurs independently of IP(3) receptors. J Physiol 2001; 536: 3– 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yazdanpanah B, Wiegmann K, Tchikov V, Krut O, Pongratz C, Schramm M, Kleinridders A, Wunderlich T, Kashkar H, Utermöhlen O, Brüning JC, Schütze S, Krönke M: Riboflavin kinase couples TNF receptor 1 to NADPH oxidase. Nature 2009; 460: 1159– 1163 [DOI] [PubMed] [Google Scholar]
- 18.Naftalin RJ, Rist RJ: The relationship between sugar metabolism, transport and superoxide radical production in rat peritoneal macrophages. Biochim Biophys Acta 1993; 1148: 39– 50 [DOI] [PubMed] [Google Scholar]
- 19.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M: Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339: 229– 234 [DOI] [PubMed] [Google Scholar]
- 20.Brownlee M: Biochemistry and molecular cell biology of diabetic complications. Nature 2001; 414: 813– 820 [DOI] [PubMed] [Google Scholar]
- 21.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M: Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000; 404: 787– 790 [DOI] [PubMed] [Google Scholar]
- 22.Kiritoshi S, Nishikawa T, Sonoda K, Kukidome D, Senokuchi T, Matsuo T, Matsumura T, Tokunaga H, Brownlee M, Araki E: Reactive oxygen species from mitochondria induce cyclooxygenase-2 gene expression in human mesangial cells: potential role in diabetic nephropathy. Diabetes 2003; 52: 2570– 2577 [DOI] [PubMed] [Google Scholar]
- 23.Shanmugam N, Reddy MA, Guha M, Natarajan R: High glucose-induced expression of proinflammatory cytokine and chemokine genes in monocytic cells. Diabetes 2003; 52: 1256– 1264 [DOI] [PubMed] [Google Scholar]
- 24.Gleissner CA, Sanders JM, Nadler J, Ley K: Upregulation of aldose reductase during foam cell formation as possible link among diabetes, hyperlipidemia, and atherosclerosis. Arterioscler Thromb Vasc Biol 2008; 28: 1137– 1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balzer M, Lintschinger B, Groschner K: Evidence for a role of Trp proteins in the oxidative stress-induced membrane conductances of porcine aortic endothelial cells. Cardiovasc Res 1999; 42: 543– 549 [DOI] [PubMed] [Google Scholar]
- 26.Poteser M, Graziani A, Rosker C, Eder P, Derler I, Kahr H, Zhu MX, Romanin C, Groschner K: TRPC3 and TRPC4 associate to form a redox-sensitive cation channel: evidence for expression of native TRPC3-TRPC4 heteromeric channels in endothelial cells. J Biol Chem 2006; 281: 13588– 13595 [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto S, Shimizu S, Kiyonaka S, Takahashi N, Wajima T, Hara Y, Negoro T, Hiroi T, Kiuchi Y, Okada T, Kaneko S, Lange I, Fleig A, Penner R, Nishi M, Takeshima H, Mori Y: TRPM2-mediated Ca2+influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat Med 2008; 14: 738– 747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu D, Maier A, Scholze A, Rauch U, Boltzen U, Zhao Z, Zhu Z, Tepel M: High glucose enhances transient receptor potential channel canonical type 6-dependent calcium influx in human platelets via phosphatidylinositol 3-kinase-dependent pathway. Arterioscler Thromb Vasc Biol 2008; 28: 746– 751 [DOI] [PubMed] [Google Scholar]
- 29.Hu G, Oboukhova EA, Kumar S, Sturek M, Obukhov AG: Canonical transient receptor potential channels expression is elevated in a porcine model of metabolic syndrome. Mol Endocrinol 2009; 23: 689– 699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graham S, Ding M, Sours-Brothers S, Yorio T, Ma JX, Ma R: Downregulation of TRPC6 protein expression by high glucose, a possible mechanism for the impaired Ca2+ signaling in glomerular mesangial cells in diabetes. Am J Physiol Renal Physiol 2007; 293: F1381– F1390 [DOI] [PubMed] [Google Scholar]
- 31.Nilius B, Mahieu F, Prenen J, Janssens A, Owsianik G, Vennekens R, Voets T: The Ca2+-activated cation channel TRPM4 is regulated by phosphatidylinositol 4,5-biphosphate. EMBO J 2006; 25: 467– 478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saleh SN, Albert AP, Large WA: Obligatory role for phosphatidylinositol 4,5-bisphosphate in activation of native TRPC1 store-operated channels in vascular myocytes. J Physiol 2009; 587: 531– 540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tong Q, Chu X, Cheung JY, Conrad K, Stahl R, Barber DL, Mignery G, Miller BA: Erythropoietin-modulated calcium influx through TRPC2 is mediated by phospholipase Cgamma and IP3R. Am J Physiol Cell Physiol 2004; 287: C1667– C1678 [DOI] [PubMed] [Google Scholar]