Abstract
OBJECTIVE
The proinflammatory cytokines/adipokines produced from adipose tissue act in an autocrine and/or endocrine manner to perpetuate local inflammation and to induce peripheral insulin resistance. The present study investigates whether lipocalin-2 deficiency or replenishment with this adipokine has any impact on systemic insulin sensitivity and the underlying mechanisms.
METHODS AND RESULTS
Under conditions of aging or dietary-/genetic-induced obesity, lipocalin-2 knockout (Lcn2-KO) mice show significantly decreased fasting glucose and insulin levels and improved insulin sensitivity compared with their wild-type littermates. Despite enlarged fat mass, inflammation and the accumulation of lipid peroxidation products are significantly attenuated in the adipose tissues of Lcn2-KO mice. Adipose fatty acid composition of these mice varies significantly from that in wild-type animals. The amounts of arachidonic acid (C20:4 n6) are elevated by aging and obesity and are paradoxically further increased in adipose tissue, but not skeletal muscle and liver of Lcn2-KO mice. On the other hand, the expression and activity of 12-lipoxygenase, an enzyme responsible for metabolizing arachidonic acid, and the production of tumor necrosis factor-α (TNF-α), a critical insulin resistance–inducing factor, are largely inhibited by lipocalin-2 deficiency. Lipocalin-2 stimulates the expression and activity of 12-lipoxygenase and TNF-α production in fat tissues. Cinnamyl-3,4-dihydroxy-α-cyanocinnamate (CDC), an arachidonate lipoxygenase inhibitor, prevents TNF-α expression induced by lipocalin-2. Moreover, treatment with TNF-α neutralization antibody or CDC significantly attenuated the differences of insulin sensitivity between wild-type and Lcn2-KO mice.
CONCLUSIONS
Lipocalin-2 deficiency protects mice from developing aging- and obesity-induced insulin resistance largely by modulating 12-lipoxygenase and TNF-α levels in adipose tissue.
The prevalence of obesity increases dramatically and has attained the characteristics of an epidemic (1). Studies in both humans and animals demonstrate that obesity is a state of low-grade, chronic inflammation, characterized by elevated circulating proinflammatory molecules produced predominantly from enlarged adipocytes and activated macrophages in adipose tissue (2–4). In fact, chronic inflammation in adipose tissue per se plays a key role in the development of obesity and associated metabolic disorders, such as type 2 diabetes. Various proinflammatory adipokines, including tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), resistin, retinol-binding protein 4, and plasminogen activator inhibitor-1, directly antagonize the metabolic actions of insulin and cause decreased insulin sensitivity (5,6).
Lipocalin-2, also called growth factor–stimulated superinducible protein 24 (7), neutrophil gelatinase-associated lipocalin (8), 24p3, or oncogene neu-related lipocalin (9,10), belongs to the lipocalin superfamily consisting of more than 20 small secretory proteins, including retinol-binding protein 4, adipocyte fatty acid binding protein, apolipoprotein D, and prostaglandin D synthase (11). Members of the lipocalin family share a highly conserved structural homology (12). By forming a cup-shaped hydrophobic cavity, lipocalins bind and transport a variety of small lipophilic substances such as retinoids, arachidonic acid, and various steroids. Although lipocalin-2 can bind weakly to some common ligands of lipocalins, including leukotriene B4 and platelet activating factor, its high-affinity endogenous ligand(s) remain to be identified.
Lipocalin-2 is abundantly produced from adipocytes (13–15). The expression and secretion of this protein increases sharply after conversion of preadipocytes to mature adipocytes. Its expression can be induced by various inflammatory stimuli, including lipopolysaccharide and IL-1β (16,17). The proinflammatory transcription factor nuclear factor-κB transactivates lipocalin-2 expression through binding to the consensus motif within its promoter (16,18). This evidence suggests that lipocalin-2 may participate in inflammation-related disorders. Expression of lipocalin-2 in adipose tissue is elevated in various experimental models of obesity and in obese humans (19–23). Moreover, this increase can be reversed by the insulin-sensitizing drug rosiglitazone. In human subjects, serum concentrations of lipocalin-2 are associated closely with obesity-related anthropometric and biochemical variables (20). The positive correlations of serum lipocalin-2 with fasting glucose, homeostasis model assessment of insulin resistance (HOMA-IR) index, and the inflammatory marker high-sensitivity C-reactive protein are significant even after adjustment for BMI, suggesting that it is an independent risk factor for insulin resistance, diabetes, and inflammation. The present study has used a knockout mouse model to evaluate the impact of lipocalin-2 loss-of-function on systematic energy homeostasis and insulin sensitivities under both basal and obese conditions. The results demonstrate that lipocalin-2 plays a causal role in the development of insulin resistance, at least partly through modulating the inflammatory responses in adipose tissue.
RESEARCH DESIGN AND METHODS
Experimental animals.
Male mice were used for this study. C57BL/6J and C57BL/6J db/db diabetic mice were from The Jackson Laboratory (Bar Harbor, ME). The lipocalin-2 knockout (Lcn2-KO) mice were generated as reported (24). The mRNA and protein levels of lipocalin-2 were undetectable in all tissues evaluated including liver, fat, and muscle. The mice were backcrossed to C57BL/6J mice for more than 20 generations. Leptin receptor−/−/lipocalin-2−/− double knockout (DKO) mice were established by cross-breeding male C57BL/6J db/+ mice with female Lcn2-KO mice. The mice were housed in a room under controlled temperature (23 ± 1°C) and 12-h light-dark cycle, with free access to water and standard chow (LabDiet 5053; Purina Mills, Richmond, IN). Dietary obesity was induced in wild-type and Lcn2-KO mice by allowing free access to a high-fat diet (D12451; Research Diet, New Brunswick, NJ) from the age of 4 weeks onward. The comparisons throughout this study are between wild-type and knockout littermates from heterocrosses. Intraperitoneal glucose tolerance test (ipGTT) and insulin tolerance test (ITT) were performed using mice that were fasted overnight and for 6 h, respectively, as described (25). For drug treatment, 8 mg/kg of cinnamyl-3,4-dihydroxy-α-cyanocinnamate (CDC; BIOMOL Research Laboratories, Plymouth Meeting, PA) mixed with sesame oil was injected intraperitoneally three times per week for 2 weeks. The control mice were injected with diluent sesame oil. The TNF-α neutralization experiment was performed by injecting the TNF-α–neutralizing antibody (50 μg · mouse · day i.p.; Sigma-Aldrich, St. Louis, MO) or control IgG during the 2-week treatment period. The animal experimental procedures were approved by the Committee on the Use of Live Animals for Teaching and Research, University of Hong Kong, and were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (26).
Production of recombinant adenoviruses and lipocalin-2 for in vivo treatment.
The adenovirus vector encoding FLAG-tagged murine lipocalin-2 was generated using the Adeno-X Expression System (Clontech, Mountain View, CA). The recombinant adenovirus was injected into the tail vein of mice 2 weeks prior to tissue collection (25). The amount of injected adenovirus (108 plaque-forming units) caused no toxicity in the mice. The increased expression level of lipocalin-2 was confirmed by both Western blotting and enzyme-linked immunosorbent assay (ELISA; supplementary Fig. 1, available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db09-1541/DC1). Recombinant murine lipocalin-2 was expressed and purified, and endotoxin was removed as described (20). The purity of the protein was confirmed by SDS-PAGE and mass spectrometry analysis. No siderophore or iron was found to bind to the protein.
Measurement of insulin and lipid levels.
Fasting serum insulin concentrations were determined with a commercial ELISA kit (Mercodia AB, Uppsala, Sweden). The amounts of triglyceride (TG), total cholesterol, and free fatty acids (FFAs) in tissues and serum samples were analyzed as described elsewhere (27). Fatty acid compositions of the epididymal adipose tissue, liver, and muscle were analyzed by gas chromatography–mass spectrometry (GC-MS) (28). Nonadecanoic acid C19:0 and tridecanoic acid methyl ester C13:0 were added as internal controls during sample processing. The standard curve was generated using the fatty acid standard Supelco 37 Component FAME mix (10 mg/ml, Sigma-Aldrich).
ELISA quantification of lipocalin-2, adiponectin, TNF-α, and 12(S)-HETE.
Total serum lipocalin-2 and adiponectin levels were measured using in-house ELISAs (20,29). Serum TNF-α concentrations were quantified using a high-sensitivity TNF-α Quantikine ELISA System (R&D Systems, Minneapolis, MN). Mouse adipose TNF-α levels were measured using immunoassay kit from Invitrogen (Camarillo, CA). Tissue membrane and soluble fractions were prepared as described (30). Equal amounts (500 μg) of samples were used for analysis. 12(S)-hydroperoxy tetraenoic eicosatetraenoic acid [12(S)-HETE] in different tissues was measured using an enzyme immunosorbent assay (Assay Designs, Ann Arbor, MI) as described (31).
Measurement of glucose uptake.
Fat pads or skeletal muscle strips were stimulated with or without insulin, and the glucose uptake was determined as described (32).
Evaluation of in vivo insulin signaling.
After overnight fasting, mice were anesthetized and 1 IU per kg insulin (Novo Nordisk, Novo Allé, Denmark) or an equal volume of vehicle was administered through the portal vein. Adipose tissue (epididymal fat pads), liver, and soleus muscle were collected 120 s after the injection and immediately stored in liquid nitrogen for subsequent Western blotting analysis.
Quantitative RT-PCR analysis.
Quantitation of target genes was performed using SYBR Green PCR Master Mix (Qiagen) and an ABI PRISM 7900 HT Sequence Detection System (Applied Biosystems, Foster City, CA). The primer sequences are listed in supplementary Table 1.
Western blotting.
Antibodies against total or phosphorylated Akt and insulin receptor-β (IR-β) were purchased from Cell Signaling Technology. Proteins (100 μg) derived from cell or tissue lysates were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes. The immune complexes were detected with the enhanced chemiluminescence reagents from GE Healthcare (Uppsala, Sweden).
Thiobarbituric acid reactive substance assays.
The concentrations of the lipid peroxidation product malondialdehyde (MDA) were determined with a commercial thiobarbituric acid reactive substance assay kit (Cayman Chemical, Ann Arbor, MI). The results were calculated against the total protein contents.
TOBEC measurement.
The total body electrical conductivity (TOBEC) was measured in an EM-SCAN SA-3203-type chamber (EM-SCAN, Springfield, IL). Briefly, mice were anesthetized and placed in the middle of the Plexiglas cylinder. A 10-HMz oscillating magnetic field was applied and the energy dissipation was detected and expressed as E-value. At least five measurements were taken for each mouse each time. The fat-free body mass was calculated by the formula: −3.732 + 0.578 × body wt (g) + 2.967 × E0.5.
Histologic analysis.
Paraffin sections (5 μm) were prepared for hematoxylin and eosin staining and analyzed under a microscope (Leica Microsystems, Bensheim, Germany). The sizes of adipocytes were measured using ImageJ software. Histologic staining of a macrophage-specific marker was performed as described (33,34).
Data analysis.
All results were derived from at least three sets of repeated experiments. The statistical calculations were performed with SPSS 11.5 statistical software package. Differences between groups were determined by Student t test. All values were presented as means ± SD. In all statistical comparisons, P < 0.05 was used to indicate significant differences.
RESULTS
Improved systemic insulin sensitivity in mice without lipocalin-2 under conditions of aging and dietary- or genetic-induced obesity.
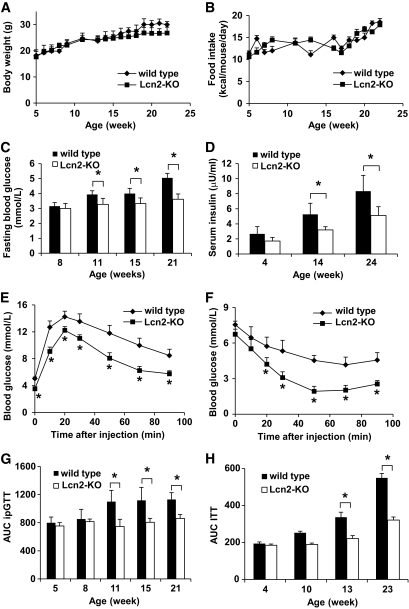
Mice lacking lipocalin-2 had similar growth rates and food intake compared with their wild-type littermates (Fig. 1A and B). However, starting from the age of 11 weeks, the fasting glucose levels of Lcn2-KO mice were significantly lower than those of wild-type mice (Fig. 1C). Moreover, the fasting serum insulin levels were constantly lower by ∼45% in Lcn2-KO mice compared with wild-type mice at all time points (Fig. 1D). At the end of the monitoring period, wild-type mice were much more glucose intolerant and insulin resistant than Lcn2-KO mice (Fig. 1E and F). In fact, the values of ipGTT area under the curve (AUC) in Lcn2-KO mice at ages 11, 15, and 21 weeks were significantly reduced than those in wild-type mice (Fig. 1G). Similar results had also been observed for ITT, showing that insulin sensitivity was greatly improved in Lcn2-KO mice at 13 and 23 weeks (Fig. 1H).
FIG. 1.
Lipocalin-2 deficiency ameliorates age-associated deterioration of insulin sensitivity. Age-matched wild-type and Lcn2-KO mice were fed with normal chow. Their body weight (A) and food intake (B) were monitored from 5 to 24 weeks. Fasting blood glucose (C) and serum insulin concentrations (D) were measured in blood samples collected from the tail vein. At the end of the period, Lcn2-KO mice showed significantly improved insulin sensitivity as evaluated by ipGTT (E) and ITT (F). The AUC of ipGTT (G) and ITT (H) were calculated for each set of experiments to demonstrate the progressive development of aging-associated insulin resistance, which was attenuated by lipocalin-2 deficiency. *P < 0.05 Lcn2-KO mice vs. wild-type controls, n = 6–8.
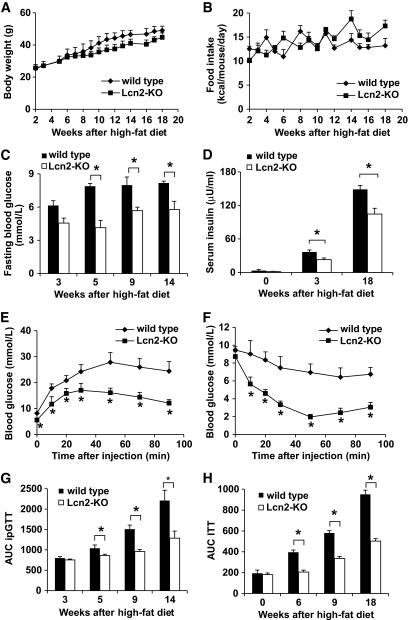
Dietary obesity was induced by feeding the mice with 18 weeks of high-fat diet. Compared with wild-type animal, the percentage body weight gain of Lcn2-KO mice was slightly lower (116.4 ± 0.2 and 96.8 ± 0.12%, respectively), despite a similar food intake (Fig. 2A and B). The fasting glucose levels of Lcn2-KO mice were lower (4.0 ± 0.67 to 5.6 ± 1.18 mmol/l) than those of the wild-type littermates (6.2 ± 0.22 to 8.4 ± 1.51 mmol/l) throughout the monitoring period (Fig. 2C). Although hyperinsulinemia was observed in both types of animals, the values remained much lower in Lcn2-KO mice than those of the wild-type littermates (Fig. 2D). At the end of the treatment, severe glucose intolerance and insulin resistance developed in wild-type mice (Fig. 2E and F). Lipocalin-2 deficiency significantly alleviated high-fat diet–induced insulin resistance, and the effect could be observed as early as 5 weeks after high-fat diet feeding (Fig. 2G and H).
FIG. 2.
Mice without lipocalin-2 are partly protected from high-fat diet–induced insulin resistance. Age-matched wild-type and Lcn2-KO mice were fed with high-fat diet for 18 weeks. Body weight (A) and food intake (B) were monitored on a weekly basis. Fasting blood glucose levels (C) and serum insulin concentrations (D) were evaluated as in Fig. 1. At the end of the treatment, mice deficient in lipocalin-2 showed greatly improved insulin sensitivity as demonstrated by ipGTT (E) and ITT (F). The AUC of ipGTT (G) and ITT (H) were calculated for monitoring the development of insulin resistance induced by high-fat diet feeding. *P < 0.05 Lcn2-KO mice vs. wild-type controls, n = 6–8.
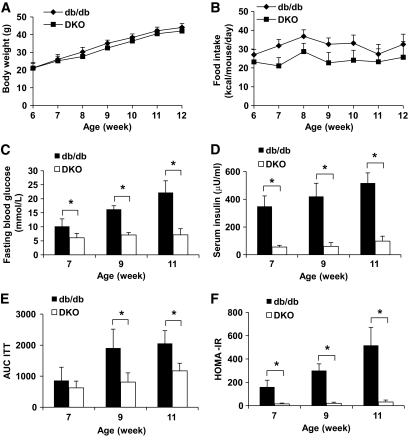
Next, leptin receptor–deficient db/db mice lacking the expression of lipocalin-2 (DKO) were generated. Both db/db and DKO mice showed early-onset obesity (Fig. 3A). The food intake of db/db mice was slightly higher compared with DKO mice (Fig. 3B). At 7 weeks of age, db/db mice developed hyperglycemia (fasting glucose levels: 10.16 ± 2.67 mmol/l, Fig. 3C). By contrast, both fasting and fed blood glucose levels (data not shown) of DKO mice were maintained at a much lower level throughout the observation period. The db/db mice displayed a severe and progressive hyperinsulinemia during the course of the study (348.421 ± 75.716, 420.826 ± 94.706, and 516.778.421 ± 73.225 μU/ml at 7, 9, and 11 weeks, respectively) (Fig. 3D), whereas DKO mice showed a significantly lower fasting plasma insulin levels (55.18 ± 12.8, 60.48 ± 26.21, and 97.67 ± 35.63 μU/ml at 7, 9, and 11 weeks, respectively). The results from both ITT and HOMA-IR calculations confirmed that systemic insulin sensitivity was significantly higher in DKO mice compared with db/db controls (Fig. 3E and F).
FIG. 3.
Insulin resistance caused by genetic obesity is attenuated in mice lacking lipocalin-2. Body weight (A) and food intake (B) were measured regularly for db/db and DKO mice between 6 and 12 weeks. Fasting blood glucose levels (C), fasting serum insulin concentrations (D), AUC of ITT (E), and HOMA-IR indexes (F) were determined for 7-, 9-, and 11-week-old animals. *P < 0.05 DKO vs. db/db mice, n = 3–6.
Recombinant adenoviruses were used for administration of exogenous murine lipocalin-2 into Lcn2-KO mice and the wild-type littermates. Overexpressing this adipokine for 2 weeks significantly elevated fasting glucose levels and HOMA-IR indexes in both types of animals (supplementary Fig. 1). The serum insulin levels were significantly augmented in Lcn2-KO mice, but only slightly increased in wild-type controls, compared with those treated with recombinant adenoviruses encoding luciferase. On the other hand, acute treatment with lipocalin-2 recombinant protein by intraperitoneal injection into both types of animals at different dosages had no effects on circulating glucose and insulin levels during the short period of treatment (up to 24 h, data not shown).
Despite enlarged mass, the fat tissues of Lcn2-KO mice show attenuated inflammation and increased insulin sensitivity.
Circulating lipid profiles were analyzed in wild-type and lipocalin-2–null mice under four different conditions (supplementary Table 2). Although elevated serum FFA levels could contribute to the development of systemic insulin resistance, no significant changes were detected. Serum total cholesterol levels were reduced in lipocalin-2–deficient mice. However, overexpression of lipocalin-2 did not increase the circulating total cholesterol concentrations. Individual tissue sample analyses revealed that compared with wild-type mice, the amount of all three major lipid species (TG, FFA, and total cholesterol) was increased by 1.5- to 1.8-fold in epididymal fat of Lcn2-KO mice fed with either normal chow or high-fat diet. Moreover, overexpression of lipocalin-2 significantly reduced the lipid content in fat tissues.
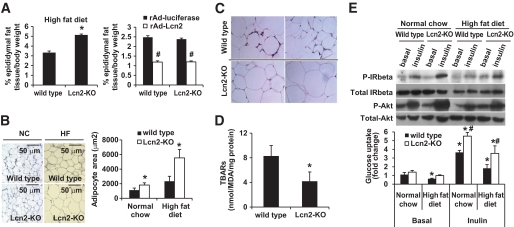
In obese Lcn2-KO mice, an expansion of the epididymal adipose tissue by ∼50% was observed compared with wild-type mice (Fig. 4A). In DKO mice, the net weight of epididymal fat pad was also increased by ∼25% compared with db/db mice (data not shown). Conversely, adenovirus-mediated overexpression of lipocalin-2 reduced the epididymal adipose tissue mass by ∼55% in wild-type mice and ∼48% in Lcn2-KO mice. Compared with the wild-type mice, lipogenesis was significantly increased and lipolysis decreased in the adipose tissues of Lcn2-KO mice (supplementary Fig. 2). Histologic examination revealed that the average area of adipocytes derived from epididymal fat pads of obese Lcn2-KO mice was about threefold larger than that of obese wild-type mice (Fig. 4B). When expressed on a per-organ basis, the total lipid contents in epididymal fat pads of high-fat diet–fed Lcn2-KO obese mice were even more markedly augmented (FFA: 44.8 ± 8.29 mmol; TG: 137.174 ± 25.39 mg; total cholesterol: 1.7 ± 0.32 mg) compared with wild-type obese mice (FFA: 19.8 ± 4.12 mg; TG: 55.9 ± 11.62 mg; total cholesterol: 0.76 ± 0.16 mg). The average cell size of epididymal adipocytes of Lcn2-KO mice fed with normal chow was also significantly larger compared with wild-type littermates (Fig. 4B). Increased subcutaneous fat mass had also been observed for obese Lcn2-KO mice compared with wild-type littermates (data not shown). Body composition analysis using TOBEC, which reflects total body fat mass (35), revealed that 15 weeks of high-fat diet induced an increase of 40 and 24% fat mass in Lcn2-KO mice and wild-type mice, respectively, whereas the values were not significantly different from those fed with normal chow (wild-type mice: 12.32 ± 2.921; Lcn2-KO mice: 13.42 ± 1.8309).
FIG. 4.
Increased adipocyte sizes, reduced inflammation, and improved insulin sensitivity of the epididymal fat tissues derived from high-fat diet–fed Lcn2-KO mice. A: The percentages of epididymal fat mass to body weights are shown for wild-type and Lcn2-KO mice fed high-fat diet for 18 weeks (left panel) and those treated with recombinant adenovirus overexpressing luciferase or lipocalin-2 (right panel). *P < 0.05 vs. wild-type controls; #P < 0.05 vs. those treated with recombinant adenovirus overexpressing luciferase, n = 5–6. B: The sizes of adipocytes were measured under microscope (magnification, ×200) and calculated using image analysis software (Image J). Ten fields were randomly chosen and sizes of 20 cells in each field were measured. *P < 0.05 vs. wild-type controls. C: Immunohistochemical analysis suggested that local inflammation occurred in epididymal fat tissues of high-fat diet–fed wild-type animals but not those of Lcn2-KO mice. Infiltrated macrophages were visualized by staining with a monoclonal anti-F4/80 antibody (magnification, ×400). D: MDA contents were significantly lower in epididymal fat tissue of Lcn2-KO mice than those of wild-type mice, after high-fat diet feeding for 18 weeks. *P < 0.05 vs. wild-type control group, n = 5–6. E: Epididymal adipose tissues were collected from normal chow, or high-fat diet–fed wild-type and Lcn2-KO mice, which were acutely injected with insulin as described in research design and methods. Both basal and insulin-stimulated phosphorylations of IR-β and Akt were evaluated by Western blotting analysis (upper panel). Proteins (100 μg) were loaded for each sample and same membranes were stripped and blotted for monitoring total IR-β and total Akt levels. Basal and insulin (100 nmol/l)–stimulated glucose uptake was measured in isolated fat pads derived from wild-type and Lcn2-KO mice (bottom panel). *P < 0.05 vs. those of wild-type mice fed with standard chow; #P < 0.05 vs. wild-type littermates of the same treatment group, n = 3–6. (A high-quality digital representation of this figure is available in the online issue.)
Immunohistochemical staining revealed that a large number of F4/80-positive macrophages were accumulated in the epididymal fat tissues from high-fat diet–fed wild-type mice, whereas the macrophages were virtually undetectable in Lcn2-KO mice, despite the enlargement of the fat cells (Fig. 4C). The concentrations of MDA, markers of oxidative stress, were lower by 50% in Lcn2-KO mice compared with wild-type mice (Fig. 4D). The total protein levels of inhibitor of κBα were increased in the adipose tissues of Lcn2-KO mice (data not shown). Quantitative PCR analysis revealed that the expressions of TNF-α, monocyte chemoattractant protein 1, F4/80, and CD14 were significantly lower in high-fat diet–fed Lcn2-KO mice compared with wild-type animals (supplementary Table 3). Insulin-induced phosphorylation of insulin receptor and Akt was examined in adipose tissue. Whereas high-fat diet–fed mice showed a much lower magnitude of response to portal vein injection of insulin (Fig. 4E), both insulin receptor and Akt phosphorylations were enhanced significantly in lean and obese Lcn2-KO mice compared with wild-type animals. Moreover, the insulin-stimulated glucose uptake was significantly higher in epididymal fat pad of Lcn2-KO mice, under both normal and high-fat diet conditions than that of wild-type mice (Fig. 4E). Compared with fat tissue, the phosphorylations of insulin receptor and Akt in skeletal muscle and liver tissues showed less prominent changes between mice with and without lipocalin-2. Insulin-stimulated glucose uptake was not significantly different in soleus muscle of Lcn2-KO mice from that of the wild-type littermates (supplementary Fig. 3A). Of note is that the expressions of key genes involved in gluconeogenesis were much lower in obese Lcn2-KO mice (supplementary Fig. 3B).
Lipocalin-2 treatment stimulates TNF-α expression in adipose tissue partly through upregulating 12-lipoxygenase expression and activity.
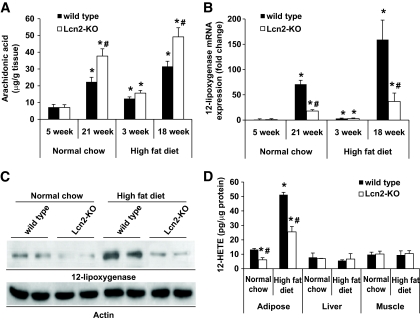
GC-MS analysis revealed that fatty acid composition in the epididymal adipose tissue of Lcn2-KO mice, but not in the liver and skeletal muscle, varied significantly from those of wild-type littermates, under both standard chow and high-fat diet conditions (supplementary Fig. 4). One of the significantly increased fatty acid species was arachidonic acid (C20:4 n6) (Fig. 5A). Aging and high-fat diet elevated arachidonic acid contents in adipose tissues, which were found to be further elevated in Lcn2-KO mice. Quantitative real-time PCR was performed to measure the expression levels of enzymes involved in arachidonic acid metabolic pathways. The results demonstrated that although cycloxygenase-1 and -2 were not obviously different between the two types of animals (data not shown), lipocalin-2 deficiency dramatically attenuated both aging- and dietary obesity–induced upregulation of 12-lipoxygenase (Fig. 5B and C). The activity of 12-lipoxygenase, indicated by the total amount of its metabolite 12(S)-HETE, was also largely reduced in the adipose tissues of obese Lcn2-KO mice (Fig. 5D). Note that in liver and skeletal muscle tissues, the gene expression (data not shown) and activity of 12-lipoxygenase were not different between mice with and without lipocalin-2.
FIG. 5.
Aging- and dietary obesity–associated upregulation of 12-lipoxygenase in adipose tissue is blocked largely by lipocalin-2 deficiency. A: GC-MS analysis revealed that the arachidonic acid amounts in epididymal fat tissues of aged or obese Lcn2-KO mice were much higher than those of wild-type mice. B: Quantitative PCR analysis of 12-lipoxygenase mRNA levels in adipose tissues showed significant difference between the wild-type and Lcn2-KO group. C: The protein expression of 12-lipoxygenase was much lower in Lcn2-KO mice (21 weeks old fed with standard chow or high-fat diet) compared with the age-matched wild-type controls. D: The 12(S)-HETE metabolites were reduced in the epididymal adipose tissues of mice without lipocalin-2. The amounts of 12(S)-HETE were not different in muscle or liver tissues compared with those of wild-type littermates (21 weeks old fed with standard chow or high-fat diet). *P < 0.05 vs. 5-week-old (A and B) or 21-week-old (D) wild-type mice fed with standard chow; #P < 0.05 vs. wild-type mice of the same treatment group, n = 5.
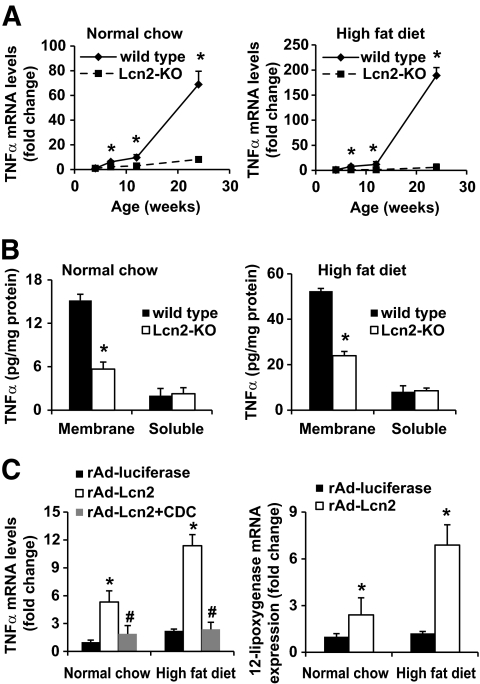
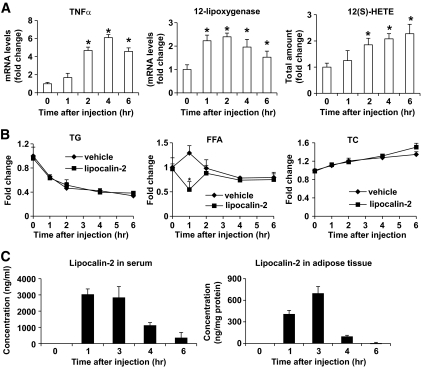
The above results showed that lipocalin-2 deficiency decreased TNF-α expression in adipose tissue (supplementary Table 3). Further analysis using tissues derived from different ages of animals revealed that the increased TNF-α mRNA levels associated with both aging and obesity were blocked in Lcn2-KO mice, and the significant differences could be observed in animals as young as 7 weeks (Fig. 6A). Similarly, the protein levels of TNF-α were also decreased in adipose tissues of Lcn2-KO mice, especially in the membrane fractions, with a reduction of ∼70% (Fig. 6B). Administration of recombinant adenovirus expressing lipocalin-2 promoted TNF-α expression by ∼5-fold and ∼11-fold in Lcn2-KO mice fed with standard chow and high-fat diet, respectively (Fig. 6C). These effects were largely reversed by treatment with CDC, a small molecular inhibitor of 12-lipoxygenase. Furthermore, overexpression of lipocalin-2 resulted in a significant increase of 12-lipoxygenase expression (Fig. 6C) and 12(S)-HETE production (data not shown) in adipose tissue. Acute treatment with lipocalin-2 significantly increased the mRNA levels of both 12-lipoxygenase and TNF-α at 1 and 2 h, respectively, in Lcn2-KO mice (Fig. 7A), but not in those treated with CDC (data not shown). In the meantime, a transient but significant decrease of serum FFA was observed in mice treated with lipocalin-2 (Fig. 7B). The 12(S)-HETE production was steadily elevated from 2 h after injection. These data indicated that arachidonate lipoxygenase pathway was involved in lipocalin-2–mediated TNF-α production from adipose tissue. Note that a large amount of lipocalin-2 rapidly entered into the adipose tissues (Fig. 7C). However, the levels of both serum and adipose lipocalin-2 gradually decreased and could not be detected at 12 h after the treatment.
FIG. 6.
Lipocalin-2 deficiency prevents aging- and obesity-induced TNF-α expression, whereas lipocalin-2 treatment stimulates TNF-α expression in adipose tissues. A: Quantitative PCR analysis of TNF-α mRNA levels in the adipose tissues derived from different ages of mice fed with normal chow (left panel) or high-fat diet (right panel). *P < 0.05 vs. Lcn2-KO mice, n = 5. B: The protein levels of TNF-α were assayed in membrane and soluble fractions of adipose tissues using the commercial ELISA kit. *P < 0.05 vs. wild-type mice, n = 5. C: Quantitative PCR analysis of TNF-α (left panel) and 12-lipoxygenase (right panel) levels in epididymal fat pad collected from Lcn2-KO mice (six-week-old) treated with adenovirus overexpressing luciferase (rAd-luciferase) or lipocalin-2 (rAd-Lcn2). The latter group was injected with vehicle or CDC as described in research design and methods. Vehicle treatment did not cause any changes on TNF-α or 12-lipoxygenase expression (data not shown). The fold changes were calculated by comparing with rAd-luciferase–treated standard chow-fed wild-type mice. *P < 0.05 vs. rAd-luciferase mice fed with standard chow; #P < 0.05 vs. rAd-Lcn2 group, n = 5.
FIG. 7.
Acute lipocalin-2 treatment rapidly induces TNF-α and 12-lipoxygenase expression in the adipose tissues of Lcn2-KO mice. Mice fed with high-fat diet for 6 weeks were treated with lipocalin-2 (800 μg/mouse) or vehicle (a bacterial-expressed unrelated protein purified following the same procedure as lipocalin-2) by intraperitoneal injection. A: TNF-α and 12-lipoxygenase mRNA levels were evaluated by quantitative PCR and the 12(S)-HETE metabolites measured by enzyme immunosorbent assay. Vehicle treatment had no effects on these parameters (data not shown). *P < 0.05 vs. time zero, n = 6. B: The circulating lipid levels (TG, FFA, and total cholesterol) were measured using the serum collected at different time points before and after injection. C: Lipocalin-2 contents in serum and adipose tissue were quantified using an in-house ELISA. *P < 0.05 vs. vehicle, n = 6. Note that the vehicle treatment had similar results as those mice injected with PBS (data not shown).
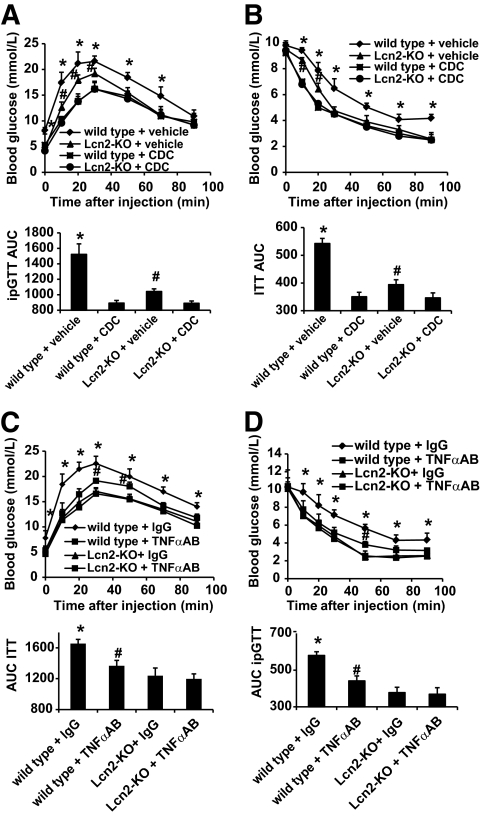
To investigate whether there was any relationship between the decreased 12-lipoxygenase activity/TNF-α production and the improved insulin sensitivity in Lcn2-KO mice, CDC or specific TNF-α neutralization antibody was administered into mice that were fed a high-fat diet (Fig. 8). Two weeks of treatment with CDC significantly attenuated the progression of insulin resistance in both wild-type and Lcn2-KO animals and abolished the differences between the two groups (Fig. 8A and B). On the other hand, similar treatment with TNF-α neutralization antibody improved insulin sensitivity in wild-type littermates, but had no significant effects on Lcn2-KO mice (Fig. 8C and D).
FIG. 8.
Both CDC treatment and neutralization of TNF-α abolish the differences of insulin sensitivity between wild-type and Lcn2-KO mice. Mice had been fed with high-fat diet for 6 weeks before starting the treatment with CDC (A and B) or TNF-α neutralization antibody (C and D) for another 2 weeks. ipGTT (A and C) or ITT (B and D) were performed at the end of the experiment. AUC was calculated and displayed at the bottom of each panel. *P < 0.05 vs. all other groups; #P < 0.05 vs. CDC-treated mice (A and B) or Lcn2-KO mice (C and D), n = 3.
DISCUSSION
Although lipocalin-2 has been identified for nearly two decades, its physiological function remains poorly understood. Studies have focused on its role in innate immune response to bacterial infection (24) and cancer progression (36). It has been considered as an early marker of acute kidney damage (37). In human obese subjects, like other insulin resistance–inducing adipokines and cytokines, circulating lipocalin-2 levels are markedly elevated (20–22). In db/db obese mice, increased serum levels of lipocalin-2 are mainly due to the selective augmentation of its expression in adipose tissue and liver (20,21). Both stimulatory and inhibitory effects of lipocalin-2 on insulin sensitivities in 3T3-L1 adipocytes have been reported (21,22). The present study has used a knockout mouse model to evaluate the physiological functions of lipocalin-2 on systematic energy homeostasis and insulin sensitivities. The results suggest that lipocalin-2 deficiency attenuates the development of aging- and obesity-associated insulin resistance, hyperglycemia, and hyperinsulinemia. Lipocalin-2 elicits its adverse effects at least partly by activating the arachidonate 12-lipoxygenase metabolic pathway and stimulating adipose expression of TNF-α, which may in turn magnify the local inflammation and cause impaired energy homeostasis and systemic insulin resistance.
TNF-α has been proposed as a link between obesity and insulin resistance because it is highly expressed in adipose tissues of obese animals and humans and can directly impair insulin signaling in both cultured cells and experimental animals (38). Obese mice lacking either TNF-α or TNF-α receptors are protected against insulin resistance (39,40). Infusion of TNF-α to adult rats reduces systemic insulin sensitivity, which is associated with major changes of gene expression in adipose tissue (30,41). Direct exposure of isolated cells to TNF-α induces a state of insulin resistance in several systems, including adipocytes and myocytes (42). In addition to obesity and type 2 diabetes, insulin resistance is associated with many other pathological conditions including aging, cancer, and infections (43). A decline in fat-free mass and a relative increase in fat mass are common findings in aged subjects and are associated with a rise in TNF-α concentration and a deterioration of insulin action (44,45). Neutralization of TNF-α reverses age-induced impairment of insulin responsiveness (46). Although these pharmacologic studies have attributed most of the action of TNF-α to the pathogenesis of insulin resistance, the molecular basis underlying increased TNF-α expression in the obese state is largely unknown. The present study provides evidence suggesting that lipocalin-2 plays critical roles in regulating TNF-α expressions in fat tissues, at least partly through upregulating 12-lipoxygenase expression and activity. First, increased levels of lipocalin-2 are found to be associated with both aging (data not shown) and obesity (20) in wild-type mice. Second, mice lacking lipocalin-2 are protected from aging- and obesity-induced upregulation of TNF-α and activation of 12-lipoxygenase in adipose tissue. Third, lipocalin-2 treatment increases TNF-α levels and 12-lipoxygenase expression and activity. Fourth, blockage of arachidonate lipoxygenase pathway by CDC treatment prevents the induction of TNF-α expression by both high-fat diet (data not shown) and lipocalin-2 treatment. Taken together, the presence of lipocalin-2 may be indispensable for TNF-α induction by various pathologic conditions.
Consistent with the findings on TNF-α production, insulin resistance is largely prevented in aged and obese Lcn2-KO mice. This improvement of insulin sensitivity is correlated mainly with attenuated inflammation in adipose tissues of mice lacking lipocalin-2. Both the total protein and adipose membrane fraction of TNF-α are significantly decreased in obese Lcn2-KO mice compared with wild-type mice. Membrane TNF-α is a precursor form of soluble TNF-α and exerts proinflammatory functions in a cell-to-cell contact manner. It has been demonstrated that macrophages in fat pads of obese mice and humans are localized to dead adipocytes and are often coincident with increased TNF-α expression (47). This information suggests that lipocalin-2 may exert adverse metabolic and inflammatory actions, locally and systemically, partly through upregulating the expression of TNF-α. This has been further verified by introducing neutralization antibodies to high-fat diet–fed wild-type and Lcn2-KO mice. TNF-α neutralization attenuates insulin resistance in wild-type mice, whereas lipocalin-2–deficient mice do not show reduced insulin sensitivity. Of note is that CDC treatment, which attenuates TNF-α expression and 12-lipoxygenase activity induced by lipocalin-2, improves insulin sensitivity in both wild-type and Lcn2-KO mice. Because CDC at higher concentrations also inhibits other lipoxygenases, it is highly possible that some unidentified inflammatory mediators may play a role in causing insulin resistance in both wild-type and Lcn2-KO mice, which could not be prevented by lipocalin-2 deficiency. In fact, our unpublished observation suggests that CDC treatment attenuates the expression of a wide range of inflammatory adipokines, including TNF-α, IL-6, and IL-1β in adipose tissue of high-fat diet–fed mice (J.T.C.L. and Y.W.).
12-Lipoxygenase has been linked to inflammation and insulin resistance partly through the production of biologically active lipid species, such as 12(S)-HETE (31,48). Mice deficient in this gene are resistant to inflammatory effects induced by Western diet. Treatment with its product 12(S)-HETE enhances the expression of proinflammatory cytokine genes and impairs insulin signaling in 3T3-L1 adipocytes. Stimulators of 12-lipoxygenase gene expression include saturated fatty acids, such as palmitate (48). In addition, the expression levels of this enzyme can be upregulated by iron deficiency (49), in which the overall effect is a perturbation of lipid homeostasis. Using inductively coupled plasma mass spectrometry analyses, we have found that lipocalin-2 deficiency is associated with a higher level of iron contents in adipose tissues of Lcn2-KO mice than in wild-type animals (supplementary Fig. 5). However, the iron levels are decreased by high-fat feeding in both types of animals to a similar extent, suggesting that other factors in addition to iron may be involved in causing the different expression levels of 12-lipoxygenase in mice with or without lipocalin-2. Although lipocalin-2 belongs to a family of proteins that can bind to lipids, its endogenous ligands have not been identified. Acute lipocalin-2 treatment causes a rapid but transient reduction of the circulating FFA levels. It can also enhance fatty acid uptake into fat tissue, suggesting that the inducing effect of this adipokine on 12-lipoxygenase may also involve transportation of lipid species into the adipocytes.
Excessive ectopic lipid accumulation plays an important role in inducing peripheral insulin resistance (50). Note that lipid accumulation in liver can be markedly abolished by lipocalin-2 deficiency. Moreover, the lipid contents in skeletal muscle are lower in Lcn2-KO mice and can be augmented by replacement with lipocalin-2, suggesting that it may promote lipid remobilization from fat to peripheral tissues. Indeed, irrespective of obesity conditions induced by the diet or the genetic mutations, the absence of lipocalin-2 enhances lipid storage in fat tissue and treatment with this adipokine reduces the adipose fat content, which may explain the phenomenon that excess ectopic lipid accumulation is attenuated in Lcn2-KO mice. Nevertheless, whether lipocalin-2 could promote peripheral insulin resistance through its lipid-binding activities needs to be further addressed but is beyond the scope of this study.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from Hong Kong Research Grant Council HKU 778007 (Y.W.) and HKU 7645/06M (A.X.), the Collaborative Research Fund (HKU 2/07C), and the Area of Excellent Scheme (AoE/P-10-01) established under the University Grants Committee, HKSAR.
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Runge CF: Economic consequences of the obese. Diabetes 2007; 56: 2668– 2672 [DOI] [PubMed] [Google Scholar]
- 2.Tataranni PA, Ortega E: A burning question: does an adipokine-induced activation of the immune system mediate the effect of overnutrition on type 2 diabetes? Diabetes 2005; 54: 917– 927 [DOI] [PubMed] [Google Scholar]
- 3.Wellen KE, Hotamisligil GS: Inflammation, stress, and diabetes. J Clin Invest 2005; 115: 1111– 1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tilg H, Moschen AR: Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 2006; 6: 772– 783 [DOI] [PubMed] [Google Scholar]
- 5.Fantuzzi G: Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol 2005; 115: 911– 919 [DOI] [PubMed] [Google Scholar]
- 6.Hotamisligil GS: Inflammatory pathways and insulin action. Int J Obes Relat Metab Disord 2003; 27( Suppl 3.): S53– S55 [DOI] [PubMed] [Google Scholar]
- 7.Davis TR, Tabatabai L, Bruns K, Hamilton RT, Nilsen-Hamilton M: Basic fibroblast growth factor induces 3T3 fibroblasts to synthesize and secrete a cyclophilin-like protein and beta 2-microglobulin. Biochim Biophys Acta 1991; 1095: 145– 152 [DOI] [PubMed] [Google Scholar]
- 8.Kjeldsen L, Johnsen AH, Sengeløv H, Borregaard N: Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem 1993; 268: 10425– 10432 [PubMed] [Google Scholar]
- 9.Hraba-Renevey S, Türler H, Kress M, Salomon C, Weil R: SV40-induced expression of mouse gene 24p3 involves a post-transcriptional mechanism. Oncogene 1989; 4: 601– 608 [PubMed] [Google Scholar]
- 10.Stoesz SP, Gould MN: Overexpression of neu-related lipocalin (NRL) in neu-initiated but not ras or chemically initiated rat mammary carcinomas. Oncogene 1995; 11: 2233– 2241 [PubMed] [Google Scholar]
- 11.Lögdberg L, Wester L: Immunocalins: a lipocalin subfamily that modulates immune and inflammatory responses. Biochim Biophys Acta 2000; 1482: 284– 297 [DOI] [PubMed] [Google Scholar]
- 12.Flower DR, North AC, Sansom CE: The lipocalin protein family: structural and sequence overview. Biochim Biophys Acta 2000; 1482: 9– 24 [DOI] [PubMed] [Google Scholar]
- 13.Jessen BA, Stevens GJ: Expression profiling during adipocyte differentiation of 3T3-L1 fibroblasts. Gene 2002; 299: 95– 100 [DOI] [PubMed] [Google Scholar]
- 14.Kratchmarova I, Kalume DE, Blagoev B, Scherer PE, Podtelejnikov AV, Molina H, Bickel PE, Andersen JS, Fernandez MM, Bunkenborg J, Roepstorff P, Kristiansen K, Lodish HF, Mann M, Pandey A: A proteomic approach for identification of secreted proteins during the differentiation of 3T3-L1 preadipocytes to adipocytes. Mol Cell Proteomics 2002; 1: 213– 222 [DOI] [PubMed] [Google Scholar]
- 15.Lin Y, Rajala MW, Berger JP, Moller DE, Barzilai N, Scherer PE: Hyperglycemia-induced production of acute phase reactants in adipose tissue. J Biol Chem 2001; 276: 42077– 42083 [DOI] [PubMed] [Google Scholar]
- 16.Cowland JB, Muta T, Borregaard N: IL-1beta-specific up-regulation of neutrophil gelatinase-associated lipocalin is controlled by IkappaB-zeta. J Immunol 2006; 176: 5559– 5566 [DOI] [PubMed] [Google Scholar]
- 17.Meheus LA, Fransen LM, Raymackers JG, Blockx HA, Van Beeumen JJ, Van Bun SM, Van de Voorde A: Identification by microsequencing of lipopolysaccharide-induced proteins secreted by mouse macrophages. J Immunol 1993; 151: 1535– 1547 [PubMed] [Google Scholar]
- 18.Shen F, Hu Z, Goswami J, Gaffen SL: Identification of common transcriptional regulatory elements in interleukin-17 target genes. J Biol Chem 2006; 281: 24138– 24148 [DOI] [PubMed] [Google Scholar]
- 19.van Dam RM, Hu FB: Lipocalins and insulin resistance: etiological role of retinol-binding protein 4 and lipocalin-2? Clin Chem 2007; 53: 5– 7 [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Lam KS, Kraegen EW, Sweeney G, Zhang J, Tso AW, Chow WS, Wat NM, Xu JY, Hoo RL, Xu A: Lipocalin-2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. Clin Chem 2007; 53: 34– 41 [DOI] [PubMed] [Google Scholar]
- 21.Yan QW, Yang Q, Mody N, Graham TE, Hsu CH, Xu Z, Houstis NE, Kahn BB, Rosen ED: The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes 2007; 56: 2533– 2540 [DOI] [PubMed] [Google Scholar]
- 22.Zhang J, Wu Y, Zhang Y, Leroith D, Bernlohr DA, Chen X: The role of lipocalin 2 in the regulation of inflammation in adipocytes and macrophages. Mol Endocrinol 2008; 22: 1416– 1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Catalán V, Gómez-Ambrosi J, Rodríguez A, Ramírez B, Silva C, Rotellar F, Gil MJ, Cienfuegos JA, Salvador J, Frühbeck G: Increased adipose tissue expression of lipocalin-2 in obesity is related to inflammation and matrix metalloproteinase-2 and metalloproteinase-9 activities in humans. J Mol Med 2009; 87: 803– 813 [DOI] [PubMed] [Google Scholar]
- 24.Berger T, Togawa A, Duncan GS, Elia AJ, You-Ten A, Wakeham A, Fong HE, Cheung CC, Mak TW: Lipocalin 2-deficient mice exhibit increased sensitivity to Escherichia coli infection but not to ischemia-reperfusion injury. Proc Natl Acad Sci U S A 2006; 103: 1834– 1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Lam KS, Chan L, Chan KW, Lam JB, Lam MC, Hoo RC, Mak WW, Cooper GJ, Xu A: Post-translational modifications of the four conserved lysine residues within the collagenous domain of adiponectin are required for the formation of its high molecular weight oligomeric complex. J Biol Chem 2006; 281: 16391– 16400 [DOI] [PubMed] [Google Scholar]
- 26.National Research Council Guide for the Care and Use of Laboratory Animals Washington, DC: National Academy Press; 1996 [Google Scholar]
- 27.Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ: The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest 2003; 112: 91– 100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haan GJ, van der Heide S, Wolthers BG: Analysis of fatty acids from human lipids by gas chromatography. J Chromatogr 1979; 162: 261– 271 [DOI] [PubMed] [Google Scholar]
- 29.Xu A, Yin S, Wong L, Chan KW, Lam KS: Adiponectin ameliorates dyslipidemia induced by the human immunodeficiency virus protease inhibitor ritonavir in mice. Endocrinology 2004; 145: 487– 494 [DOI] [PubMed] [Google Scholar]
- 30.Xu H, Uysal KT, Becherer JD, Arner P, Hotamisligil GS: Altered tumor necrosis factor-alpha (TNF-alpha) processing in adipocytes and increased expression of transmembrane TNF-alpha in obesity. Diabetes 2002; 51: 1876– 1883 [DOI] [PubMed] [Google Scholar]
- 31.Nunemaker CS, Chen M, Pei H, Kimble SD, Keller SR, Carter JD, Yang Z, Smith KM, Wu R, Bevard MH, Garmey JC, Nadler JL: 12-Lipoxygenase-knockout mice are resistant to inflammatory effects of obesity induced by Western diet. Am J Physiol Endocrinol Metab 2008; 295: E1065– E1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Xu A, Ye J, Kraegen EW, Tse CA, Cooper GJ: Alteration in phosphorylation of P20 is associated with insulin resistance. Diabetes 2001; 50: 1821– 1827 [DOI] [PubMed] [Google Scholar]
- 33.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H: Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003; 112: 1821– 1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW, Jr: Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003; 112: 1796– 1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stenger J, Bielajew C: Comparison of TOBEC-derived total body fat with fat pad weights. Physiol Behav 1995; 57: 319– 323 [DOI] [PubMed] [Google Scholar]
- 36.Yang J, Bielenberg DR, Rodig SJ, Doiron R, Clifton MC, Kung AL, Strong RK, Zurakowski D, Moses MA: Lipocalin 2 promotes breast cancer progression. Proc Natl Acad Sci U S A 2009; 106: 3913– 3918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P: Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 2005; 365: 1231– 1238 [DOI] [PubMed] [Google Scholar]
- 38.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM: Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest 1995; 95: 2409– 2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS: Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature 1997; 389: 610– 614 [DOI] [PubMed] [Google Scholar]
- 40.Ventre J, Doebber T, Wu M, MacNaul K, Stevens K, Pasparakis M, Kollias G, Moller DE: Targeted disruption of the tumor necrosis factor-alpha gene: metabolic consequences in obese and nonobese mice. Diabetes 1997; 46: 1526– 1531 [DOI] [PubMed] [Google Scholar]
- 41.Ruan H, Miles PD, Ladd CM, Ross K, Golub TR, Olefsky JM, Lodish HF: Profiling gene transcription in vivo reveals adipose tissue as an immediate target of tumor necrosis factor-alpha: implications for insulin resistance. Diabetes 2002; 51: 3176– 3188 [DOI] [PubMed] [Google Scholar]
- 42.Lorenzo M, Fernández-Veledo S, Vila-Bedmar R, Garcia-Guerra L, De Alvaro C, Nieto-Vazquez I: Insulin resistance induced by tumor necrosis factor-alpha in myocytes and brown adipocytes. J Anim Sci 2008; 86: E94– E104 [DOI] [PubMed] [Google Scholar]
- 43.Hotamisligil GS: The role of TNFalpha and TNF receptors in obesity and insulin resistance. J Intern Med 1999; 245: 621– 625 [DOI] [PubMed] [Google Scholar]
- 44.Morin CL, Pagliassotti MJ, Windmiller D, Eckel RH: Adipose tissue-derived tumor necrosis factor-alpha activity is elevated in older rats. J Gerontol A Biol Sci Med Sci 1997; 52: B190– B195 [DOI] [PubMed] [Google Scholar]
- 45.Paolisso G, Tagliamonte MR, Rizzo MR, Giugliano D: Advancing age and insulin resistance: new facts about an ancient history. Eur J Clin Invest 1999; 29: 758– 769 [DOI] [PubMed] [Google Scholar]
- 46.Borst SE, Lee Y, Conover CF, Shek EW, Bagby GJ: Neutralization of tumor necrosis factor-alpha reverses insulin resistance in skeletal muscle but not adipose tissue. Am J Physiol Endocrinol Metab 2004; 287: E934– E938 [DOI] [PubMed] [Google Scholar]
- 47.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS: Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res 2005; 46: 2347– 2355 [DOI] [PubMed] [Google Scholar]
- 48.Chakrabarti SK, Cole BK, Wen Y, Keller SR, Nadler JL: 12/15-Lipoxygenase products induce inflammation and impair insulin signaling in 3T3-L1 adipocytes. Obesity (Silver Spring) 2009; 17: 1657– 1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Collins JF, Hu Z, Ranganathan PN, Feng D, Garrick LM, Garrick MD, Browne RW: Induction of arachidonate 12-lipoxygenase (Alox15) in intestine of iron-deficient rats correlates with the production of biologically active lipid mediators. Am J Physiol Gastrointest Liver Physiol 2008; 294: G948– G962 [DOI] [PubMed] [Google Scholar]
- 50.Consitt LA, Bell JA, Houmard JA: Intramuscular lipid metabolism, insulin action, and obesity. IUBMB Life 2009; 61: 47– 55 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.