Abstract
OBJECTIVE
Because adipose tissue is highly vascularized, modifying adipose tissue vasculature may provide a novel method for reducing body fat. A peptide sequence that elicits apoptosis of endothelium in white fat potently reduced body weight. We sought to determine how inhibiting adipose tissue vasculature changes key aspects of energy balance regulation and the neuroendocrine system that maintains energy balance.
RESEARCH DESIGN AND METHODS
Lean and obese mice or rats were treated with proapoptotic peptide for 4 or 27 days. Daily energy intake and expenditure were measured in mice on a low- (LFD) or high-fat diet (HFD) and in rats on a HFD. A conditioned taste aversion test was performed to assess whether proapoptotic peptide produces visceral illness. Hypothalamic neuropeptide Y, agouti-related peptide, and proopiomelanocoritin (POMC) mRNA expression and plasma leptin levels were evaluated.
RESULTS
Proapoptotic peptide completely reversed HFD-induced obesity in mice and reduced body weight in mice and rats on a HFD but not in those on a LFD. Fat loss occurred with no change of energy expenditure but reduced food intake that occurred without signs of illness and despite reduced circulating leptin and reduced hypothalamic POMC gene expression, indicating that the decrease in food intake is independent of the action of leptin.
CONCLUSIONS
These experiments provide compelling evidence for a previously unknown relationship between the status of adipose tissue vasculature and the regulation of food intake.
The prevalence of overweight and obesity among adults and children is increasing throughout the developed world (1,2), highlighting the enormous need to find new therapeutic strategies that allow for safe and durable weight loss. However, chronic weight loss is difficult to achieve because of elaborate neuroendocrine mechanisms that work against sustained negative energy balance (3–5). We herein present data on a promising strategy to induce significant weight loss by inhibiting angiogenesis in adipose tissue (6–8). White adipose tissue is highly vascularized, and both the expansion and maintenance of adipose tissue depend on a continued ability to build the necessary vasculature to support a large volume of tissue (9,10). In this way, increasing adipose mass can be compared with an expanding tumor because both tissues require rapid angiogenesis. Given this dependence on the capacity to make new blood vessels, one successful strategy for reducing the size of tumors has been to inhibit angiogenesis and thereby starve tumors (6–8).
Pharmacological inhibitors of angiogenesis such as O-(chloracetyl-carbamoyl) fumagillol (TNP-470) and angiostatin reduce fat mass in obese and leptin-deficient mice (6,8). Adopting an alternative strategy, Kolonin et al. (7) used phage display to identify a peptide sequence that specifically homes to endothelial cells in white adipose tissue by binding to the cell-surface molecule prohibitin. This peptide sequence was then fused to a proapoptotic sequence that, when internalized in a cell, initiates apoptosis. Thus, they developed a “proapoptotic peptide” that specifically reduces endothelium and vasculature in white adipose tissue. When administered over a 4-week period, the proapoptotic peptide reduced body weight and fat mass of ob/ob mice and mice with diet-induced obesity without eliciting abnormal fat absorption. That report did not identify how the energy-balance equation was modified to achieve the dramatic weight loss (3,4). Thus, the present experiments sought to determine the relationship between adipose tissue vasculature and regulation of energy balance by identifying mechanisms of energy balance by which apoptosis selectively in endothelium of white adipose tissue results in profound weight loss.
RESEARCH DESIGN AND METHODS
Male C57Bl/6 mice obtained from The Jackson Laboratories at 8 weeks of age or adult male Long-Evans rats from Harlan (Indianapolis, IL) were housed individually in standard mouse or rat cages with a 12-h light/12-h dark cycle. They had ad lib access to either a high-fat diet (HFD) providing 40% calories as fat (D03082706; Research Diet, NJ) or a low-fat diet (LFD) with 4% fat (D03082705) for 8 weeks as previously described (11). After 8 weeks on the HFD, half of those mice were administered 3 mg/kg of proapoptotic peptide [CKGGRAKDC-GG-D(KLAKLAK)2] in 0.5% DMSO/saline, and half were administered the same dose of the control peptide (CKGGRAKDC) subcutaneously daily just before dark for 27 days as previously described (7). Lean mice were injected with 0.5% DMSO/saline. A second cohort of comparable C57Bl/6 mice was made obese as a result of 127 days on a HFD (D012451; Research Diet). Eight mice were treated with control peptide or proapoptotic peptide once daily (>44 g) on the HFD in calorimetry chambers for 4 days, and another eight mice were injected with with vehicle or proapoptotic peptide in the short-term study. To determine the differential effect of the proapoptotic peptide on energy intake of mice on the LFD and HFD, a third, fourth, and fifth cohort of comparable C57Bl/6 mice were prepared by being fed the HFD (D03082706; Research Diet) or the LFD (D03082705) for 3 or 4 months. For the experiments with rats, adult male Long-Evans rats were injected with 1.5 mg/kg of proapoptotic peptide or vehicle (saline) for 4 days after they were put on the HFD for 3 months. All animal protocols were approved by the University of Cincinnati Institutional Animal Care and Use Committee.
Food intake and body-weight measurements.
Food intake and body weight were measured daily. The biological kinetics of the proapoptotic peptide targeting adipose tissue are unusual. Other treatments that have used the (KLAKLAK)2 with other targeting sequences have elicited apoptosis after 24 h (12).
Determination of energy expenditure and respiratory quotient.
Energy expenditure was measured by indirect calorimetry. For the 27-day experiment, obese mice of each group (n = 8/group) were placed individually into calorimetry chambers (Oxymax System; Columbus Instruments) with food and water available for determining O2 consumption and CO2 production. Mice were monitored for 22 h on days 10, 11, and 12. For the acute study, obese mice of each group (n = 4/group) were acclimated to the metabolic chamber (Physioscan Metabolic System; Accuscan Instruments) for 4 days prior to treatment. O2 consumption and CO2 production were then determined for 22 h/day throughout the experiment. VO2 consumption was adjusted for lean body mass as determined by nuclear magnetic resonance, and the VCO2-to-VO2 ratio (RQ ratio) was calculated by dividing VCO2 production by VO2 consumption.
Conditioned taste aversion protocol.
To determine whether the proapoptotic peptide elicits visceral illness, we used a conditioned taste aversion (CTA) paradigm as previously described (13). Mice ad libitum fed a HFD diet (vehicle, n = 6; control peptide, n = 6; and proapoptotic peptide, n = 12) were allowed to drink water or Kool-Aid for 1 h at the same time each day. On training days 1, 2, and 4, all animals had access to two bottles of water for 1 h/day. On training day 3, all animals had 1-h access to one of two novel flavors (1% saccharin flavored with either grape or cherry Kool-Aid). Immediately after access to the flavor, each mouse was subcutaneously injected with 0.15 mol/l NaCl at a volume of 1% of body weight. On training day 5, each mouse had 1 h access to the alternate novel flavor in both bottles and received a subcutaneous injection of vehicle (0.5% DMSO/saline), control peptide, or proapoptotic peptide at the same volume. The 2-day training with saline or one of the test solutions was repeated, and on the subsequent 3 days the mice had 1 h access to two bottles of water. On the test day, the mice were given both flavors in separate bottles. Intake was recorded at 30, 60, 90, and 120 min and at 24 h. The relative positions of the two flavors were changed at each measurement time point to ensure that a side preference would not influence the data. The preference ratio was calculated as the ratio of fluid intake of the flavor paired with a test solution over total fluid intake. We used 0.15 mol/l LiCl-treated mice as a positive control.
Intracerebral third-ventricular injection of proapoptotic peptide.
Under ketamine and xylazine anesthesia, 14 rats received cannula in the third cerebral ventricle (14). On the test day, food was removed 3 h before dark, and the rats received 30 μg proapoptotic peptide in 2 μl of 0.9% physiological saline or vehicle. Food was returned just before dark and intake measured at 2, 4, 6, 24, and 48 h. Body weight was measured at 24 and 48 h.
Body composition analysis.
Body composition was analyzed with a quantitative nuclear magnetic resonance (Echo MRI Whole Body Composition Analyzer; Echo Medical Systems) (15) on days 12 and 23 for the longer-term study and on day 4 in the acute study.
Leptin, adiponectin, and resistin measurement.
Blood samples were taken by cardiac puncture from mice fasted for 4 h. Leptin was determined using a rat leptin RIA kit (Linco, St. Louis, MO). Adiponectin was determined using a mouse/rat adiponectin ELISA kit (B-Bridge International). Resistin was measured using a mouse resistin ELISA kit (Millipore).
Hypothalamic gene expression.
As previously described (16), mice were killed during the light phase after a 4-h fast. The brain was quickly removed and stored in RNA later (Ambion, Austin, TX). Total RNA was isolated and cDNA synthesized using iScript and verified by L32 amplification products. All RT-PCR were performed using Failsafe PCR kits (EPICENTRE Biotechnologies). Mouse quantitative PCR primer sequences are in Table 1. Each primer set was optimized such that the correlation was 0.99–1.0 and the PCR efficiency was 90–100%. PCR was performed in triplicated using an iCycler and the iQ SYBR Green Supermix (Bio-rad Laboratories) with two-step amplification (95°C for 10 sec and annealing temperature for 30 sec) for 40 cycles. L32 was amplified from every sample for use as an endogenous control. For the data analysis, the threshold cycle (CT) of each set of triplicates was calculated. To normalize the data, the ΔCT was calculated for each sample by subtracting the average CT of L32 from the average CT of the gene of interest. The ΔCT was averaged for the control group and was then subtracted from the ΔCT of each experimental sample to generate the ΔΔCT. The ΔΔCT was then used to calculate the fold difference, 2ΔΔCT (16,17).
TABLE 1.
Mouse quantitative PCR primer sequences
| Gene | Mouse Q-PCR primer sequences | Temperature (°C) |
|---|---|---|
| L32 | 61.2 | |
| Forward | 5′-GCCAGGAGACGACAAAAAT | |
| Reverse | 5′-AATCCTCTTGCCCTGATCC | |
| AgRP | 61.2 | |
| Forward | 5′-TGTGTAAGGCTGCACGAGTC | |
| Reverse | 5′-GGCAGTAGCAAAAGGCATTG | |
| NPY | 61.2 | |
| Forward | 5′-AGGCTTGAAGACCCTTCCAT | |
| Reverse | 5′-ACAGGCAGACTGGTTTCAGG | |
| POMC | 61.2 | |
| Forward | 5′-GAGTTCAAGAGGGAGCTGGA | |
| Reverse | 5′-GGTCATGAAGCCACCGTAAC | |
| Lep | 61.2 | |
| Forward | 5′-TTCACACACGCAGTCGGTAT | |
| Reverse | 5′-GCTGGTGAGGACCTGTTGAT | |
| LPL | 55 | |
| Forward | 5′-TTCCAGCCAGGATGCAACA | |
| Reverse | 5′-GGTCCACGTCTCCGAGTCC | |
| FABP4 | 55.8 | |
| Forward | 5′-CATCAGCGTAAATGGGGATT | |
| Reverse | 5′-CTTGTGGAAGTCACGCCTTT | |
| FASN | 58.7 | |
| Forward | 5′-TTGCTGGCACTACAGAATGC | |
| Reverse | 5′-AACAGCCTCAGAGCGACAAT | |
| ACACA | 61.4 | |
| Forward | 5′-GAGAGGGGTCAAGTCCTTCC | |
| Reverse | 5′-CTGCTGCCGTCATAAGACAA |
Determination of adipose leptin mRNA expression.
Adipose tissue was quickly removed and stored in RNA later. Total RNA was isolated using Tri Reagent (Medical Research Center) and repurified using an RNeasy MinElute Cleanup Kit (Qiagen). After verifying the integrity of the RNA, target RNA was prepared using the Ovation Biotin RNA Amplification and Labeling System (Nugen) from 20 ng total RNA from each mouse and hybridized to Affymetirx Mouse Genome 430 2.0 microarrays. The data were analyzed to identify differentially expressed genes among vehicle-injected LFD (LF-V) mice, control peptide–injected HFD (HF-CP) mice, and proapoptotic peptide–treated HFD (HF-PP) mice. Specifically, the differences were estimated for HF-PP versus HF-CP, HF-CP versus LF-V, and HF-PP versus LF-V. Analysis was performed using R statistical software and the limma Bioconductor package (18). All steps of data preprocessing, including background correction, normalization, and expression set summaries, were performed using robust multichip analysis (RMA). Estimated fold changes were calculated using ANOVA, and resulting t statistics from each comparison were modified using an intensity-based empirical Bayes method (19).
Statistical analyses.
All data are expressed as means ± SEM. Body weight, food intake, O2 consumption, and RQ ratio in the short- and long-term studies were analyzed by repeated two-way ANOVA. CTA data, O2 consumption, and leptin were analyzed by one-way ANOVA. Differences in gene expression, leptin, adiponectin, and resistin were determined by Student's t test. Significant ANOVAs were followed by post hoc Tukey's test. P values <0.05 were considered significant.
RESULTS
Proapoptotic peptide and energy intake.
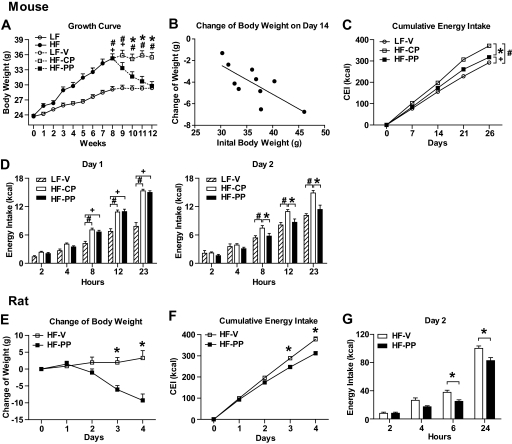
C57Bl/6 mice were placed on a LFD or beginning at 8 weeks of age. HFD-fed mice were 20% heavier than mice on the LFD after 8 weeks. HFD-fed mice were subcutaneously injected with control peptide or proapoptotic peptide, and LFD-fed mice were injected with vehicle. Consistent with a previous report, HF-PP mice lost significant body weight over 27 days of treatment compared with HF-CP mice. Body weight of HF-PP mice was not significantly different from that of LF-V mice (Fig. 1A). The loss of body weight induced by the proapoptotic peptide was positively correlated with initial body weight (r2 = 0.550; P < 0.05) (Fig. 1B). Thus, the heavier mice lost more weight.
FIG. 1.
Effect of 27 days of proapoptotic peptide administration on energy balance in mice and of 4 days' proapoptotic peptide administration in rats. A: Change (growth curve) in body weight of LFD- or HFD-fed C57Bl/6 mice. The body weight of HFD-fed C57Bl/6 mice was greatly decreased after 27 days of subcutaneous treatment with proapoptotic peptide (150 μg) compared with that of mice treated with equimolar amount of control peptide and not significantly different from that of LFD-fed C57Bl/6 mice injected with vehicle (0.5% DMSO/saline). B: Negative correlation between initial body weight and body weight gain over 14 days' treatment. C: Cumulative energy intake was significantly decreased in HF-PP mice throughout the treatment compared with that in HF-CP mice. D: Change over 24 h in energy intake on the first (left panel) and second (right panel) days of treatment. The energy intake was significantly different in HF-PP mice from 8 h after treatment on the second day of treatment compared with that in HF-CP mice but not on the first day. LF-V, n = 8; HF-CP, n = 8; HF-PP, n = 8. E–G: Effect of the proapoptotic peptide on body weight (E), cumulative energy intake (F), and 24-h change in energy intake on the second day of treatment (G) in rats. HF-V, n = 12; HF-PP, n = 12.
A key question pertains to the cause of the reduction in body weight. ANOVA revealed a significant interaction between treatment and time on cumulative energy intake, and Tukey's post hoc test indicated that cumulative energy intake was significantly decreased in HF-PP mice compared with HF-CP mice throughout the experiment and beginning at day 7 (Fig. 1C). A closer analysis revealed that the energy intake of HF-PP mice was significantly lower than that of HF-CP mice beginning 8 h after the injection on the second day of treatment but not on the first day of treatment (Fig. 1D).
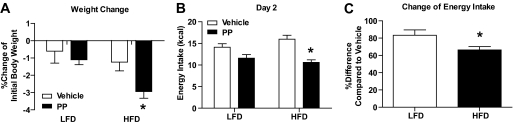
In a separate experiment comparing mice on both diets (LFD vs. HFD 34.0 ± 1.1 g vs. 44.5 ± 0.6 g of initial body weight), the effect of the proapoptotic peptide on energy intake and body weight was greater in HFD mice than in LFD mice on day two, suggesting that the peptide reduces energy intake by directly acting on adipose tissue (Fig. 2).
FIG. 2.
Differential effect of the proapoptotic peptide on change of body weight (A), energy intake (B), and change of energy intake (C) of mice on a LFD (n = 10 in each group) or HFD (n = 10 in each group) on day 2. All data are represented as means ± SEM. *P < 0.05.
To determine whether rats respond comparably, rats fed the HFD for 3 months were treated with the proapoptotic peptide for 4 days. Energy intake and body weight of PP-treated rats were significantly decreased compared with those of vehicle-injected rats in a pattern similar to what occurred in mice (Fig. 1D, F, and G), indicating that both mice and rats are sensitive to the weight- and food intake–reducing effects of PP (Fig. 2B).
Direct effect of proapoptotic peptide in hypothalamus on food intake.
To determine whether the proapoptotic peptide directly affects the central nervous system (CNS), we compared food intake and change of body weight after administration of 30 μg proapoptotic peptide or vehicle into the third ventricle of rats. There was no difference in food intake or body weight after proapoptotic peptide administration compared with vehicle (supplemental Fig. 1A and B, available in an online appendix [http://diabetes.diabetesjournals.org/cgi/content/full/db09-1141/DC1]). We further explored whether the PP might target the CNS by examining transferase-mediated dUTP nick-end labeling (TUNEL) staining in brains of proapoptotic peptide–treated mice. We observed no TUNEL staining in key brain regions, indicating that there was no obvious apoptosis in the hypothalamus.
CTA.
To determine whether the reduction of food intake following administration of the proapoptotic peptide is associated with visceral illness, we assessed the ability of proapoptotic peptide to produce a CTA using the toxin LiCl as a positive control. The preference ratio of Kool-aid intake among LF-V, HF-CP, and HF-PP mice was not different at any time point. In contrast, LiCl caused a robust CTA (P < 0.001) (Fig. 3A). There are limitations to CTAs with the proapoptotic peptide because the effect on food intake takes time to become apparent. However, there were clear differences between LiCl and proapoptotic peptide. Further, proapoptotic peptide did not significantly reduce intake in LFD mice (Fig. 2).
FIG. 3.
Proapoptotic peptide treatment did not produce a CTA at the same dose that results in reduced energy intake and weight loss. A: Preference ratio over 24 h. Subcutaneous administration of 0.15 mol/l LiCl resulted in reduced intake of the flavor paired with LiCl compared with mice treated with equiosmotic NaCl. All data are represented as means ± SEM. Saline, n = 8; LiCl, n = 8; LF-V, n = 6; HF-CP, n = 6; HF-PP, n = 12. *P < 0.05. B: Effect of proapoptotic peptide on energy expenditure during the second week of treatment. O2 consumption was adjusted for lean mass as determined by nuclear magnetic resonance. LF-V, n = 8; HF-CP, n = 8; HF-PP, n = 8.
Proapoptotic peptide and energy expenditure.
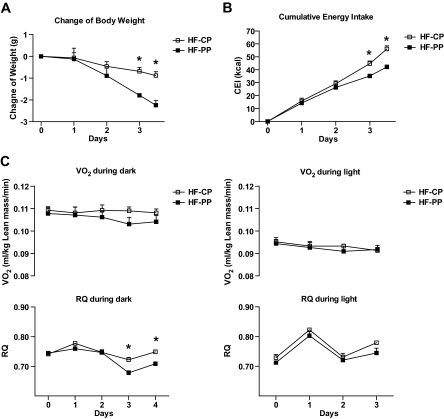
Energy expenditure in the indirect calorimeter of HF-PP mice expressed as O2 consumption adjusted for lean mass was not significantly different from that of HF-CP mice during the second week of treatment (Fig. 3B). To determine a possible effect of proapoptotic peptide on energy balance earlier in treatment, a heavier cohort of HFD mice (44.6 ± 0.6 g at the start) was acclimated to indirect calorimetry chambers before receiving the proapototic or control peptide for 4 days. Body-weight loss was significantly greater in HF-PP mice on days three and four of treatment than in HF-CP mice (Fig. 4A). Cumulative energy intake of HF-PP mice was also significantly decreased on days three and four, with no change in energy expenditure, compared with that of HF-CP mice (Fig. 4B and C). However, the nocturnal RQ ratio was significantly reduced on days three and four, consistent with the reduction in energy intake, indicating that HF-PP mice oxidized a greater proportion of fat than HF-CP mice (Fig. 4C).
FIG. 4.
Effect of 4-day treatment with proapoptotic peptide on energy balance. A: Change in body weight. B: Change in cumulative energy intake. C: Change in energy expenditure (VO2 [top panel]) and respiratory coefficient (RQ) ratio (bottom panel) at the nocturnal (left panel) or diurnal (right panel) period during the treatment after 4 days of acclimation to the indirect calorimetry chambers. All data are represented as means ± SEM. □, HF-CP (n = 4); ■, HF-PP (n = 4).
Proapoptotic peptide and adiposity.
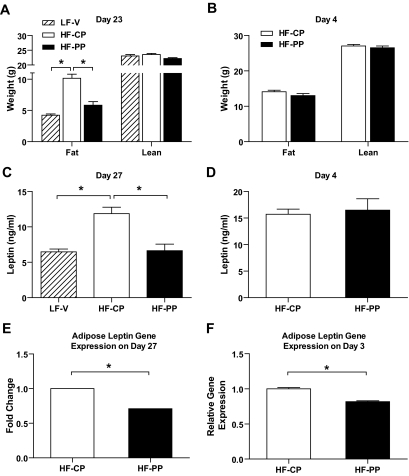
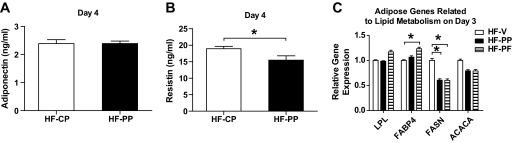
The decreased body weight was mainly attributable to loss of fat mass in the longer-term study; however, there was no significant difference of fat and lean mass in the four-day study (Fig. 5A and B). Consistent with the reduction in fat mass, serum leptin levels were significantly decreased in the longer-term study, as was leptin mRNA expression in adipose tissue by 1.3-fold as measured by Affymetrix gene chip analysis (intensity-based empirical Bayes method [0.0011]) (Fig. 5C and E). Adipose leptin mRNA expression was significantly decreased (P < 0.01) after 3 days of treatment, whereas serum leptin level was not changed in the 4-day study (Fig. 5D and F). There was no difference in serum adiponectin level and significant decrease in serum resistin level in the 4-day study, eliminating them as factors that affect food intake (20–25) (Fig. 6A and B).
FIG. 5.
Body composition was analyzed using the mouse nuclear magnetic resonance on the 23rd day of treatment in the long-term study (A) and on the 4th day of treatment in the short-term study (B). C and D: Serum leptin levels after 27 (C) and 4 (D) days of treatment with proapoptotic peptide. E and F: relative leptin mRNA expression in adipose tissue after 27 days using a microarray (E) and after 3 days of treatment with proapoptotic peptide by quantitative PCR (F). All samples were collected via cardiac puncture after a 4-h fast at the end of treatment. All data are represented as means ± SEM. LF-V, n = 8; HF-CP, n = 8; HF-PP, n = 8. *P < 0.05.
FIG. 6.
Effect of proapoptotic peptide on serum adiponectin (A) and resistin (B) levels after 4 days of treatment and expression of genes related to lipid metabolism in adipose tissue after 3 days of treatment (C). Pair feeding was performed every 12 h for 3 days. All data are represented as means ± SEM. HF-V, n = 8; HF-CP, n = 8; HF-PP, n = 8; HF-PF, n = 8. *P < 0.05.
To determine how adipose tissue lipid metabolism is changed by targeting adipose tissue vasculature, we measured gene expression levels of lipoprotein lipase (LPL), fatty acid–binding protein 4 (FABP4), fatty acid synthase (FASN), and acetyl-CoA carboxylase α (ACACA) in adipose tissue after 3 days of treatment in a separate cohort of HF-PF mice. The pair feeding was performed every 12 h to better match the pattern of caloric intake between groups. There was a significant decrease in FASN of HF-PP mice and HF-PF mice compared with that of HF-V mice and a nonsignificant trend toward a reduction in ACACA compared with HF-V mice (P = 0.06). FABP4 of HF-PF mice was significantly increased compared with that of HF-V mice, and LPL of HF-PF mice had a trend to be increased compared with that of HF-PP mice (P = 0.08), whereas there was no significant difference in LPL and FABP4 between HF-V mice and HF-PP mice (Fig. 6C). These data suggest that the changes in lipid metabolism caused by the proapoptotic peptide are not just the result of reduced food intake.
Proapoptotic peptide and hypothalamic gene expression.
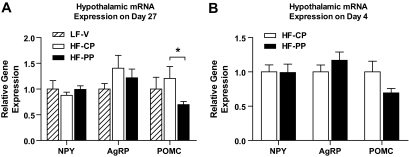
Many genes in the hypothalamus are regulated by energy balance and are thought to play a key role in regulating food intake and energy expenditure. We therefore evaluated hypothalamic neuropeptide Y (NPY), agouti-related peptide (AgRP), and proopiomelanocoritin (POMC) gene expression after 4 and 27 days of treatment with the proapoptotic peptide. POMC gene expression was significantly decreased in HF-PP mice with no change in NPY or AgRP gene expression after 27 days, although there were no overall significant differences among LF-V, HF-CP, and HF-PP mice by one-way ANOVA (Fig. 7A). Consistent with this, there was a tendency toward decreased POMC gene expression in the hypothalamus after 4 days of treatment with the proapoptotic peptide (P = 0.06) and, again, no change of NPY or AgRP gene expression (Fig. 7B).
FIG. 7.
Effect of 27-day (A) or 4-day (B) treatment with proapoptotic peptide on hypothalamic NPY, AgRP, and POMC mRNA. All data are represented as means ± SEM. LF-V, n = 8; HF-CP, n = 8; HF-PP, n = 8. *P < 0.05.
DISCUSSION
These results demonstrate that administering a peptide that produces apoptosis uniquely in the endothelium of white adipose tissue can produce profound reductions in body weight that are primarily in the form of reduced body fat (Fig. 5A). This reduction in body weight occurs through a decrease in food intake without signs of illness. In particular, mice made obese on a HFD can be returned to the body weight and nearly the body fat of mice maintained on a LFD. The ability to completely reverse diet-induced obesity is a result achieved by few other treatments (26–30) and replicates the original reports using this proapoptotic peptide (7).
In the original description of the effect of the proapoptotic peptide, the large weight loss could not be accounted for by increased energy expenditure, decreased energy intake, or a decreased ability to extract calories from food (7). While we observed no change in energy expenditure in either the short or the long term, by the second week of treatment, HF-PP mice were ingesting fewer calories than HF-CP mice (Figs. 1 and 2). In fact, most (76%) of the reduction in body mass can be accounted for by reduced energy intake over the 27-day study when the difference in ingested calories between HP-CP mice and HF-PP mice is compared with the calculated calories from the difference in body composition.
The pattern of changes in intake was distinctive. There was no reduction in food intake in the HF-PP mice during the first day of treatment. However, beginning 8 h after the second daily administration, hypophagia was apparent and lasted for the duration of that day and thereafter, and a similar pattern of hypophagia and weight loss occurred in rats. The effect of the proapoptotic peptide to reduce food intake and body weight was more potent in mice on a HFD than in those on a LFD (Fig. 2A) and was not due to a direct action in the hypothalamus (supplemental Fig. 1). These data suggest that the enlarged adipose tissue mass caused by consumption of the HFD is a primary target of the proapoptotic peptide. Interestingly, this robust decrease in intake occurred despite lower adiposity and lower circulating leptin level (Fig. 5). Low leptin levels, such as after fasting leptin deficiency or genetic lipodystrophy, are associated with increased food intake (31,32) Some data link other adipokines such as adiponectin and resistin to the regulation of food intake (20–25). However, those adipokines also do not appear to be critical to the observed effects on food intake. Thus, we propose that there is a signal or signals originating from adipose tissue vasculature, which override the effects of low-circulating leptin levels. These results are in contrast to the original report with the proapoptotic peptide, where no changes in intake were observed (7). However, we have observed this phenomenon clearly in multiple experiments and multiple species and that the failure to observe this in the original report was likely due to not measuring food intake with sufficient accuracy at the most important time points.
Whenever animals eat less food and lose body weight following administration of a novel compound, it is important to determine whether the reduced intake is secondary to visceral illness. While the overall pattern of intake elicited by the proapoptotic peptide suggests that this is unlikely, this possibility was evaluated directly by comparing the effect of the peptide to produce a CTA with that of an anorexic dose of lithium chloride. Neither the proapoptotic peptide nor the control peptide influenced preference for the flavor with which it had been paired (Fig. 3A). Combined with the present data showing that the proapoptotic peptide is less effective in lean mice, this suggests that the anorexia is not secondary to toxic effects of the treatment.
Interestingly, the effect of the proapoptotic peptide on expression of genes related to lipid metabolism in adipose tissue was different than that in pair-fed mice. Fasting decreases adipose tissue LPL in lean rats, and fasting-induced decrease in adipose LPL is blunted in obese rats (33). Adipose tissue LPL is increased in obese rats and humans, and weight reduction further increases or cannot normalize adipose tissue LPL in obese rats or humans (34–38). HF-PF mice had a tendency toward increased adipose tissue LPL expression compared with HF-PP mice. mRNA expression of FABP4 and LPL in HF-PP mice was similar to that in HF-V mice. Genes such as FASN and ACACA, which are linked to lipogenesis, decreased equivalently in HF-PP mice and HF-PF mice.
Given that reduced food intake appears responsible for most of the weight loss, we assessed the effect of the apoptotic peptide on CNS circuits that influence food ingestion. A key component of these circuits is a group of neuropeptides in the arcuate nucleus of the hypothalamus that are direct targets of leptin (3,5). There are two distinct populations of these arcuate neurons: one synthesizing the orexigenic peptides NPY and AgRP and the other synthesizing the anorexic peptide α-melanocyte–stimulating hormone from the precursor molecule POMC. Expression of NPY and AgRP is inhibited by leptin (39,40), whereas expression of POMC is increased by leptin (41–43). There was no change in either NPY or AgRP gene expression at either time point assessed. In contrast, POMC gene expression was significantly reduced chronically and there was a trend toward a decrease after just 4 days of peptide administration. Reduced POMC gene expression is likely secondary to reduced intake, body weight, and leptin and therefore cannot account for the reduced food intake. The leptin-independent action of the adipose tissue–specific angiogenesis inhibitor proapoptotic peptide is consistent with a previous finding that a systemic angiogenesis inhibitor such as TNP-470 reduces body weight and intake in ob/ob mice (8).
Obesity remains a daunting problem. The current results, however, support the notion that starving adipose tissue of its necessary vasculature results in profound reductions in body weight and body fat. Of particular interest in the present studies is that administering a peptide designed to cause apoptosis in endothelial cells uniquely in white adipose tissue results in a reduction in caloric intake. Although the mechanism by which food intake is reduced remains unclear, it occurs despite falling levels of leptin. The phenomenon extends to changes in hypothalamic gene expression that are consistent with the overall negative energy balance and cannot explain the effect of the proapoptotic peptide to reduce food intake. Consequently, these data imply the existence of a novel component of the regulation of energy balance that reflects the status of white adipose tissue vasculature and is sufficiently potent to override signals derived from low-circulating leptin levels. Understanding these novel signals may provide entirely new approaches to producing safe and efficacious weight loss.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK54080, DK56863, and DK073505.
R.J.S. has received research support and consulting fees from and has served on the speaker's bureau for Amylin Pharmaceuticals; has served on the scientific advisory board and speakers bureau for Eli Lilly; has received research support and consulting fees from and has served on the scientific advisory board for Johnson & Johnson; has served on the scientific advisory board for and has received research support from Zafgen; and has served on the scientific advisory board and speakers bureau for Merck. No other potential conflicts of interest relevant to this article were reported.
We thank Kay Ellis and David D'Alessio for measurement of leptin, Kathleen Smith and Lucas De for performing the CTA study, and Joyce Sorrell for technical assistance.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM: Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 2006; 295: 1549– 1555 [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Flegal KM: High body mass index for age among US children and adolescents, 2003–2006. JAMA 2008; 299: 2401– 2405 [DOI] [PubMed] [Google Scholar]
- 3.Seeley RJ, Woods SC: Monitoring of stored and available fuel by the CNS: implications for obesity. Nat Rev Neurosci 2003; 4: 901– 909 [DOI] [PubMed] [Google Scholar]
- 4.Spiegelman BM, Flier JS: Obesity and the regulation of energy balance. Cell 2001; 104: 531– 543 [DOI] [PubMed] [Google Scholar]
- 5.Flier JS: Obesity wars: molecular progress confronts an expanding epidemic. Cell 2004; 116: 337– 350 [DOI] [PubMed] [Google Scholar]
- 6.Brakenhielm E, Cao R, Gao B, Angelin B, Cannon B, Parini P, Cao Y: Angiogenesis inhibitor, TNP-470, prevents diet-induced and genetic obesity in mice. Circ Res 2004; 94: 1579– 1588 [DOI] [PubMed] [Google Scholar]
- 7.Kolonin MG, Saha PK, Chan L, Pasqualini R, Arap W: Reversal of obesity by targeted ablation of adipose tissue. Nat Med 2004; 10: 625– 632 [DOI] [PubMed] [Google Scholar]
- 8.Rupnick MA, Panigrahy D, Zhang CY, Dallabrida SM, Lowell BB, Langer R, Folkman MJ: Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci U S A 2002; 99: 10730– 10735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crandall DL, Hausman GJ, Kral JG: A review of the microcirculation of adipose tissue: anatomic, metabolic, and angiogenic perspectives. Microcirculation 1997; 4: 211– 232 [DOI] [PubMed] [Google Scholar]
- 10.Hausman GJ, Richardson RL: Adipose tissue angiogenesis. J Anim Sci 2004; 82: 925– 934 [DOI] [PubMed] [Google Scholar]
- 11.Woods SC, Seeley RJ, Rushing PA, D'Alessio D, Tso P: A controlled high-fat diet induces an obese syndrome in rats. J Nutr 2003; 133: 1081– 1087 [DOI] [PubMed] [Google Scholar]
- 12.Ellerby HM, Arap W, Ellerby LM, Kain R, Andrusiak R, Rio GD, Krajewski S, Lombardo CR, Rao R, Ruoslahti E, Bredesen DE, Pasqualini R: Anti-cancer activity of targeted pro-apoptotic peptides. Nat Med 1999; 5: 1032– 1038 [DOI] [PubMed] [Google Scholar]
- 13.Lachey JL, D'Alessio DA, Rinaman L, Elmquist JK, Drucker DJ, Seeley RJ: The role of central glucagon-like peptide-1 in mediating the effects of visceral illness: differential effects in rats and mice. Endocrinology 2005; 146: 458– 462 [DOI] [PubMed] [Google Scholar]
- 14.Chavez M, Kaiyala K, Madden LJ, Schwartz MW, Woods SC: Intraventricular insulin and the level of maintained body weight in rats. Behav Neurosci 1995; 109: 528– 531 [DOI] [PubMed] [Google Scholar]
- 15.Taicher GZ, Tinsley FC, Reiderman A, Heiman ML: Quantitative magnetic resonance (QMR) method for bone and whole-body-composition analysis. Anal Bioanal Chem 2003; 377: 990– 1002 [DOI] [PubMed] [Google Scholar]
- 16.Reed JA, Clegg DJ, Smith KB, Tolod-Richer EG, Matter EK, Picard LS, Seeley RJ: GM-CSF action in the CNS decreases food intake and body weight. J Clin Invest 2005; 115: 3035– 3044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ: Hypothalamic mTOR signaling regulates food intake. Science 2006; 312: 927– 930 [DOI] [PubMed] [Google Scholar]
- 18.Smyth GK: Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 2004; 3: Article3 [DOI] [PubMed] [Google Scholar]
- 19.Sartor MA, Tomlinson CR, Wesselkamper SC, Sivaganesan S, Leikauf GD, Medvedovic M: Intensity-based hierarchical Bayes method improves testing for differentially expressed genes in microarray experiments. BMC Bioinformatics 2006; 7: 538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masaki T, Chiba S, Yasuda T, Tsubone T, Kakuma T, Shimomura I, Funahashi T, Matsuzawa Y, Yoshimatsu H: Peripheral, but not central, administration of adiponectin reduces visceral adiposity and upregulates the expression of uncoupling protein in agouti yellow (Ay/a) obese mice. Diabetes 2003; 52: 2266– 2273 [DOI] [PubMed] [Google Scholar]
- 21.Qi Y, Takahashi N, Hileman SM, Patel HR, Berg AH, Pajvani UB, Scherer PE, Ahima RS: Adiponectin acts in the brain to decrease body weight. Nat Med 2004; 10: 524– 529 [DOI] [PubMed] [Google Scholar]
- 22.Kubota N, Yano W, Kubota T, Yamauchi T, Itoh S, Kumagai H, Kozono H, Takamoto I, Okamoto S, Shiuchi T, Suzuki R, Satoh H, Tsuchida A, Moroi M, Sugi K, Noda T, Ebinuma H, Ueta Y, Kondo T, Araki E, Ezaki O, Nagai R, Tobe K, Terauchi Y, Ueki K, Minokoshi Y, Kadowaki T: Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab 2007; 6: 55– 68 [DOI] [PubMed] [Google Scholar]
- 23.Shklyaev S, Aslanidi G, Tennant M, Prima V, Kohlbrenner E, Kroutov V, Campbell-Thompson M, Crawford J, Shek EW, Scarpace PJ, Zolotukhin S: Sustained peripheral expression of transgene adiponectin offsets the development of diet-induced obesity in rats. Proc Natl Acad Sci U S A 2003; 100: 14217– 14222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vazquez MJ, Gonzalez CR, Varela L, Lage R, Tovar S, Sangiao-Alvarellos S, Williams LM, Vidal-Puig A, Nogueiras R, Lopez M, Dieguez C: Central resistin regulates hypothalamic and peripheral lipid metabolism in a nutritional-dependent fashion. Endocrinology 2008; 149: 4534– 4543 [DOI] [PubMed] [Google Scholar]
- 25.Tovar S, Nogueiras R, Tung LY, Castaneda TR, Vazquez MJ, Morris A, Williams LM, Dickson SL, Dieguez C: Central administration of resistin promotes short-term satiety in rats. Eur J Endocrinol 2005; 153: R1– R5 [DOI] [PubMed] [Google Scholar]
- 26.Pierroz DD, Ziotopoulou M, Ungsunan L, Moschos S, Flier JS, Mantzoros CS: Effects of acute and chronic administration of the melanocortin agonist MTII in mice with diet-induced obesity. Diabetes 2002; 51: 1337– 1345 [DOI] [PubMed] [Google Scholar]
- 27.Hamilton BS, Doods HN: Chronic application of MTII in a rat model of obesity results in sustained weight loss. Obes Res 2002; 10: 182– 187 [DOI] [PubMed] [Google Scholar]
- 28.Van Heek M, Compton DS, France CF, Tedesco RP, Fawzi AB, Graziano MP, Sybertz EJ, Strader CD, Davis HR, Jr: Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J Clin Invest 1997; 99: 385– 390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Widdowson PS, Upton R, Buckingham R, Arch J, Williams G: Inhibition of food response to intracerebroventricular injection of leptin is attenuated in rats with diet-induced obesity. Diabetes 1997; 46: 1782– 1785 [DOI] [PubMed] [Google Scholar]
- 30.Levin BE, Dunn-Meynell AA: Sibutramine alters the central mechanisms regulating the defended body weight in diet-induced obese rats. Am J Physiol Regul Integr Comp Physiol 2000; 279: R2222– R2228 [DOI] [PubMed] [Google Scholar]
- 31.Moitra J, Mason MM, Olive M, Krylov D, Gavrilova O, Marcus-Samuels B, Feigenbaum L, Lee E, Aoyama T, Eckhaus M, Reitman ML, Vinson C: Life without white fat: a transgenic mouse. Genes Dev 1998; 12: 3168– 3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ebihara K, Ogawa Y, Masuzaki H, Shintani M, Miyanaga F, Aizawa-Abe M, Hayashi T, Hosoda K, Inoue G, Yoshimasa Y, Gavrilova O, Reitman ML, Nakao K: Transgenic overexpression of leptin rescues insulin resistance and diabetes in a mouse model of lipoatrophic diabetes. Diabetes 2001; 50: 1440– 1448 [DOI] [PubMed] [Google Scholar]
- 33.Llado I, Pons A, Palou A: Effects of fasting on lipoprotein lipase activity in different depots of white and brown adipose tissues in diet-induced overweight rats. J Nutr Biochem 1999; 10: 609– 614 [DOI] [PubMed] [Google Scholar]
- 34.Lemonnier D, de Gasquet P, Mackay S, Planche E, Alexiu A, Rosselin G, Loiseau A: Different levels of food restriction have opposite effects on adipocyte cellularity and lipoprotein-lipase activity in obese rats. Diabete Metab 1989; 15: 394– 402 [PubMed] [Google Scholar]
- 35.Eckel RH, Yost TJ: Weight reduction increases adipose tissue lipoprotein lipase responsiveness in obese women. J Clin Invest 1987; 80: 992– 997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bessesen DH, Robertson AD, Eckel RH: Weight reduction increases adipose but decreases cardiac LPL in reduced-obese Zucker rats. Am J Physiol 1991; 261: E246– E251 [DOI] [PubMed] [Google Scholar]
- 37.Schwartz RS, Brunzell JD: Increase of adipose tissue lipoprotein lipase activity with weight loss. J Clin Invest 1981; 67: 1425– 1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kern PA, Ong JM, Saffari B, Carty J: The effects of weight loss on the activity and expression of adipose-tissue lipoprotein lipase in very obese humans. N Engl J Med 1990; 322: 1053– 1059 [DOI] [PubMed] [Google Scholar]
- 39.Schwartz MW, Baskin DG, Bukowski TR, Kuijper JL, Foster D, Lasser G, Prunkard DE, Porte D, Jr, Woods SC, Seeley RJ, Weigle DS: Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide Y gene expression in ob/ob mice. Diabetes 1996; 45: 531– 535 [DOI] [PubMed] [Google Scholar]
- 40.Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG: Identification of targets of leptin action in rat hypothalamus. J Clin Invest 1996; 98: 1101– 1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwartz MW, Seeley RJ, Weigle DS, Burn P, Campfield LA, Baskin DG: Leptin increases hypothalamic proopiomelanocoritin (POMC) mRNA expression in the rostral arcuate nucleus. Diabetes 1997; 46: 2119– 2123 [DOI] [PubMed] [Google Scholar]
- 42.Cheung CC, Clifton DK, Steiner RA: Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology 1997; 138: 4489– 4492 [DOI] [PubMed] [Google Scholar]
- 43.Mizuno T, Kleopoulos S, Bergen H, Roberts J, Priest C, Mobbs C: Hypothalamic pro-opiomelanocortin mRNA is reduced by fasting and in ob/ob and db/db mice, but is stimulated by leptin. Diabetes 1998; 47: 294– 297 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.