Abstract
OBJECTIVE
The beneficial effects of the inactivation of endocannabinoid system (ECS) by administration of antagonists of the cannabinoid receptor (CB) 1 on several pathological features associated with obesity is well demonstrated, but the relative contribution of central versus peripheral mechanisms is unclear.
We examined the impact of CB1 antagonism on liver and adipose tissue lipid metabolism in a mouse model of diet-induced obesity.
RESEARCH DESIGN AND METHODS
Mice were fed either with a standard diet or a high-sucrose high-fat (HSHF) diet for 19 weeks and then treated with the CB1-specific antagonist SR141716 (10 mg · kg−1 · day−1) for 6 weeks.
RESULTS
Treatment with SR141716 reduced fat mass, insulin levels, and liver triglycerides primarily increased by HSHF feeding. Serum adiponectin levels were restored after being reduced in HSHF mice. Gene expression of scavenger receptor class B type I and hepatic lipase was induced by CB1 blockade and associated with an increase in HDL-cholesteryl ether uptake. Concomitantly, the expression of CB1, which was strongly increased in the liver and adipose tissue of HSHF mice, was totally normalized by the treatment. Interestingly, in visceral but not subcutaneous fat, genes involved in transport, synthesis, oxidation, and release of fatty acids were upregulated by HSHF feeding, while this effect was counteracted by CB1 antagonism.
CONCLUSIONS
A reduction in the CB1-mediated ECS activity in visceral fat is associated with a normalization of adipocyte metabolism, which may be a determining factor in the reversion of liver steatosis induced by treatment with SR141716.
Obesity results from an imbalance between energy intake and expenditure and is characterized by increased body weight and abnormal development of adipose tissue with excessive fat storage (1). Recently, evidence has accumulated for the overactivity of the endocannabinoid system (ECS) during conditions of unbalanced energy homeostasis (2). The ECS consists of the cannabinoid receptors (CBs), their endogenous ligands (the endocannabinoids), and the enzyme proteins catalyzing the endocannabinoid formation and degradation (3). Activation of central CB1 receptors clearly promotes food intake and weight gain (4–6). Accordingly, pharmacological antagonism of CB1 has been shown to improve several pathological features associated with obesity, including overweight, hyperinsulinemia, insulin resistance, hyperglycemia, and dyslipidemia in obese rodents (7–9) and humans (10,11).
Even if the reduction in food intake induced by central CB1 blockade may be the main initial cause of body weight loss and associated beneficial effects, several data collected from animal and human studies indicate that peripheral CB1 may also directly control lipid metabolism (12–14). Thus, an activation of ECS has been recently reported in peripheral tissues of animal models of obesity (15,16) and associated with visceral fat obesity in humans (17,18). Consequently, it has been proposed that the long-term effects of CB1 antagonism are resolved by stimulation of energy expenditure and by peripheral effects related to adipose tissue, liver, skeletal muscle, and pancreas physiology (19–21).
In the present work, we tested the effects of CB1 antagonism on the regulation of the liver and adipose tissue lipid metabolism in a mouse model of diet-induced obesity. We first examined the global impact of CB1 antagonism on plasma parameters and liver steatosis, which were primarily altered by long-term feeding of a high-sucrose high-fat (HSHF) diet. Next, we examined whether CB1 inactivation was associated with biochemical and molecular alterations in the liver and adipose tissue (distinguishing visceral and subcutaneous fat depots) that could account for an improvement of liver lipid metabolism.
RESEARCH DESIGN AND METHODS
Official French regulations (no. 87848) for the use and care of laboratory animals were followed throughout the experimental period. The experimental protocol was approved by the local ethic committee for animal experimentation (no. BX0622). Four-week-old C57BL/6 male mice (Elevage Janvier, Le Genest Saint Isle, France) were housed in individual plastic cages and adapted to a standard diet (AO4; UAR, Epinay-sur-Orge, France) for 1 week. A series of mice was maintained on the standard diet (CON group; n = 5), while another series was subjected to an HSHF diet containing casein 20%, corn starch 13%, sucrose 29.3%, cellulose 5%, maltodextrin 2.2%, lard 20%, soya oil 2.5%, mineral 205B SAFE 7%, vitamin 200 SAFE 1% (ref. 235HF SAFE; Augy, France). After 19 weeks, HSHF animals that were not both overweight and hyperinsulinemic were excluded from the study. Selected mice were maintained on an HSHF diet and received orally either 10 mg · kg−1 · day−1 of SR141716 (HSHF+SR series; n = 14) or vehicle (HSHF series; n = 10). The CB1-specific antagonist SR141716 (Rimonabant) was supplied by sanofi-aventis (Paris, France). Animals had free access to fresh food and water throughout the experimental period. Mice were food deprived 4 h before anesthesia with ketamine/xylazine (7.5 mg · 1 mg−1 · 100 g body wt−1) and tissue handling. Epididymal and inguinal fat were surgically removed as representatives of visceral and subcutaneous fat, respectively (22). Tissue samples were frozen in liquid nitrogen pending further analyses.
Serum and tissue parameters.
Serum parameters were determined using commercial kits (glucose RTU, TG PAP150, and cholesterol RTU from BioMérieux [Marcy l'Etoile, France] for glucose, triglycerides, and cholesterol assay, respectively; nonesterified fatty acid C from Wako Pure Chemical Industries [Richmond, VA] for free fatty acid [FFA] assay; and mouse insulin and adiponectine enzyme-linked immunosorbent assay kits from AbCys [Paris, France]). Liver malonyl-CoA concentration was determined by high-peformance liquid chromatography as previously described (23). Liver total lipids were extracted according to the method of Folch et al. (24). After mixing thoroughly, 1.0 ml of organic phase was transferred to a clean tube containing 1 ml of 1% Triton X-100 in chloroform and dried using nitrogen. The residue was resolubilized in 0.25 ml distilled water and used for the determination of triglycerides and cholesterol as in serum. For determination of adiponectin content in adipose tissue, samples were homogenized in 10 volume of PBS. After centrifugation (10 min at 12,000g, 4°C), the supernatants were carefully collected through the fat cake, diluted to 1/40,000 in PBS, and used for adiponectine measurements as in serum.
Fatty acid oxidation and apolipoprotein A and B secretion.
Freshly removed livers from five HSHF and five HSHF+SR mice were sliced using a Brendel/Vitron slicer (Tucson, AZ), and thin slices were used to measure [1-14C] palmitic acid oxidation and apolipoprotein (apo) A and B secretion as previously described (25).
[3H]-cholesteryl ether-HDL uptake.
Liver slices, prepared as described above, were also intended for HDL uptake. First, an HDL fraction was isolated from human plasma by sequential flotation ultracentrifugations (26). HDL was radiolabeled with [3H]-cholesteryl ether (CE) combining [3H]cholesteryl hedacyl ether with l-a-phosphatidylcholine and butylhydroxytoluene in a 500:1:6 molar ratio and sonicating to form liposomes. HDL-[3H]CE was obtained by addition of liposomes to the HDL fraction in presence of lipoprotein-free plasma, as a source of CE transfer protein, after an overnight incubation at 37°C under light agitation. Labeled HDL was separated from remaining liposomes by an other sequential flotation ultracentrifugation and washed twice in a solution of potassium bromide (density 1.21). Finally, HDL-[3H]CE was aliquoted and stored at −80°C until used. Measurement of the uptake was carried out at 37°C by incubating two liver slices in 1 ml of William's medium E containing 40 μg proteins (0.3 mCi of HDL-[3H]CE) under slight agitation. After 3 h, slices were removed from medium, washed three times, and homogenized in 400 ml PBS with a mini-beadbeater (BioSpec Products, Bartlesville, OK). The radioactivity recovered in the homogenate was finally estimated, representing the amount of HDL uptaken by the liver cells.
Gene expression.
Total mRNA from liver and adipose tissue were extracted with Tri-Reagent (Euromedex, Souffelweyersheim, France) and reverse transcripted using the Iscript cDNA kit (Bio-Rad, Marnes-La-Coquette, France). Real-time PCR was performed as described previously (27) using a Bio-Rad iCycler iQ. The sequences of forward and reverse primers used for the amplification are presented in the online supplemental Table 1 (available at http://diabetes.diabetesjournals.org/cgi/content/full/db09-1482/DC1).
Statistical analysis.
Results are expressed as means ± SE. Data were analyzed statistically using the Kruskal-Wallis nonparametric test. Differences were considered significant at P < 0.05.
RESULTS
Body and organ weights.
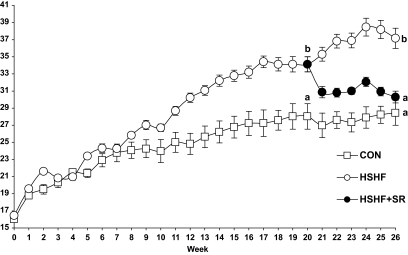
Baseline body weights of the three groups of mice were comparable. After 19 weeks, body weights of HSHF animals were significantly higher than that of control mice (Fig. 1). From week 20 to 26, the body weight of HSHF+SR mice rapidly decreased to become similar to that of CON mice at week 26 (Fig. 1). Consistent with that, the masses of both epididymal and inguinal fat pads differed in the order HSHF > HSHF+SR > CON mice (Table 1). Concomitantly, the liver weight was greater in HSHF and less in HSHF+SR than in CON mice (Table 1).
FIG. 1.
Evolution of body weight during the induction period of obesity and during the treatment with CB1 antagonist. C57BL/6J mice were fed an HSHF diet (42.3% carbohydrates, 22.5% lipids) for 25 weeks receiving orally during the last 6 weeks either 10 mg · kg−1 · day−1 of the CB1-specific antagonist SR141716 (HSHF+SR; n = 14) or the vehicle (HSHF; n = 10). In parallel, a series of mice was maintained on a control diet (CON; n = 5). Results are expressed as means ± SE. Results of statistical analysis were indicated at weeks 20 and 26, values with different superscript letters (a, b, c) are statistically different at P < 0.05.
TABLE 1.
Effects of CB1 antagonism on body composition, serum, and liver parameters
| CON group | HSHF group | HSHF+SR group | |
|---|---|---|---|
| n | 5 | 10 | 14 |
| Organ weight | |||
| Liver (g) | 1.38 ± 0.03* | 1.49 ± 0.06† | 1.21 ± 0.03‡ |
| Epididymal fat (g) | 0.67 ± 0.14* | 2.04 ± 0.16† | 0.98 ± 0.07‡ |
| Inguinal fat (g) | 0.31 ± 0.06* | 1.10 ± 0.10† | 0.41 ± 0.03* |
| Serum | |||
| Glucose (mg/ml) | 2.79 ± 0.16 | 2.72 ± 0.21 | 2.53 ± 0.08 |
| Insulin (ng/ml) | 0.40 ± 0.03* | 0.59 ± 0.07† | 0.44 ± 0.03* |
| Adiponectin (μg/ml) | 63.05 ± 3.24* | 42.31 ± 7.21† | 59.82 ± 3.95* |
| FFAs (mmol/l) | 0.30 ± 0.04* | 0.54 ± 0.06† | 0.43 ± 0.03‡ |
| Triglycerides (mg/ml) | 0.74 ± 0.06* | 0.36 ± 0.03† | 0.31 ± 0.02† |
| Total cholesterol (mg/ml) | 1.09 ± 0.04* | 2.05 ± 0.07† | 1.87 ± 0.06† |
| Liver | |||
| Glycogen (mg/g) | 66.7 ± 8.1* | 49.2 ± 4.2† | 37.4 ± 3.0‡ |
| Triglycerides (mg/g) | 24.4 ± 4.5* | 118.7 ± 17.6† | 46.4 ± 4.9‡ |
| Total cholesterol (mg/g) | 16.07 ± 0.94 | 17.98 ± 1.63 | 15.72 ± 0.7 |
| Malonyl CoA (nmol/g) | 1.67 ± 0.29* | 3.43 ± 0.52† | 2.41 ± 0.18‡ |
Data are means ± SE. Mice were fed an HSHF diet for 25 weeks, receiving during the last 6 weeks either 10 mg · kg−1 · day−1 of the CB1-specific antagonist SR141716 (HSHF+SR) or the vehicle (HSHF diet). In parallel, a series of mice was maintained on a control diet (CON group). Mice were food deprived 4 h before tissue handling.
*,†,‡Statistically different at P < 0.05.
Serum and liver parameters.
At the end of the experiment, serum glucose concentration of HSHF mice was not different from CON mice despite an increase in insulin levels, indicating that the HSHF mice were in the early stage of developing insulin resistance (Table 1). Likewise, FFAs and total cholesterol levels were higher in HSHF than in CON mice. Surprisingly, HSHF mice had 50% lower plasma triglyceride levels than CON mice, suggesting an increase in triglyceride clearance by the liver and adipose tissue. In parallel with fat mass expansion, serum adiponectin levels were less in mice fed with the HSHF diet than in control mice. Interestingly, insulin and adiponectin levels in HSHF+SR mice were not different from the control group (Table 1). Serum FFA concentration was less in HSHF+SR than in HSHF mice, while glucose, triglycerides, and cholesterol levels did not differ between these groups. In the liver, administration of HSHF diet induced a steatosis with a fivefold increase in triglyceride content, while total cholesterol content remained unchanged. The malonyl-CoA content, a potent inhibitor of fatty acid β-oxidation, was also markedly increased. Meanwhile, glycogen stores were less in HSHF than in CON mice, reflecting a stimulation of glycogenolysis. Interestingly, triglyceride and malonyl-CoA accumulations were partially reversed and glycogen concentration further decreased by CB1 antagonism (Table 1).
Adiponectin content in visceral and subcutaneous fat.
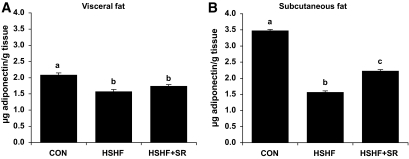
To explore whether the variations of serum adiponectin levels could correspond to a different production of the adipokine by visceral and subcutaneous fats, we determined the adiponectin content in epididymal and inguinal fat, respectively. The adiponectin content was less in both fat depots of HSHF than CON mice with a more marked effect in inguinal fat. Remarkably, after treatment with CB1 antagonist, the adiponectin content significantly increased in inguinal fat only (Fig. 2).
FIG. 2.
Effect of CB1 antagonism on adiponectin concentration in visceral (A) and subcutaneous (B) fat. Mice were fed an HSHF diet for 25 weeks receiving during the last 6 weeks either 10 mg · kg−1 · day−1 of the CB1-specific antagonist SR141716 (HSHF+SR; n = 14) or the vehicle (HSHF; n = 10). In parallel, a series of mice was maintained on a control diet (CON; n = 5). Adiponectin concentration was measured in adipose tissue homogenates prepared as described in research design and methods. Results are expressed as means ± SE. Values with different superscript letters (a, b, c) are statistically different at P < 0.05.
Fatty acid oxidation and parameters related to lipoprotein metabolism.
The ability of SR141716 to partially reverse HSHF-induced liver steatosis prompted us to verify whether this effect was related to an increased capacity of hepatocytes to β-oxidize fatty acid or to produce lipids and lipoproteins. Palmitic acid β-oxidation rates measured in liver explants did not differ between HSHF and HSHF+SR mice (Table 2). ApoB secretion was less with HSHF+SR than HSHF explants, while apoA secretion did not differ between the two groups. This model was also used to determine whether the blockade of CB1 affected HDL-CE uptake. The recovery of HDL-CE was greater in the liver explants from HSHF+SR than HSHF mice, suggesting interesting metabolic adaptations that could affect lipid and lipoprotein metabolism (Table 2).
TABLE 2.
Effects of CB1 antagonism on fatty acid oxidation and parameters related to lipoprotein metabolism in liver explants
| HSHF | HSHF+SR | |
|---|---|---|
| Palmitic acid oxidation (nmol · h−1 · g protein−1) | 23.0 ± 13 | 22.0 ± 2.4 |
| ApoB secretion (μg · h−1 · g protein−1) | 155 ± 17* | 123 ± 8† |
| ApoA secretion (μg · h−1 · g protein−1) | 141 ± 2 | 140 ± 3 |
| HDL-CE uptake ([3H]-CE dpm · h−1 · μg protein−1) | 430 ± 119* | 1,075 ± 139† |
Data are means ± SE. Thin liver slices (∼200 μm) were obtained from mice fed an HSHF diet and treated either with 10 mg · kg−1 · day−1 of SR141716 (HSHF+SR; n = 5) or vehicle (HSHF; n = 5). For fatty acid oxidation and apo secretion, slices were incubated at 37°C in oxygenated William's medium E supplemented with l-carnitine (0.5 mmol/l) in the presence of 0.2 mmol/l of [1-14C] palmitic acid (55.5 GBq/mol) complexed to albumin (fatty acid/BSA molar ratio 2.5/1). After 4 h of incubation, slices were rinsed with cold PBS and immediately submitted to lipid extraction for counting of labelled CO2 and acid-soluble products, while the incubation medium was used for determination of apoB and apoA secreted. Measurement of HDL uptake was carried out at 37°C by incubating liver slices with [3H]-CE-HDL under slight agitation for 3 h. Then, slices were washed and homogenized in PBS. Radioactivity recovered in the homogenate represented the amount of HDL uptaken by the liver cells.
*,†Statistically different at P < 0.05.
Gene expression in liver and adipose tissue
Liver.
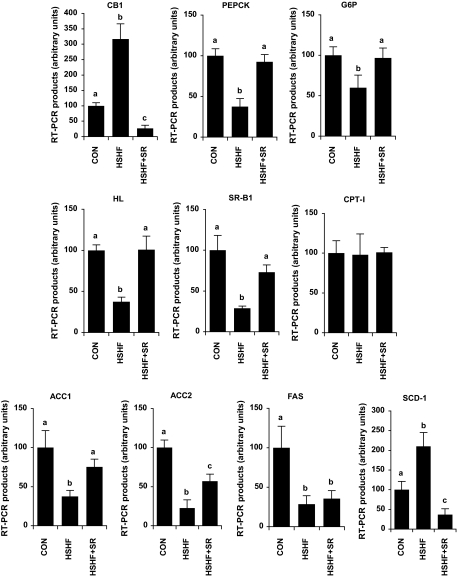
We first tested the impact of the diet and of the treatment with SR141716 on CB1 gene expression as an indicator of ECS activity (Fig. 3). CB1 mRNA was the greatest in HSHF mice and less in HSHF+SR than in CON mice, reflecting a stimulation of ECS in our mice model of obesity and an effective inhibition of this pathway after CB1 antagonist treatment. Then, mRNA levels of phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6P) were measured as an indicator of liver insulin resistance. The inverse relationship existing between insulin levels and expression levels of this two enzymes suggested that insulin still had the ability to control their transcription. In line with a possible impact of CB1 antagonism on lipoprotein and cholesterol metabolism, we measured the mRNA expression of hepatic lipase and scavenger receptor class B type I (SR-BI) in the liver. The expression levels of these two genes were the lowest in HSHF mice and did not differ from CON in HSHF+SR mice.
FIG. 3.
Effect of CB1 antagonism on the mRNA expression of CB1 and genes involved in carbohydrate and lipid metabolism in the liver. Mice were fed an HSHF diet for 25 weeks receiving during the last 6 weeks either 10 mg · kg−1 · day−1 of the CB1-specific antagonist SR141716 (HSHF+SR) or the vehicle (HSHF). In parallel, a series of mice was maintained on a control diet (CON). For each gene, a standard curve was established from four cDNA dilutions (1/10–1/10,000) and used to determine the relative gene expression after normalization with a geometric average of 18S and TATA box binding protein expression. Results are expressed as means ± SE (n = 5 per group). Values with different superscript letters (a, b, c) are statistically different at P < 0.05.
Besides, mRNA levels of the lipogenic enzymes acetyl-CoA carboxylase (ACC) 1 and 2, and fatty acid synthase (FAS) were all markedly less in HSHF than in CON mice. Interestingly, the expression of both ACC isoforms was higher in the liver of HSHF+SR than HSHF mice, while antagonism of CB1 had no effect on FAS expression. The expression of stearoyl-CoA desaturase (SCD)-1, which converts saturated fatty acids into monounsaturated fatty acids, was higher in HSHF and less in HSHF+SR than in CON mice, suggesting that the inhibition of ECS could have limited the high production of monounsaturated fatty acids primarily induced by HSHF feeding. Besides, neither the diet nor the treatment modified the transcript levels of carnitine palmitoyltransferase (CPT)-I, the rate-limiting enzyme of long-chain fatty acid β-oxidation.
Adipose tissue.
CB1 expression was also induced in both visceral and subcutaneous adipose tissue of HSHF compared with CON mice (Fig. 4A and B). In obese mice, SR141716 treatment was efficient in reducing CB1 expression in the two tissues. The expression of tumor necrosis factor (TNF)-α, an inflammatory cytokine associated with insulin resistance, was higher in both visceral and subcutaneous fat of HSHF than CON mice. It is noteworthy that the TNF-α induction was dramatically higher in visceral than in subcutaneous fat (24-fold vs. 4.8-fold, respectively). Likewise, in HSHF+SR mice, TNF-α mRNA levels were not different from CON mice in subcutaneous fat and were only half that of CON mice in visceral fat. In addition, compared with CON feeding, HSHF feeding gave rise to a fourfold increase in the expression of the γ2 isoform of the peroxisome proliferator–activated receptor (PPAR) γ2 in visceral fat, while this induction was only twofold in HSHF+SR mice (Fig. 4A). In subcutaneous adipocytes, PPARγ2 expression was surprisingly the highest in HSHF+SR mice, while the two other groups did not differ each other (Fig. 4B). Concomitantly, the expression of genes related to uptake (fatty acid translocase [FAT]/CD36), lipolysis (hormone-sensitive lipase [HSL]), β-oxidation (CPT-I), and lipogenesis (FAS and ACC1) was strongly higher in visceral fat of HSHF than CON mice (Fig. 4A). Conversely, in subcutaneous deposits of HSHF mice, the expression of these genes was either less than CON mice (FAS and ACC1) or unchanged (FAT/CD36, HSL, and CPT-I), suggesting different sensitivity and function of subcutaneous versus visceral adipocytes in conditions of insulin resistance (Fig. 4B). Interestingly, in visceral fat, all genes that were upregulated by HSHF feeding were significantly downregulated after treatment with CB1 antagonist except FAT/CD36 (Fig. 4A).
FIG. 4.
Effect of CB1 antagonism treatment on the mRNA expression of CB1 and genes involved in adipocyte metabolism in epididymal (A) and inguinal (B) fat. Mice were fed an HSHF diet for 25 weeks receiving during the last 6 weeks either 10 mg · kg−1 · day−1 of the CB1-specific antagonist SR141716 (HSHF+SR) or the vehicle (HSHF). In parallel, a series of mice was maintained on a control diet (CON). For each gene, a standard curve was established from four cDNA dilutions (1/10–1/10,000) and used to determine the relative gene expression after normalization with a geometric average of 18S and TATA box binding protein expression. Results are expressed as means ± SE (n = 5 per group). Values with different superscript letters (a, b, c) are statistically different at P < 0.05.
DISCUSSION
In this study, the effects of CB1 antagonism were tested on mice previously exposed to a long-term HSHF diet (19 weeks) with a lipid content and fatty acid composition nearly similar to the human Western diet. Administration of HSHF diet–induced obesity, liver fat accumulation and peripheral insulin resistance as indicated by the elevation of plasma insulin and FFA levels. A significant number of experimental reports describe beneficial effects of CB1 antagonism on insulin resistance and fatty liver in mice and humans, and these data strongly indicate that the ECS has a major role in the regulation of lipid metabolism not only at the central but also at the peripheral level (rev. in 28). From our mouse model of obesity, we provided further evidence that CB1 blockade causes peripheral metabolic and molecular changes in liver and adipose tissue associated with the reversion of fatty liver. We particularly showed that the lipid metabolism of visceral and subcutaneous adipocytes was differently regulated in response to diet-induced obesity and to CB1 antagonism.
Effects of CB1 antagonism on liver lipid metabolism.
Our findings clearly indicate that the strong upregulation of liver CB1 primarily induced by an HSHF diet is fully reversed by the treatment with SR141716, suggesting that the metabolic improvements observed could be mediated by the blockade of these receptors. This concept is supported by other studies using CB1−/− mice, demonstrating that ECS overactivity occurs in the liver of animals fed a high-fat diet and that hepatic CB1 are required for the development of diet induced steatosis (14,29). In line with this, the normalization of liver parameters related to carbohydrate and lipid metabolism such as PEPCK, G6P, ACC, and SCD-1 mRNA levels after treatment with CB1 antagonist strongly suggests that these adaptations correspond to a normalization of liver insulin responsiveness as evoked in muscles of rimonabant-treated ob/ob mice (21). The activation of hepatic CB1 receptors has been recently associated with an increase in de novo lipogenesis, suggesting that this metabolic pathway participates to steatosis development in conditions of ECS overactivity (30). Unlike this finding, we observed no stimulation of the liver expression of ACC and FAS in HSHF animals, which were quite hyperinsulinemic. In the works of Osei-Hyiaman et al. (30), the stimulation of ECS consisted of an acute injection of CB1 agonist to control animals, while in our study, ECS activation was induced for a much longer period using an HSHF diet. Since the diet contained high proportions of saturated fatty acids, it can be hypothesized that the provision of a diet rich in preformed saturated fatty acids led to the reduced expression of mRNA for lipogenic genes. The inhibitory effect of palmitoyl-CoA on ACC demonstrated by Ogiwara et al. (31) supports this concept. The induction of the SCD-1 gene and the increase in monounsaturated fatty acid content in the liver of HSHF mice (data not shown) indicate that saturated fatty acid delivery to the liver was increased. Indeed, in HSHF mice, the liver steatosis appears to be mainly due to an enhanced delivery of FFAs to the liver rather to an increase in de novo lipogenesis. Aside from direct effects on the liver, steatosis might have also been reduced indirectly by the limitation of the influx of fatty acids originating from adipose tissue. The gene expression profile of visceral adipose tissue is consistent with an hyperactivation of lipid metabolism as suggested by the strong upregulation of genes involved in transport, synthesis, oxidation, and release of fatty acids. Altogether, these data suggest that the reversion of liver steatosis induced by the treatment with CB1 antagonist was associated with an improvement of adipose tissue metabolism.
In line with an improvement of cardiovascular risk in type 2 diabetic patients treated with rimonabant (11,32), our findings support the possibility that CB1 antagonism is associated with an alteration of liver HDL catabolism. Previous studies (33) showed that overexpression of SR-BI in the liver, while reducing plasma HDL cholesterol levels, reduced atherosclerosis in mice, suggesting that hepatic SR-BI overexpression may promote reverse cholesterol transport. Accordingly, the increase in SR-BI and hepatic lipase expression induced by CB1 antagonism may be associated with a modification of HDL size and kinetics (34) and thereby explain the increase in HDL-CE uptake observed in our model of liver slices. Additional studies are currently under investigation to clearly identify the direct effects of CB1 antagonism on liver lipid metabolism.
Effects of CB1 antagonism on visceral fat.
Recently, evidence has accumulated from animal and human studies (17,35–37) that obesity is also associated with overactivation of ECS in visceral fat. Concordant findings from this study and from literature support the view that CB1 blockade exerts specific effects on visceral fat metabolism that could be associated with the reduction of liver triglyceride content. Hence, the coordinated upregulation of genes acting at different levels of the lipogenic pathway and that of the nuclear activator PPARγ strongly suggested that an HSHF diet favored triglyceride synthesis and thereby formation of enlarged visceral fat deposits. Adipocyte hypertrophy in obesity is consecutive to a deficit in adipogenesis (38), and the limitation of fat stores would promote ectopic lipid deposition in liver and skeletal muscle, leading to decreased insulin action in these tissues (39). Remarkably, the fact that CB1 antagonism totally or partially normalized the expression levels of lipogenic genes in adipocytes may limit the accumulation of intracellular lipid droplets and give rise to smaller cells and reduction of visceral fat mass as also suggested in (40). The decrease in HSL expression consecutive to CB1 antagonism is of particular importance since excessive HSL-dependent fat lipolysis leads to an increased release of FFAs into the circulation, which in turn has deleterious effects on insulin sensitivity (41).
It has been suggested recently that obesity-induced inflammation of adipose tissue may directly activate ECS (42). This could result in a protective response against inflammation as described in colon (43). From our research, it appears that ECS activation induced by an HSHF diet is also associated with an increase in TNF-α in adipose tissue. This interaction between inflammation and ECS needs to be further explored to determine whether inflammation causes ECS activation or vice versa. However, the concentration-dependent stimulation of lipolysis by TNF-α demonstrated in rodent and human fat cells is considered to be an important pathogenetic factor in the development of insulin resistance and type 2 diabetes (44). Therefore, it is reasonable to suggest that the reduction of TNF-α expression in visceral adipose tissue of HSHF mice treated with CB1 antagonist is linked to the normalization of adipocyte metabolism and to underlying effects on lipid and carbohydrate metabolism.
Effects of an HSHF diet and CB1 antagonism in subcutaneous versus visceral fat.
This study also provides new information regarding the impact of HSHF diet and subsequent CB1 antagonism on the regulation of lipid metabolism in subcutaneous compared with visceral adipose tissue. Taken together, our findings give molecular evidence that 1) an HSHF diet causes deleterious effects in visceral adipose tissue that were not observed in subcutaneous fat and 2) CB1 blockade is able to reverse the molecular changes primarily induced by an HSHF diet in visceral adipose tissue and to exert specific effects on subcutaneous adipocytes. These discrepancies in gene regulation between visceral and subcutaneous adipocytes in response to high-fat diet and CB1 antagonism are consistent with a different degree of ECS activation in these tissues. This consideration is supported by several recent findings (36,37,45,46) indicating differences in endocannabinoid levels between epididymal and subcutaneous fat. In addition, the overexpression of PPARγ2, FAS, and ACC gene in epididymal fat of obese mice is also in favor of the activation of ECS in this tissue since it has been reported that CB1 activation stimulates lipogenesis by increasing PPARγ and lipogenic enzyme expression in adipocytes and liver (30,36,47). Collectively, data suggest that ECS is more activated in epididymal than in subcutaneous fat in our mice model of obesity and it can be predicted that antagonism of CB1 was more effective in the tissue presenting elevated levels of endocannabinoids (36,45).
Interestingly, an HSHF diet or CB1 antagonist treatment induced nearly similar effects on the amounts of epididymal and subcutaneous fat, suggesting that molecular and metabolic differences observed are not solely related to the modification of the fat depot size. In contrast, the induction of TNF-α expression by an HSHF diet was far less important in subcutaneous than in visceral fat, and the treatment of obese mice with CB1 antagonist induced the complete normalization of TNF-α expression only in subcutaneous fat, whereas inflammation remains high in visceral adipocytes. Concomitantly, the normalization of TNF-α mRNA levels in subcutaneous fat is associated with an increase in adiponectin content in this tissue. An inverse relationship between circulating adiponectin and TNF-α has already been evoked (48), suggesting that adipose tissue inflammation could alter adiponectin production. The increased expression of PPARγ2 induced by CB1 antagonism in subcutaneous fat may also correspond to an activation of adipocyte differentiation (49) and thereby of adiponectin secretion (50). In addition, our findings regarding the adiponectin content in visceral and subcutaneous fat suggest that the normalization of adiponectin plasma levels induced by CB1 antagonism may be exclusively associated with an increased production of this adipokine by subcutaneous adipocytes.
In conclusion, this study indicates that treating obese mice with a CB1 antagonist exerts beneficial effects on liver steatosis and various lipid parameters, providing supportive evidence that the hyperactivity of ECS associated with obesity was adjusted by the antagonism of CB1. This notion is further supported by data from an ongoing study indicating that CB1 antagonism exerts no effects on body weight, fat mass, and liver triglyceride content in control mice (T.J., L.Dj., L.De., J.G., B.V., and P.D.; personal data). Our findings are also consistent with a contribution of peripheral CB1 and suggest different degrees of ECS activity in visceral and subcutaneous fat. In this way, the improvement of visceral adipose tissue metabolism appears to be a determining factor for the normalization of plasma parameters and the reversion of liver steatosis. Therefore, future studies should investigate the direct effects of CB1 antagonism on the liver to precise the respective implication of ECS and products secreted by adipose tissue in the regulation of lipid metabolism.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from sanofi-aventis and the Région Bourgogne.
No other potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Trayhurn P, Beattie JH: Physiological role of adipose tissue: white adipose tissue as an endocrine and secretory organ. Proc Nutr Soc 2001; 60: 329– 339 [DOI] [PubMed] [Google Scholar]
- 2.Matias I, Di Marzo V: Endocannabinoids and the control of energy balance. Trends Endocrinol Metab 2007; 18: 27– 37 [DOI] [PubMed] [Google Scholar]
- 3.Di Marzo V, Matias I: Endocannabinoid control of food intake and energy balance. Nat Neurosci 2005; 8: 585– 589 [DOI] [PubMed] [Google Scholar]
- 4.Di Marzo V, Goparaju SK, Wang L, Liu J, Batkai S, Jarai Z, Fezza F, Miura GI, Palmiter RD, Sugiura T, Kunos G: Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature 2001; 410: 822– 825 [DOI] [PubMed] [Google Scholar]
- 5.Jamshidi N, Taylor DA: Anandamide administration into the ventromedial hypothalamus stimulates appetite in rats. Br J Pharmacol 2001; 134: 1151– 1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams CM, Kirkham TC: Anandamide induces overeating: mediation by central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 1999; 143: 315– 317 [DOI] [PubMed] [Google Scholar]
- 7.Gary-Bobo M, Elachouri G, Gallas JF, Janiak P, Marini P, Ravinet-Trillou C, Chabbert M, Cruccioli N, Pfersdorff C, Roque C, Arnone M, Croci T, Soubrie P, Oury-Donat F, Maffrand JP, Scatton B, Lacheretz F, Le Fur G, Herbert JM, Bensaid M: Rimonabant reduces obesity-associated hepatic steatosis and features of metabolic syndrome in obese Zucker fa/fa rats. Hepatology 2007; 46: 122– 129 [DOI] [PubMed] [Google Scholar]
- 8.Poirier B, Bidouard JP, Cadrouvele C, Marniquet X, Staels B, O'Connor SE, Janiak P, Herbert JM: The anti-obesity effect of rimonabant is associated with an improved serum lipid profile. Diabetes Obes Metab 2005; 7: 65– 72 [DOI] [PubMed] [Google Scholar]
- 9.Ravinet Trillou C, Arnone M, Delgorge C, Gonalons N, Keane P, Maffrand JP, Soubrie P: Anti-obesity effect of SR141716, a CB1 receptor antagonist, in diet-induced obese mice. Am J Physiol Regul Integr Comp Physiol 2003; 284: R345– R353 [DOI] [PubMed] [Google Scholar]
- 10.Despres JP, Golay A, Sjostrom L: Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med 2005; 353: 2121– 2134 [DOI] [PubMed] [Google Scholar]
- 11.Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S: Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet 2005; 365: 1389– 1397 [DOI] [PubMed] [Google Scholar]
- 12.Engeli S, Jordan J: The endocannabinoid system: body weight and metabolic regulation. Clin Cornerstone 2006; 8( Suppl. 4): S24– S35 [DOI] [PubMed] [Google Scholar]
- 13.Nogueiras R, Veyrat-Durebex C, Suchanek PM, Klein M, Tschop J, Caldwell C, Woods SC, Wittmann G, Watanabe M, Liposits Z, Fekete C, Reizes O, Rohner-Jeanrenaud F, Tschop MH: Peripheral, but not central, CB1 antagonism provides food intake-independent metabolic benefits in diet-induced obese rats. Diabetes 2008; 57: 2977– 2991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osei-Hyiaman D, Liu J, Zhou L, Godlewski G, Harvey-White J, Jeong WI, Batkai S, Marsicano G, Lutz B, Buettner C, Kunos G: Hepatic CB1 receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J Clin Invest 2008; 118: 3160– 3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bensaid M, Gary-Bobo M, Esclangon A, Maffrand JP, Le Fur G, Oury-Donat F, Soubrie P: The cannabinoid CB1 receptor antagonist SR141716 increases Acrp30 mRNA expression in adipose tissue of obese fa/fa rats and in cultured adipocyte cells. Mol Pharmacol 2003; 63: 908– 914 [DOI] [PubMed] [Google Scholar]
- 16.Matias I, Petrosino S, Racioppi A, Capasso R, Izzo AA, Di Marzo V: Dysregulation of peripheral endocannabinoid levels in hyperglycemia and obesity: effect of high fat diets. Mol Cell Endocrinol 2008; 286: S66– S78 [DOI] [PubMed] [Google Scholar]
- 17.Cote M, Matias I, Lemieux I, Petrosino S, Almeras N, Despres JP, Di Marzo V: Circulating endocannabinoid levels, abdominal adiposity and related cardiometabolic risk factors in obese men. Int J Obes (Lond) 2007; 31: 692– 699 [DOI] [PubMed] [Google Scholar]
- 18.Di Marzo V, Cote M, Matias I, Lemieux I, Arsenault BJ, Cartier A, Piscitelli F, Petrosino S, Almeras N, Despres JP: Changes in plasma endocannabinoid levels in viscerally obese men following a 1 year lifestyle modification programme and waist circumference reduction: associations with changes in metabolic risk factors. Diabetologia 2009; 52: 213– 217 [DOI] [PubMed] [Google Scholar]
- 19.Duvivier VF, Delafoy-Plasse L, Delion V, Lechevalier P, Le Bail JC, Guillot E, Pruniaux MP, Galzin AM: Beneficial effect of a chronic treatment with rimonabant on pancreatic function and beta-cell morphology in Zucker Fatty rats. Eur J Pharmacol 2009; 616: 314– 320 [DOI] [PubMed] [Google Scholar]
- 20.Getty-Kaushik L, Richard AM, Deeney JT, Krawczyk S, Shirihai O, Corkey BE: The CB1 antagonist rimonabant decreases insulin hypersecretion in rat pancreatic islets. Obesity (Silver Spring) 2009; 17: 1856– 1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu YL, Connoley IP, Wilson CA, Stock MJ: Effects of the cannabinoid CB1 receptor antagonist SR141716 on oxygen consumption and soleus muscle glucose uptake in Lep(ob)/Lep(ob) mice. Int J Obes (Lond) 2005; 29: 183– 187 [DOI] [PubMed] [Google Scholar]
- 22.Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE: Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest 2007; 117: 2621– 2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Degrace P, Demizieux L, Gresti J, Chardigny JM, Sebedio JL, Clouet P: Hepatic steatosis is not due to impaired fatty acid oxidation capacities in C57BL/6J mice fed the conjugated trans-10,cis-12-isomer of linoleic acid. J Nutr 2004; 134: 861– 867 [DOI] [PubMed] [Google Scholar]
- 24.Folch J, Lees M, Sloane Stanley GH: A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 1957; 226: 497– 509 [PubMed] [Google Scholar]
- 25.Jourdan T, Djaouti L, Demizieux L, Gresti J, Verges B, Degrace P: Liver carbohydrate and lipid metabolism of insulin-deficient mice is altered by trans-10, cis-12 conjugated linoleic acid. J Nutr 2009 [DOI] [PubMed] [Google Scholar]
- 26.Persegol L, Verges B, Foissac M, Gambert P, Duvillard L: Inability of HDL from type 2 diabetic patients to counteract the inhibitory effect of oxidised LDL on endothelium-dependent vasorelaxation. Diabetologia 2006; 49: 1380– 1386 [DOI] [PubMed] [Google Scholar]
- 27.Degrace P, Moindrot B, Mohamed I, Gresti J, Du ZY, Chardigny JM, Sebedio JL, Clouet P: Upregulation of liver VLDL receptor and FAT/CD36 expression in LDLR-/- apoB100/100 mice fed trans-10,cis-12 conjugated linoleic acid. J Lipid Res 2006; 47: 2647– 2655 [DOI] [PubMed] [Google Scholar]
- 28.Vettor R, Pagano C: The role of the endocannabinoid system in lipogenesis and fatty acid metabolism. Best Pract Res Clin Endocrinol Metab 2009; 23: 51– 63 [DOI] [PubMed] [Google Scholar]
- 29.Ravinet Trillou C, Delgorge C, Menet C, Arnone M, Soubrie P: CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int J Obes Relat Metab Disord 2004; 28: 640– 648 [DOI] [PubMed] [Google Scholar]
- 30.Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Batkai S, Harvey-White J, Mackie K, Offertaler L, Wang L, Kunos G: Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest 2005; 115: 1298– 1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogiwara H, Tanabe T, Nikawa J, Numa S: Inhibition of rat-liver acetyl-coenzyme-A carboxylase by palmitoyl-coenzyme A: formation of equimolar enzyme-inhibitor complex. Eur J Biochem 1978; 89: 33– 41 [DOI] [PubMed] [Google Scholar]
- 32.Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J: Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA 2006; 295: 761– 775 [DOI] [PubMed] [Google Scholar]
- 33.Kozarsky KF, Donahee MH, Glick JM, Krieger M, Rader DJ: Gene transfer and hepatic overexpression of the HDL receptor SR-BI reduces atherosclerosis in the cholesterol-fed LDL receptor-deficient mouse. Arterioscler Thromb Vasc Biol 2000; 20: 721– 727 [DOI] [PubMed] [Google Scholar]
- 34.Nijstad N, Wiersma H, Gautier T, van der Giet M, Maugeais C, Tietge UJ: Scavenger receptor BI-mediated selective uptake is required for the remodeling of high density lipoprotein by endothelial lipase. J Biol Chem 2009; 284: 6093– 6100 [DOI] [PubMed] [Google Scholar]
- 35.Bluher M, Engeli S, Kloting N, Berndt J, Fasshauer M, Batkai S, Pacher P, Schon MR, Jordan J, Stumvoll M: Dysregulation of the peripheral and adipose tissue endocannabinoid system in human abdominal obesity. Diabetes 2006; 55: 3053– 3060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matias I, Gonthier MP, Orlando P, Martiadis V, De Petrocellis L, Cervino C, Petrosino S, Hoareau L, Festy F, Pasquali R, Roche R, Maj M, Pagotto U, Monteleone P, Di Marzo V: Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. J Clin Endocrinol Metab 2006; 91: 3171– 3180 [DOI] [PubMed] [Google Scholar]
- 37.Starowicz KM, Cristino L, Matias I, Capasso R, Racioppi A, Izzo AA, Di Marzo V: Endocannabinoid dysregulation in the pancreas and adipose tissue of mice fed with a high-fat diet. Obesity (Silver Spring) 2008; 16: 553– 565 [DOI] [PubMed] [Google Scholar]
- 38.Nadler ST, Stoehr JP, Schueler KL, Tanimoto G, Yandell BS, Attie AD: The expression of adipogenic genes is decreased in obesity and diabetes mellitus. Proc Natl Acad Sci U S A 2000; 97: 11371– 11376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heilbronn L, Smith SR, Ravussin E: Failure of fat cell proliferation, mitochondrial function and fat oxidation results in ectopic fat storage, insulin resistance and type II diabetes mellitus. Int J Obes Relat Metab Disord 2004; 28( Suppl. 4): S12– S21 [DOI] [PubMed] [Google Scholar]
- 40.Jbilo O, Ravinet-Trillou C, Arnone M, Buisson I, Bribes E, Peleraux A, Penarier G, Soubrie P, Le Fur G, Galiegue S, Casellas P: The CB1 receptor antagonist rimonabant reverses the diet-induced obesity phenotype through the regulation of lipolysis and energy balance. Faseb J 2005; 19: 1567– 1569 [DOI] [PubMed] [Google Scholar]
- 41.Arner P: Insulin resistance in type 2 diabetes: role of fatty acids. Diabetes Metab Res Rev 2002; 18( Suppl. 2): S5– S9 [DOI] [PubMed] [Google Scholar]
- 42.Kempf K, Hector J, Strate T, Schwarzloh B, Rose B, Herder C, Martin S, Algenstaedt P: Immune-mediated activation of the endocannabinoid system in visceral adipose tissue in obesity. Horm Metab Res 2007; 39: 596– 600 [DOI] [PubMed] [Google Scholar]
- 43.Massa F, Marsicano G, Hermann H, Cannich A, Monory K, Cravatt BF, Ferri GL, Sibaev A, Storr M, Lutz B: The endogenous cannabinoid system protects against colonic inflammation. J Clin Invest 2004; 113: 1202– 1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruan H, Lodish HF: Insulin resistance in adipose tissue: direct and indirect effects of tumor necrosis factor-alpha. Cytokine Growth Factor Rev 2003; 14: 447– 455 [DOI] [PubMed] [Google Scholar]
- 45.D'Eon TM, Pierce KA, Roix JJ, Tyler A, Chen H, Teixeira SR: The role of adipocyte insulin resistance in the pathogenesis of obesity-related elevations in endocannabinoids. Diabetes 2008; 57: 1262– 1268 [DOI] [PubMed] [Google Scholar]
- 46.Izzo AA, Piscitelli F, Capasso R, Aviello G, Romano B, Borrelli F, Petrosino S, Di Marzo V: Peripheral endocannabinoid dysregulation in obesity: relation to intestinal motility and energy processing induced by food deprivation and re-feeding. Br J Pharmacol 2009; 158: 451– 461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pagano C, Pilon C, Calcagno A, Urbanet R, Rossato M, Milan G, Bianchi K, Rizzuto R, Bernante P, Federspil G, Vettor R: The endogenous cannabinoid system stimulates glucose uptake in human fat cells via phosphatidylinositol 3-kinase and calcium-dependent mechanisms. J Clin Endocrinol Metab 2007; 92: 4810– 4819 [DOI] [PubMed] [Google Scholar]
- 48.Lau DC, Dhillon B, Yan H, Szmitko PE, Verma S: Adipokines: molecular links between obesity and atheroslcerosis. Am J Physiol Heart Circ Physiol 2005; 288: H2031– H2041 [DOI] [PubMed] [Google Scholar]
- 49.Schoonjans K, Staels B, Auwerx J: The peroxisome proliferator activated receptors (PPARS) and their effects on lipid metabolism and adipocyte differentiation. Biochim Biophys Acta 1996; 1302: 93– 109 [DOI] [PubMed] [Google Scholar]
- 50.Gustafson B, Jack MM, Cushman SW, Smith U: Adiponectin gene activation by thiazolidinediones requires PPAR gamma 2, but not C/EBP alpha-evidence for differential regulation of the aP2 and adiponectin genes. Biochem Biophys Res Commun 2003; 308: 933– 939 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.