Abstract
OBJECTIVE
To investigate if recurrent autoimmunity explained hyperglycemia and C-peptide loss in three immunosuppressed simultaneous pancreas-kidney (SPK) transplant recipients.
RESEARCH DESIGN AND METHODS
We monitored autoantibodies and autoreactive T-cells (using tetramers) and performed biopsy. The function of autoreactive T-cells was studied with in vitro and in vivo assays.
RESULTS
Autoantibodies were present pretransplant and persisted on follow-up in one patient. They appeared years after transplantation but before the development of hyperglycemia in the remaining patients. Pancreas transplant biopsies were taken within ∼1 year from hyperglycemia recurrence and revealed β-cell loss and insulitis. We studied autoreactive T-cells from the time of biopsy and repeatedly demonstrated their presence on further follow-up, together with autoantibodies. Treatment with T-cell–directed therapies (thymoglobulin and daclizumab, all patients), alone or with the addition of B-cell–directed therapy (rituximab, two patients), nonspecifically depleted T-cells and was associated with C-peptide secretion for >1 year. Autoreactive T-cells with the same autoantigen specificity and conserved T-cell receptor later reappeared with further C-peptide loss over the next 2 years. Purified autoreactive CD4 T-cells from two patients were cotransplanted with HLA-mismatched human islets into immunodeficient mice. Grafts showed β-cell loss in mice receiving autoreactive T-cells but not control T-cells.
CONCLUSIONS
We demonstrate the cardinal features of recurrent autoimmunity in three such patients, including the reappearance of CD4 T-cells capable of mediating β-cell destruction. Markers of autoimmunity can help diagnose this underappreciated cause of graft loss. Immune monitoring during therapy showed that autoimmunity was not resolved by the immunosuppressive agents used.
Type 1 diabetes is an autoimmune disease characterized by the lymphocytic infiltration of the pancreatic islets (insulitis), β-cell destruction, and loss of insulin secretion (1). Autoreactive CD4 and CD8 T-cells and autoantibodies to islet cell autoantigens are detected in patients and pre-diabetic subjects, often preceding diabetes onset by months to years. Insulin, GAD (GAD, 65-kDa isoform), the tyrosine-like phosphatase protein IA-2, the islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP), and the recently identified cation efflux transporter ZnT8 are well characterized and commonly targeted autoantigens (2–8).
Simultaneous pancreas-kidney (SPK) transplantation from deceased donors restores insulin secretion in patients and corrects end-stage renal disease (9). Immunological failures occur in a minority of transplant recipients and are usually categorized as chronic rejection. Another possible cause of immunological failure is recurrence of type 1 diabetes. This was initially reported a few weeks after transplantation in recipients of the tail of the pancreas from living-related HLA-identical twins or siblings who, because of HLA matching, received either no or reduced immunosuppression (10–13). However, diabetes recurrence was <10% in a large series of recipients of deceased donor grafts given immunosuppression sufficient to prevent rejection (14). Further studies associated islet cell autoantibodies with graft failure (15–19) but lacked biopsy data, and rejection was not excluded. Two SPK recipients had partial evidence for diabetes recurrence (20), including limited biopsy data showing selective β-cell loss and/or insulitis and limited autoantibody data (20). None of these studies assessed autoantigen-specific T-cells in the context of graft loss. Islet autoimmunity is considered rare and is not routinely monitored in SPK recipients. Thus, recurrence of type 1 diabetes in SPK recipients remains incompletely characterized.
We investigated whether recurrent islet autoimmunity explained the hyperglycemia and loss of insulin secretion observed in three immunosuppressed SPK recipients in the absence of rejection. The immunological assessment included both retrospective and prospective testing for autoantibodies and prospective testing for autoantigen-specific T-cells. Monitoring was continued on extended follow-up after patients were diagnosed with recurrence of type 1 diabetes and received additional immunotherapy to antagonize the autoimmune process. We also characterized the functional features of the autoreactive T-cells detected in these patients in the context of recurring diabetes, using both in vitro and in vivo experimental assays to test the pathogenic effects of the autoreactive T-cells.
RESEARCH DESIGN AND METHODS
The three SPK recipients studied (two males, one female) had type 1 diabetes for many years and no C-peptide response to a Sustacal test before transplantation. Pancreas transplants were bladder drained (exocrine) with systemic venous effluent, so that urine amylase reflects exocrine pancreas transplant function. The patients were identified after the occurrence of hyperglycemia, years after transplantation, in the absence of rejection and changes in pancreas transplant exocrine function. All three recipients received immunosuppression with tacrolimus, mycophenolate mofetil, and steroids (for maintenance). They all reversed diabetes and normalized kidney function after SPK transplantation. Patients signed informed consent to participate in research to characterize their diabetes recurrence. The study was approved by the University of Miami Institutional Review Board (protocol no. 20053039).
Analysis of pancreas transplant biopsies.
Transplant biopsies were performed based on clinical indication and with written informed consent. Formalin-fixed, paraffin-embedded sections were stained with hematoxylin and eosin (H&E) and with specific antibodies to insulin, glucagon, CD3, CD4, CD20, CD8, CD68, Fas, active caspase-3, human monocyte chemoattractant protein-1 (MCP-1), chemokine (C-X-C motif) ligand 10 (CXCL10 or IP-10), interferon-α (IFN-α), the proliferation marker Ki-67, and the enterovirus protein VP-1 using immunohistochemistry and immunofluorescence, as detailed in the supplemental methods section in the online appendix, available at http://diabetes.diabetesjournals.org/cgi/content/full/db09-0498/DC1.
Monitoring allograft function.
C-peptide was assessed by radioimmunoassay (Diagnostic Products, Deerfield, IL; intra- and interassay coefficients of variation 4.8 and 8.1%, respectively). The normal range is 0.8–4.0 ng/ml. C-peptide levels are higher in SPK recipients because of the systemic venous drainage of the transplanted pancreas. The exocrine function of the transplanted pancreas was monitored by measuring urine amylase levels. Kidney allograft function was assessed by monitoring creatinine levels.
Autoantibody monitoring.
Autoantibodies to GAD, IA-2, and ZnT8 were measured using radioimmunoassays validated in the proficiency workshops of the Immunology of Diabetes Society and Centers for Disease Control and Prevention (21). Autoantibody levels are expressed as the ratio of the autoantibody index levels of the patient over the cutoff index of each assay. A ratio >1 denotes a positive result. Autoantibodies were measured retrospectively in stored samples collected before the occurrence of diabetes symptoms, after which autoantibodies were measured prospectively.
In vitro T-cell studies.
The prospective evaluation of circulating autoreactive T-cells began after the onset of hyperglycemia. There were no frozen cells for retrospective testing. We used tetramer-based assays to analyze autoreactive T-cells with defined autoantigen specificity (22,23). Tetramers are fluorescent-labeled, chimeric molecules consisting of four HLA-DR molecules loaded with autoantigenic peptide. The HLA-DR specificity is matched to patients' HLA-DR type, and tetramer-peptide complexes bind to the T-cell receptor (TCR) of autoreactive T-cells, allowing the enumeration of tetramer-stained cells by flow cytometry. Culture and antigen stimulation is required to expand the antigen-responsive populations to detectable levels (∼0.1–2%), given their extremely low frequency in the circulation (1:40,000–1:100,000). The following peptides were used: 1) the modified GAD 555–567 peptide (557i; NFIRMVISNPAAT); 2) the hemagglutinin (HA) 306–318 peptide (PKYVKQNTLKLAT), a positive control peptide from the influenza hemagglutinin; and 3) the OspA 161–175 peptide (VLKSYVLEGTLTAEK), derived from the causative agent of Lyme disease. Responses to this antigen are uncommon. After 12–14 days in culture, cells were stained with specific tetramers and anti-CD4 and CD25 antibodies and analyzed by flow cytometry. The antigens were chosen based on the availability of HLA class II tetramers that matched the HLA types of our patients and on the previously reported association of T-cells reacting against these epitopes in patients with type 1 diabetes and pre-diabetic subjects (7,8,22,23). Patient 2 was tested for IGRP-reactive CD8 T-cells using a class I HLA-A2 (A*0201) pentamer, since she carried HLA class II genes for which there were no relevant tetramers. Further methodological details are provided in supplemental methods section.
In vivo assessment of the pathogenicity of GAD-specific, autoreactive CD4 T-cells.
With approval of the Animal Care and Use Committee of the University of Miami, islet transplantation was performed in athymic, immunodeficient mice (FoxN1 null; Harlan Laboratories), which do not reject human islet grafts, using a previously reported procedure (24). After tetramer staining, GAD-autoreactive CD4 T-cells were purified by fluorescence-activated cell sorting, selecting the cells that were positive for CD4 and the tetramer. Control T-cells were incubated with the HA or OspA peptides, depending on HLA type and tetramer availability. The sorted cells were directly cotransplanted with human islets under the kidney capsule using a polyethylene catheter and precision syringe (Hamilton, Reno, NV). For mice rendered diabetic with streptozotocin (200 mg/kg i.v.), post-transplantation monitoring included assessment of nonfasting blood glucose levels. Nephrectomy of the graft-bearing kidney was performed in animals achieving normoglycemia to observe a prompt return to hyperglycemia and exclude the effects of residual function of the native pancreas. Mice were killed 1–2 weeks after transplantation, and the grafts were examined for islet damage and β-cell loss. Formalin-fixed paraffin-embedded sections of the grafts were stained with H&E or with antibodies to insulin, glucagon, CD3, CD68, Fas, caspase 3, MCP-1, IP-10, and IFN-α by immunohistochemistry and immunofluorescence (see supplemental methods).
RESULTS
Recurrence of type 1 diabetes in SPK recipients, in the absence of rejection.
Figures 1–3 depict the clinical history of the SPK recipients. All reversed diabetes after transplantation. Insulin secretion became impaired and hyperglycemia requiring insulin therapy ensued 5 (patient 1), 9 (patient 2), and 5 (patient 3) years after transplantation, respectively. They maintained normal exocrine pancreas graft function (secretion of urine amylase) and normal kidney graft function throughout the follow-up, even beyond the time of diabetes recurrence. Thus, the patients had no clinical signs of rejection. They also lacked donor-specific HLA antibodies at the time of diabetes recurrence and on further follow-up (supplemental Tables S3–S5). Patient 1 eventually developed donor-specific antibodies about 5 years after diabetes recurrence, while patient 3 developed donor-specific antibodies in connection with the rejection of his second pancreas transplant. Thus, there was no clinical or laboratory evidence of rejection in relation to the development of recurrent diabetes. The selective loss of insulin secretion in the absence of clinical signs of allorejection led to the suspicion of recurrence of type 1 diabetes.
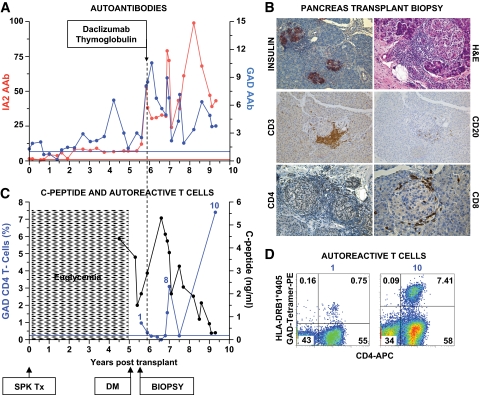
FIG. 1.
Clinical course, autoimmunity assessment, and biopsy in patient 1. Patient 1 was a 41-year-old Caucasian male [HLA A2/A3, B57/B60, DR4 (DRB1*0405)/DR6] who developed type 1 diabetes at age 7 years. He received an SPK transplant from an HLA A2/A30, B41/B60, DR4/DRX donor at age 32 years. The transplant reversed diabetes, but the patient returned to insulin dependence 5 years later, while kidney and exocrine pancreas allografts had normal function. A: Autoantibody levels before transplant and on follow-up. The patient had GAD and IA-2 autoantibodies before transplantation, which persisted despite immunosuppression, and titers increased on follow-up. Color-matched, horizontal lines represent the cutoff level for each autoantibody. For all autoantibodies, a value >1 denotes a positive result. B: Pancreas transplant biopsy stained as labeled, obtained ∼6 months after the recurrence of hyperglycemia. Insulitis and β-cell loss are shown. C: Serum C-peptide levels and % of GAD tetramer–positive T-cells in the CD4 T-cell population from the time of hyperglycemia recurrence. C-peptide was still detectable at diagnosis, confirming the function of residual β-cells observed at biopsy. Autoreactive T-cells were detected at the time of biopsy, ∼6 months after the recurrence of hyperglycemia on two samples, and again at several time points ∼1 year after treatment. The horizontal blue line represents the cutoff of the tetramer assay (0.25%). D: Flow cytometry plots demonstrating GAD-autoreactive CD4 T-cells. The numbers above the plots identify the same sample in C. Tetramer staining with irrelevant peptide was <0.1% (not shown). DM, diabetes; Tx, treatment. (A high-quality digital representation of this figure is available in the online issue.)
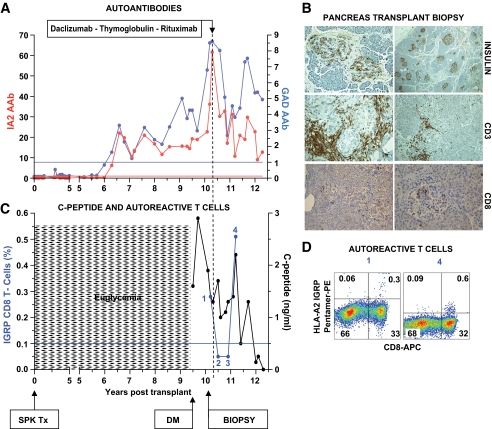
FIG. 2.
Clinical course, autoimmunity assessment, and biopsy in patient 2. Patient 2 is a Caucasian female (HLA A2/A24, B44/B56, DR5/DR9) who developed type 1 diabetes at age 8 years. She received an SPK transplant from an HLA A2/A3, B7/B14, DR7/DR9 donor at age 30 years. Her pancreas transplant successfully reversed diabetes. After approximately 9 years, the patient developed hyperglycemia requiring insulin therapy, while the function of the kidney and exocrine pancreas allografts remained unchanged. A: Autoantibody levels before transplant and on follow-up. The patient converted to GAD and IA-2 autoantibody positivity 6 years after transplantation. Hyperglycemia ensued 3.5 years after autoantibody conversion. B: Pancreas transplant biopsy stained as labeled, obtained ∼7 months after the recurrence of hyperglycemia. There was evidence for insulitis and β-cell loss. C: C-peptide levels from the time of hyperglycemia recurrence and % of IGRP tetramer–positive T-cells in the CD8 T-cell population. The horizontal blue line represents the cutoff of the T-cell assay (0.1%). Percentage of cells plotted is the specific staining value shown in D minus the background staining with control peptide. Circulating CD8 T-cells reacting against IGRP were found in a sample obtained at the time of biopsy and again ∼1 year after treatment. D: Flow cytometry plots showing IGRP-specific autoreactive CD8 T-cells. Staining with tetramers loaded with a control peptide yielded 0.1% background staining levels, gating on PBMC (not shown). The numbers above the plots identify the IGRP T-cell measurements in C, thus corresponding to the samples measured closest to the onset of hyperglycemia and over 1 year after treatment. DM, diabetes; Tx, treatment. (A high-quality digital representation of this figure is available in the online issue.)
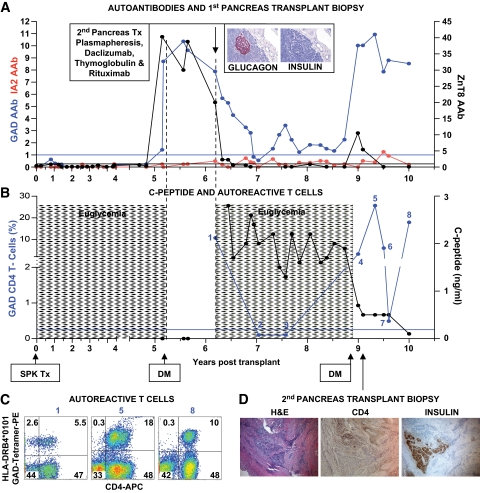
FIG. 3.
Clinical course, autoimmunity assessment, and biopsy in patient 3. Patient 3 is a 38-year-old Caucasian male [HLA A26/A30, B38/B58, DR3/DR4 (DRB1*0402)] who developed type 1 diabetes at age 12 years. He received an SPK transplant from an HLA-A23/A33, B7/B52, DR2/DR10 donor at age 27 years. The pancreas transplant successfully reversed diabetes. Five years later, the patient developed hyperglycemia requiring insulin therapy with unchanged function of the kidney and exocrine pancreas allografts. A: Autoantibody levels before transplant and on follow-up. Color-matched blue and black horizontal lines represent cutoffs for GAD/IA-2 and ZnT8 autoantibodies, respectively. The patient had been autoantibody negative before transplant and for almost 5 years on follow-up, but converted to GAD and ZnT8 autoantibody positivity about 3 months before the recurrence of hyperglycemia. At the time, there was a sharp rise in ZnT8 autoantibodies, shortly thereafter followed by a similar rise in GAD autoantibody levels, peaking at levels that were 40-fold and 10-fold higher than the upper limit of normal, respectively. Inset: Hormone stains in the first pancreas transplant biopsy obtained at retransplantation demonstrate β-cell loss. B: Serum C-peptide levels and % of GAD tetramer–positive T-cells in the CD4 T-cell population from the time of hyperglycemia recurrence. Patient 3 had no residual C-peptide secretion in the fasting state and no response to a Sustacal meal test (not shown) at the onset of hyperglycemia. C-peptide secretion was restored by retransplantation but was lost again after rejection of the second pancreas transplant. GAD-specific autoreactive CD4 T-cells were first studied in the sample obtained before the immunosuppression required for the second transplant. Autoreactive T-cells became undetectable after immunosuppression, but eventually rebounded and were detected on multiple occasions. The horizontal blue line represents the cutoff of the tetramer assay (0.25%). C: Flow cytometry plots demonstrating strong responses of GAD autoreactive, CD4 T-cells. Numbers above the plots correspond to those in B. Tetramer staining with irrelevant peptide was <0.1% (not shown). D: Biopsy of the second pancreas graft showing rejection. CD4 infiltrates are seen near residual insulin-stained areas. DM, diabetes; Tx, treatment. (A high-quality digital representation of this figure is available in the online issue.)
We performed retrospective autoantibody testing. In patient 1, GAD and IA-2 autoantibodies were present before transplantation (time 0), persisted despite immunosuppression, and titers increased on follow-up (Fig. 1A). Patient 2 converted to GAD and IA-2 autoantibody positivity 6 years after transplantation (Fig. 2A). ZnT8 autoantibodies were never detected in patients 1 and 2 (not shown). Patient 3 had been autoantibody-negative before transplant and for almost 5 more years and converted to GAD and ZnT8 autoantibody positivity ∼3 months before the recurrence of hyperglycemia (Fig. 3A). At that time, there was a sharp rise in ZnT8 autoantibodies, closely followed by a similar rise of GAD autoantibodies. Insulin autoantibodies were also detected during that period, before reinstitution of insulin therapy (not shown).
All patients underwent a pancreas transplant biopsy within a year from the recurrence of hyperglycemia. There was no evidence of rejection in either pancreas or kidney transplants. However, there was β-cell loss and insulitis, varying from severe to minimal. Patients 1 and 2 (Figs. 1B and 2B) had peri-insulitis to severe insulitis affecting many islets with infiltrating T-cells (CD3, CD4, CD8) and B-cells (CD20) detected by immunostaining. Many islets no longer stained for insulin and many more appeared damaged with reduced insulin content. Yet several islets appeared healthy with normal proportions of β-cells. In contrast, patient 3 had minimal insulitis, but there was dramatic loss of β-cells (Fig. 3A, inset), suggesting that the insulitis process had mostly run its course. In all patients, infiltrates consisted mostly of CD8 T-cells, whereas CD4 T-cells appeared much less frequent. B-cells were less represented than T-cells. Additional biopsy data are reported in the supplemental data, and Figs. S5–S17, S18–S27, and S28–S35 for patients 1–3, respectively. As we previously reported (25), biopsies of these patients demonstrated ductal cells expressing insulin (supplemental Figs. S17, S27, and S34), rarely expressing the proliferation marker Ki-67 (Fig. S35), suggesting a potential mechanism of pancreas remodeling and perhaps β-cell regeneration. Ki-67 expression was not observed in residual β-cells within the islets (not shown) in the biopsy material available. We detected VP-1 protein in the islets of patient 2 (Fig. S26), which was colocalized with insulin by confocal microscopy and indicated β-cell infection with Coxsackie B virus, which has been associated with type 1 diabetes (26,27). We did not detect VP-1 expression in the islets of the other two patients and in biopsies from three additional SPK recipients who were normoglycemic and autoantibody negative; two other normoglycemic and autoantibody-negative SPK recipients expressed VP-1 in either β-cells or α-cells (not shown).
Prospective testing for autoreactive T-cells began at the time of biopsy for all patients. In patients 1 and 3, GAD-autoreactive CD4 T-cells were detected from peripheral blood samples using HLA class II tetramer-based assays: these were restricted by HLA-DR4 (DRB1*0405) (patient 1, Fig. 1C and D) and by HLA-DRB4*0101 (patient 3, Fig. 3B and C, who also had DRB1*0402-restricted GAD-autoreactive CD4 T-cells, not shown). Autoreactive T-cells were detected on multiple occasions in both patients. In patient 2, we detected IGRP-specific autoreactive CD8 T-cells using an HLA-A2 (A*0201) class I pentamer from a blood sample obtained at the time of biopsy (Fig. 2C and D).
Autoreactive T-cells are temporarily and nonspecifically inhibited by T-cell– and B-cell–directed immunosuppression, and their reappearance is followed by further loss of insulin secretion.
In patient 1, detectable C-peptide levels (Fig. 1C) confirmed the function of the residual β-cells identified at biopsy (Fig. 1B). To salvage the residual β-cells, the patient received daclizumab (1 mg/kg, ×2, 2 weeks apart) and thymoglobulin (1 mg · kg−1 · day−1, ×5 days). After treatment, GAD-autoreactive CD4 T-cells became undetectable (Fig. 1C), similar to T-cells responding to a control antigen (not shown), consistent with nonspecific immunosuppression. Autoantibody levels fluctuated but persisted on follow-up (Fig. 1A). While the patient remained insulin-dependent throughout, C-peptide levels increased and remained detectable for 1 year after treatment. C-peptide secretion declined over the subsequent 2 years, following the return of circulating GAD-autoreactive CD4 T-cells (Fig. 1C and D), and were undetectable 3 years after treatment (Fig. 1C).
Patient 2 also had detectable C-peptide (Fig. 2C) and residual β-cells at biopsy (Fig. 2B). The patient received daclizumab (1 mg/kg, ×2, 2 weeks apart), thymoglobulin (1 mg · kg−1 · day−1, ×5 days), and rituximab (375 mg/m2, ×1) after the first thymoglobulin dose. After treatment, HLA-A2–restricted IGRP-autoreactive CD8-T-cells were not detected on two separate occasions (Fig. 2C), while the patient was T-cell depleted. T-cell counts normalized 6 months after therapy and IGRP-autoreactive CD8 T-cells reappeared 1 year after treatment (Fig. 2C and D). Autoantibody levels fell after therapy but rebounded after the reappearance of IGRP-autoreactive CD8 T-cells (Fig. 2A). C-peptide persisted after therapy but declined after the reappearance of IGRP-autoreactive CD8 T-cells (Fig. 2C and D) and the subsequent elevation of autoantibody levels (Fig. 2A). The patient remained insulin dependent thereafter.
Patient 3 had no residual insulin secretion and received a second pancreas transplant from an HLA-A1/A29, B8/B44, DR2/DR3 donor. The first pancreas transplant was biopsied at retransplantation and demonstrated severe β-cell loss in the absence of pancreas rejection (Fig. 3A, inset). The immunosuppression for the second transplant consisted of daclizumab (1 mg/kg, ×2, 2 weeks apart), thymoglobulin (1 mg · kg−1 · day−1, ×5 days), rituximab (375 mg/m2, ×1), and plasmapheresis (pretransplant and every other day, ×3). Maintenance doses of tacrolimus, mycophenolate mofetil, and steroids were continued. GAD and ZnT8 autoantibodies were declining before retransplantation and continued to decline afterward (Fig. 3A). Serial measurements performed during a plasmapheresis course initiated at the time of the second pancreas transplant showed no effect on GAD autoantibodies, while ZnT8 autoantibody levels declined (supplemental Fig. S1). After the immunosuppression, GAD-autoreactive CD4 T-cells became undetectable (Fig. 3B and C). The second pancreas transplant restored insulin secretion (Fig. 3B) and the patient became normoglycemic. Fifteen months later, there was a mild rejection episode, documented by percutaneous biopsy, which resolved with steroids. The patient became hyperglycemic ∼1.5 years later. At biopsy, the second pancreas transplant appeared as a 1.5-cm calcified fibrotic remnant with evidence of both acute and chronic rejection (Fig. 3D), and donor-specific antibodies against the second donor were demonstrated (supplemental Table S5). Rejection was associated with reactivation of autoimmunity, as it was preceded by another steep elevation of GAD autoantibodies and to a lesser extent of ZnT8 autoantibodies (Fig. 3A). GAD-autoreactive CD4 T-cells reappeared as well and continued to be detected in multiple peripheral blood samples (Fig. 3B and C) and in peri-pancreatic lymph nodes (not shown) from the first pancreas transplant obtained at the time of the second pancreas transplant biopsy. The patient eventually lost stimulated C-peptide secretion 3 years after re-transplantation (Fig. 3B) and became insulin dependent.
In vitro functional assessment of autoreactive T-cells.
We studied GAD-specific CD4 T-cell clones from patients 1 and 3 (supplemental data and Fig. S2 and S3). For both patients, clones derived from before and after treatment samples displayed Th1 bias with predominant IFN-γ secretion (supplemental Tables S1 and S2). For patient 1, all clones expressed the V β 5.1 TCR chain, although the CDR3 sequence between pre- and post-treatment samples was not identical (Table S1). For patient 3, TCR analysis of several DRB1*0401-restricted clones from before and after treatment showed the same TCR V β 9 chain and identical CDR3 sequence (Table S2). For patient 2, we tested the specificity and cytotoxic effects of the IGRP-autoreactive CD8 T-cells obtained from the sample closest to the recurrence of hyperglycemia (sample no. 1, Fig. 2C and D) and found that they specifically targeted peptide-loaded cell lines (Fig. S4). Thus, the IGRP-autoreactive CD8 T-cells from patient 2 were functional and mediated antigen-specific cytotoxicity.
Assessing the pathogenicity of GAD-autoreactive CD4 T-cells using an in vivo transplant model.
We cotransplanted human islets from unrelated donors under the kidney capsule of immunodeficient mice together with patient-derived, tetramer-positive, fluorescence-activated cell sorting–purified, GAD-specific CD4 T-cells. In the first experiment, we studied a sample (no. 10, Fig. 1C and D) from patient 1, which yielded a very strong CD4 T-cell response to GAD. The grafts were examined after 1 week. We observed damaged islets and β-cell loss only in the mouse that received the GAD-specific CD4 T-cells (Fig. 4A) but not in the mice that received islet and control T-cells directed against the HA antigen (Fig. 4B) or islets alone (Fig. 4C). To assess specificity, we counted β- and α-cells in sections from the whole grafts. We observed β/α-cell ratios of 1.0 (islets alone; 1,025 β-cells and 951 α-cells counted), 1.7 (islet + control T-cells; 582 β-cells and 342 α-cells), and 0.6 (islets + GAD-autoreactive CD4 T-cells, 278 β-cells and 446 α-cells). While the ratios observed for the control grafts are close to that reported in human islets (28,29), the ratio observed in the graft with autoreactive T-cells suggests preferential β-cell loss. We could not identify expression of MCP-1, IP-10, IFN-α, Fas, and caspase-3 in the transplanted islet grafts (not shown).
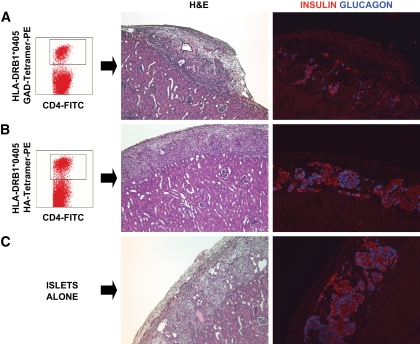
FIG. 4.
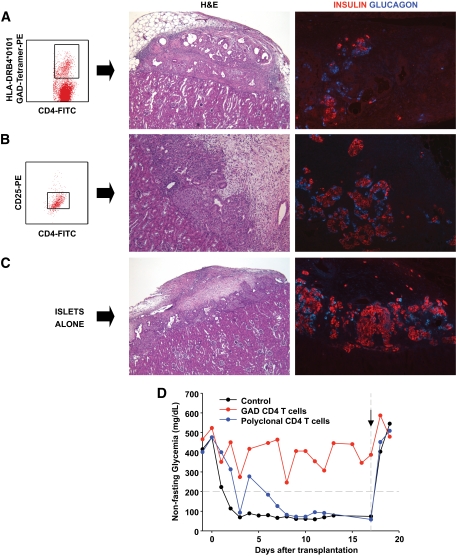
In vivo assessment of the autoreactive potential of GAD-autoreactive CD4 T-cells from patient 1. A peripheral blood sample (sample no. 10, Fig. 1C and D) from patient 1 yielded a very strong CD4 T-cell response to GAD; ∼7% of the CD4 T-cells were GAD autoreactive after in vitro stimulation and stained specifically with the DRB1*0405-GAD 555–567 tetramer (A). Approximately 15,000 tetramer-positive, GAD-autoreactive CD4 T-cells were purified by fluorescence-activated cell sorting and cotransplanted with human islets (1,300 islet equivalents), freshly isolated from an unrelated, deceased donor [HLA-A30, A33, B42, B70, DR8, DR17(3)], under the kidney capsule of a nondiabetic immunodeficient mouse. Control mice received islets with 15,000 CD4 T-cells from the same patient, which were stimulated with the HA control peptide and sorted after staining with a DRB1*0405-HA tetramer (B) or islets alone (C). H&E and insulin and glucagon stains representing the same areas of the graft reveal damaged islets and loss of insulin staining in the graft that received GAD-specific CD4 T-cells (A). Normal graft morphology and hormone staining patterns are seen in control mice receiving HA-specific CD4 T-cells (B) or islets alone (C). (A high-quality digital representation of this figure is available in the online issue.)
In the second experiment, autoreactive T-cells from patient 3 were cotransplanted with islets in immunodeficient mice that had been previously rendered diabetic with streptozotocin. Grafts were retrieved after ∼2 weeks. Once again, we observed severe islet damage and β-cell loss only in the graft receiving the GAD-reactive CD4 T-cells (Fig. 5A) and not in the control mice (Fig. 5B and C) receiving islets alone or islets together with activated CD4 T-cells that did not respond to the OspA control peptide, in essence representing a polyclonal population. We observed β/α-cell ratios of 1.3 (islet alone, 415 β-cells and 318 α-cells), 2.0 (islet + control T-cells, 294 β-cells and 145 α-cells), and 0.7 (islet + GAD-autoreactive CD4 T-cells, 43 β-cells and 61 α-cells), again suggesting preferential β-cell loss. Expression of MCP-1, IP-10, IFN-α, and Fas was not observed in the retrieved islet grafts (not shown). However, we identified rare β-cells expressing the active form of caspase-3, indicating apoptosis, in the graft that received GAD T-cells from patient 3 (Fig. S37). Caspase-3–expressing β-cells were not observed in the control grafts (not shown). Both control mice reversed their diabetes after islet transplantation, while the mouse that received the GAD autoreactive CD4 T-cells remained hyperglycemic for the entire duration of the experiment (Fig. 5D).
FIG. 5.
In vivo assessment of the autoreactive potential of GAD-autoreactive CD4 T-cells from patient 3. Immunodeficient mice with streptozotocin-induced diabetes were transplanted with human islets (2,000 islets equivalents) from an HLA-A1, A29, B8, B44, DR17(3)/DRX donor, which had been cultured for 24 h, and T-cells purified from blood obtained from patient 3 at 9.5 years of follow-up (sample no. 6, Fig. 3B). Specifically, we purified and cotransplanted islets with 6,000 tetramer-positive, GAD-autoreactive CD4 T-cells (6% of the CD4 T-cells, A). Control diabetic mice received islets and 30,000 CD4 T-cells after stimulation with the OspA control peptide (B) or islets alone (C). There was no response to this negative control peptide. Thus, this was a polyclonal T-cell population, which could have included alloreactive T-cells. CD25 staining confirmed that these T-cells had been activated in vitro (B). On metabolic follow-up (D), both control mice reversed their diabetes after islet transplantation, while the mouse that received the GAD-autoreactive CD4 T-cells remained hyperglycemic for the entire duration of the experiment. Control mice reverted to diabetes when the grafts were removed by nephrectomy (arrow) after ∼2 weeks. H&E stains from the islet-only control mouse showed mostly normal islets with some fibrosis in the surrounding tissue, as sometimes observed in these grafts (C). Insulin and glucagon stains in the same graft area revealed normal immunoreactivity for the islet-only control (C) and in the control mouse that received polyclonal, activated CD4 T-cells (B). However, H&E-stained sections revealed more fibrosis and inflammation, which could have been in part mediated by the polyclonal T-cells. H&E staining revealed severe islet destruction in the graft of the mouse transplanted with GAD-autoreactive CD4 T-cells, and hormone stains highlighted β-cell loss (A). (A high-quality digital representation of this figure is available in the online issue.)
In both experiments, there was no evidence of human lymphocytes or antigen-presenting cells persisting in the graft after 1–2 weeks by CD3 and CD68 staining (not shown), respectively. We also analyzed spleen, pancreas, lymph nodes (pancreatic and inguinal), and blood from the transplanted mice for the presence of human T-cells. These were undetectable by flow cytometry analysis and by CD3 staining of pancreas tissue sections (not shown). Because a very small number of T-cells were transplanted, surviving cells could have been diluted below current detection limits. After prolonged culture and in vitro activation, transplanted lymphocytes were likely to die in the absence of further stimuli and growth factors.
DISCUSSION
Our findings demonstrate the cardinal features of disease recurrence in immunosuppressed SPK recipients, in the absence of rejection, either clinically or on biopsy: 1) hyperglycemia without functional impairment of the exocrine pancreas or kidney allografts, associated with selective loss of insulin secretion; 2) insulitis and/or β-cell loss at biopsy; 3) persistence or reappearance of autoantibodies preceding diabetes recurrence; and 4) presence of circulating autoreactive CD4 or CD8 T-cells, assessed with autoantigen-specific tetramer assays, around the time of diabetes recurrence and on further follow-up. Specifically, we demonstrated GAD-autoreactive CD4 T-cells in the circulation of patients 1 and 3. Of note, patient 3 also harbored such cells in the local lymph node of the first pancreas transplant, which further suggests involvement in the autoimmune process. Patient 2 had IGRP-autoreactive CD8 T-cells. Thus, markers of islet autoimmunity as used in our study can help in diagnosing disease recurrence. Although measures of T-cell reactivity derived from stimulated in vitro responses are semiquantitative and response levels fluctuate, there often were consistent responses at multiple time points that reflected autoimmunity and the impact of immunotherapy. Thus, tetramer-based assays can be informative in the context of intervention studies. Unpublished data from ongoing studies in our cohort of SPK recipients suggest that autoantibody conversion increases the risk of developing recurrent type 1 diabetes (odds ratio 15.33, P < 0.000002, comparing to autoantibody negative in an analysis of 200 SPK recipients); preliminary studies suggest that autoreactive T-cells tend to be roughly twice as frequent in SPK recipients with recurrent type 1 diabetes compared with normoglycemic recipients, noting that most normoglycemic recipients with autoreactive T-cells were also autoantibody positive. Further studies will help determine whether assessment of autoreactive T-cells improves autoantibody-based prediction.
Autoantibodies preceded hyperglycemia by several years in patients 1 and 2, suggesting that the recurrent autoimmunity in immunosuppressed patients may progress slower than in nonimmunosuppressed recipients of HLA-matched living-related donors, who developed disease recurrence within weeks of transplant (11,12,14,30). A slower course was also reported in the two other immunosuppressed patients with disease recurrence reported in the literature (20). However, patient 3 developed hyperglycemia within a few months from the autoantibody conversion, which involved the almost simultaneous appearance of very high titers of ZnT8 and GAD autoantibodies. Overall, autoantibodies were good markers of autoimmunity, showing conversions or level fluctuations that reflected clinical course and response to treatment.
Using HLA class I pentamers and class II tetramers, respectively, we purified IGRP-specific CD8 T-cells and GAD-specific autoreactive CD4 T-cells for phenotypic and functional studies. The IGRP-specific autoreactive CD8 T-cells identified in patient 2 recognize the same epitope described in patients with new-onset type 1 diabetes and at-risk subjects (7,8). These cells had antigen-specific cytotoxic effects, which further implicate an IGRP-responsive CD8 T-cell population in disease recurrence. Associations of type 1 diabetes recurrence with autoreactive CD8 T-cells (31,32) and autoantibodies (33) are also emerging in islet transplant recipients. A role for memory phenotypes is suggested in both spontaneous disease (34,35) and its recurrence in islet transplant recipients (36). Our preliminary analysis of TCR clonotypes in patients 1 and 3 (37) and the additional data presented here (Tables S1 and S2) reveal that GAD-autoreactive CD4 T-cells expressing identical or similar V β chains (5.1 and 9) and CDR3 sequences reappeared after immunosuppression in patients 1 and 3. The usage of V β 5.1 is common by GAD-autoreactive, HLA-DRB1*04-restricted CD4 T-cells in patients who respond to the same GAD 555–567 peptide (22,23). The persistence of autoreactive T-cells against the same autoantigen, expressing the same or closely related TCR V β chains, is consistent with a memory response associated with recurrent autoimmunity in our SPK recipients. The activation and memory status could not be assessed ex vivo, since these autoreactive T-cells were identified after in vitro culture and stimulation.
We took advantage of the simultaneous availability of sizable populations of highly purified, GAD-autoreactive CD4 T-cells from our patients and of human islets from unrelated donors to test the pathogenic effects of the autoreactive lymphocytes in vivo using a cotransplantation model into immunodeficient mice. In the first experiment, we transplanted highly purified CD4 T-cells with single autoantigen specificity, reacting against GAD or the HA control antigen derived from patient 1. We observed preferential β-cell loss in the mice receiving the GAD-specific T-cells, but not in the mice receiving HA-specific, control T-cells. We had a similar outcome in the second experiment, in which the control T-cells were a polyclonal population that had not responded to the OspA antigen, as expected in most subjects. Of note, some loss of α-cells is reported in mouse models during the development of autoimmune diabetes (38) and the GAD autoantigen is also expressed by α-cells in human islets (39). Importantly, the pathology observed is consistent with the metabolic follow-up data of the transplanted mice. We interpret these findings as collectively showing that GAD-specific CD4 T-cells from patients 1 and 3 directly mediated significant β-cell damage in our in vivo transplantation model.
Because of their extemporaneous nature, these experiments offer limited information about the mechanisms involved in the observed β-cell destruction, but some speculations are possible. The transplanted CD4 T-cells were already activated in vitro, as shown by CD25 expression. In addition, human CD4 T-cells can present antigen to each other through their HLA class II molecules, which could help with maintaining an activated status (40). Whereas we did not have fresh antigen-presenting cells from these patients at the time of the experiments, we cannot exclude that residual antigen-presenting cells contained in the purified T-cell inoculum could have contributed to T-cell activation. Moreover, antigen-presenting cells resident in the islets could have presented GAD peptides but not HA antigen, which could help explain why only the GAD-autoreactive T-cells mediated significant damage. Presentation could occur despite HLA mismatch, similar to the direct presentation pathway described in transplantation (41,42). Accordingly, the Gill group reported that MHC-mismatched islet allografts are vulnerable to autoimmune destruction by autoreactive CD4 T-cells, using a mouse model but not testing human cells (41). In these experiments, we transplanted primary autoreactive CD4 T-cells immediately after purification. Thus, we could not assess their specific cytokine profiles. However, the autoreactive CD4 T-cell clones reported here for patients 1 and 3 predominantly produced IFN-γ and TNF-α upon antigen-specific stimulation. Increased β-cell sensitivity to cytokines compared with other islet cell types may partially explain the preferential β-cell loss observed (43). The finding that significant and preferential β-cell destruction was seen only in the mice transplanted with the GAD-specific T-cells is further evidence that β-cell destruction was mediated by an autoimmune rather than allogeneic response and did not result from nonspecific inflammation caused by T-cells responding to a control antigen. Although limited, these data offer an experimental assessment of human autoreactive T-cells that is rarely feasible in clinical studies. By using a model that recapitulates the transplant setting, the data support the concept that autoreactive T-cells played a role in the recurrent autoimmunity observed in our patients.
We treated recurrent autoimmunity to salvage residual β-cells in patients 1 and 2. The third patient had no residual C-peptide and was re-transplanted. As patients were diagnosed at different times, we used a stepwise approach based on the experience from each patient and tested progressively more sophisticated immunosuppressive therapies to target T-cells, B-cells, and autoantibodies (thymoglobulin, daclizumab, rituximab, and plasmapheresis). C-peptide secretion was maintained for up to 3 years in patient 1 and patient 2, although both remained insulin-dependent throughout the follow-up. Treatment depleted T-cells nonspecifically, including the autoreactive ones. However, autoreactive T-cells reappeared ∼1 year after treatment. Their reappearance was followed by a progressive decline in C-peptide levels. Both patient 1 and 2 had rising autoantibodies mirroring the reappearance of autoreactive T-cells. At the time of re-transplantation, patient 3 received immunosuppression and plasmapheresis. Although GAD and ZnT8 autoantibody levels were declining in patient 3 before re-transplantation, plasmapheresis did not affect GAD autoantibodies but lowered ZnT8 autoantibodies. This finding is consistent with earlier studies showing that plasmapheresis lowers islet cell antibodies other than GAD autoantibodies in patients with newly diagnosed type 1 diabetes (44). Thus, ZnT8 autoantibodies may be responsive to plasmapheresis. Patient 3 eventually rejected the second transplant, but also experienced reactivation of islet autoimmunity marked by the re-expression of ZnT8 and GAD autoantibodies and the return of circulating GAD-autoreactive CD4 T-cells with the same TCR clonotype observed when disease recurred in the first pancreas transplant. The treatment with T-cell– and B-cell–directed immunosuppression affected autoimmunity temporarily and nonspecifically.
While limited to three patients, our clinical experience is consistent with results from clinical trials in recent-onset type 1 diabetes, as temporary persistence of C-peptide secretion is followed by eventual decline after treatment with immunosuppressive agents (45–47). Our study provides an assessment of autoreactive T-cells and autoantibodies in relation to immunosuppressive therapy for islet autoimmunity, noting that rituximab and thymoglobulin are currently being tested in clinical trials of new-onset patients (48) (www.diabetestrialnet.org). Our intensive monitoring suggests that nonspecific immunosuppression may not achieve long-lasting inhibition of islet autoimmunity. Future combinatorial regimens could attempt to tolerize newly emerging T-cells and memory cells during post-depletion recovery (49,50).
Supplementary Material
ACKNOWLEDGMENTS
These studies were supported by grants from the National Institutes of Health (5RO1-DK-070011 and AI-50864), the American Diabetes Association (1-09-RA-413, 1-05-RA-105), the Juvenile Diabetes Research Foundation (JDRF 1-2005-257), and the Diabetes Research Institute Foundation, Hollywood, FL. F.V. was supported by a JDRF Postdoctoral Research Fellowship (3-2008-32). We acknowledge support from the Diabetes Research Institute Cell Transplant Center and the Islet Cell Resource Center (funded by National Center for Research Resources grant 3U42RR016603-06S1 and M01RR16587), the University of Miami Analytical Imaging Core Facility (Dr. George McNamara) and the Histology Core (Kevin Johnson), partially supported by JDRF Center Grant 4-2008-811; the Cell Transplant Center Preclinical Cell Processing and Translational Laboratory (Elsie Zahr, Judith Molina, Yelena Gadea, and Irayme Labrada), both partially funded by JDRF Center Grant JDRF-4-2004-361; and the Sylvester Cancer Center Flow Cytometry Core (Jim Phillips).
No potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in abstract form at the 64th Scientific Sessions of the American Diabetes Association, Orlando, Florida, 4–8 June 2004, and Immunology of Diabetes Society/American Diabetes Association joint meetings, Miami, Florida, 14–18 November 2007.
We are indebted to members of the University of Miami Transplant Program: Dr. Robert Cirocco, Lissett Tueros, Lois Hanson, Anne Rosen, Sandra Flores, Felisa Flores, Folasade Amole, Larisa Riveron, Carmen Gomez, and Dayami Rodriguez.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Eisenbarth GS: Type I diabetes mellitus: a chronic autoimmune disease. N Engl J Med 1986; 314: 1360– 1368 [DOI] [PubMed] [Google Scholar]
- 2.Lieberman SM, DiLorenzo TP: A comprehensive guide to antibody and T-cell responses in type 1 diabetes. Tissue Antigens 2003; 62: 359– 377 [DOI] [PubMed] [Google Scholar]
- 3.Wenzlau JM, Juhl K, Yu L, Moua O, Sarkar SA, Gottlieb P, Rewers M, Eisenbarth GS, Jensen J, Davidson HW, Hutton JC: The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci U S A 2007; 104: 17040– 17045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Lorenzo TP, Peakman M, Roep BO: Translational mini-review series on type 1 diabetes: systematic analysis of T cell epitopes in autoimmune diabetes. Clin Exp Immunol 2007; 148: 1– 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarchum I, Nichol L, Trucco M, Santamaria P, DiLorenzo TP: Identification of novel IGRP epitopes targeted in type 1 diabetes patients. Clin Immunol 2008; 127: 359– 365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinuzzi E, Novelli G, Scotto M, Blancou P, Bach JM, Chaillous L, Bruno G, Chatenoud L, van Endert P, Mallone R: The frequency and immunodominance of islet-specific CD8+ T-cell responses change after type 1 diabetes diagnosis and treatment. Diabetes 2008; 57: 1312– 1320 [DOI] [PubMed] [Google Scholar]
- 7.Standifer NE, Ouyang Q, Panagiotopoulos C, Verchere CB, Tan R, Greenbaum CJ, Pihoker C, Nepom GT: Identification of novel HLA-A*0201-restricted epitopes in recent-onset type 1 diabetic subjects and antibody-positive relatives. Diabetes 2006; 55: 3061– 3067 [DOI] [PubMed] [Google Scholar]
- 8.Ouyang Q, Standifer NE, Qin H, Gottlieb P, Verchere CB, Nepom GT, Tan R, Panagiotopoulos C: Recognition of HLA class I-restricted beta-cell epitopes in type 1 diabetes. Diabetes 2006; 55: 3068– 3074 [DOI] [PubMed] [Google Scholar]
- 9.White SA, Shaw JA, Sutherland DE: Pancreas transplantation. Lancet 2009; 373: 1808– 1817 [DOI] [PubMed] [Google Scholar]
- 10.Sutherland DE, Sibley R, Xu XZ, Michael A, Srikanta AM, Taub F, Najarian J, Goetz FC: Twin-to-twin pancreas transplantation: reversal and reenactment of the pathogenesis of type I diabetes. Trans Assoc Am Physicians 1984; 97: 80– 87 [PubMed] [Google Scholar]
- 11.Sibley RK, Sutherland DE, Goetz F, Michael AF: Recurrent diabetes mellitus in the pancreas iso- and allograft: a light and electron microscopic and immunohistochemical analysis of four cases. Lab Invest 1985; 53: 132– 144 [PubMed] [Google Scholar]
- 12.Sutherland DE, Goetz FC, Sibley RK: Recurrence of disease in pancreas transplants. Diabetes 1989; 38( Suppl. 1): 85– 87 [DOI] [PubMed] [Google Scholar]
- 13.Santamaria P, Nakhleh RE, Sutherland DE, Barbosa JJ: Characterization of T lymphocytes infiltrating human pancreas allograft affected by isletitis and recurrent diabetes. Diabetes 1992; 41: 53– 61 [DOI] [PubMed] [Google Scholar]
- 14.Sibley RK, Sutherland DE: Pancreas transplantation: an immunohistologic and histopathologic examination of 100 grafts. Am J Pathol 1987; 128: 151– 170 [PMC free article] [PubMed] [Google Scholar]
- 15.Bosi E, Bottazzo GF, Secchi A, Pozza G, Shattock M, Saunders A, Gelet A, Touraine JL, Traeger J, Dubernard JM: Islet cell autoimmunity in type I diabetic patients after HLA-mismatched pancreas transplantation. Diabetes 1989; 38( Suppl. 1): 82– 84 [DOI] [PubMed] [Google Scholar]
- 16.Esmatjes E, Rodríguez-Villar C, Ricart MJ, Casamitjana R, Martorell J, Sabater L, Astudillo E, Fernández-Cruz L: Recurrence of immunological markers for type 1 (insulin-dependent) diabetes mellitus in immunosuppressed patients after pancreas transplantation. Transplantation 1998; 66: 128– 131 [DOI] [PubMed] [Google Scholar]
- 17.Petruzzo P, Andreelli F, McGregor B, Lefrançois N, Dawahra M, Feitosa LC, Dubernard JM, Thivolet C, Martin X: Evidence of recurrent type I diabetes following HLA-mismatched pancreas transplantation. Diabetes Metab 2000; 26: 215– 218 [PubMed] [Google Scholar]
- 18.Thivolet C, Abou-Amara S, Martin X, Lefrancois N, Petruzzo P, McGregor B, Bosshard S, Dubernard JM: Serological markers of recurrent beta cell destruction in diabetic patients undergoing pancreatic transplantation. Transplantation 2000; 69: 99– 103 [DOI] [PubMed] [Google Scholar]
- 19.Braghi S, Bonifacio E, Secchi A, Di Carlo V, Pozza G, Bosi E: Modulation of humoral islet autoimmunity by pancreas allotransplantation influences allograft outcome in patients with type 1 diabetes. Diabetes 2000; 49: 218– 224 [DOI] [PubMed] [Google Scholar]
- 20.Tydén G, Reinholt FP, Sundkvist G, Bolinder J: Recurrence of autoimmune diabetes mellitus in recipients of cadaveric pancreatic grafts. N Engl J Med 1996; 335: 860– 863 [DOI] [PubMed] [Google Scholar]
- 21.Bingley PJ, Bonifacio E, Mueller PW: Diabetes antibody standardization program: first assay proficiency evaluation. Diabetes 2003; 52: 1128– 1136 [DOI] [PubMed] [Google Scholar]
- 22.Reijonen H, Novak EJ, Kochik S, Heninger A, Liu AW, Kwok WW, Nepom GT: Detection of GAD65-specific T-cells by major histocompatibility complex class II tetramers in type 1 diabetic patients and at-risk subjects. Diabetes 2002; 51: 1375– 1382 [DOI] [PubMed] [Google Scholar]
- 23.Reijonen H, Mallone R, Heninger AK, Laughlin EM, Kochik SA, Falk B, Kwok WW, Greenbaum C, Nepom GT: GAD65-specific CD4+ T-cells with high antigen avidity are prevalent in peripheral blood of patients with type 1 diabetes. Diabetes 2004; 53: 1987– 1994 [DOI] [PubMed] [Google Scholar]
- 24.Pileggi A, Molano RD, Berney T, Cattan P, Vizzardelli C, Oliver R, Fraker C, Ricordi C, Pastori RL, Bach FH, Inverardi L: Heme oxygenase-1 induction in islet cells results in protection from apoptosis and improved in vivo function after transplantation. Diabetes 2001; 50: 1983– 1991 [DOI] [PubMed] [Google Scholar]
- 25.Martin-Pagola A, Sisino G, Allende G, Dominguez-Bendala J, Gianani R, Reijonen H, Nepom GT, Ricordi C, Ruiz P, Sageshima J, Ciancio G, Burke GW, Pugliese A: Insulin protein and proliferation in ductal cells in the transplanted pancreas of patients with type 1 diabetes and recurrence of autoimmunity. Diabetologia 2008; 51: 1803– 1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dotta F, Censini S, van Halteren AG, Marselli L, Masini M, Dionisi S, Mosca F, Boggi U, Muda AO, Prato SD, Elliott JF, Covacci A, Rappuoli R, Roep BO, Marchetti P: Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc Natl Acad Sci U S A 2007; 104: 5115– 5120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG: The prevalence of enteroviral capsid protein vp1 immunostaining in pancreatic islets in human type 1 diabetes. Diabetologia 2009; 52: 1143– 1151 [DOI] [PubMed] [Google Scholar]
- 28.Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A: The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A 2006; 103: 2334– 2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brissova M, Fowler MJ, Nicholson WE, Chu A, Hirshberg B, Harlan DM, Powers AC: Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem 2005; 53: 1087– 1097 [DOI] [PubMed] [Google Scholar]
- 30.Sutherland DE, Sibley R, Xu XZ, Michael A, Srikanta AM, Taub F, Najarian J, Goetz FC: Twin-to-twin pancreas transplantation: reversal and reenactment of the pathogenesis of type I diabetes. Trans Assoc Am Physicians 1984; 97: 80– 87 [PubMed] [Google Scholar]
- 31.Huurman VA, Hilbrands R, Pinkse GG, Gillard P, Duinkerken G, van de LP, van der Meer-Prins PM, Versteeg-van der Voort Maarschalk MF, Verbeeck K, Alizadeh BZ, Mathieu C, Gorus FK, Roelen DL, Claas FH, Keymeulen B, Pipeleers DG, Roep BO: Cellular islet autoimmunity associates with clinical outcome of islet cell transplantation. PLoS One 2008; 3: e2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinkse GG, Tysma OH, Bergen CA, Kester MG, Ossendorp F, van Veelen PA, Keymeulen B, Pipeleers D, Drijfhout JW, Roep BO: Autoreactive CD8 T cells associated with beta cell destruction in type 1 diabetes. Proc Natl Acad Sci U S A 2005; 102: 18425– 18430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, Alejandro R, Ryan EA, DiMercurio B, Morel P, Polonsky KS, Reems JA, Bretzel RG, Bertuzzi F, Froud T, Kandaswamy R, Sutherland DE, Eisenbarth G, Segal M, Preiksaitis J, Korbutt GS, Barton FB, Viviano L, Seyfert-Margolis V, Bluestone J, Lakey JR: International trial of the Edmonton protocol for islet transplantation. N Engl J Med 2006; 355: 1318– 1330 [DOI] [PubMed] [Google Scholar]
- 34.Monti P, Scirpoli M, Rigamonti A, Mayr A, Jaeger A, Bonfanti R, Chiumello G, Ziegler AG, Bonifacio E: Evidence for in vivo primed and expanded autoreactive T cells as a specific feature of patients with type 1 diabetes. J Immunol 2007; 179: 5785– 5792 [DOI] [PubMed] [Google Scholar]
- 35.Waid DM, Wagner RJ, Putnam A, Vaitaitis GM, Pennock ND, Calverley DC, Gottlieb P, Wagner DH, Jr: A unique T cell subset described as CD4loCD40+ T cells (TCD40) in human type 1 diabetes. Clin Immunol 2007; 124: 138– 148 [DOI] [PubMed] [Google Scholar]
- 36.Monti P, Scirpoli M, Maffi P, Ghidoli N, De TF, Bertuzzi F, Piemonti L, Falcone M, Secchi A, Bonifacio E: Islet transplantation in patients with autoimmune diabetes induces homeostatic cytokines that expand autoreactive memory T cells. J Clin Invest 2008; 118: 1806– 1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laughlin E, Burke G, Pugliese A, Falk B, Nepom G: Recurrence of autoreactive antigen-specific CD4+ T cells in autoimmune diabetes after pancreas transplantation. Clin Immunol 2008; 128: 23– 30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pechhold K, Zhu X, Harrison VS, Lee J, Chakrabarty S, Koczwara K, Gavrilova O, Harlan DM: Dynamic changes in pancreatic endocrine cell abundance, distribution, and function in antigen-induced and spontaneous autoimmune diabetes. Diabetes 2009; 58: 1175– 1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mally MI, Cirulli V, Otonkoski T, Soto G, Hayek A: Ontogeny and tissue distribution of human GAD expression. Diabetes 1996; 45: 496– 501 [DOI] [PubMed] [Google Scholar]
- 40.Holling TM, Schooten E, van Den Elsen PJ: Function and regulation of MHC class II molecules in T-lymphocytes: of mice and men. Hum Immunol 2004; 65: 282– 290 [DOI] [PubMed] [Google Scholar]
- 41.Kupfer TM, Crawford ML, Pham K, Gill RG: MHC-mismatched islet allografts are vulnerable to autoimmune recognition in vivo. J Immunol 2005; 175: 2309– 2316 [DOI] [PubMed] [Google Scholar]
- 42.Afzali B, Lechler RI, Hernandez-Fuentes MP: Allorecognition and the alloresponse: clinical implications. Tissue Antigens 2007; 69: 545– 556 [DOI] [PubMed] [Google Scholar]
- 43.Rabinovitch A: Immunoregulation by cytokines in autoimmune diabetes. Adv Exp Med Biol 2003; 520: 159– 193 [DOI] [PubMed] [Google Scholar]
- 44.Sundkvist G, Hagopian WA, Landin-Olsson M, Lernmark A, Ohlsson L, Ericsson C, Ahlmén J: Islet cell antibodies, but not glutamic acid decarboxylase antibodies, are decreased by plasmapheresis in patients with newly diagnosed insulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1994; 78: 1159– 1165 [DOI] [PubMed] [Google Scholar]
- 45.Herold KC, Gitelman SE, Masharani U, Hagopian W, Bisikirska B, Donaldson D, Rother K, Diamond B, Harlan DM, Bluestone JA: A single course of anti-CD3 monoclonal antibody hOKT3gamma1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes 2005; 54: 1763– 1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, Gitelman SE, Harlan DM, Xu D, Zivin RA, Bluestone JA: Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med 2002; 346: 1692– 1698 [DOI] [PubMed] [Google Scholar]
- 47.Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, Gorus F, Goldman M, Walter M, Candon S, Schandene L, Crenier L, De Block C, Seigneurin JM, De Pauw P, Pierard D, Weets I, Rebello P, Bird P, Berrie E, Frewin M, Waldmann H, Bach JF, Pipeleers D, Chatenoud L: Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med 2005; 352: 2598– 2608 [DOI] [PubMed] [Google Scholar]
- 48.Staeva-Vieira T, Peakman M, von Herrath M: Translational mini-review series on type 1 diabetes: immune-based therapeutic approaches for type 1 diabetes. Clin Exp Immunol 2007; 148: 17– 31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bresson D, Togher L, Rodrigo E, Chen Y, Bluestone JA, Herold KC, von Herrath M: Anti-CD3 and nasal proinsulin combination therapy enhances remission from recent-onset autoimmune diabetes by inducing Tregs. J Clin Invest 2006; 116: 1371– 1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.von Herrath M: Can we learn from viruses how to prevent type 1 diabetes? The role of viral infections in the pathogenesis of type 1 diabetes and the development of novel combination therapies. Diabetes 2009; 58: 2– 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.